A New Class of Chiral Polyethers and Polyesters Based on the [2.2]Paracyclophane Scaffold
Abstract
1. Introduction
2. Materials and Methods
2.1. General Remarks
2.1.1. Reaction Monitoring
2.1.2. Melting Point
2.1.3. Optical Rotation
2.1.4. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.1.5. Infrared Spectroscopy (IR)
2.1.6. Mass Spectrometry (MS)
2.1.7. Gel Permeation Chromatography (GPC)
2.2. Synthetic Procedures
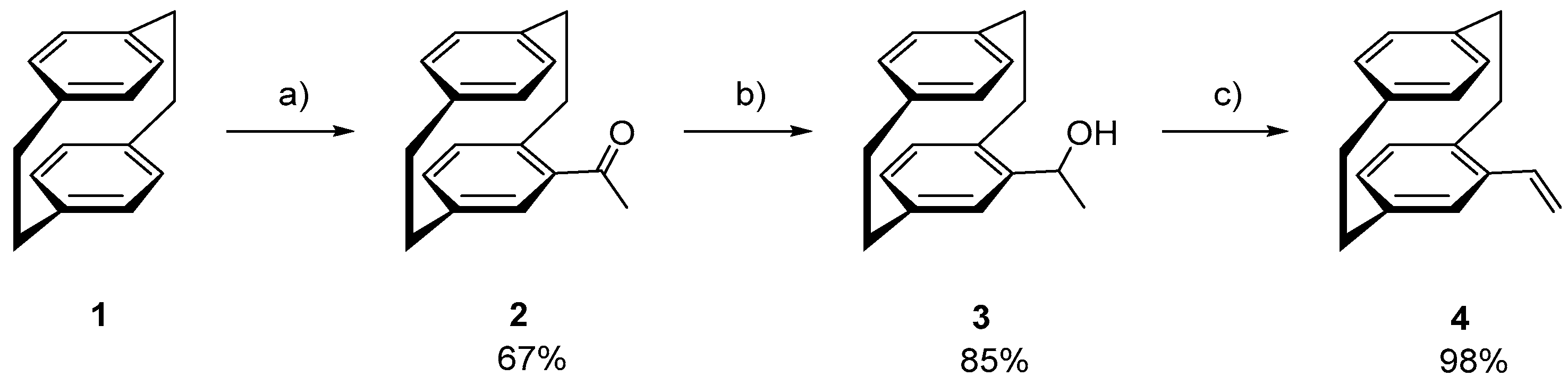
- (rac)-4-Acetyl [2.2]paracyclophane (2) [15]
- 1H NMR (400 MHz, CDCl3 [7.26 ppm], ppm) δ = 6.93 (d, J = 1.9 Hz, 1H), 6.66 (dd, J = 7.9, 1.9 Hz, 1H), 6.58–6.45 (m, 4 H), 6.38 (dd, J = 7.9, 1.9 Hz, 1H), 4.05–3.90 (m, 1H), 3.26–3.10 (m, 4 H), 3.09–2.96 (m, 2 H), 2.90–2.78 (m, 1H), 2.47 (s, 3H).
- 13C{1H} NMR (101 MHz, CDCl3 [77.16 ppm], ppm) δ = 200.4 (Cq), 141.7 (Cq), 140.4 (Cq), 139.9 (Cq), 139.3 (Cq), 138.0 (Cq), 136.5 (+, CH), 136.5 (+, CH), 134.3 (+, CH), 133.2 (+, CH), 133.0 (+, CH), 132.2 (+, CH), 131.3 (+, CH), 36.2 (−, CH2), 35.3 (−, CH2), 35.3 (−, CH2), 35.0 (−, CH2), 28.9 (+, CH3).
- MS (EI, 70 eV, 30 °C), m/z (%): 250 (89) [M]+, 146 (100), 145 (62), 131 (16), 105 (26), 104 (77), 103 (31), 78 (18), 77 (18).
- HRMS–EI (m/z): [M]+ calcd for C18H18O 250.1352; found 250.1351.
- IR (ATR, ṽ) = 3029 (w), 3007 (w), 2985 (w), 2951 (w), 2921 (m), 2887 (w), 2849 (w), 2776 (w), 2766 (w), 1679 (vs), 1643 (w), 1589 (w), 1551 (w), 1494 (w), 1483 (w), 1446 (w), 1431 (w), 1409 (w), 1349 (s), 1320 (w), 1296 (w), 1265 (vs), 1239 (m), 1203 (m), 1184 (m), 1176 (s), 1162 (m), 1126 (w), 1092 (w), 1065 (w), 1018 (w), 983 (w), 952 (m), 941 (w), 918 (w), 901 (s), 853 (vs), 805 (m), 792 (s), 730 (s), 710 (s), 686 (w), 647 (s), 615 (vs), 599 (s), 562 (w), 534 (w), 510 (vs), 493 (m), 458 (w), 431 (w), 419 (w), 395 (m), 384 (w) cm−1.
- (rac)/(Sp)-1-(4-[2.2]Paracyclophanyl)ethanol (3) [15]
- 1H NMR (400 MHz, CDCl3 [7.26 ppm], ppm) δ = 6.66–6.59 (m, 1H), 6.57–6.33 (m, 6 H), 4.94 (q, J = 6.4 Hz, 0.5H), 4.87 (q, J = 6.5 Hz, 0.5H), 3.66 (ddd, J = 13.0, 10.0, 2.5 Hz, 0.5H), 3.34 (ddd, J = 13.6, 10.0, 2.1 Hz, 0.5H), 3.22–2.97 (m, 6 H), 2.96–2.78 (m, 1H), 1.76 (br, s, 0.5H), 1.6 (d, J = 6.5 Hz, 1.5H), 1.42 (br, s, 0.5H), 1.3 (d, J = 6.4 Hz, 1.5H).
- 13C{1H} NMR (101 MHz, CDCl3 [77.16 ppm], ppm) δ = 144.9 (Cq), 142.3 (Cq), 140.5 (Cq), 140.4 (Cq), 139.8 (Cq), 139.6 (Cq), 139.6 (Cq), 139.5 (Cq), 138.7 (Cq), 135.8 (+, CH), 135.2 (+, CH), 135.0 (Cq), 133.8 (+, CH), 133.5 (+, CH), 133.2 (+, CH), 133.2 (+, CH), 132.7 (+, CH), 132.3 (+, CH), 132.1 (+, CH), 131.7 (+, CH), 130.1 (+, CH), 130.0 (+, CH), 129.8 (+, CH), 128.3 (+, CH), 68.1 (+, CH), 67.6 (+, CH), 35.4 (−, CH2), 35.4 (−, CH2), 35.4 (−, CH2), 35.3 (−, CH2), 34.9 (−, CH2), 34.5 (−, CH2), 33.5 (−, CH2), 33.3 (−, CH2), 25.9 (+, CH3), 21.3 (+, CH3).
- MS (EI, 70 eV, 50 °C), m/z (%): 252 (62) [M]+, 237 (31), 130 (100), 129 (67), 105 (92), 104 (64).
- HRMS–EI (m/z): [M]+ calcd for C18H20O 252.1509; found 252.1510.
- IR (ATR, ṽ) = 3332 (m), 3010 (w), 2983 (w), 2968 (w), 2945 (w), 2924 (m), 2890 (w), 2850 (w), 1592 (w), 1497 (w), 1485 (w), 1443 (w), 1412 (s), 1401 (m), 1367 (m), 1353 (w), 1290 (w), 1266 (w), 1224 (w), 1205 (w), 1183 (w), 1153 (w), 1146 (w), 1120 (w), 1077 (vs), 1060 (m), 1027 (vs), 956 (w), 935 (w), 901 (m), 887 (m), 850 (vs), 792 (m), 755 (w), 734 (w), 714 (vs), 681 (w), 653 (vs), 635 (s), 609 (vs), 565 (m), 526 (s), 504 (vs), 484 (vs), 460 (w), 435 (w), 422 (w), 399 (w), 384 (w) cm−1.
- (Sp)-4-Vinyl [2.2]paracyclophane (4) [15]
- 1H NMR (400 MHz, CDCl3 [7.26 ppm], ppm) δ = 6.82 (dd, J = 17.4, 10.9 Hz, 1H), 6.73 (dd, J = 7.8, 1.8 Hz, 1H), 6.58–6.47 (m, 4 H), 6.47–6.39 (m, 2 H), 5.56 (dd, J = 17.4, 1.4 Hz, 1H), 5.30 (dd, J = 10.9, 1.4 Hz, 1H), 3.50 (ddd, J = 13.6, 9.9, 1.9 Hz, 1H), 3.19–2.91 (m, 6 H), 2.82 (ddd, J = 13.5, 10.3, 6.7 Hz, 1H).
- 13C{1H} NMR (101 MHz, CDCl3 [77.16 ppm], ppm) δ = 139.9 (Cq), 139.5 (Cq), 139.5 (Cq), 138.1 (Cq), 137.9 (+, CH), 135.3 (+, CH), 134.9 (+, CH, CAr), 133.2 (+, CH, CAr, 2C), 132.1 (+, CH), 131.9 (+, CH), 130.3 (+, CH), 129.7 (+, CH), 114.4 (−, CH2), 35.6 (−, CH2), 35.3 (−, CH2), 34.8 (−, CH2), 33.8 (−, CH2).
- MS (EI, 70 eV, 30 °C), m/z (%): 234 (33) [M]+, 130 (49), 129 (100), 115 (28), 104 (20).
- HRMS–EI (m/z): [M]+ calcd for C18H18 234.1403; found 234.1404.
- IR (ATR, ṽ) = 3030 (w), 3007 (m), 2948 (w), 2925 (s), 2918 (s), 2890 (m), 2847 (s), 1619 (w), 1589 (w), 1497 (w), 1482 (m), 1434 (m), 1417 (m), 1409 (m), 1203 (w), 1183 (w), 986 (s), 938 (w), 905 (vs), 880 (w), 864 (vs), 810 (m), 793 (s), 748 (vs), 715 (vs), 688 (w), 670 (vs), 628 (s), 569 (m), 555 (m), 507 (vs), 460 (w), 439 (w), 422 (w), 387 (w) cm−1.
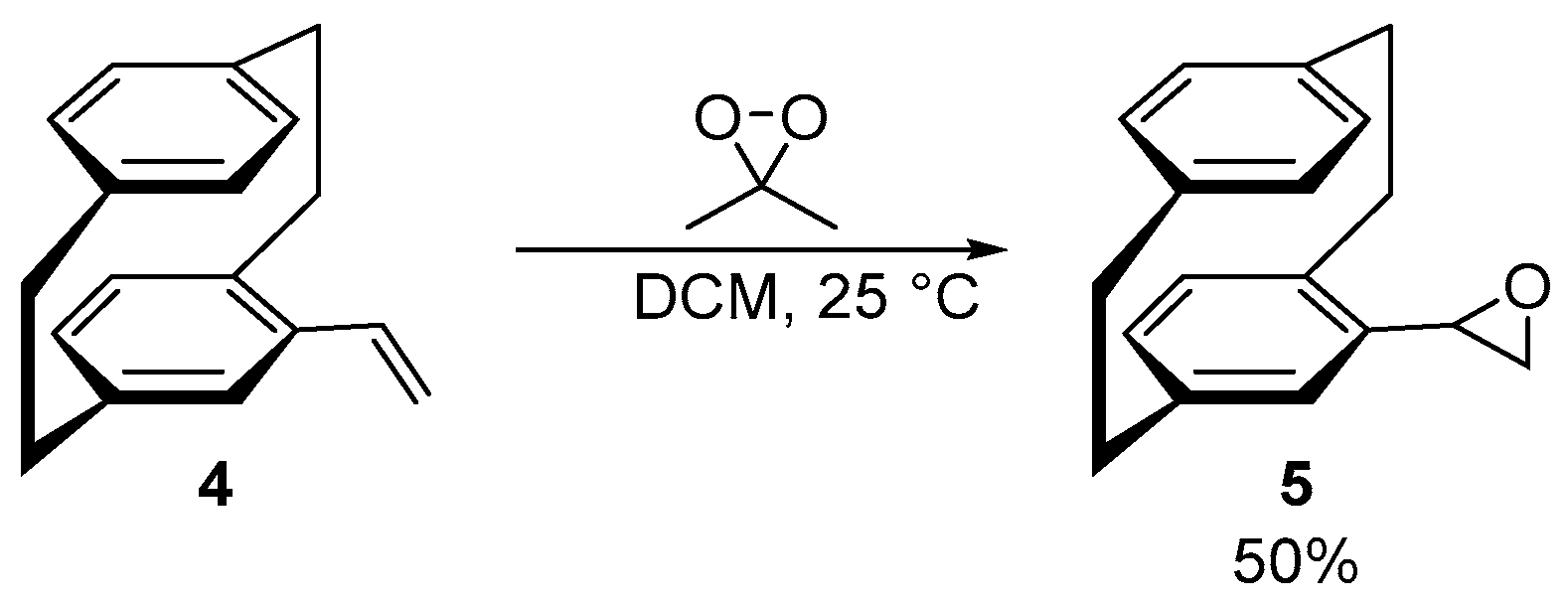
- (Sp)-(4-[2.2]Paracyclophanyl)oxirane (5) [16]
- 1H NMR (400 MHz, CDCl3 [7.26 ppm], ppm) δ = 6.81–6.75 (m, 0.5H), 6.62 (dd, J = 8.0, 1.8 Hz, 0.5H), 6.59–6.46 (m, 4 H), 6.44 (d, J = 6.4 Hz, 0.5H), 6.36 (dd, J = 7.8, 1.8 Hz, 0.5H), 6.28 (d, J = 1.9 Hz, 0.5H), 6.16 (d, J = 1.8 Hz, 0.5H), 3.93–3.83 (m, 1H), 3.53 (dddd, J = 25.0, 12.9, 10.0, 2.5 Hz, 1H), 3.24 (td, J = 4.9, 4.2, 3.1 Hz, 1H), 3.21–2.83 (m, 7.5H), 2.53 (dd, J = 5.7, 2.6 Hz, 0.5H).
- 13C{1H} NMR (101 MHz, CDCl3 [77.16 ppm], ppm) δ = 13C NMR (101 MHz, CDCl3 [77.16 ppm], ppm) δ = 140.7 (Cq, 0.5 C), 140.3 (Cq, 0.5 C), 139.7 (Cq, 0.5 C), 139.6 (Cq, 0.5 C), 139.5 (Cq, 0.5 C), 139.1 (Cq, 0.5 C), 139.0 (Cq, 0.5 C), 138.3 (Cq, 0.5 C), 136.9 (Cq, 0.5 C), 135.8 (Cq, 0.5 C), 135.2 (+, CH, 0.5 C), 134.8 (+, CH, 0.5 C), 133.4 (+, CH), 133.2 (+, CH, 0.5 C), 132.9 (+, CH, 0.5 C), 132.8 (+, CH, 0.5 C), 132.4 (+, CH, 0.5 C), 132.3 (+, CH, 0.5 C), 132.0 (+, CH, 0.5 C), 131.5 (+, CH, 0.5 C), 130.1 (+, CH, 0.5 C), 129.3 (+, CH, 0.5 C), 129.1 (+, CH, 0.5 C), 51.4 (+, CH), 50.7 (−, CH2, 0.5 C), 50.3 (−, CH2, 0.5 C), 35.5 (−, CH2, 0.5 C), 35.5 (−, CH2, 0.5 C), 35.4 (−, CH2, 0.5 C), 35.3 (−, CH2, 0.5 C), 35.3 (−, CH2, 0.5 C), 34.9 (−, CH2, 0.5 C), 33.4 (−, CH2, 0.5 C), 33.2 (−, CH2, 0.5 C).
- MS (EI, 70 eV, 70 °C), m/z (%): 250 (18), 118 (92), 117 (27), 115 (23), 105 (19), 104 (100), 103 (24), 91 (18), 78 (18).
- HRMS–EI (m/z): [M]+ calcd for C18H18O 250.1352; found 250.1353.
- IR (ATR, ṽ) = 3030 (w), 3007 (w), 2986 (w), 2922 (vs), 2893 (m), 2850 (s), 2704 (w), 1720 (vs), 1686 (w), 1672 (w), 1657 (w), 1592 (w), 1499 (m), 1492 (m), 1453 (w), 1435 (m), 1412 (m), 1385 (w), 1289 (w), 1261 (w), 1239 (w), 1217 (w), 1207 (w), 1183 (w), 1154 (w), 1142 (w), 1128 (w), 1101 (w), 1088 (w), 1078 (w), 1038 (w), 1007 (w), 984 (w), 960 (w), 939 (m), 928 (w), 905 (s), 882 (vs), 877 (vs), 858 (s), 847 (s), 796 (vs), 745 (m), 730 (s), 715 (vs), 681 (w), 647 (vs), 591 (m), 568 (m), 514 (vs), 499 (vs), 465 (m), 452 (m), 432 (w), 414 (w), 387 (w) cm−1.
- Dimethyldioxirane (DMDO) [16]
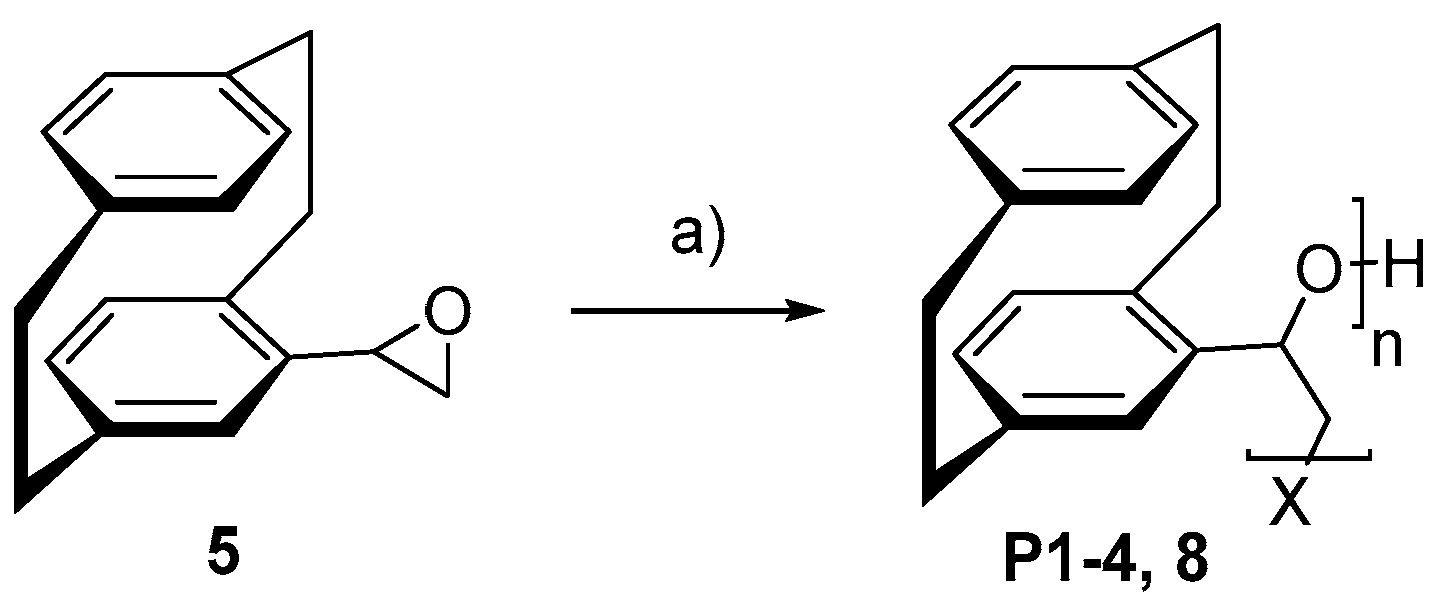
- Homopolymerization for poly(4-[2.2]paracyclophanyl)oxirane) P1-P8 [17]
- 1H NMR (400 MHz, CDCl3 [7.26 ppm], ppm) δ = 7.00–5.96 (m), 3.58–2.38 (m), 2.45–1.98 (m).
- IR (ATR, ṽ) = 3439 (w), 3432 (w), 3427 (w), 3398 (w), 3390 (w), 3383 (w), 3374 (w), 3369 (w), 3357 (w), 3352 (w), 2952 (w), 2918 (vs), 2871 (w), 2850 (m), 1720 (w), 1591 (s), 1458 (vs), 1453 (vs), 1411 (s), 1364 (m), 1323 (m), 1261 (m), 1179 (w), 1156 (w), 1084 (s), 1057 (s), 1024 (vs), 959 (m), 939 (m), 899 (m), 873 (m), 798 (vs), 756 (m), 717 (vs), 697 (vs), 636 (vs), 625 (vs), 589 (vs), 579 (vs), 555 (vs), 548 (vs), 535 (vs), 513 (vs), 459 (vs), 452 (vs), 441 (vs), 425 (vs), 418 (vs), 408 (vs), 398 (vs), 384 (vs) cm−1.
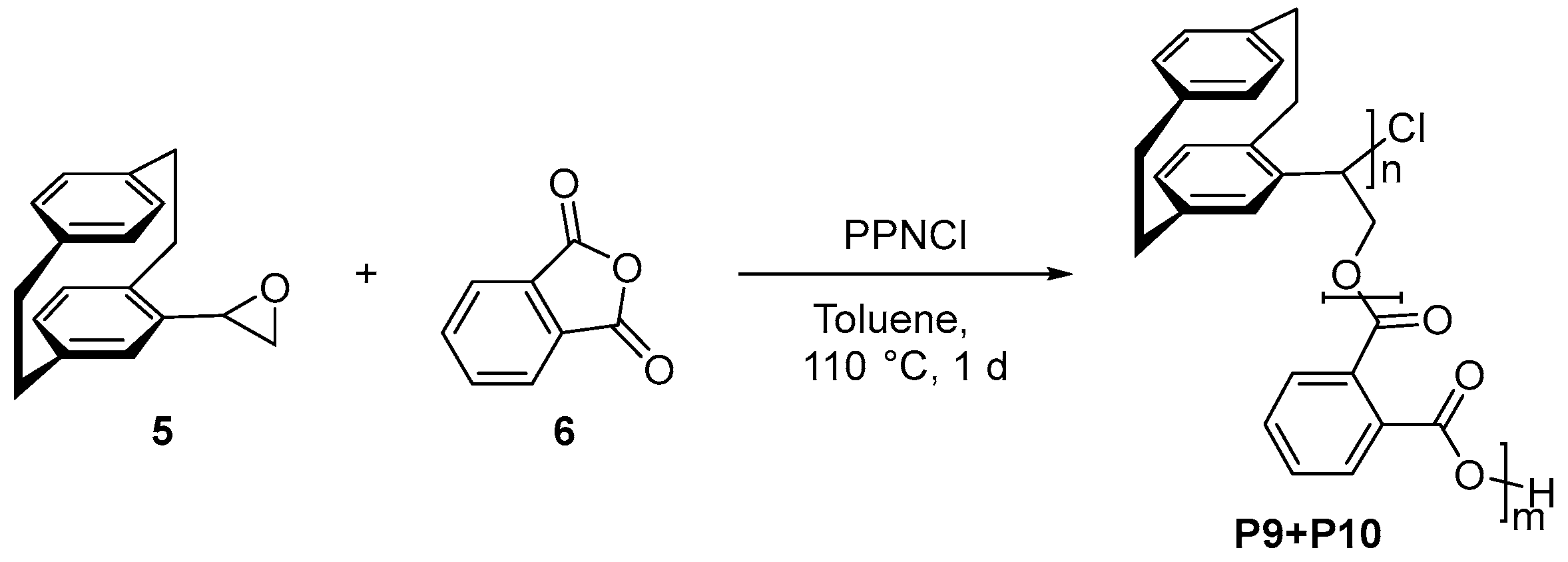
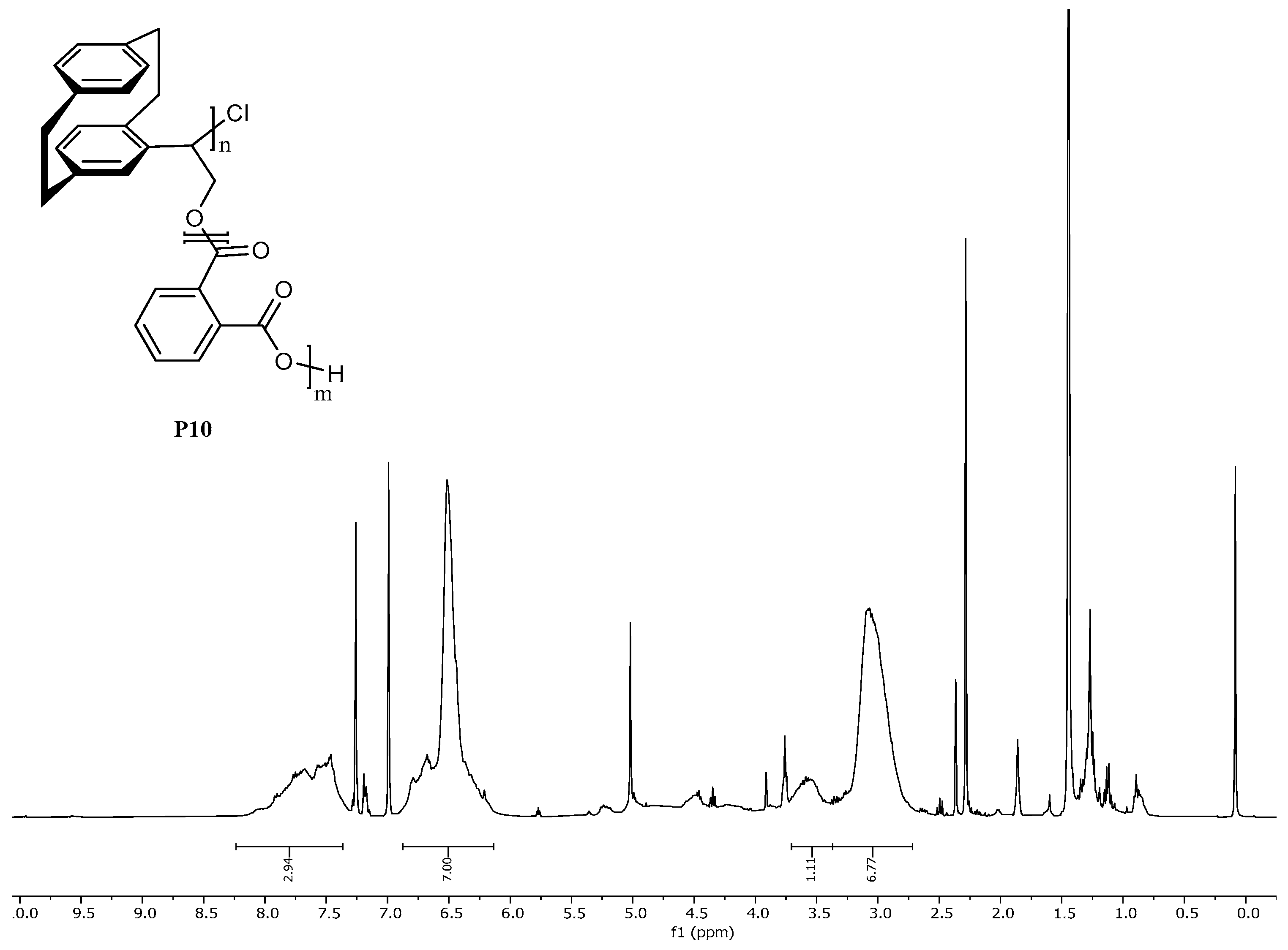
- Copolymerization for poly(4-[2.2]paracyclophanyl)oxirane-co-phtalic anhydrid) P9+P10 [18]
- 1H NMR (400 MHz, CDCl3 [7.26 ppm], ppm) δ = 8.15–7.34 (m), 6.94–6.07 (m), 3.69–3.41 (m), 3.41–2.70 (m).
- IR (ATR, ṽ) = 3010 (w), 2924 (m), 2851 (w), 1718 (vs), 1595 (w), 1581 (w), 1489 (w), 1446 (w), 1411 (w), 1383 (vw), 1256 (vs), 1119 (vs), 1064 (vs), 1038 (s), 965 (w), 899 (w), 796 (m), 734 (s), 717 (s), 704 (m), 646 (w), 588 (w), 511 (m), 504 (m) cm−1.
2.3. Additional Information
- Determination of the DMDO Concentration using 1H NMR Spectroscopy
3. Results
3.1. Synthesis and Stereochemical Analysis of the Planar Chiral PCP-derived Monomer
3.2. Homopolymerization
3.3. Copolymerization
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okamoto, Y. Chiral polymers. Prog. Polym. Sci. 2000, 25, 159–162. [Google Scholar] [CrossRef]
- Bonner, W.A. Chirality and life. Orig. Life Evol. Biosph. 1995, 25, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Okamoto, Y. Efficient separation of enantiomers using stereoregular chiral polymers. Chem. Rev. 2016, 116, 1094–1138. [Google Scholar] [CrossRef] [PubMed]
- Long, G.; Sabatini, R.; Saidaminov, M.I.; Lakhwani, G.; Rasmita, A.; Liu, X.; Sargent, E.H.; Gao, W. Chiral-perovskite optoelectronics. Nat. Rev. Mater. 2020, 5, 423–439. [Google Scholar] [CrossRef]
- Itsuno, S. Chiral polymer synthesis by means of repeated asymmetric reaction. Prog. Polym. Sci. 2005, 30, 540–558. [Google Scholar] [CrossRef]
- Hassan, Z.; Spuling, E.; Knoll, D.M.; Brase, S. Regioselective Functionalization of [2.2]Paracyclophanes: Recent Synthetic Progress and Perspectives. Angew. Chem. Int. Ed. Engl. 2020, 59, 2156–2170. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.; Spuling, E.; Knoll, D.M.; Lahann, J.; Bräse, S. Planar chiral [2.2] paracyclophanes: From synthetic curiosity to applications in asymmetric synthesis and materials. Chem. Soc. Rev. 2018, 47, 6947–6963. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.E.; Knight, J.D. [2.2] Paracyclophane derivatives in asymmetric catalysis. Org. Biomol. Chem. 2003, 1, 1256–1269. [Google Scholar] [CrossRef]
- Paradies, J. [2.2] Paracyclophane derivatives: Synthesis and application in catalysis. Synthesis 2011, 23, 3749–3766. [Google Scholar] [CrossRef]
- Rowlands, G.J. The synthesis of enantiomerically pure [2.2] paracyclophane derivatives. Org. Biomol. Chem. 2008, 6, 1527–1534. [Google Scholar] [CrossRef]
- Dahmen, S.; Bräse, S. The asymmetric dialkylzinc addition to imines catalyzed by [2.2] paracyclophane-based N, O-ligands. J. Am. Chem. Soc. 2002, 124, 5940–5941. [Google Scholar] [CrossRef] [PubMed]
- Knoll, D.M.; Zippel, C.; Hassan, Z.; Nieger, M.; Weis, P.; Kappes, M.M.; Bräse, S. A highly stable, Au/Ru heterobimetallic photoredox catalyst with a [2.2] paracyclophane backbone. Dalton Trans. 2019, 48, 17704–17708. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, S.; Itoh, T.; Kubo, M.; Okuno, H. Synthesis and polymerization of 4-vinyl [2.2] paracyclophane. Polym. Bull. 1994, 32, 27–34. [Google Scholar] [CrossRef]
- Morisaki, Y.; Chujo, Y. Novel through-space conjugated polymers consisting of alternate [2.2] paracyclophane and fluorene. Bull. Chem. Soc. Jpn. 2005, 78, 288–293. [Google Scholar] [CrossRef]
- Zippel, C.; Hassan, Z.; Parsa, A.Q.; Hohmann, J.; Bräse, S. Multigram-Scale Kinetic Resolution of 4-Acetyl [2.2] Paracyclophane via Ru-Catalyzed Enantioselective Hydrogenation: Accessing [2.2] Paracyclophanes with Planar and Central Chirality. Adv. Synth. Catal. 2021, 363, 2861–2865. [Google Scholar] [CrossRef]
- Mikula, H.; Svatunek, D.; Lumpi, D.; Glöcklhofer, F.; Hametner, C.; Fröhlich, J. Practical and efficient large-scale preparation of dimethyldioxirane. Org. Process Res. Dev. 2013, 17, 313–316. [Google Scholar] [CrossRef]
- Brocas, A.-L.; Gervais, M.; Carlotti, S.; Pispas, S. Amphiphilic diblock copolymers based on ethylene oxide and epoxides bearing aliphatic side chains. Polym. Chem. 2012, 3, 2148–2155. [Google Scholar] [CrossRef]
- Hošťálek, Z.; Trhlikova, O.; Walterová, Z.; Martinez, T.; Peruch, F.; Cramail, H.; Merna, J. Alternating copolymerization of epoxides with anhydrides initiated by organic bases. Eur. Polym. J. 2017, 88, 433–447. [Google Scholar] [CrossRef]
- Taber, D.F.; DeMatteo, P.W.; Hassan, R.A. Simplified preparation of dimethyldioxirane (DMDO). Org. Synth. 2003, 90, 350–357. [Google Scholar]
- Klaus, S.; Lehenmeier, M.W.; Anderson, C.E.; Rieger, B. Recent advances in CO2/epoxide copolymerization—New strategies and cooperative mechanisms. Coord. Chem. Rev. 2011, 255, 1460–1479. [Google Scholar] [CrossRef]
- Zhang, C.J.; Yang, J.L.; Hu, L.F.; Zhang, X.H. Anionic copolymerization of carbonyl sulfide with epoxides via alkali metal alkoxides. Chin. J. Chem. 2018, 36, 625–629. [Google Scholar] [CrossRef]
- Jurrat, M.; Pointer-Gleadhill, B.J.; Ball, L.T.; Chapman, A.; Adriaenssens, L. Polyurethanes and polyallophanates via sequence-selective copolymerization of epoxides and isocyanates. J. Am. Chem. Soc. 2020, 142, 8136–8141. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 2015, 51, 6459–6479. [Google Scholar] [CrossRef]
- Longo, J.M.; Sanford, M.J.; Coates, G.W. Ring-opening copolymerization of epoxides and cyclic anhydrides with discrete metal complexes: Structure–property relationships. Chem. Rev. 2016, 116, 15167–15197. [Google Scholar] [CrossRef] [PubMed]
| Entry | Initiator | Catalyst | Mn [kDa] | D | Yield [%] |
|---|---|---|---|---|---|
| P1 | NBu4Cl | AliBu3 | 1.85 | 1.12 | 18 |
| P2 | NOct4Br | AliBu3 | 1.78 | 1.08 | 19 |
| P3 | NBu4Cl | AlEt3 | 1.92 | 1.08 | 20 |
| P4 | NOct4Br | AlEt3 | 1.79 | 1.09 | 17 |
| P5 | NBu4Cl | AliBu3 | - | - | - |
| P6 | sec-BuLi | AliBu3 | - | - | - |
| P7 | NOct4Br | Co(II)-salen * | - | - | - |
| P8 (chiral) | NOct4Br | AliBu3 | 1.90 | 1.19 | 13 |
| Entry | Mn [kDa] | D | Yield [%] |
|---|---|---|---|
| P9 | 1.85 | 1.12 | 18 |
| P10 (chiral) | 1.29 | 1.14 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kern, P.; Tappert, H.; Bräse, S. A New Class of Chiral Polyethers and Polyesters Based on the [2.2]Paracyclophane Scaffold. Polymers 2024, 16, 1603. https://doi.org/10.3390/polym16111603
Kern P, Tappert H, Bräse S. A New Class of Chiral Polyethers and Polyesters Based on the [2.2]Paracyclophane Scaffold. Polymers. 2024; 16(11):1603. https://doi.org/10.3390/polym16111603
Chicago/Turabian StyleKern, Patrick, Henrik Tappert, and Stefan Bräse. 2024. "A New Class of Chiral Polyethers and Polyesters Based on the [2.2]Paracyclophane Scaffold" Polymers 16, no. 11: 1603. https://doi.org/10.3390/polym16111603
APA StyleKern, P., Tappert, H., & Bräse, S. (2024). A New Class of Chiral Polyethers and Polyesters Based on the [2.2]Paracyclophane Scaffold. Polymers, 16(11), 1603. https://doi.org/10.3390/polym16111603






