Isolation and Characterization of Spherical Cellulose Nanocrystals Extracted from the Higher Cellulose Yield of the Jenfokie Plant: Morphological, Structural, and Thermal Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
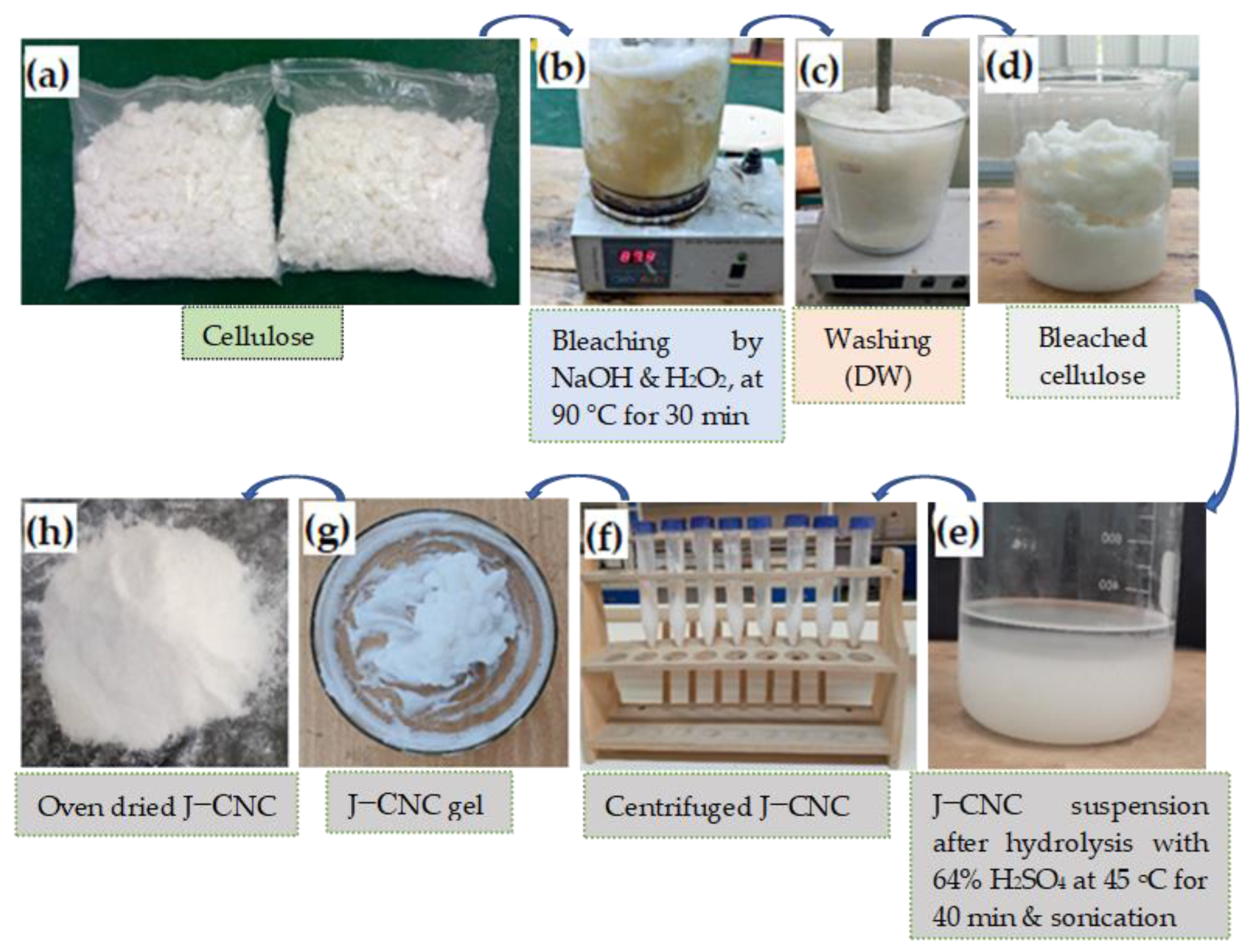
2.2. Micro- and Nanocrystalline Cellulose Preparation
2.3. Characterization of Nanocellulose
2.3.1. Yield Measurement of J–CNC
2.3.2. J–CNC’s Specific Surface Area
2.3.3. Morphological Properties
2.3.4. Fourier Transform Infrared (FT–IR) Spectroscopy Analysis
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Crystallinity Index by X-ray Diffraction (XRD)
3. Results and Discussion
3.1. Analysis of J–CNC Yield and Specific Surface Area
3.2. Morphological Properties
3.2.1. Scanning Electron Microscope
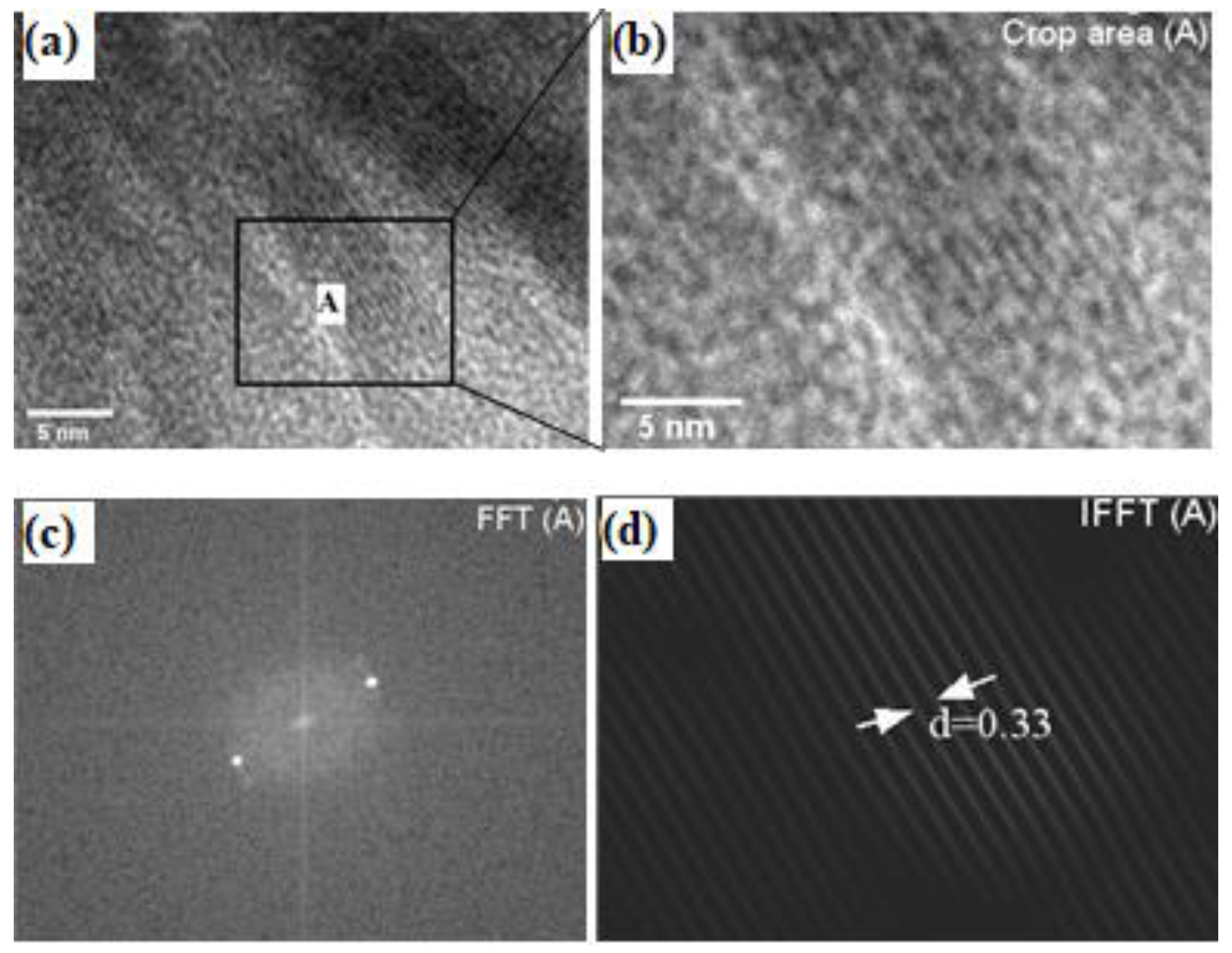
3.2.2. Transmission Electron Microscope
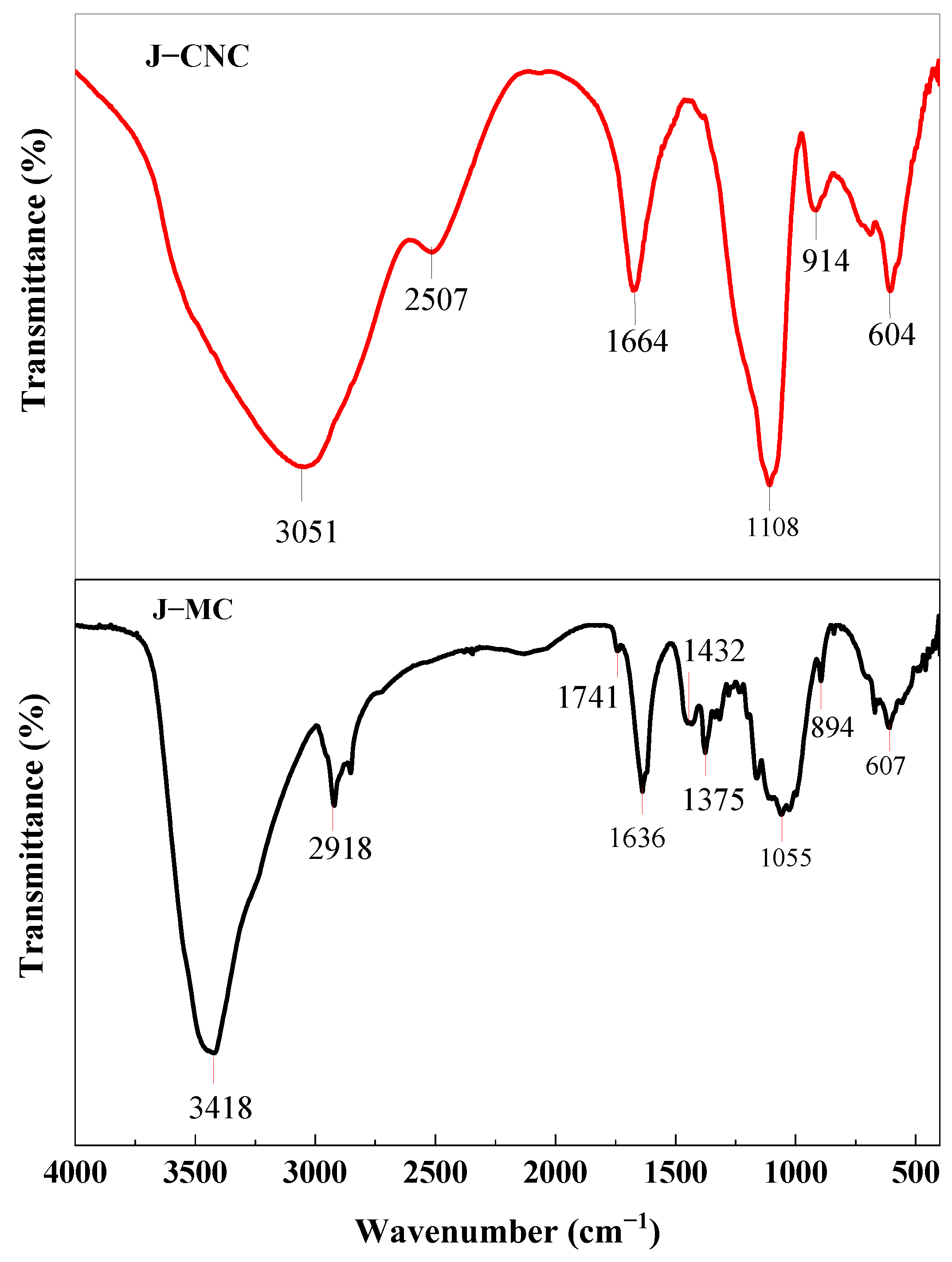
3.3. Fourier Transform Infrared (FT–IR) Spectroscope Analysis
3.4. Thermogravimetric Analysis (TGA)
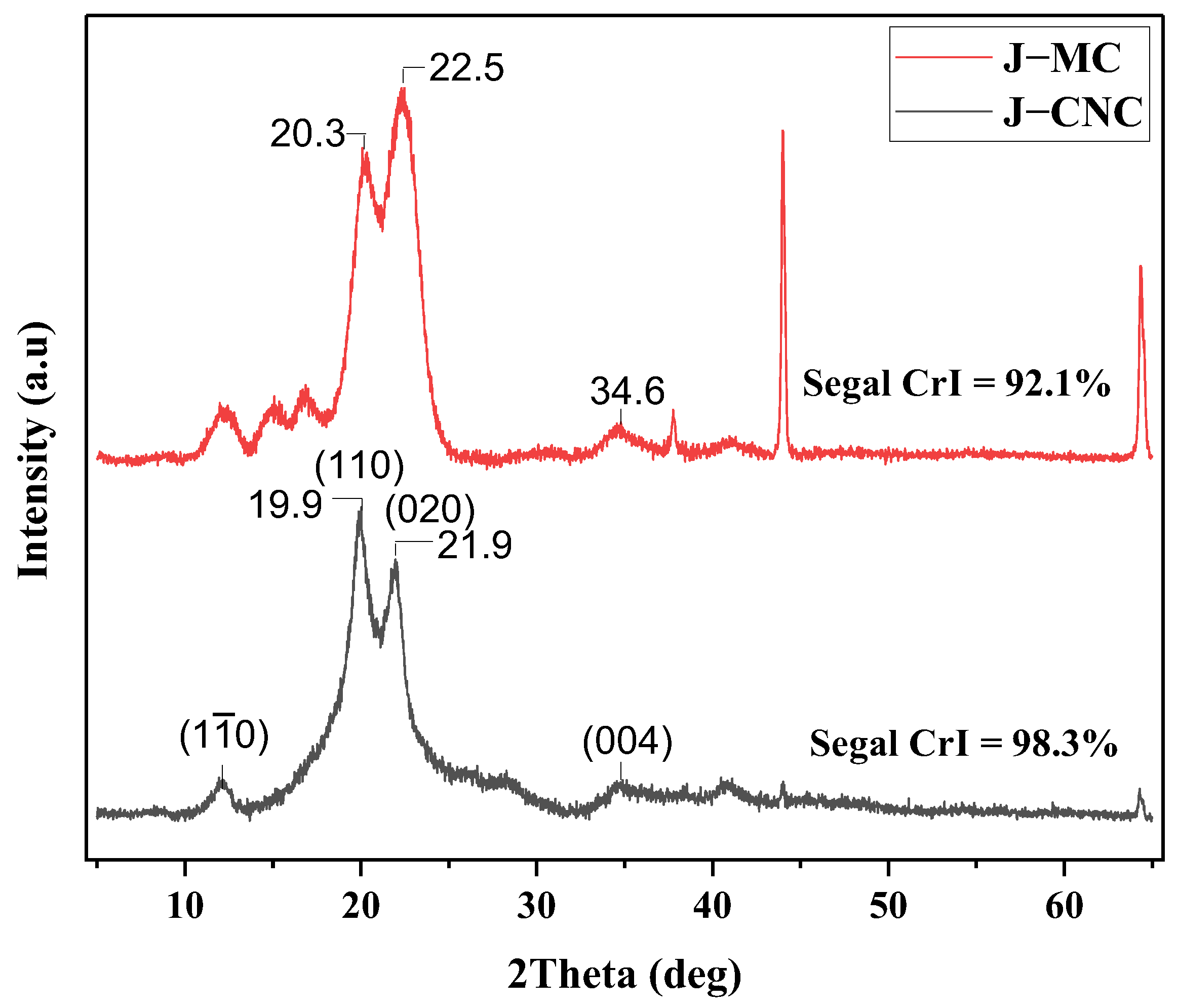
3.5. Crystalline Structure Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Yui, T.; Hayashi, S. Structural stability of the solvated cellulose iiii crystal models: A molecular dynamics study. Cellulose 2008, 16, 151–165. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a versatile green platform: From biosources to materials and their applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Misra, M.A.; Hinrichsen, G.I. Biofibres, biodegradable polymers and biocomposites: An overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Hamawand, I.; Seneweera, S.; Kumarasinghe, P.; Bundschuh, J. Nanoparticle technology for separation of cellulose, hemicellulose and lignin nanoparticles from lignocellulose biomass: A short review. Nano-Struct. Nano-Objects 2020, 24, 100601. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced functional materials based on nanocellulose for pharmaceutical/medical applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef]
- Zhu, P.; Feng, L.; Ding, Z.; Bai, X. Preparation of spherical cellulose nanocrystals from microcrystalline cellulose by mixed acid hydrolysis with different pretreatment routes. Int. J. Mol. Sci. 2022, 23, 10764. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Xu, H.; Balea, A.; Negro, C.; Blanco, A. Nanocellulose from a colloidal material perspective. Front. Mater. Sci. 2023, 10, 1231404. [Google Scholar] [CrossRef]
- Mokhena, T.C.; John, M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2019, 27, 1149–1194. [Google Scholar] [CrossRef]
- Yadav, C.; Saini, A.; Zhang, W.; You, X.; Chauhan, I.; Mohanty, P.; Li, X. Plant-based nanocellulose: A review of routine and recent preparation methods with current progress in its applications as rheology modifier and 3d bioprinting. Int. J. Biol. Macromol. 2018, 166, 1586–1616. [Google Scholar] [CrossRef] [PubMed]
- Fornari, A.; Rossi, M.; Rocco, D.; Mattiello, L. A review of applications of nanocellulose to preserve and protect cultural heritage wood, paintings, and historical papers. Appl. Sci. 2022, 12, 12846. [Google Scholar] [CrossRef]
- Pereda, M.; Dufresne, A.; Aranguren, M.I.; Marcovich, N.E. Polyelectrolyte films based on chitosan/olive oil and reinforced with cellulose nanocrystals. Carbohydr. Polym. 2014, 101, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Habibi, Y.; Adhikari, B. Surface modifications of nanocellulose: From synthesis to high-performance nanocomposites. Prog. Polym. Sci. 2021, 119, 101418. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent advances in nanocellulose-based different biomaterials: Types, properties, and emerging applications. J. Mater. Res. Technol. 2021, 14, 2601–2623. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Kumar, R.; Rai, B.; Gahlyan, S.; Kumar, G. A comprehensive review on production, surface modification and characterization of nanocellulose derived from biomass and its commercial applications. Express Polym. Lett. 2021, 15, 104–120. [Google Scholar] [CrossRef]
- Díaz-Cruz, C.A.; Caicedo, C.; Jiménez-Regalado, E.J.; de León, R.D.; López-González, R.; Aguirre-Loredo, R.Y. Evaluation of the antimicrobial, thermal, mechanical, and barrier properties of corn starch-chitosan biodegradable films reinforced with cellulose nanocrystals. Polymers 2022, 14, 2166. [Google Scholar] [CrossRef]
- Chausali, N.; Saxena, J.; Prasad, R. Recent trends in nanotechnology applications of bio-based packaging. J. Agric. Food Res. 2022, 7, 100257. [Google Scholar] [CrossRef]
- Ilyas, R.; Sapuan, S.; Atikah, M.; Asyraf, M.; Rafiqah, S.A.; Aisyah, H.; Nurazzi, N.M.; Norrrahim, M. Effect of hydrolysis time on the morphological, physical, chemical, and thermal behavior of sugar palm nanocrystalline cellulose (Arenga pinnata (Wurmb.) merr). Text. Res. J. 2020, 91, 152–167. [Google Scholar] [CrossRef]
- Fiorello, I.; Del Dottore, E.; Tramacere, F.; Mazzolai, B. Taking inspiration from climbing plants: Methodologies and benchmarks—A review. Bioinspir. Biomim. 2020, 15, 031001. [Google Scholar] [CrossRef] [PubMed]
- Manso, M.; Castro-Gomes, J. Green wall systems: A review of their characteristics. Renew. Sustain. Energy Rev. 2015, 41, 863–871. [Google Scholar] [CrossRef]
- Gentry, A.H. The distribution and evolution of climbing plants. Biol. Vines 1992, 351, 3–50. [Google Scholar]
- Naik, R.K. Studies on physical properties of sisal (Agave sisalana) plant leaves. Int. J. Agric. Sci. 2016, 8, 2004–2007. [Google Scholar]
- Berhanu, H.; Kiflie, Z.; Miranda, I.; Lourenço, A.; Ferreira, J.; Feleke, S.; Yimam, A.; Pereira, H. Characterization of crop residues from false banana/ensete ventricosum/in ethiopia in view of a full-resource valorization. PLoS ONE 2018, 13, e0199422. [Google Scholar] [CrossRef] [PubMed]
- Nkhabu, K.; Liphoto, M.; Ntahane, T.; Senoko, K. Plant Sciences, and Phytology, Genetic diversity of stinging nettle (Urtica dioica) by agro morphological markers. Eur. J. Bot. Plant Sci. Phytol. 2021, 6, 51–68. [Google Scholar]
- Goudenhooft, C.; Alméras, T.; Bourmaud, A.; Baley, C. The remarkable slenderness of flax plant and pertinent factors affecting its mechanical stability. Biosyst. Eng. 2019, 178, 1–8. [Google Scholar] [CrossRef]
- Masum, S.; Ali, M.; Islam, M.; Sultana, S.J.H. Influence of plant spacing and post emergence herbicide on the yield of white jute (Corchorus capsularis). Hand 2011, 18, 5. [Google Scholar]
- Wossine, S.E.; Thothadri, G.; Tufa, H.B. Extraction and characterization of cellulosic fibers from jenfokie and doby stems: Effect of extraction methods on physicochemical, mechanical, and thermal properties. J. Nat. Fibers 2023, 20, 2229518. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Jin, T.; He, H.; Liu, L. Preparation of nanocellulose and its potential in reinforced composites: A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 919–946. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, S.; Zhang, N.; Zhang, J. Preparation and characterization of nanocrystalline cellulose via low-intensity ultrasonic-assisted sulfuric acid hydrolysis. Cellulose 2013, 21, 335–346. [Google Scholar] [CrossRef]
- Mendez, J.C.; Hiemstra, T.; Koopmans, G.F. Assessing the reactive surface area of soils and the association of soil organic carbon with natural oxide nanoparticles using ferrihydrite as proxy. Environ. Sci. Technol. 2020, 54, 11990–12000. [Google Scholar] [CrossRef] [PubMed]
- Panicker, A.M.; Rajesh, K.A.; Varghese, T.O. Mixed morphology nanocrystalline cellulose from sugarcane bagasse fibers/poly(lactic acid) nanocomposite films: Synthesis, fabrication and characterization. Iran. Polym. J. 2017, 26, 125–136. [Google Scholar] [CrossRef]
- Kalhori, F.; Yazdyani, H.; Khademorezaeian, F.; Hamzkanloo, N.; Mokaberi, P.; Hosseini, S.; Chamani, J. Enzyme activity inhibition properties of new cellulose nanocrystals from citrus medica l. Pericarp: A perspective of cholesterol lowering. J. Lumin. 2022, 37, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, Y.; Wu, Q.; Wei, Z.; Lin, X.; Chen, Y. Preparation and characterization of cellulose nanocrystal extraction from pennisetum hydridum fertilized by municipal sewage sludge via sulfuric acid hydrolysis. Front. Energy Res. 2021, 9, 774783. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Ravishankar, K.; Dhamodharan, R. Preparation of nanofibrillated cellulose and nanocrystalline cellulose from surgical cotton and cellulose pulp in hot-glycerol medium. Cellulose 2019, 26, 3127–3141. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, B.; Xia, Q.; Meng, J.; Chen, W.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient cleavage of strong hydrogen bonds in cotton by deep eutectic solvents and facile fabrication of cellulose nanocrystals in high yields. ACS Sustain. Chem. Eng. 2017, 5, 7623–7631. [Google Scholar] [CrossRef]
- Kasiri, N.; Fathi, M. Production of cellulose nanocrystals from pistachio shells and their application for stabilizing pickering emulsions. Int. J. Biol. Macromol. 2018, 106, 1023–1031. [Google Scholar] [CrossRef]
- Darpentigny, C.; Molina-Boisseau, S.; Nonglaton, G.; Bras, J.; Jean, B. Ice-templated freeze-dried cryogels from tunicate cellulose nanocrystals with high specific surface area and anisotropic morphological and mechanical properties. Cellulose 2019, 27, 233–247. [Google Scholar] [CrossRef]
- Sekar, S.; Ahmed, A.T.A.; Inamdar, A.I.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Activated carbon-decorated spherical silicon nanocrystal composites synchronously-derived from rice husks for anodic source of lithium-ion battery. Nanomaterials 2019, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, Y.; Zhao, H.; Chen, X.; Jiang, C.; Bi, S.; Liu, Y. Preparation of porous composite bio-carriers from lignin-carbohydrate complexes and cellulose nanocrystals, and their application in the culture of human hepatocytes. BioResources 2019, 14, 6465–6484. [Google Scholar] [CrossRef]
- Singh, G.P.; Madiwale, P.V.; Adivarekar, R.V. Preparation and characterization of microcrystalline cellulose (mcc) from renewable source. Curr. Appl. Polym. Sci. 2018, 1, 152–158. [Google Scholar] [CrossRef]
- Ye, S.; Yu, H.-Y.; Wang, D.; Zhu, J.; Gu, J. Green acid-free one-step hydrothermal ammonium persulfate oxidation of viscose fiber wastes to obtain carboxylated spherical cellulose nanocrystals for oil/water pickering emulsion. Cellulose 2018, 25, 5139–5155. [Google Scholar] [CrossRef]
- Dong, H.; Ding, Q.; Jiang, Y.; Li, X.; Han, W. Pickering emulsions stabilized by spherical cellulose nanocrystals. Carbohydr. Polym. 2021, 265, 118101. [Google Scholar] [CrossRef] [PubMed]
- Sadare, O.O.; Mabunda, N.; Ikegwu, U.M.; Keitemoge, M.K.; Daramola, M.O.; Moothi, K. Parametric optimization of the production of cellulose nanocrystals (cncs) from south african corncobs via an empirical modelling approach. Sci. Rep. 2022, 12, 18665. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.K.; Wesley, O.N.; Nathan, O.; Moloto, M.J. Chemically purified cellulose and its nanocrystals from sugarcane baggase: Isolation and characterization. Heliyon 2019, 5, e02635. [Google Scholar] [CrossRef]
- Bao, C.; Chen, X.; Liu, C.; Liao, Y.; Huang, Y.; Hao, L.; Yan, H.; Lin, Q. Extraction of cellulose nanocrystals from microcrystalline cellulose for the stabilization of cetyltrimethylammonium bromide-enhanced pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125442. [Google Scholar] [CrossRef]
- Hashaikeh, R.; Abushammala, H. Acid mediated networked cellulose: Preparation and characterization. Carbohydr. Polym. 2011, 83, 1088–1094. [Google Scholar] [CrossRef]
- Lu, Q.; Lu, L.; Li, Y.; Huang, B. Facile manufacture of cellulose nanoparticles in high yields by efficient cleavage of hydrogen bonds via mechanochemical synergy. Cellulose 2019, 26, 7741–7751. [Google Scholar] [CrossRef]
- Trilokesh, C.; Bavadharani, P.; Mahapriyadarshini, M.; Janani, R.; Uppuluri, K.B. Recycling baby diaper waste into cellulose and nanocellulose. Waste Biomass Valorization 2020, 12, 4299–4306. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, S.; Jiang, H.; Liu, S.; Yao, Y.; Zhang, T.; Li, Q. Synthesis and characterization of amine-modified spherical nanocellulose aerogels. J. Mater. Sci. 2018, 53, 13304–13315. [Google Scholar] [CrossRef]
- Xu, J.-T.; Chen, X.-Q. Preparation and characterization of spherical cellulose nanocrystals with high purity by the composite enzymolysis of pulp fibers. Bioresour. Technol. 2019, 291, 121842. [Google Scholar] [CrossRef] [PubMed]
- Sainorudin, M.H.; Abdullah, N.A.; Rani, M.S.A.; Mohammad, M.; Mahizan, M.; Shadan, N.; Kadir, N.H.A.; Yaakob, Z.; El-Denglawey, A.; Alam, M. Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. Leaves: Cytocompatibility and molecular docking studies. Nanotechnol. Rev. 2021, 10, 793–806. [Google Scholar] [CrossRef]
- Thakur, M.; Sharma, A.; Ahlawat, V.; Bhattacharya, M.; Goswami, S. Process optimization for the production of cellulose nanocrystals from rice straw derived α-cellulose. Mater. Sci. Energy Technol. 2020, 3, 328–334. [Google Scholar] [CrossRef]
- de Souza, A.G.; Junqueira, M.T.; de Lima, G.F.; Rangari, V.K.; Rosa, D.S. A new proposal of preparation of different polymorphs of nanocellulose from eucalyptus citriodora. J. Polym. Environ. 2020, 28, 1150–1159. [Google Scholar] [CrossRef]
- Gond, R.K.; Gupta, M.K.; Jawaid, M. Extraction of nanocellulose from sugarcane bagasse and its characterization for potential applications. Polym. Compos. 2021, 42, 5400–5412. [Google Scholar] [CrossRef]
- Pandi, N.; Sonawane, S.H.; Kishore, K.A. Synthesis of cellulose nanocrystals (cncs) from cotton using ultrasound-assisted acid hydrolysis. Ultrason. Sonochem. 2021, 70, 105353. [Google Scholar] [CrossRef]
- Shahabi-Ghahafarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Preparation and characterization of nanocellulose from beer industrial residues using acid hydrolysis/ultrasound. Fibers Polym. 2015, 16, 529–536. [Google Scholar] [CrossRef]
- França, D.; de Barros, J.R.S.; Faez, R. Spray-dried cellulose nanofibrils microparticles as a vehicle for enhanced efficiency fertilizers. Cellulose 2021, 28, 1571–1585. [Google Scholar] [CrossRef]
- Li, M.; He, B.; Chen, Y.; Zhao, L. Physicochemical properties of nanocellulose isolated from cotton stalk waste. ACS Omega 2021, 6, 25162–25169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Qin, Z.; Hu, J.; Liu, Q.; Wei, T.; Li, W.; Ling, Y.; Liu, B. Facile and rapid one-step extraction of carboxylated cellulose nanocrystals by h(2)so(4)/hno(3) mixed acid hydrolysis. Carbohydr. Polym. 2020, 231, 115701. [Google Scholar] [CrossRef] [PubMed]
- Worku, L.A.; Bachheti, R.K.; Tadesse, M.G. Preparation and characterization of carboxylated cellulose nanocrystals from Oxytenanthera abyssinica (Ethiopian lowland bamboo) cellulose via citric acid anhydrous hydrolysis catalyzed by sulfuric acid. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Merlini, A.; Claumann, C.; Zibetti, A.W.; Coirolo, A.; Rieg, T.; Machado, R.A.F. Kinetic study of the thermal decomposition of cellulose nanocrystals with different crystal structures and morphologies. Ind. Eng. Chem. Res. 2020, 59, 13428–13439. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, E.d.M.; Corrêa, A.C.; Manzoli, A.; Leite, F.d.L.; de Oliveira, C.R.; Mattoso, L.H.C. Cellulose nanofibers from white and naturally colored cotton fibers. Cellulose 2010, 17, 595–606. [Google Scholar] [CrossRef]
- Anwar, B.; Bundjali, B.; Sunarya, Y.; Arcana, I.M. Properties of bacterial cellulose and its nanocrystalline obtained from pineapple peel waste juice. Fibers Polym. 2021, 22, 1228–1236. [Google Scholar] [CrossRef]
- Özkan, B.; Güner, M. Isolation, characterization, and comparison of nanocrystalline cellulose from solid wastes of horse chestnut and chestnut seed shell. Cellulose 2022, 29, 6629–6644. [Google Scholar] [CrossRef]
- Doan, T.K.Q.; Chiang, K.Y. Characteristics and kinetics study of spherical cellulose nanocrystal extracted from cotton cloth waste by acid hydrolysis. Sustain. Environ. Res. 2022, 32, 26. [Google Scholar] [CrossRef]
- Nair, S.S.; Yan, N. Effect of high residual lignin on the thermal stability of nanofibrils and its enhanced mechanical performance in aqueous environments. Cellulose 2015, 22, 3137–3150. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Reid, M.S.; Bras, J.; Heux, L.; Godoy-Vargas, J.; Panga, M.K.R.; Cranston, E.D. Insight into thermal stability of cellulose nanocrystals from new hydrolysis methods with acid blends. Cellulose 2018, 26, 507–528. [Google Scholar] [CrossRef]
- El Achaby, M.; El Miri, N.; Hannache, H.; Gmouh, S.; Ben Youcef, H.; Aboulkas, A. Production of cellulose nanocrystals from vine shoots and their use for the development of nanocomposite materials. Int. J. Biol. Macromol. 2018, 117, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Phys. Chem. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Rana, S.; Rahim, A.; Mosharraf, P.; Tipu, F.K.; Chowdhury, J.A.; Haque, M.R.; Kabir, S.; Amran, S.; Chowdhury, A.A. Chowdhury, Morphological, spectroscopic and thermal analysis of cellulose nanocrystals extracted from waste jute fiber by acid hydrolysis. Polymers 2023, 15, 1530. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Ramana, K.; Bawa, A. Siddaramaiah, Bacterial cellulose nanocrystals exhibiting high thermal stability and their polymer nanocomposites. Int. J. Biol. Macromol. 2011, 48, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.-L.; Gunny, A.A.N.; Kasim, F.H.; Gopinath, S.C.B.; Kamaludin, N.H.I.; Arbain, D. Cellulose nanocrystals from bleached rice straw pulp: Acidic deep eutectic solvent versus sulphuric acid hydrolyses. Cellulose 2021, 28, 6183–6199. [Google Scholar] [CrossRef]
- Bahloul, A.; Semlali, F.-Z.; Oumam, M.; Hannache, H.; Kassab, Z.; El Achaby, M. Starch bio-nanocomposites based on phosphorylated and sulphated cellulose nanocrystals extracted from pepper plant residue: Effect of surface functionality on property improvements. Cellulose 2023, 30, 5051–5070. [Google Scholar] [CrossRef]
- Xing, L.; Gu, J.; Zhang, W.; Tu, D.; Hu, C. Cellulose i and ii nanocrystals produced by sulfuric acid hydrolysis of tetra pak cellulose i. Carbohydr. Polym. 2018, 192, 184–192. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Messa, L.L.; Faez, R. Spray-dried chitosan/nanocellulose microparticles: Synergistic effects for the sustained release of npk fertilizer. Cellulose 2020, 27, 10077–10093. [Google Scholar] [CrossRef]
- Michelin, M.; Gomes, D.G.; Romaní, A.; Polizeli, M.d.L.T.M.; Teixeira, J.A. Nanocellulose production: Exploring the enzymatic route and residues of pulp and paper industry. Molecules 2020, 25, 3411. [Google Scholar] [CrossRef] [PubMed]
- Akinjokun, A.I.; Petrik, L.F.; Ogunfowokan, A.O.; Ajao, J.; Ojumu, T.V. Isolation and characterization of nanocrystalline cellulose from cocoa pod husk (cph) biomass wastes. Heliyon 2021, 7, e06680. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yao, Z.; Zhou, J.; He, M.; Jiang, Q.; Li, S.; Ma, Y.; Liu, M.; Luo, S. Isolation and characterization of cellulose nanocrystals from pueraria root residue. Int. J. Biol. Macromol. 2019, 129, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Doh, H.; Lee, M.H.; Whiteside, W.S. Physicochemical characteristics of cellulose nanocrystals isolated from seaweed biomass. Food Hydrocoll. 2020, 102, 105542. [Google Scholar] [CrossRef]
- Korolovych, V.F.; Cherpak, V.; Nepal, D.; Ng, A.; Shaikh, N.R.; Grant, A.; Xiong, R.; Bunning, T.J.; Tsukruk, V.V. Cellulose nanocrystals with different morphologies and chiral properties. Polymer 2018, 145, 334–347. [Google Scholar] [CrossRef]
- Maiti, S.; Jayaramudu, J.; Das, K.; Reddy, S.M.; Sadiku, R.; Ray, S.S.; Liu, D. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr. Polym. 2013, 98, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.-H.; Ismadji, S. Nanocelluloses: Sources, pretreatment, isolations, modification, and its application as the drug carriers. Polymers 2021, 13, 2052. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Indirasetyo, N.L. Kusmono, Isolation and properties of cellulose nanocrystals fabricated by ammonium persulfate oxidation from sansevieria trifasciata fibers. Fibers 2022, 10, 61. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, Y.; Han, B.; Zhang, Y. Effect of oxidation time on the properties of cellulose nanocrystals from hybrid poplar residues using the ammonium persulfate. Carbohydr. Polym. 2017, 174, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Adel, A.; El-Shafei, A.; Ibrahim, A.; Al-Shemy, M. Extraction of oxidized nanocellulose from date palm (Phoenix dactylifera L.) sheath fibers: Influence of ci and cii polymorphs on the properties of chitosan/bionanocomposite films. Ind. Crop. Prod. 2018, 124, 155–165. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Isolation of oxidized nanocellulose from rice straw using the ammonium persulfate method. Cellulose 2018, 25, 2143–2149. [Google Scholar] [CrossRef]
- Lu, Q.; Cai, Z.; Lin, F.; Tang, L.; Wang, S.; Huang, B. Extraction of cellulose nanocrystals with a high yield of 88% by simultaneous mechanochemical activation and phosphotungstic acid hydrolysis. ACS Sustain. Chem. Eng. 2016, 4, 2165–2172. [Google Scholar] [CrossRef]
- Goh, K.Y.; Ching, Y.C.; Chuah, C.H.; Abdullah, L.C.; Liou, N.-S. Individualization of microfibrillated celluloses from oil palm empty fruit bunch: Comparative studies between acid hydrolysis and ammonium persulfate oxidation. Cellulose 2015, 23, 379–390. [Google Scholar] [CrossRef]


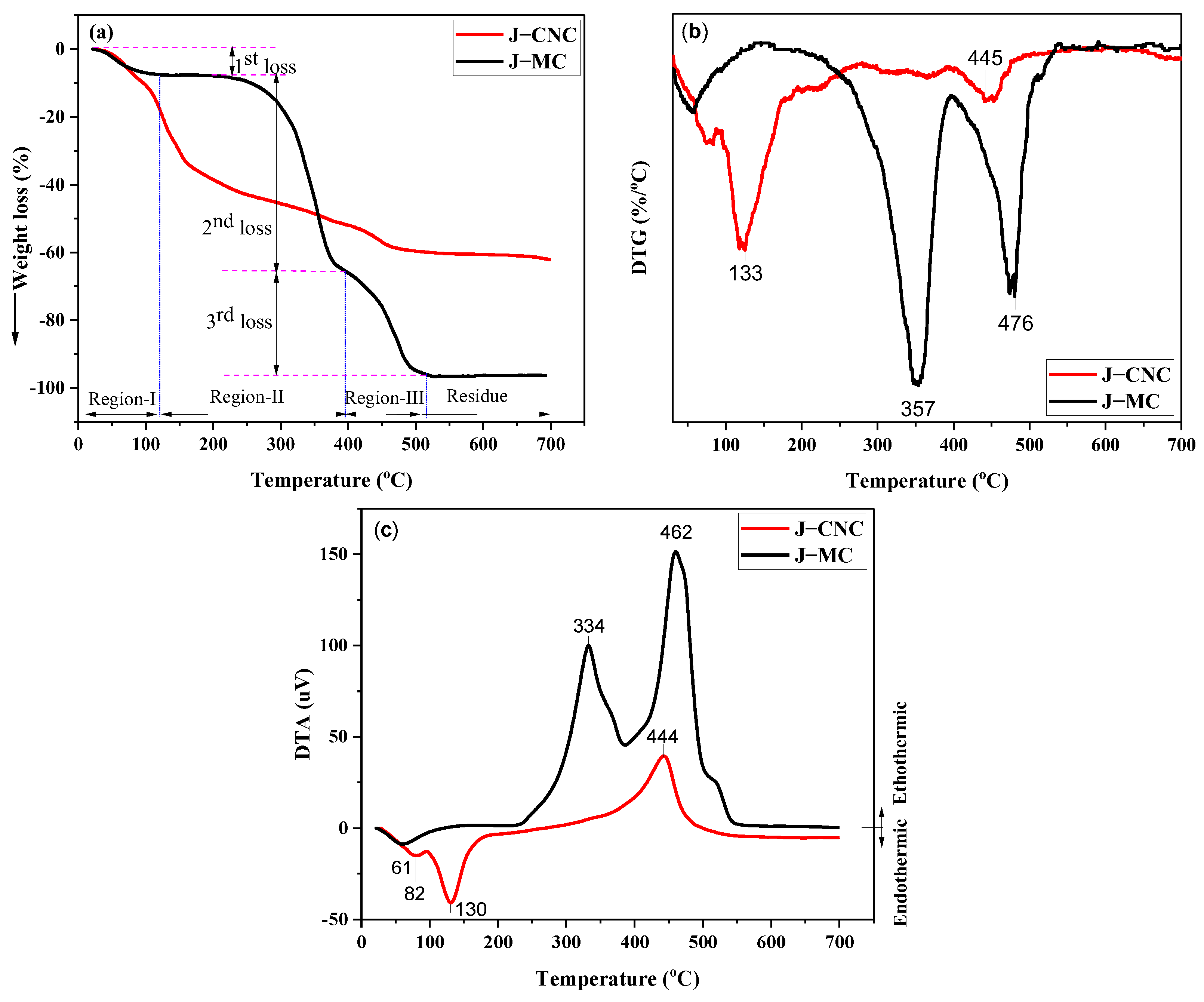
| Source | Region I | Region II | Region III | |||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Tonset (°C) | Tmax (°C) | WL (%) | Tonset (°C) | Tmax (°C) | WL (%) | Residue at 700 (°C) | |
| J–MC | RT–123 | 234 | 357 | 54 | 395 | 475 | 35 | 3 |
| J–CNC | RT–88 | 100 | 133 | 37 | 258 | 445 | 18 | 35 |
| Samples | 2theta (Degree) | d-Spacing (nm) | Crystallinity Index (%) | Mean Crystallinity Size (D, nm) | Mean Diameter J–MC (μm) | Mean Diameter J–CNC (nm) |
|---|---|---|---|---|---|---|
| J–MC | 22.3 | 0.52 | 92.1 | 4.22 | 12.80 | - |
| J–CNC | 19.9 | 0.45 | 98.4 | 6.13 | - | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wossine, S.E.; Thothadri, G.; Tufa, H.B.; Tucho, W.M.; Murtaza, A.; Edacherian, A.; Sayeed Ahmed, G.M. Isolation and Characterization of Spherical Cellulose Nanocrystals Extracted from the Higher Cellulose Yield of the Jenfokie Plant: Morphological, Structural, and Thermal Properties. Polymers 2024, 16, 1629. https://doi.org/10.3390/polym16121629
Wossine SE, Thothadri G, Tufa HB, Tucho WM, Murtaza A, Edacherian A, Sayeed Ahmed GM. Isolation and Characterization of Spherical Cellulose Nanocrystals Extracted from the Higher Cellulose Yield of the Jenfokie Plant: Morphological, Structural, and Thermal Properties. Polymers. 2024; 16(12):1629. https://doi.org/10.3390/polym16121629
Chicago/Turabian StyleWossine, Solomon Estifo, Ganesh Thothadri, Habtamu Beri Tufa, Wakshum Mekonnen Tucho, Adil Murtaza, Abhilash Edacherian, and Gulam Mohammed Sayeed Ahmed. 2024. "Isolation and Characterization of Spherical Cellulose Nanocrystals Extracted from the Higher Cellulose Yield of the Jenfokie Plant: Morphological, Structural, and Thermal Properties" Polymers 16, no. 12: 1629. https://doi.org/10.3390/polym16121629
APA StyleWossine, S. E., Thothadri, G., Tufa, H. B., Tucho, W. M., Murtaza, A., Edacherian, A., & Sayeed Ahmed, G. M. (2024). Isolation and Characterization of Spherical Cellulose Nanocrystals Extracted from the Higher Cellulose Yield of the Jenfokie Plant: Morphological, Structural, and Thermal Properties. Polymers, 16(12), 1629. https://doi.org/10.3390/polym16121629







