Preparation and Characterization of Lignin Nanoparticles from Different Plant Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Isolation of Lignin from Different Sources
2.3. Lignin Characterization
2.3.1. Biomass Analysis: Lignin and Sugars in Liquid Fraction
2.3.2. Antioxidant Activity
2.3.3. Analysis of Molecular Weight Distribution (by GPC)
2.3.4. Quantitative NMR Spectroscopy of Lignin
2.3.5. Thermogravimetric Analysis: TGA and DSC
2.3.6. FTIR Analysis
2.4. LNP Preparation and Characterization
2.4.1. Lignin Nanoparticle Preparation
2.4.2. Dynamic Light Scattering (DLS) and Zeta Potential (ζ)
2.4.3. Transmission Electron Microscopy (TEM) and Estimation of Diameter
2.4.4. Determination of Antioxidant Capacity
Scavenging Activity of H2O2
Ferric Reducing Antioxidant Power (FRAP) Activity
2.5. Statistical Analysis
3. Results
3.1. Lignin Characterization
3.1.1. Biomass Analysis: Lignin and Sugars in Liquid Fraction
3.1.2. Antioxidant Activity
3.1.3. Analysis of Molecular Weight Distribution (by GPC)
3.1.4. Quantitative NMR Spectroscopy of Lignin
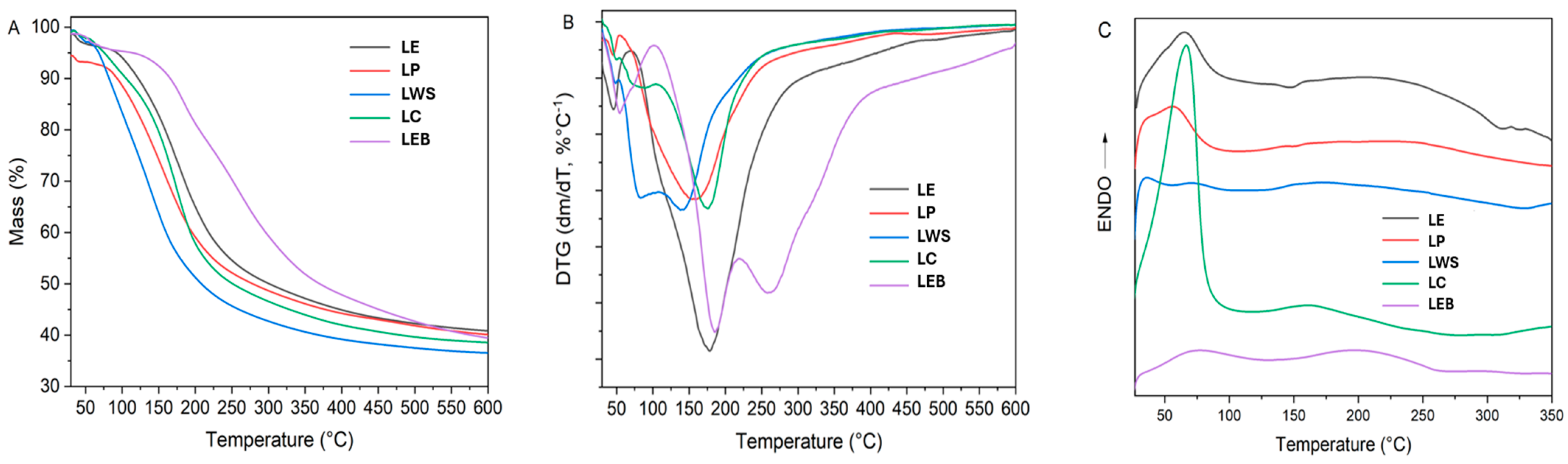
3.1.5. Thermogravimetric Analysis (TGA and DSC)
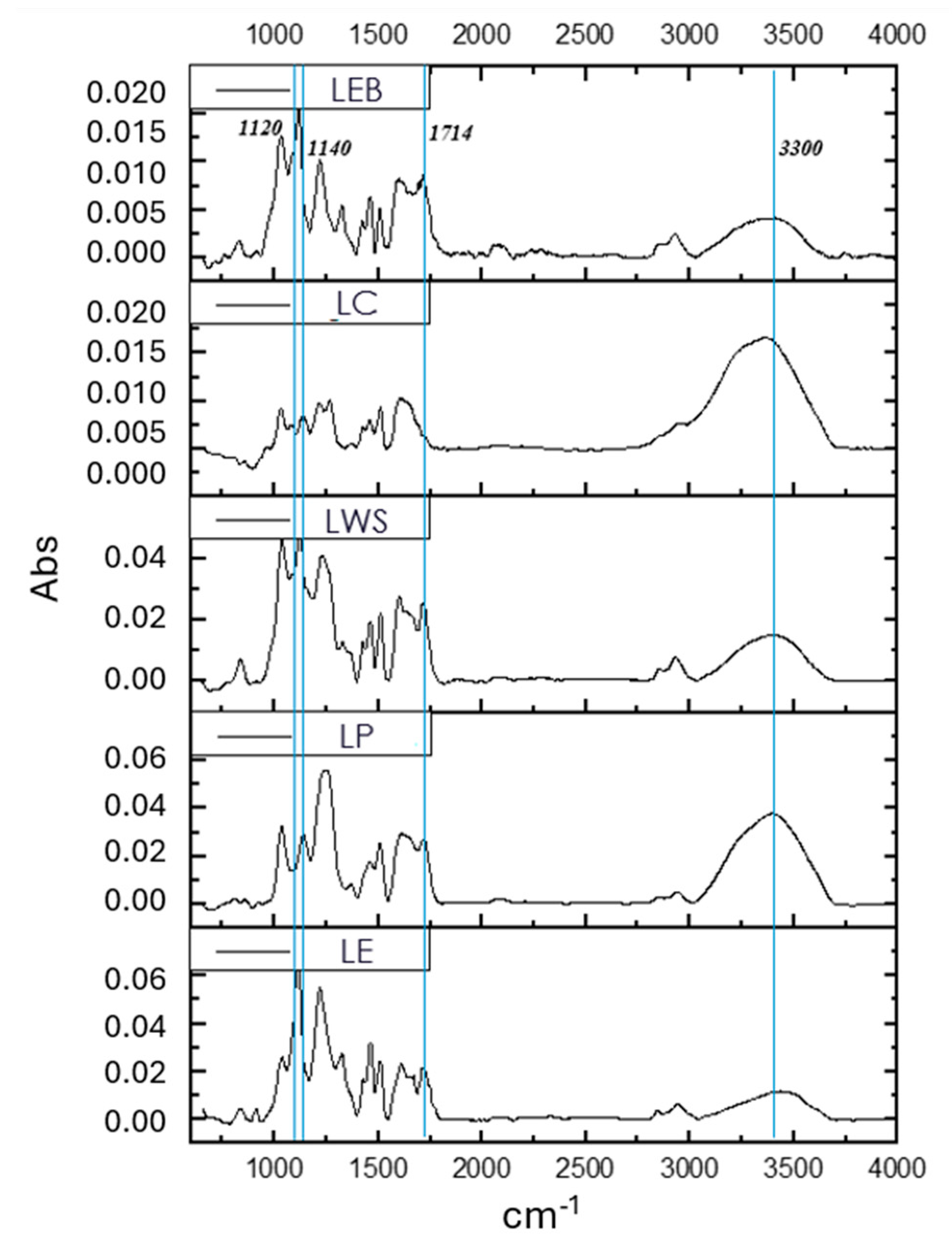
3.1.6. FTIR Analysis
3.2. Lignin Nanoparticle Characterization
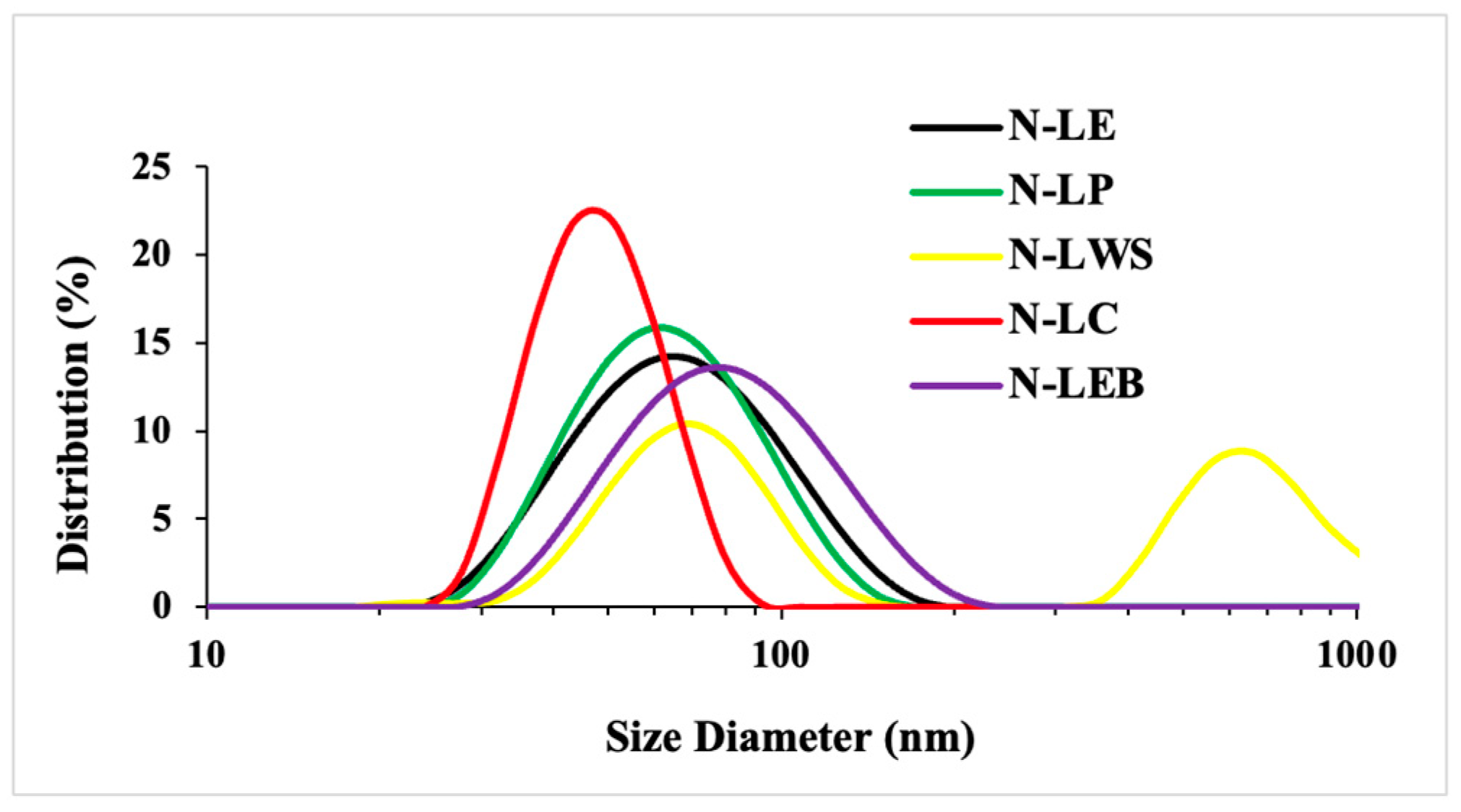
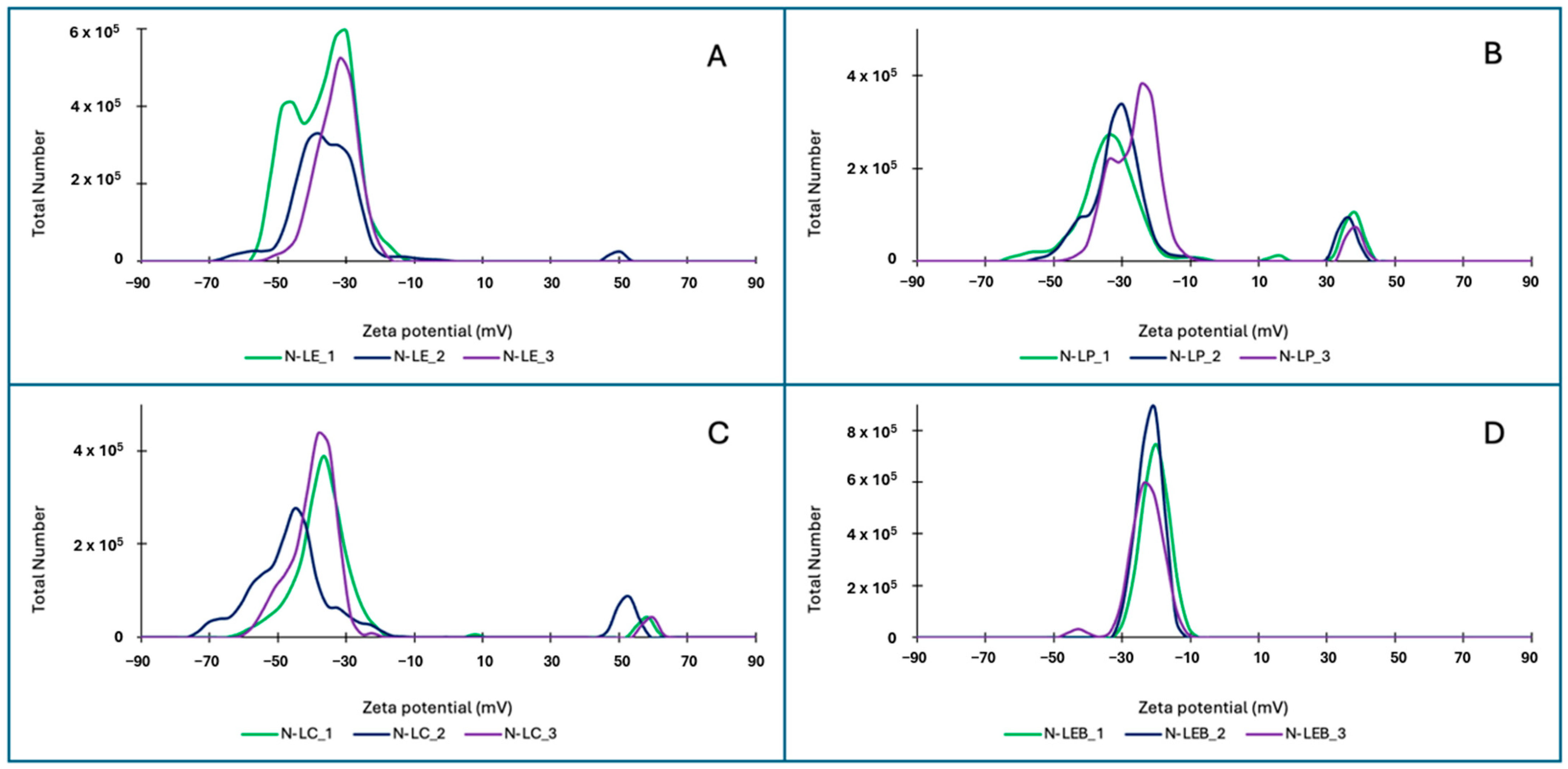
3.2.1. Dynamic Light Scattering (DLS) and Zeta Potential (ζ)
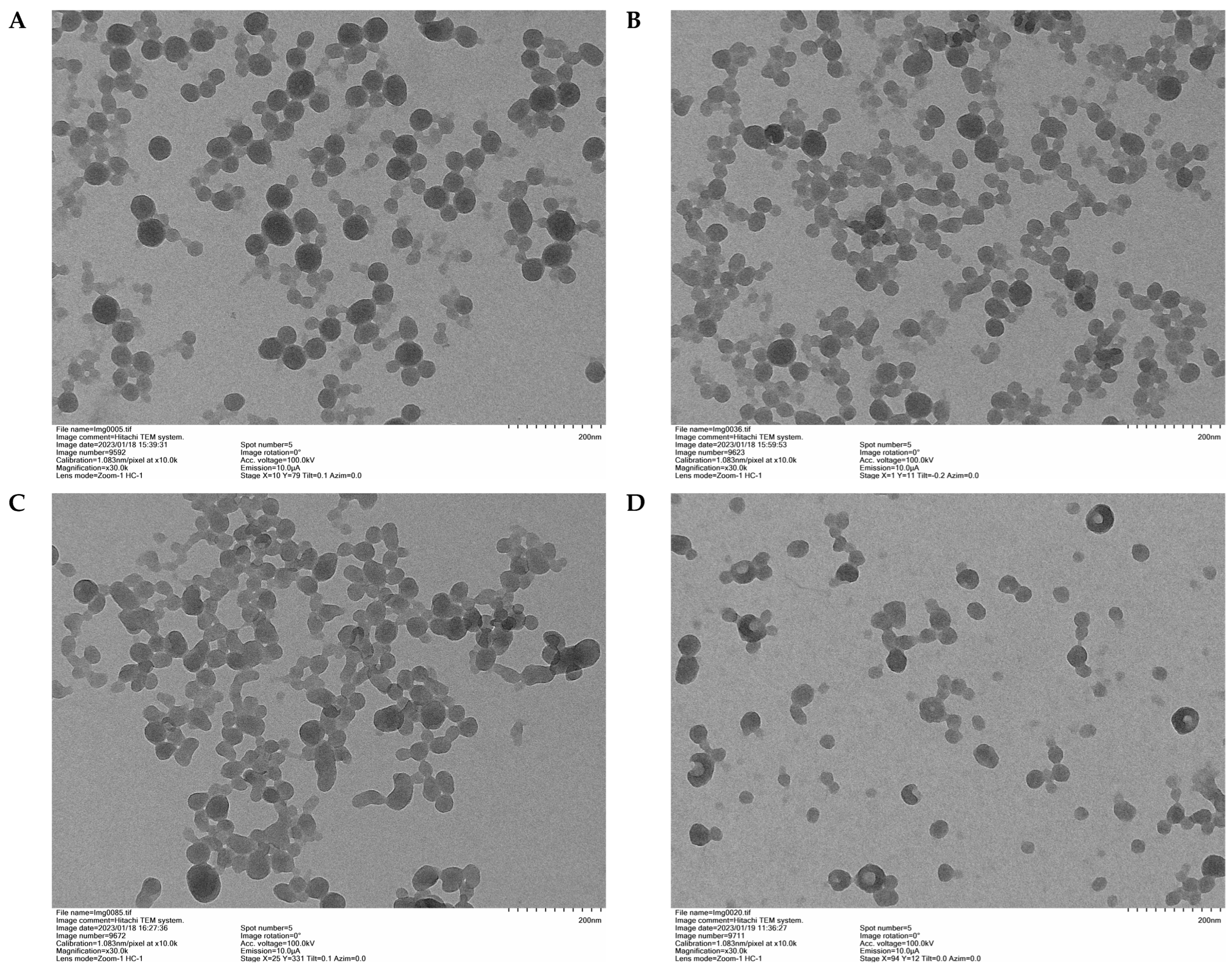
3.2.2. Transmission Electron Microscopy (TEM) and Particle Size Analysis (DSL)
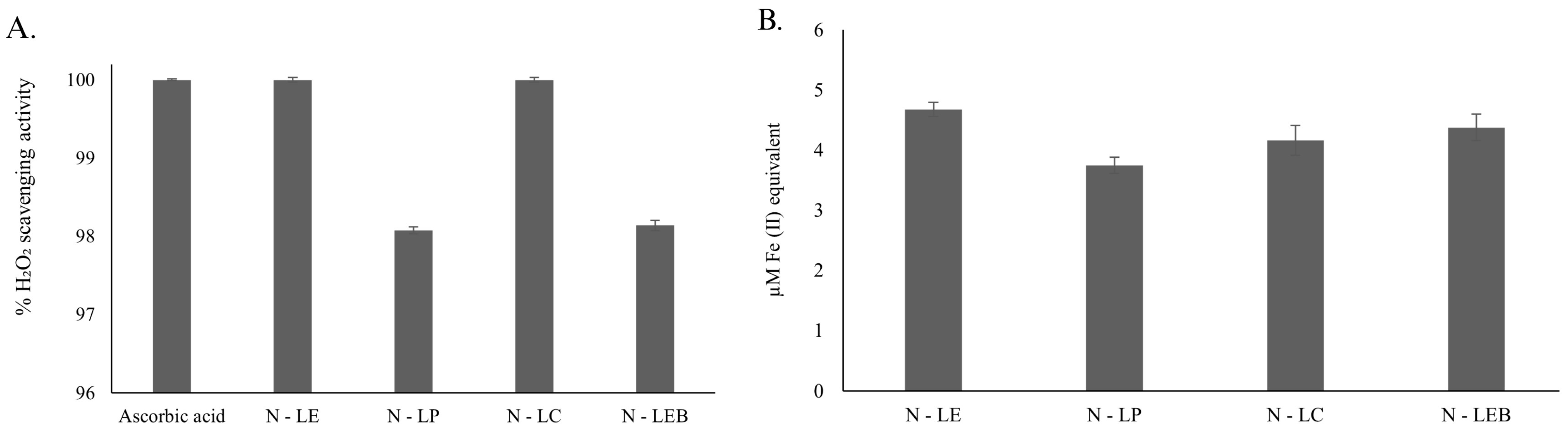
3.2.3. Determination of In Vitro Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Shi, Y.; Gao, B.; Zhao, Y.; Jing, Y.; Zha, Z.; Xue, W.; Gong, L. Lignin Nanoparticles: Green Synthesis in a γ-Valerolactone/Water Binary Solvent and Application to Enhance Antimicrobial Activity of Essential Oilet als. ACS Sustain. Chem. Eng. 2020, 8, 714–722. [Google Scholar] [CrossRef]
- Tobimatsu, Y.; Schuetz, M. Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 2019, 56, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.D.H.; Dillon, A.J.P.; Camassola, M. Lignin nanoparticles enter the scene: A promising versatile green tool for multiple applications. Biotechnol. Adv. 2021, 47, 107685. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Sixta, H. Handbook of Pulp; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; Volume 2. [Google Scholar]
- Henriksson, G. Pulp and Paper Chemistry and Technology; Gruyter, D., Ed.; De Gruyter: Estocolmo, Sweden, 2009; Volume 1. [Google Scholar]
- Yang, W.; Fortunati, E.; Gao, D.; Balestra, G.; Giovanale, G.; He, X.; Torre, L.; Kenny, J.; Puglia, D. Valorization of Acid Isolated High Yield Lignin Nanoparticles as Innovative Antioxidant/Antimicrobial Organic Materials. ACS Sustain. Chem. Eng. 2018, 6, 3502–3514. [Google Scholar] [CrossRef]
- Wang, X.; Ji, S.; Wang, X.; Bian, H.; Lin, L.; Dai, H.; Xiao, H. Thermally conductive, super flexible and flame-retardant BN-OH/PVA composite film reinforced by lignin nanoparticles. J. Mater. Chem. C 2019, 7, 14159–14169. [Google Scholar] [CrossRef]
- Pereira, A.d.E.S.; Oliveira, J.L.; Savassa, S.M.; Rogério, C.B.; Medeiros, G.A.; Fraceto, L.F. Lignin nanoparticles: New insights for a sustainable agriculture. J. Clean. Prod. 2022, 345, 131145. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Qi, G.; Li, C.; Xu, P.; Zheng, T.; Zhu, X.; Kenny, J.M.; Puglia, D.; Ma, P. Highly transparent PVA/nanolignin composite films with excellent UV shielding, antibacterial and antioxidant performance. React. Funct. Polym. 2021, 162, 104873. [Google Scholar] [CrossRef]
- Wang, B.; Sun, D.; Wang, H.M.; Yuan, T.Q.; Sun, R.C. Green and Facile Preparation of Regular Lignin Nanoparticles with High Yield and Their Natural Broad-Spectrum Sunscreens. ACS Sustain. Chem. Eng. 2019, 7, 2658–2666. [Google Scholar] [CrossRef]
- Zhang, Z.; Terrasson, V.; Guénin, E. Lignin Nanoparticles and Their Nanocomposites. Nanomaterials 2021, 11, 1336. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saito, T. Lignin-Derived Advanced Carbon Materials. ChemSusChem 2015, 8, 3941–3958. [Google Scholar] [CrossRef] [PubMed]
- Rahman, O.u.; Shi, S.; Ding, J.; Wang, D.; Ahmad, S.; Yu, H. Lignin nanoparticles: Synthesis, characterization and corrosion protection performance. New J. Chem. 2018, 42, 3415–3425. [Google Scholar] [CrossRef]
- Österberg, M.; Sipponen, M.; Mattos, B.D.; Rojas, O.J. Spherical lignin particles: A review on their sustainability and applications. Green Chem. 2020, 22, 2712–2733. [Google Scholar] [CrossRef]
- Parvathy, G.; Sethulekshmi, A.S.; Jayan, J.S.; Raman, A.; Appukuttan, S. Lignin based nano-composites: Synthesis and applications. Process Saf. Environ. Prot. 2021, 145, 395–410. [Google Scholar]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Synthesis and Characterization of Biodegradable Lignin Nanoparticles with Tunable Surface Properties. Langmuir 2016, 32, 6468–6477. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.A.; Lee, H.W.; Seo, B.; Son, W.S.; Yoon, J.; Yoon, T.J.; Park, H.J.; Yu, J.; Yoon, J.; Lee, Y.W. One pot synthesis of environmentally friendly lignin nanoparticles with compressed liquid carbon dioxide as an antisolvent. Green Chem. 2016, 18, 2129–2146. [Google Scholar] [CrossRef]
- Hussin, M.H.; Appaturi, J.N.; Poh, N.E.; Latif, N.H.A.; Brosse, N.; Ziegler-Devin, I.; Vahabi, H.; Syamani, F.A.; Fatriasara, W.; Solihat, N.N.; et al. A recent advancement on preparation, characterization and application of nanolignin. Int. J. Biol. Macromol. 2022, 200, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Lisperguer, J.; Ballerini, A.; Nuñez, M.; Palavecino, P. Analisis termico de adhesivos fenolicos modificados con lignina. Boletín Soc. Chil. Química 2000, 45, 403–408. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escalante, A.; Murillo-Vázquez, R.N.; Delgado, E.; González, F.J.; Toríz, G. Use of Agave tequilana-lignin and zinc oxide nanoparticles for skin photoprotection. J. Photochem. Photobiol. B Biol. 2016, 163, 156–161. [Google Scholar] [CrossRef]
- Ali, I.; Asim, M.; Khan, T.A. Low cost adsorbents for the removal of organic pollutants from wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, S.; Guo, X.; Huang, H. Adsorption of chromium(III) on lignin. Bioresour. Technol. 2008, 99, 7709–7715. [Google Scholar] [CrossRef] [PubMed]
- Sameni, J.; Jaffer, S.A.; Tjong, J.; Sain, M. Advanced Applications for Lignin Micro- and Nano-based Materials. Curr. For. Rep. 2020, 6, 159–171. [Google Scholar] [CrossRef]
- Nair, S.S.; Sharma, S.; Pu, Y.; Sun, Q.; Pan, S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High shear homogenization of lignin to nanolignin and thermal stability of Nanolignin-Polyvinyl alcohol blends. ChemSusChem 2014, 7, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Gilca, I.A.; Popa, V.I.; Crestini, C. Obtaining lignin nanoparticles by sonication. Ultrason. Sonochem. 2015, 23, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; He, H.; Jiang, H.; Ma, L.; Jia, D.M. Nano-lignin filled natural rubber composites: Preparation and characterization. Express Polym. Lett. 2013, 7, 480–493. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin valorization: Lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10, 192. [Google Scholar] [CrossRef] [PubMed]
- Girard, V.; Chapuis, H.; Brosse, N.; Canilho, N.; Marchal-Heussler, L.; Ziegler-Devin, I. Lignin Nanoparticles: Contribution of Biomass Types and Fractionation for an Eco-Friendly Production. ACS Sustain. Chem. Eng. 2024, 12, 7055–7068. [Google Scholar] [CrossRef]
- Berg, A. Separation of lignocellulosic components in acetic acid media and evaluation of applications. J. Sci. Technol. For. Prod. Process. 2014, 3, 27–32. [Google Scholar]
- TAPPI Test Method T222 om-02; Techinical Association for the Pulp and Paper Industries. TAPPI Press: Atlanta, GA, USA, 2002.
- Ruch, R.J.; Cheng, S.-J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Sinha, S.; Lindner, A.B. The good, the bad, and the ugly of ROS: New insights on aging and aging-related diseases from eukaryotic and prokaryotic model organisms. Oxidative Med. Cell. Longev. 2018, 2018, 1941285. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, D.S.; de Santi, A.; Oldenburger, B.; de Vries, J.; Barta, K.; Deuss, P.J. Extraction of lignin with high β-O-4 content by mild ethanol extraction and its effect on the depolymerization yield. J. Vis. Exp. 2019, 143, e58575. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gu, X.; Shi, Y. A review on lignin antioxidants: Their sources, isolations, antioxidant activities and various applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef] [PubMed]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2, 2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv Ethanol Lignin from Hybrid Poplar as a Radical Scavenger: Relationship between Lignin Structure, Extraction Conditions, and Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Wei, L.; Li, W.; Gong, D.; Qin, H.; Feng, X.; Li, G.; Ling, Z.; Wang, P.; Yin, B. Isolating High Antimicrobial Ability Lignin From Bamboo Kraft Lignin by Organosolv Fractionation. Front. Bioeng. Biotechnol. 2021, 9, 683796. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin-carbohydrate complexes: Properties, applications, analyses, and methods of extraction: A review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, D.S. Wood and Cellulosic Chemistry. Second Edition, Revised and Expanded Edited by David N.-S. Hon (Clemson University) and Nubuo Shiraishi (Kyoto University). Marcel Dekker: New York and Basel. 2001. vii + 914 pp. $250.00. ISBN 0-8247-0024-4. J. Am. Chem. Soc. 2001, 123, 8880–8881. [Google Scholar] [CrossRef]
- Peredo, K.; Escobar, D.; Vega-Lara, J.; Berg, A.; Pereira, M. Thermochemical properties of cellulose acetate blends with acetosolv and sawdust lignin: A comparative study. Int. J. Biol. Macromol. 2016, 83, 403–409. [Google Scholar] [CrossRef]
- Fodil Cherif, M.; Trache, D.; Brosse, N.; Benaliouche, F.; Tarchoun, A.F. Comparison of the physicochemical properties and thermal stability of organosolv and kraft lignins from hardwood and softwood biomass for their potential valorization. Waste Biomass Valorization 2020, 11, 6541–6553. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Sahoo, S.; Seydibeyoğlu, M.Ö.; Mohanty, A.K.; Misra, M. Characterization of industrial lignins for their utilization in future value added applications. Biomass Bioenergy 2011, 35, 4230–4237. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Zhang, H.; Xiao, R. Thermal conversion of lignin to phenols: Relevance between chemical structure and pyrolysis behaviors. Fuel 2016, 182, 864–870. [Google Scholar] [CrossRef]
- Jakab, E.; Faix, O.; Till, F. Thermal decomposition of milled wood lignins studied by thermogravimetry/mass spectrometry. J. Anal. Appl. Pyrolysis 1997, 40, 171–186. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Greener synthesis of lignin nanoparticles and their applications. Green Chem. 2020, 22, 612–636. [Google Scholar] [CrossRef]
- Hernández-García, D.; Wood, C.D.; Castro-Obregón, S.; Covarrubias, L. Reactive oxygen species: A radical role in development? Free. Radic. Biol. Med. 2010, 49, 130–143. [Google Scholar] [CrossRef]
- Riley, P. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 1–28. [Google Scholar] [CrossRef]
- Bhat, R.; Khalil, H.; Karim, A. Exploring the antioxidant potential of lignin isolated from black liquor of oil palm waste. Comptes rendus. Biologies 2009, 332, 827–831. [Google Scholar] [CrossRef]
- Trevisan, H.; Rezende, C.A. Pure, stable and highly antioxidant lignin nanoparticles from elephant grass. Ind. Crops Prod. 2020, 145, 112105. [Google Scholar] [CrossRef]

| Sample | Lignin Code | LNPs Code |
|---|---|---|
| Lignin acetosolv Eucalyptus globulus (Acetic acid 87%, 1:5 solid/liquid, 160 °C for 2 h) | LE | N-LE |
| Lignin acetosolv Pinus radiata D. Don (Acetic acid 87%, 1:5 solid/liquid, 180 °C for 2 h) | LP | N-LP |
| Lignin acetosolv Triticum spp. (wheat straw) (Acetic acid 87%, 1:8 solid/liquid, 160 °C for 2 h) | LWS | N-LWS |
| Lignin Kraft commercial Picea abies and Pinus sylvestris (Stora Enso CAS 8068-05-1) | LC | N-LC |
| Lignin organosolv bark of Eucalyptus globulus (Ethanol 60%, 1:10 solid/liquid, 160 °C for 3 h) | LEB | N-LEB |
| Sample | Lignin (%) | Cellulose (%) | Hemicellulose (%) |
|---|---|---|---|
| LE | 89.6 c ± 0.1 | 0.06 ± 0.01 | 0.22 ± 0.01 |
| LP | 91.0 b ± 0.8 | 0.09 ± 0.01 | 0.99 ± 0.12 |
| LWS | 78.7 e ± 0.4 | 0.84 ± 0.10 | 4.91 ± 0.61 |
| LC | 93.6 a ± 0.6 | 0.12 ± 0.02 | 1.70 ± 0.27 |
| LEB | 82.5 d ± 0.5 | 0.20 ± 0.02 | 5.80 ± 0.76 |
| Sample | IC50 (mg/L) | AAI |
|---|---|---|
| LE | 14.3 | 3.5 |
| LP | 41.3 | 1.2 |
| LWS | 44.0 | 1.1 |
| LC | 59.6 | 0.8 |
| LEB | 36.7 | 1.4 |
| p-value | 0.005 | 0.030 |
| Sample | Mw (g/mol) |
|---|---|
| LE | 22,024 |
| LP | 21,979 |
| LWS | 24,303 |
| LC | 29,441 |
| LEB | 35,712 |
| p-value | 0.005 |
| Sample | β-O-4 (%) | β-5 (%) | β-β (%) | S/G (%) |
|---|---|---|---|---|
| LE | 4.2 | 0 | 5.9 | 21.1 |
| LP | 9.6 | 10.7 | 2.4 | 0 |
| LWS | 35.6 | 1.9 | 10.4 | 1.2 |
| LC | 6.0 | 0.5 | 2.5 | 0 |
| LEB | 50.5 | 0 | 6.2 | 25.8 |
| Sample | Temperature (°C) | Mass Loss (%) | Char (%) | ||
|---|---|---|---|---|---|
| Tonset | Tpeak | Tend | |||
| LE | 71 | 178 | 467 | 52.3 | 40.8 |
| LP | 72 | 157 | 431 | 51.2 | 40.1 |
| LWC | 108 | 140 | 451 | 58.5 | 36.6 |
| LC | 104 | 176 | 453 | 55.7 | 38.6 |
| LEB | 103 | 185 | 218 | 18.1 | 39.4 |
| 220 | 257 | 457 | 34.9 | ||
| Sample | Size (nm) LNP 5%w/v | Size (nm) LNP 2%w/v | Polydispersity Index (PDI) LNP 2%w/v | Zeta Potential (ζ) (mV) LNP 2%w/v |
|---|---|---|---|---|
| N-LE | 113.9 ± 2.4 | 90.7 a ± 0.1 | 0.10 b ± 0.02 | −35.0 b ± 1.6 |
| +50.3 ± 0.0 | ||||
| N-LP | 123.5 ± 0.11 | 77.0 b ± 0.10 | 0.10 b ± 0.03 | −30.7 b ± 2.7 |
| +36.8 ± 1.9 | ||||
| N-LC | 145.9 ± 0.22 | 77.1 b ± 0.09 | 0.09 b ± 0.01 | −41.5 c ± 1.9 |
| +56.6 ± 4.5 | ||||
| N-LEB | 154.6 ± 7.7 | 88.1 a ± 1.0 | 0.26 a ± 0.02 | −23.5 a ± 3.0 |
| Sample | Mean (nm) | SD (nm) |
|---|---|---|
| N-LE | 362.5 | 109.9 |
| N-LP | 353.1 | 88.5 |
| N-LC | 353.1 | 100.6 |
| N-LEB | 361.0 | 126.3 |
| N-LE | 362.5 | 109.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Sanhueza, I.; Girard, V.; Ziegler-Devin, I.; Chapuis, H.; Brosse, N.; Valenzuela, F.; Banerjee, A.; Fuentealba, C.; Cabrera-Barjas, G.; Torres, C.; et al. Preparation and Characterization of Lignin Nanoparticles from Different Plant Sources. Polymers 2024, 16, 1610. https://doi.org/10.3390/polym16111610
Ortega-Sanhueza I, Girard V, Ziegler-Devin I, Chapuis H, Brosse N, Valenzuela F, Banerjee A, Fuentealba C, Cabrera-Barjas G, Torres C, et al. Preparation and Characterization of Lignin Nanoparticles from Different Plant Sources. Polymers. 2024; 16(11):1610. https://doi.org/10.3390/polym16111610
Chicago/Turabian StyleOrtega-Sanhueza, Isidora, Victor Girard, Isabelle Ziegler-Devin, Hubert Chapuis, Nicolas Brosse, Francisca Valenzuela, Aparna Banerjee, Cecilia Fuentealba, Gustavo Cabrera-Barjas, Camilo Torres, and et al. 2024. "Preparation and Characterization of Lignin Nanoparticles from Different Plant Sources" Polymers 16, no. 11: 1610. https://doi.org/10.3390/polym16111610
APA StyleOrtega-Sanhueza, I., Girard, V., Ziegler-Devin, I., Chapuis, H., Brosse, N., Valenzuela, F., Banerjee, A., Fuentealba, C., Cabrera-Barjas, G., Torres, C., Méndez, A., Segovia, C., & Pereira, M. (2024). Preparation and Characterization of Lignin Nanoparticles from Different Plant Sources. Polymers, 16(11), 1610. https://doi.org/10.3390/polym16111610










