Biopolymer Compositions Based on Poly(3-hydroxybutyrate) and Linear Polyurethanes with Aromatic Rings—Preparation and Properties Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
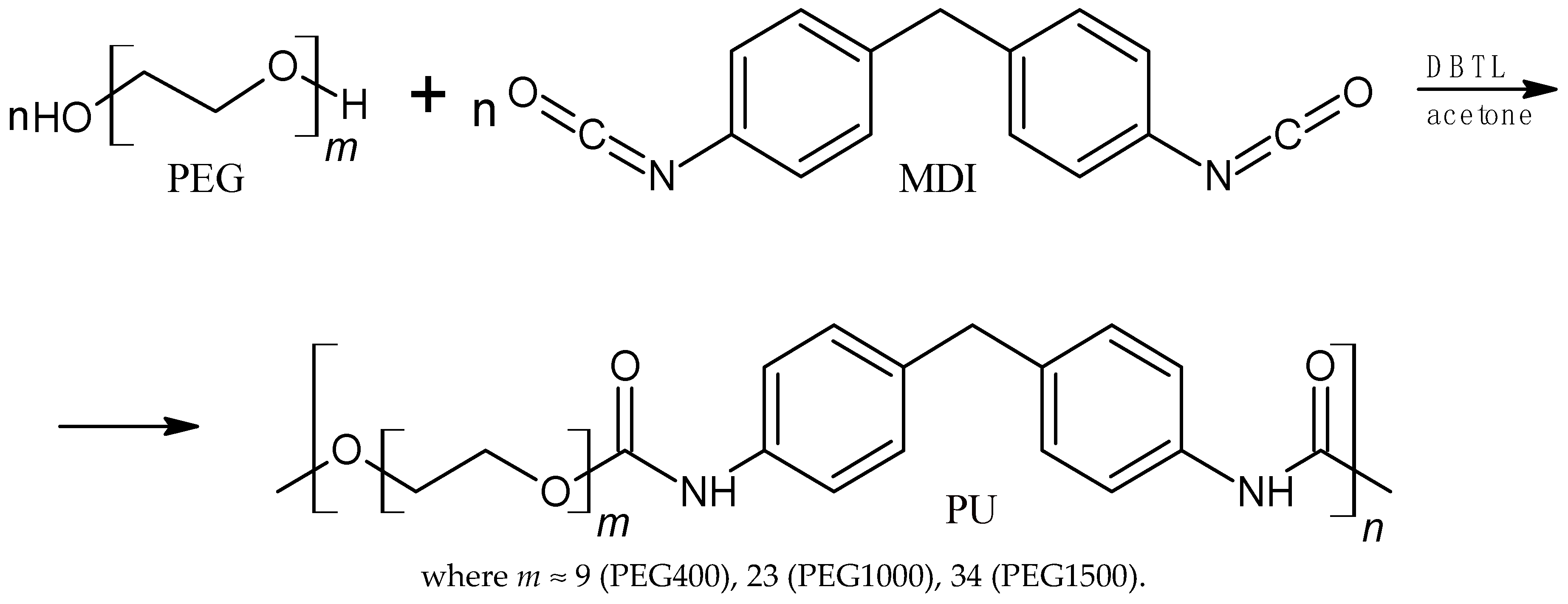
2.2. Synthesis of Linear Polyurethanes with Aromatic Rings
2.3. Preparation of Polymer Blends
2.4. Analytical Methods
2.4.1. Size-Exclusive Chromatography (SEC)
2.4.2. Scanning Electron Microscopy
2.4.3. Thermogravimetric Analysis
2.4.4. Standard Differential Scanning Calorimetry Measurements
2.4.5. FTIR Spectroscopy
2.4.6. Mechanical Properties
3. Results and Discussion
3.1. Spectral Analysis of the Prepared Polymer Biocompositions
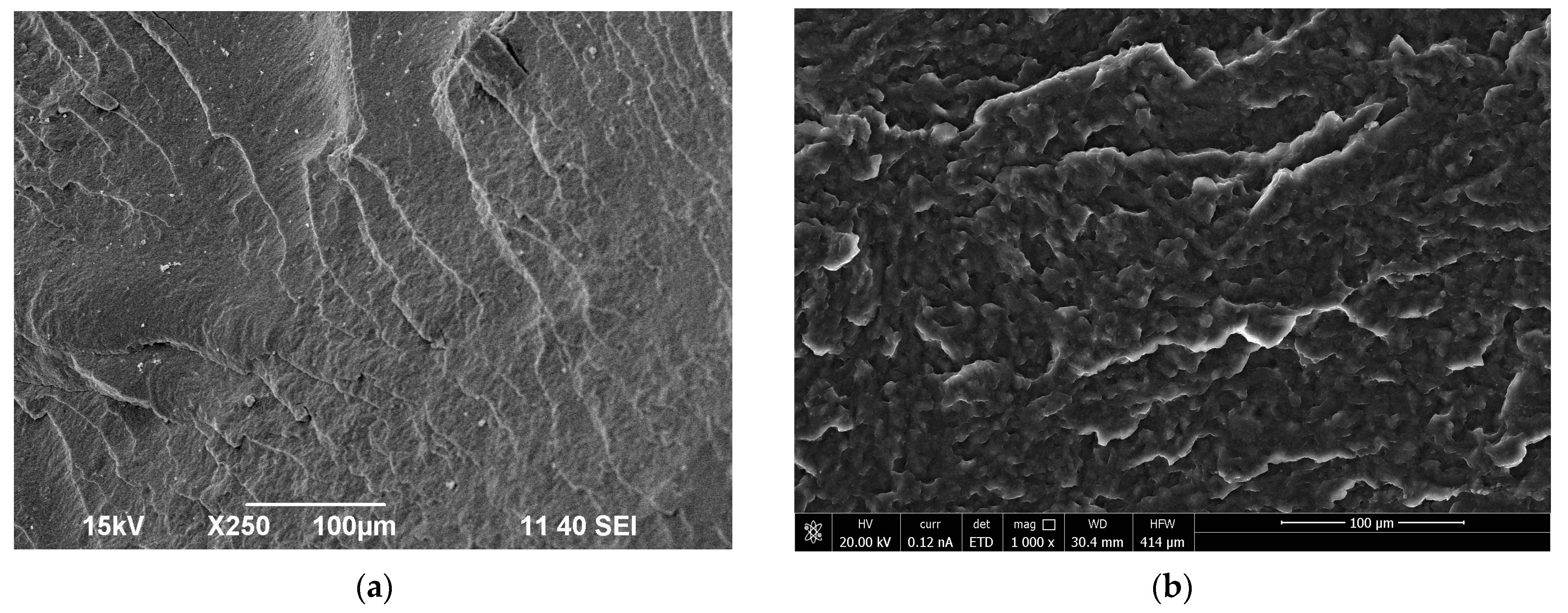
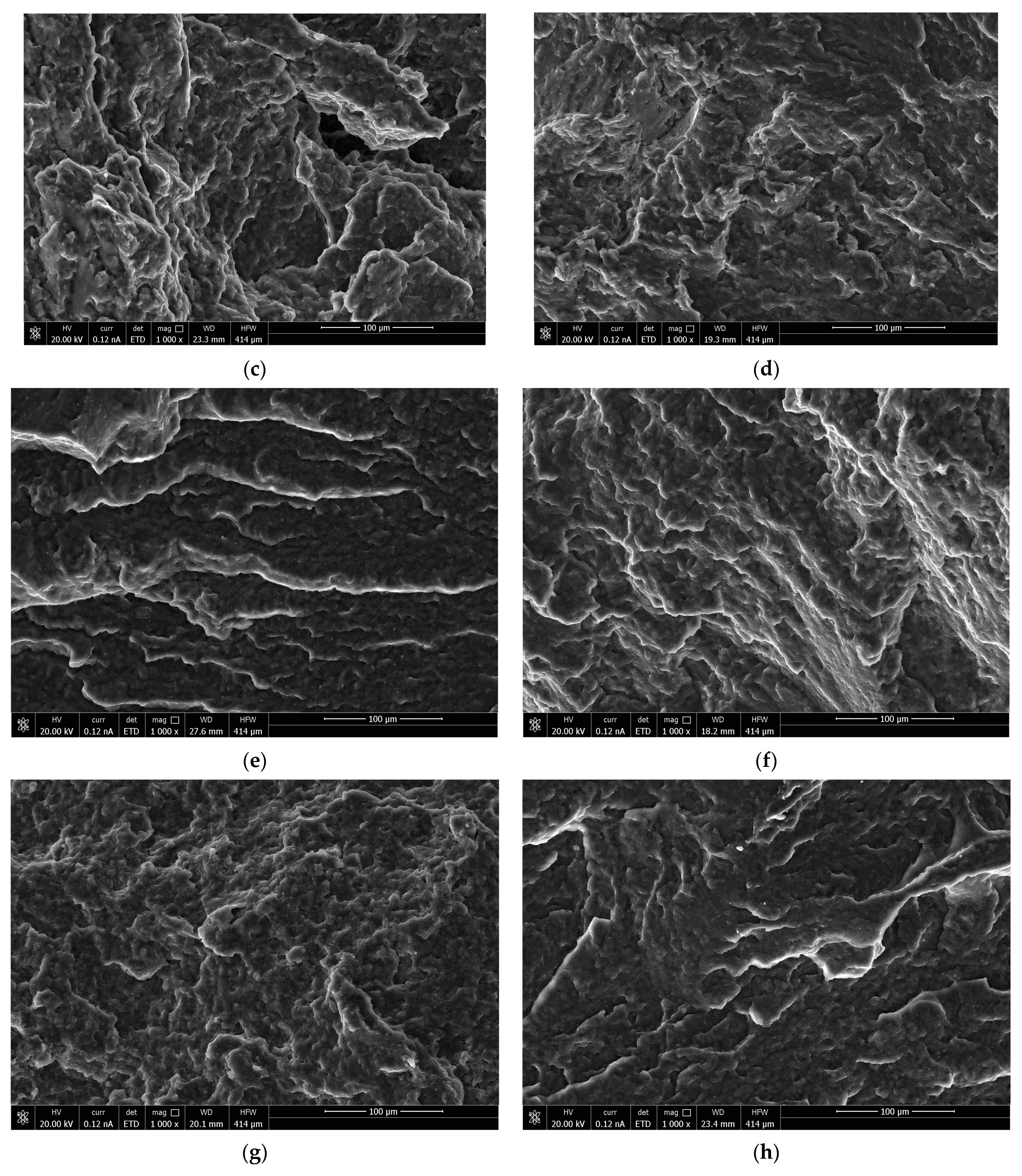
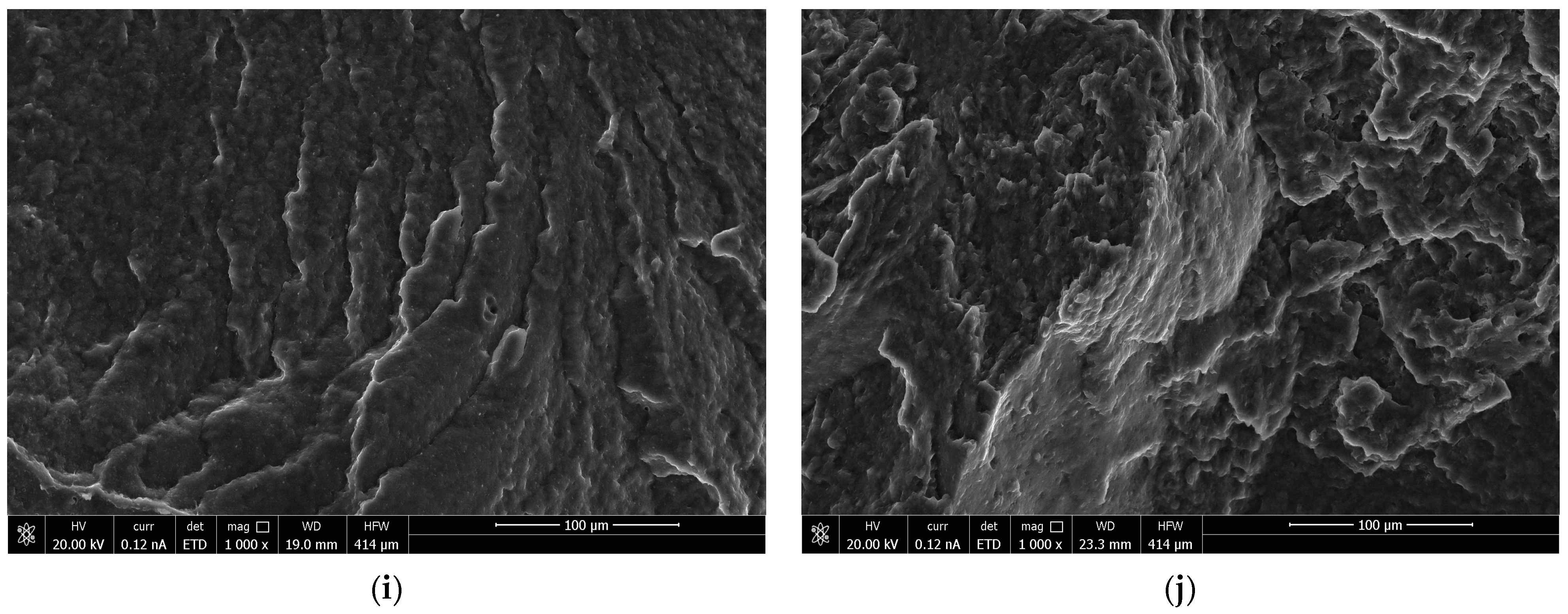
3.2. Morphology Characteristics of the Obtained Polymer Biocompositions
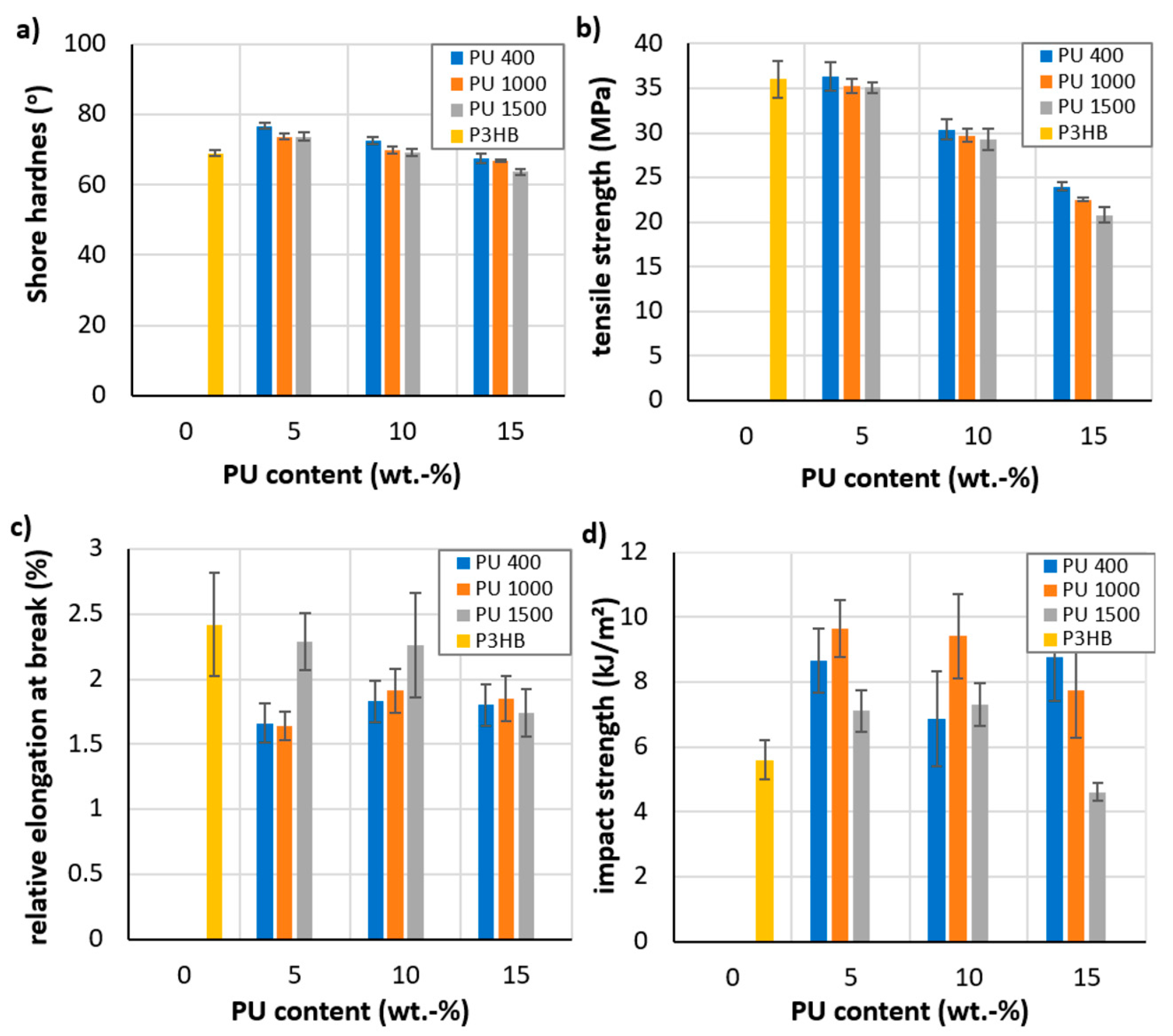
3.3. Mechanical Properties of the Produced Polymer Biocompositions
3.4. Thermal Stability of the Prepared P3HB Polymer Blends
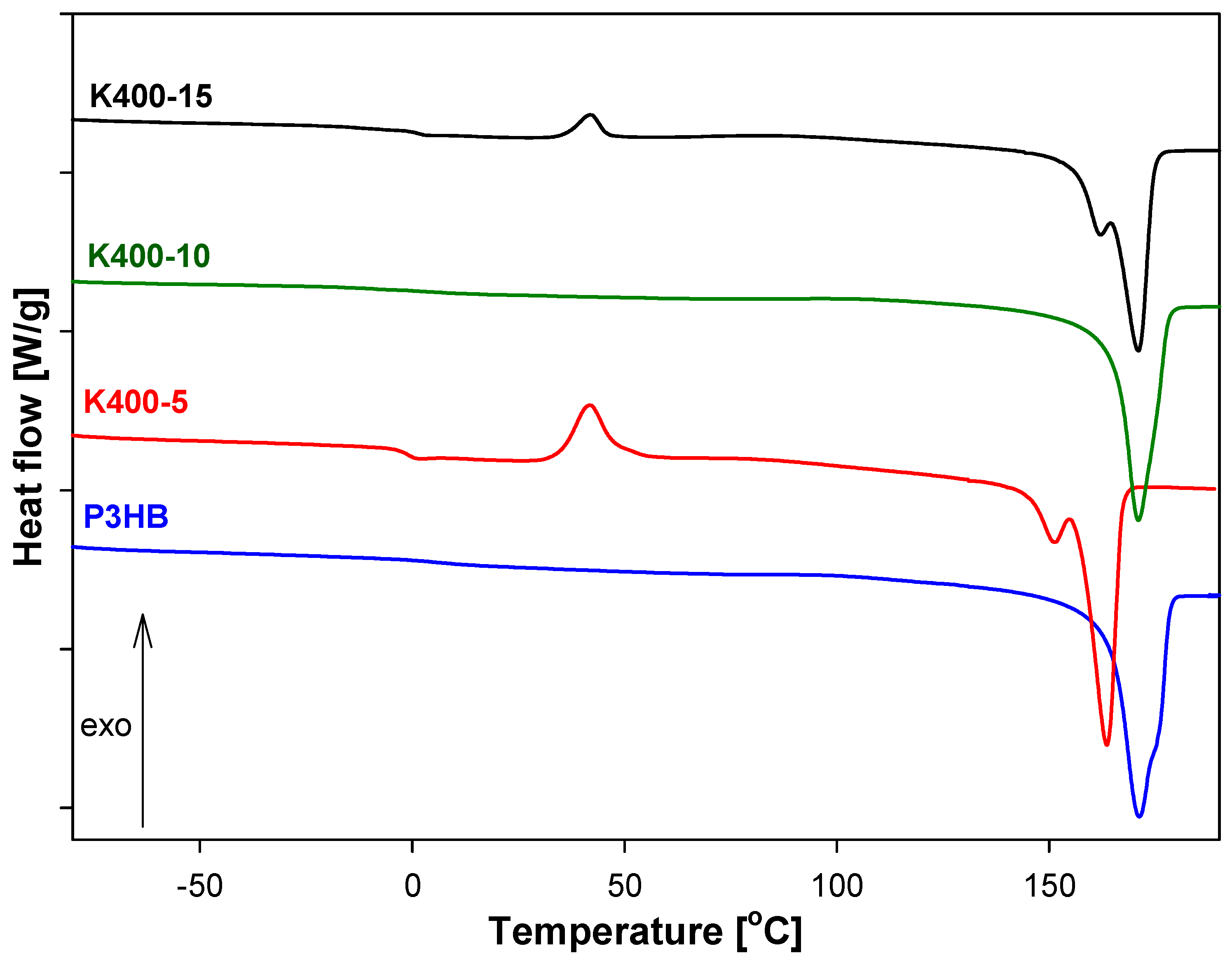
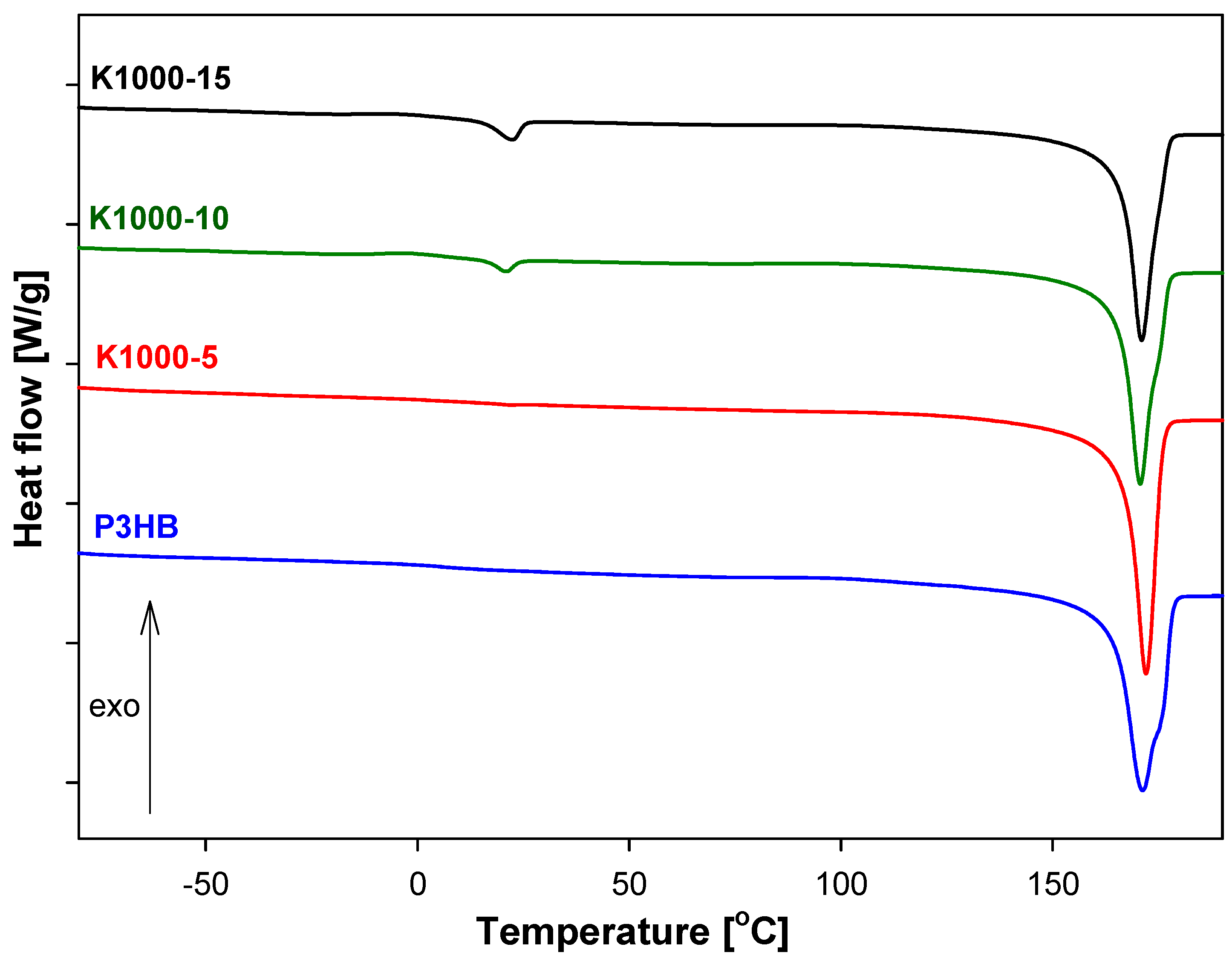
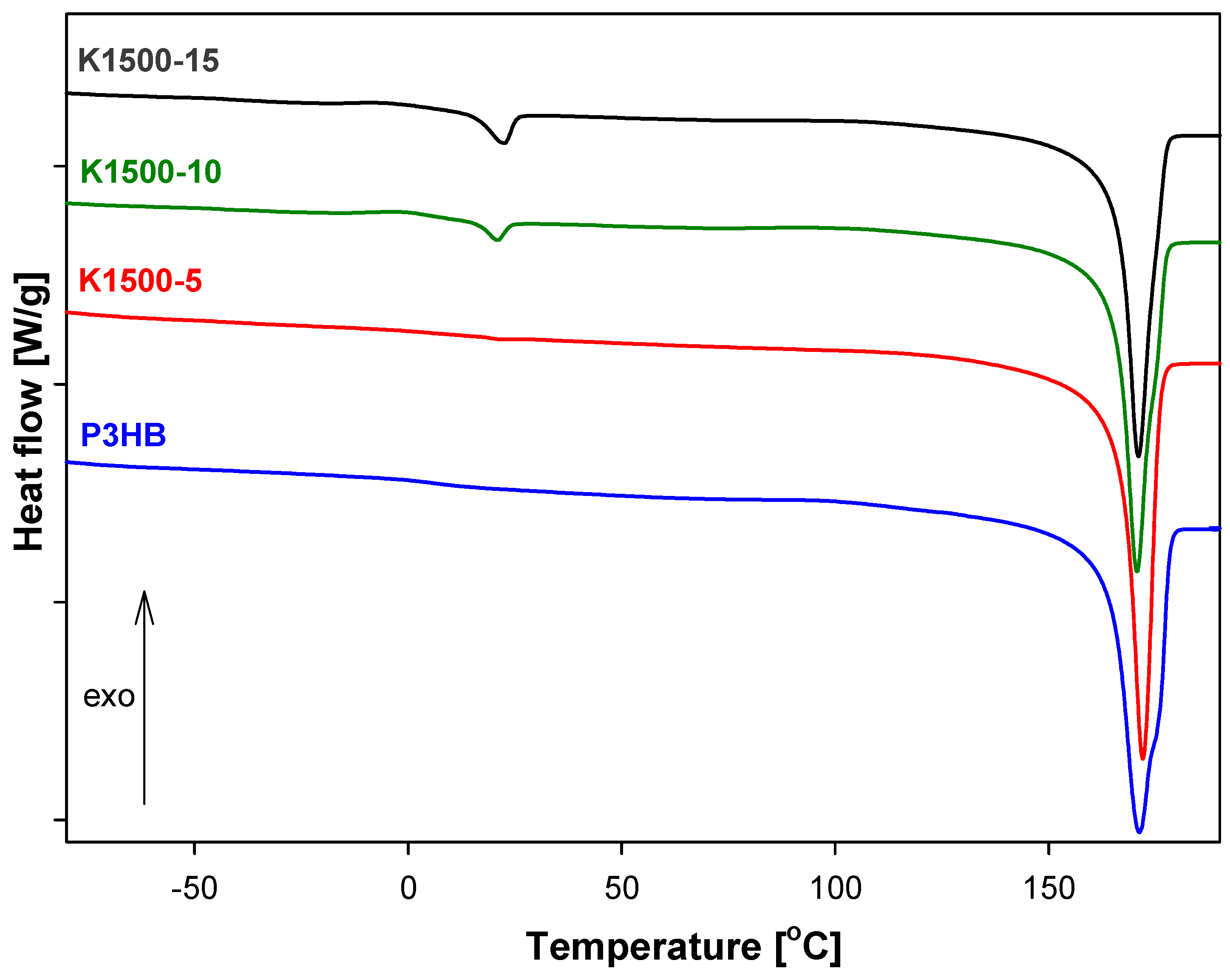
3.5. Analysis of Thermal Parameters of P3HB-PU Polymer Biocompositions
4. Conclusions
- New polymer biocompositions were prepared with poly(3-hydroxybutyrate) and aromatic linear polyurethane obtained by reacting diphenylmethane 4,4′-diisocyanate with polyethylene glycol at molecular weights of 400, 1000, and 1500 g/mol to produce the polymer compositions with polyurethane used in amounts of 5, 10, and 15 wt.%;
- Spectral analysis (FTIR) confirmed the interactions of polymers with hydrogen bonds and their compatibility in the preparation of polymer biocompositions;
- SEM analysis demonstrated that the morphology of the obtained polymer compositions showed a homogeneous structure, and the interaction of biopolyester with polyurethanes with the formation of P3HB-PU adducts easily displaced relative to each other. It resulted in the effect of P3HB plasticization, and the effect was better when the longer chain of PEG in PU was there;
- The plasticizing effect of PU resulted in better mechanical properties for the new biocompositions compared to native P3HB. The prepared polymer biocompositions were characterized by a significant desirable increase in impact strength, an acceptable decrease in tensile strength, and a relative elongation at break, which was related to the plasticizing effect of PU on the properties of P3HB. The best mechanical properties were characterized by biocompositions containing 5 and 10 wt.% polyurethane PU400 and PU1000 modifiers, as confirmed by the analysis of the morphology of these blends;
- The prepared polymer biocompositions were characterized by higher thermal stability compared to unmodified P3HB. The onset decomposition temperature of the polymer biocompositions was higher by 30 °C on average. Increasing the amount of added polyurethane modifier did not significantly affect the thermal stability of the blends obtained, as the values of the onset decomposition temperature of all the compositions tested were similar. The compositions with PU400 and PU1000 additives had the highest thermal stability;
- The study carried out showed that the preparation of P3HB polymer biocompositions with the modifier in the form of aromatic linear polyurethane had a positive effect on the thermal and mechanical properties and significantly increased the processability of this polyester. It will be used in the future application of the compositions, among others, in the production of non-woven fabric.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baran, B. Resource (in)efficiency in the EU: A case of plastic waste. Ekon. I Prawo. 2022, 21, 45–62. [Google Scholar] [CrossRef]
- Saud, S.; Yang, A.; Jiang, Z.; Ning, D.; Fahad, S. New insights in to the environmental behavior and ecological toxicity of microplastics. J. Hazard. Mater. Adv. 2023, 10, 100298–100305. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric sustainable materials and their emerging applications. J. Environ. Chem. Eng. 2022, 10, 108159–108168. [Google Scholar] [CrossRef]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Rhee, K.Y.; Park, S.J. Polyhydroxyalkanoates (PHAs): Biopolymers for biofuel and biorefineries. Polymers 2021, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Bordes, P.; Pollet, E.; Avérous, L. Nano-biocomposites: Biodegradable polyester/nanoclay systems. Progress. Polym. Sci. 2009, 34, 125–155. [Google Scholar] [CrossRef]
- Antipova, T.V.; Zhelifonova, V.P.; Zaitsev, K.V.; Nedorezova, P.M.; Aladyshev, A.M.; Klyamkina, A.N.; Kostyuk, S.V.; Danilogorskaya, A.A.; Kozlovsky, A.G. Biodegradation of Poly-ε-caprolactones and Poly-l-lactides by Fungi. J. Polym. Environ. 2018, 26, 4350–4359. [Google Scholar] [CrossRef]
- Baidurah, S. Methods of Analyses for Biodegradable Polymers: A Review. Polymers 2022, 14, 4928. [Google Scholar] [CrossRef]
- Olkhov, A.A.; Tyubaeva, P.M.; Vetcher, A.A.; Karpova, S.G.; Kurnosov, A.S.; Rogovina, S.Z.; Iordanskii, A.L.; Berlin, A.A. Aggressive Impacts Affecting the Biodegradable Ultrathin Fibers Based on Poly(3-Hydroxybutyrate), Polylactide and Their Blends: Water Sorption, Hydrolysis and Ozonolysis. Polymers 2021, 13, 941. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nanocomposites—A review. Biotechnol. Rep. 2019, 15, e00316. [Google Scholar] [CrossRef]
- Khan, A.; Wang, B.; Ni, Y. Chitosan-Nanocellulose Composites for Regenerative Medicine Applications. Curr. Med. Chem. 2020, 27, 4584–4592. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Ilyas, R.A.; Sapuan, S.M.; Jumaidin, R. Sugar Palm Starch-Based Composites for Packaging Applications. In Bionanocomposites for Packaging Applications; Jawaid, M., Swain, S., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Meléndez-Rodríguez, B.; Torres-Giner, S.; Reis, M.A.M.; Silva, F.; Matos, M.; Cabedo, L.; Lagarón, J.M. Blends of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) with Fruit Pulp Biowaste Derived Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate-co-3-Hydroxyhexanoate) for Organic Recycling Food Packaging. Polymers 2021, 13, 1155. [Google Scholar] [CrossRef]
- Sukhanova, A.A.; Murzova, A.E.; Boyandin, A.N.; Kiselev, E.G.; Sukovatyi, A.G.; Kuzmin, A.P.; Shabanov, A.V. Poly-3-hydroxybutyrate/chitosan composite films and nonwoven mats. Int. J. Biol. Macromol. 2020, 165, 2947–2956. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent progress in development and chemical modification of poly(hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef]
- Dalton, B.; Bhagabati, P.; De Micco, J.; Padamati, R.B.; O’Connor, K. A Review on Biological Synthesis of the Biodegradable Polymers Polyhydroxyalkanoates and the Development of Multiple Applications. Catalysts 2022, 12, 319. [Google Scholar] [CrossRef]
- Kalia, V.C.; Singh-Patel, S.K.; Shanmugam, R.; Lee, J.K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef]
- Fernandes, E.G.; Pietrini, M.; Chiellini, E. Thermo-mechanical and morphological characterization of plasticized poly[(R)-3-hydroxybutyric acid]. Macromol. Symp. 2004, 218, 157–164. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C. 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Villalba-Rodríguez, A.M.; Romero-Castillo, K.D.; Zavala-Yoe, R.; Bilal, M.; Ramirez-Mendoza, R.A.; Parra-Saldivar, R.; Iqbal, H.M. Poly-3-hydroxybutyrate-based constructs with novel characteristics for drug delivery and tissue engineering applications—A review. Polym. Eng. Sci. 2020, 60, 1760–1772. [Google Scholar] [CrossRef]
- Taylor, C.S.; Behbehani, M.; Glen, A.; Basnett, P.; Gregory, D.A.; Lukasiewicz, B.B.; Nigmatullin, R.; Claeyssens, F.; Roy, I.; Haycock, J.W. Aligned Polyhydroxyalkanoate Blend Electrospun Fibers as Intraluminal Guidance Scaffolds for Peripheral Nerve Repair. ACS Biomater. Sci. Eng. 2023, 9, 1472–1485. [Google Scholar] [CrossRef]
- El-Taweel, S.H.; Höhne, G.W.H.; Mansour, A.A.; Stoll, B. Glass transition and the rigid amorphous phase in semicrystalline blends of bacterial polyhydroxybutyrate PHB with low molecular mass atactic R, S-PHB-diol. Polymer 2004, 45, 983–992. [Google Scholar] [CrossRef]
- Lee, S.N.; Lee, M.Y.; Park, W.H. Thermal stabilization of poly(3-hydroxybutyrate) by poly(glycidyl methacrylate). J. Appl. Polym. Sci. 2002, 83, 2945–2952. [Google Scholar] [CrossRef]
- Bakar, M.; Kostrzewa, M.; Pawelec, Z. Preparation and properties of epoxy resin modified with polyurethane based on hexamethylene diisocyanate and different polyols. J. Thermoplast. Compos. Mater. 2014, 27, 620–631. [Google Scholar] [CrossRef]
- Bakar, M.; Kostrzewa, M.; Hausnerova, B. Effect of diisocyanates on the properties and morphology of epoxy/polyurethane interpenetrating polymer networks. J. Thermoplast. Compos. Mater. 2012, 26, 1364–1376. [Google Scholar] [CrossRef]
- Kostrzewa, M.; Hausnerova, B.; Bakar, M.; Dalka, M. Property evaluation and structure analysis of polyurethane/epoxy graft interpenetrating polymer networks. J. Appl. Polym. Sci. 2011, 122, 1722–1730. [Google Scholar] [CrossRef]
- Bakar, M.; Kostrzewa, M.; Hausnerova, B.; Sar, K. Preparation and property evaluation of nanocomposites based on polyurethane-modified epoxy/montmorillonite systems. Adv. Polym. Tech. 2010, 29, 237–248. [Google Scholar] [CrossRef]
- Bakar, M.; Kostrzewa, M.; Hausnerova, B.; Pająk, K. Preparation and characterization of an epoxy resin modified by a combination of MDI-based polyurethane and montmorillonite. J. Appl. Polym. Sci. 2011, 122, 3237–3247. [Google Scholar] [CrossRef]
- Freier, T. Biopolyesers in Tissue Engineering Applications. Adv. Polym. Sci. 2006, 203, 1–61. [Google Scholar] [CrossRef]
- Zarzyka, I.; Czerniecka-Kubicka, A.; Hęclik, K.; Dobrowolski, D.; Pyda, M.; Leś, K.; Walczak, M.; Białkowska, A.; Bakar, M. Thermally stable biopolymer composites based on poly(3-hydroxybutyrate) modified with linear aliphatic polyurethanes—Preparation and properties. Acta Bioeng. Biomech. 2021, 23, 1–15. [Google Scholar] [CrossRef]
- Zarzyka, I.; Czerniecka-Kubicka, A.; Hęclik, K.; Dobrowolski, L.; Krzykowska, B.; Białkowska, A.; Bakar, M. Biobased poly(3-hydroxybutyrate acid) composites with addition of aliphatic polyurethane based on polypropylene glycols. Acta Bioeng. Biomech. 2022, 24, 75–89. [Google Scholar] [CrossRef]
- Krzykowska, B.; Czerniecka-Kubicka, A.; Białkowska, A.; Bakar, M.; Kovarova, M.; Sedlarik, V.; Zarzyka, I. Polymer/layered clay/polyurethane nanocomposites: P3HB hybride nanobiocomposites—Preparation and properties evaluation. Nanomaterials. 2023, 13, 225. [Google Scholar] [CrossRef]
- Krzykowska, B.; Czerniecka-Kubicka, A.; Białkowska, A.; Bakar, M.; Hęclik, K.; Dobrowolski, L.; Longosz, M.; Zarzyka, I. Polymer Biocompositions and Nanobiocomposites Based on P3HB with Polyurethane and Montmorillonite. Int. J. Mol. Sci. 2023, 24, 17405. [Google Scholar] [CrossRef]
- PN-EN 1242; Adhesives—Determination of the Content of Isocyanate Groups. Polish Committee for Standardization: Warsaw, Poland, 2006.
- PN-EN ISO 527-2; Determination of Tensile Properties, Part 2: Test Conditions for Moulding and Extrusion Plastics. Polish Committee for Standardization: Warsaw, Poland, 2012.
- PN-EN ISO 179-1; Plastics—Determination of Charpy Impact Properties, Part 1: Non-Instrumented Impact Test. Polish Committee for Standardization: Warsaw, Poland, 2023.
- PN-EN ISO 868; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). Polish Committee for Standardization: Warsaw, Poland, 2003.
- ASTM D2240; Standard Test Method for Rubber Property—Durometer Hardness. Polish Committee for Standardization: Berlin, Germany, 2017.
- Eesaee, M.; Ghassemi, P.; Nguyen, D.D.; Thomas, S.; Elkoun, S.; Nguyen-Tri, P. Morphology and crystallization behaviour of polyhydroxyalkanoates-based blends and composites: A review. Biochem. Eng. J. 2022, 187, 108588. [Google Scholar] [CrossRef]
- Rodríguez-Cendal, A.I.; Gómez-Seoane, I.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Señarís-Rodríguez, J.; Díaz-Prado, S.M. Biomedical Applications of the Biopolymer Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Drug Encapsulation and Scaffold Fabrication. Int. J. Mol. Sci. 2023, 24, 11674. [Google Scholar] [CrossRef]
- Alfano, S.; Pagnanelli, F.; Martinelli, A. Rapid Estimation of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Composition Using ATR-FTIR. Polymers 2023, 15, 4127. [Google Scholar] [CrossRef]
- Łukaszewska, I.; Lalik, S.; Bukowczan, A.; Marzec, M.; Pielichowski, K.; Raftopoulos, K.N. Tailoring the physical properties of non-isocyanate polyurethanes by introducing secondary amino groups along their main chain. J. Mol. Liq. Part A 2023, 391, 123263. [Google Scholar] [CrossRef]
- Ryszkowska, J. Supermolecular structure, morphology and physical properties of urea-urethane elastomers. Polimery 2012, 58, 775–785. [Google Scholar] [CrossRef]
- Iqbal, H.M.; Kyazze, G.; Tron, T.; Keshavarz, T. “One-pot” synthesis and characterisation of novel P(3HB)–ethyl cellulose based graft composites through lipase catalysed esterification. Polym. Chem. 2014, 5, 7004–7012. [Google Scholar] [CrossRef]
- Tejada-Oliveros, R.; Balart, R.; Ivorra-Martinez, J.; Gomez-Caturla, J.; Montanes, N.; Quiles-Carrillo, L. Improvement of Impact Strength of Polylactide Blends with a Thermoplastic Elastomer Compatibilized with Biobased Maleinized Linseed Oil for Applications in Rigid Packaging. Molecules 2021, 26, 240. [Google Scholar] [CrossRef]
- Kowalska, M.; Broniatowski, M.; Mach, M.; Płachta, Ł.; Wydro, P. The effect of the polyethylene glycol chain length of a lipopolymer (DSPE-PEGn) on the properties of DPPC monolayers and bilayers. J. Mol. Liq. 2021, 335, 116529. [Google Scholar] [CrossRef]
- Fernández-Ronco, M.P.; Gradzik, B.; Gooneie, A.; Hufenus, R.; El Fray, M. Tuning poly(3 hydroxybutyrate) (P3HB) properties by tailored segmented bio copolymers. ACS Sustain. Chem. Eng. 2017, 5, 11060–11068. [Google Scholar] [CrossRef]
- Matumba, K.I.; Motloung, M.P.; Ojijo, V.; Ray, S.S.; Sadiku, E.R. Investigation of the Effects of Chain Extender on Material Properties of PLA/PCL and PLA/PEG Blends: Comparative Study between Polycaprolactone and Polyethylene Glycol. Polymers 2023, 15, 2230. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Fenollar, O.; Fombuena, V.; Lopez-Martinez, J.; Balart, R. Improvement of Mechanical Ductile Properties of Poly (3-hydroxybutyrate) by Using Vegetable Oil Derivatives. Macromol. Mater. Eng. 2017, 302, 1600330. [Google Scholar] [CrossRef]
- Mangeon, C.; Michely, L.; Rios de Anda, A.; Thevenieau, F.; Renard, E.; Langlois, V. Natural terpenes used as plasticizers for poly (3-hydroxybutyrate). ACS Sustain. Chem. Eng. 2018, 6, 16160–16168. [Google Scholar] [CrossRef]
- Jin, P.; Pang, A.; Yang, R.; Guo, X.; He, J.; Zhai, J. Study on Mechanical Properties of Polyurethane Cross-Linked P(E-co-T)/PEG Blended Polyether Elastomer. Polymers 2022, 14, 5419. [Google Scholar] [CrossRef]
- Abbasi, M.; Pokhrel, D.; Coats, E.R.; Guho, N.M.; McDonald, A.G. Effect of 3-Hydroxyvalerate Content on Thermal, Mechanical, and Rheological Properties of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Biopolymers Produced from Fermented Dairy Manure. Polymers 2022, 14, 4140. [Google Scholar] [CrossRef]
- Omura, T.; Gato, T.; Maehara, A.; Kimura, S.; Abe, H.; Iwata, T. Thermal degradation behavior of poly [(R)-3-hydroxybutyrate-co-4-hydroxybutyrate]. Polym. Degrad. Stab. 2021, 183, 109460. [Google Scholar] [CrossRef]



| Polyurethane | Mn (g/mol) | Mw (g/mol) | Mp (g/mol) | DI |
|---|---|---|---|---|
| PU400 | 5000 | 7000 | 9000 | 1.40 |
| PU1000 | 6000 | 8000 | 9000 | 1.33 |
| PU1500 | 6000 | 8000 | 10,000 | 1.33 |
| PU400 Content (wt.%) | Biocomposition Designation |
| 5 | K400-5 |
| 10 | K400-10 |
| 15 | K400-15 |
| PU1000 content (wt.%) | - |
| 5 | K1000-5 |
| 10 | K1000-10 |
| 15 | K1000-15 |
| PU1500 content (wt.%) | - |
| 5 | K1500-5 |
| 10 | K1500-10 |
| 15 | K1500-15 |
| Sample | Ton (°C) | T5% (°C) | T10% (°C) | T50% (°C) | Tmax (°C) | Residue at 600 °C (%) |
|---|---|---|---|---|---|---|
| P3HB | 221.1 | 236.2 | 245.6 | 281.2 | 282.1 | 1.10 |
| K400-5 | 252.1 | 266.5 | 271.1 | 307.6 | 283.7 | 1.23 |
| K400-10 | 252.7 | 267.1 | 271.5 | 329.3 | 285.7 | 1.40 |
| K400-15 | 253.6 | 267.3 | 271.8 | 283.8 | 285.2 | 1.90 |
| K1000-5 | 250.1 | 265.0 | 270.2 | 284.5 | 285.1 | 1.67 |
| K1000-10 | 251.7 | 265.6 | 271.0 | 285.7 | 287.1 | 1.60 |
| K1000-15 | 250.9 | 267.6 | 272.2 | 282.6 | 286.2 | 1.76 |
| K1500-5 | 250.2 | 265.5 | 267.3 | 281.3 | 286.1 | 1.57 |
| K1500-10 | 249.8 | 267.4 | 271.4 | 282.7 | 287.2 | 1.68 |
| K1500-15 | 250.8 | 266 | 270.5 | 282.7 | 282.6 | 1.78 |
| Sample | Tg (°C) | ΔCp (J·g−1·°C−1) | Tm1(Onset) (°C) | Tm1(Peak) (°C) | ΔHf1 (J·g−1) | Tm2(Onset) (°C) | Tm2(Peak) (°C) | ΔHf2 (J·g−1) |
|---|---|---|---|---|---|---|---|---|
| P3HB | 7.70 | 0.162 | 159.73 | 165.75 | 91.93 | - | - | - |
| K400-5 | −1.80 | 0.358 | 156.60 | 163.60 | 37.89 | - | - | - |
| K400-10 | 0.40 | 0.226 | 165.30 | 170.90 | 87.33 | - | - | - |
| K400-15 | 1.20 | 0.160 | 163.20 | 171.00 | 58.25 | - | - | - |
| K1000-5 | −40.74 | 0.076 | 14.80 | 21.80 | 0.535 | 166.95 | 172.00 | 92.68 |
| K1000-10 | −43.75 | 0.024 | 16.13 | 20.85 | 0.959 | 165.60 | 170.70 | 86.30 |
| K1000-15 | −39.80 | 0.098 | 15.32 | 22.30 | 5.029 | 166.11 | 171.03 | 81.27 |
| K1500-5 | 3.80 | 0.168 | 34.10 | 39.00 | 0.388 | 163.50 | 169.60 | 88.20 |
| K1500-10 | 3.50 | 0.112 | 33.10 | 41.20 | 4.833 | 163.90 | 171.20 | 86.68 |
| K1500-15 | 1.15 | 0.137 | 35.80 | 42.12 | 8.427 | 163.60 | 169.60 | 84.68 |
| PU400 | −21.32 | 0.296 | - | - | - | - | - | - |
| PU1000 | −27.85 | 0.143 | 3.42 | 49.86 | 76.82 | - | - | - |
| PU1500 | −32.70 | 0.098 | 27.05 | 47.39 | 116.05 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzykowska, B.; Czerniecka-Kubicka, A.; Białkowska, A.; Bakar, M.; Kovářová, M.; Sedlařík, V.; Hanusova, D.; Zarzyka, I. Biopolymer Compositions Based on Poly(3-hydroxybutyrate) and Linear Polyurethanes with Aromatic Rings—Preparation and Properties Evaluation. Polymers 2024, 16, 1618. https://doi.org/10.3390/polym16121618
Krzykowska B, Czerniecka-Kubicka A, Białkowska A, Bakar M, Kovářová M, Sedlařík V, Hanusova D, Zarzyka I. Biopolymer Compositions Based on Poly(3-hydroxybutyrate) and Linear Polyurethanes with Aromatic Rings—Preparation and Properties Evaluation. Polymers. 2024; 16(12):1618. https://doi.org/10.3390/polym16121618
Chicago/Turabian StyleKrzykowska, Beata, Anna Czerniecka-Kubicka, Anita Białkowska, Mohamed Bakar, Miroslava Kovářová, Vladimir Sedlařík, Dominika Hanusova, and Iwona Zarzyka. 2024. "Biopolymer Compositions Based on Poly(3-hydroxybutyrate) and Linear Polyurethanes with Aromatic Rings—Preparation and Properties Evaluation" Polymers 16, no. 12: 1618. https://doi.org/10.3390/polym16121618






