Hydrophobic Modification of Pectin Aerogels via Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Characterization of Pectin
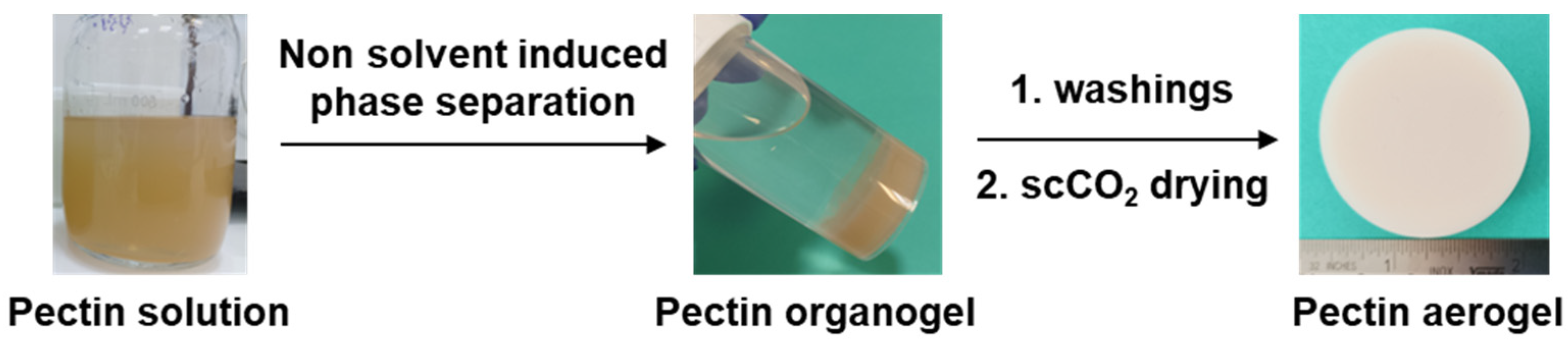
2.2.2. Preparation of Pectin Aerogels
2.2.3. MTMS Chemical Vapor Deposition on Pectin Aerogels
2.2.4. Characterization of Pectin Aerogels
3. Results
3.1. Properties of Neat Pectin Aerogels
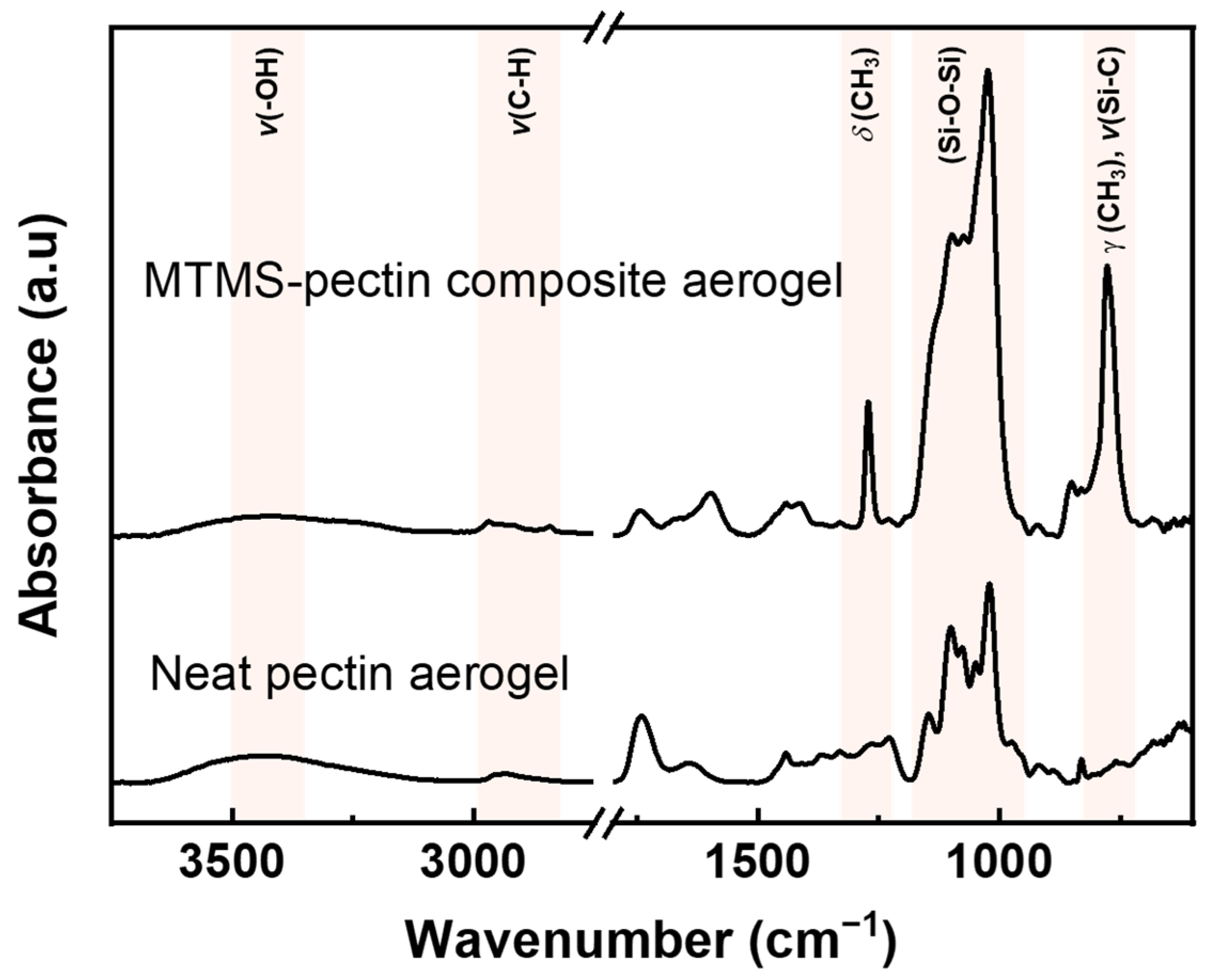
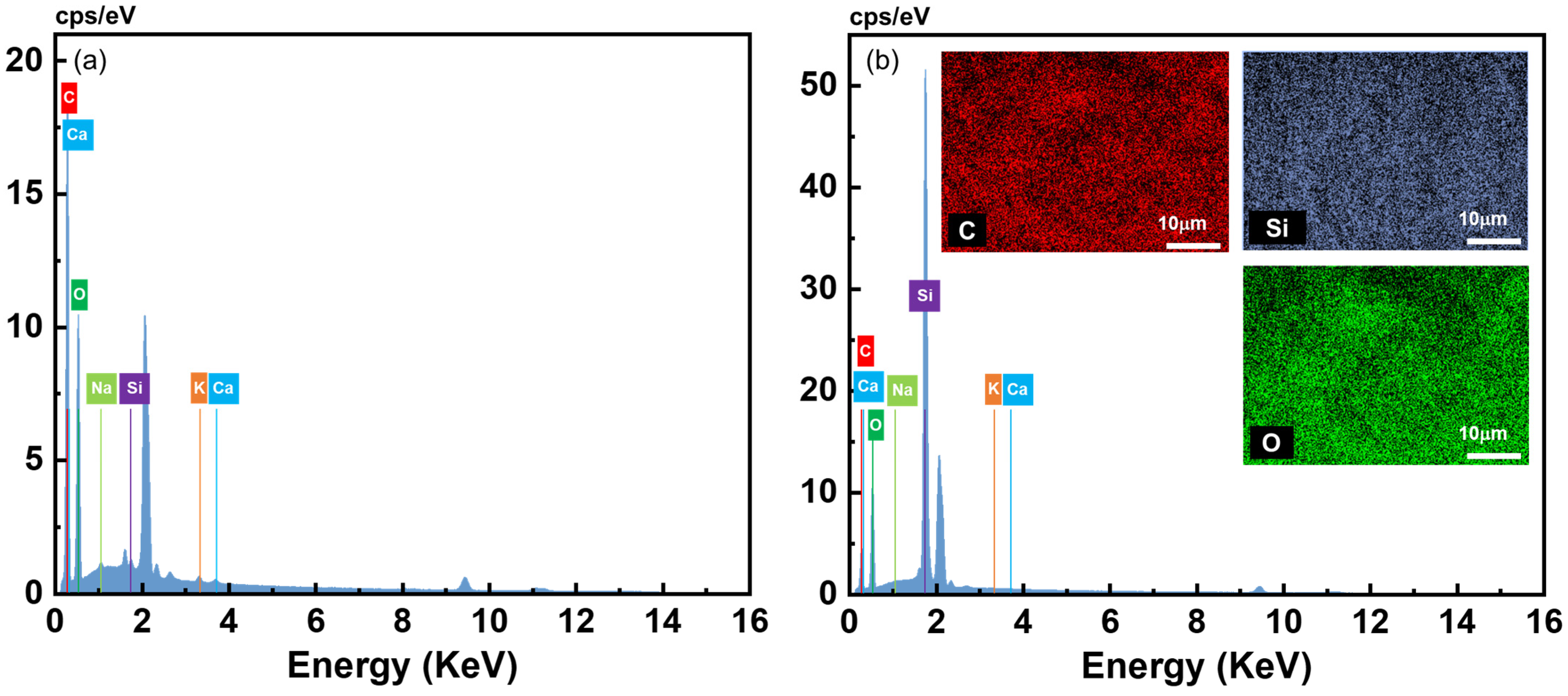
3.2. Properties of Composite Pectin Aerogels after 24 h of MTMS Vapor Deposition
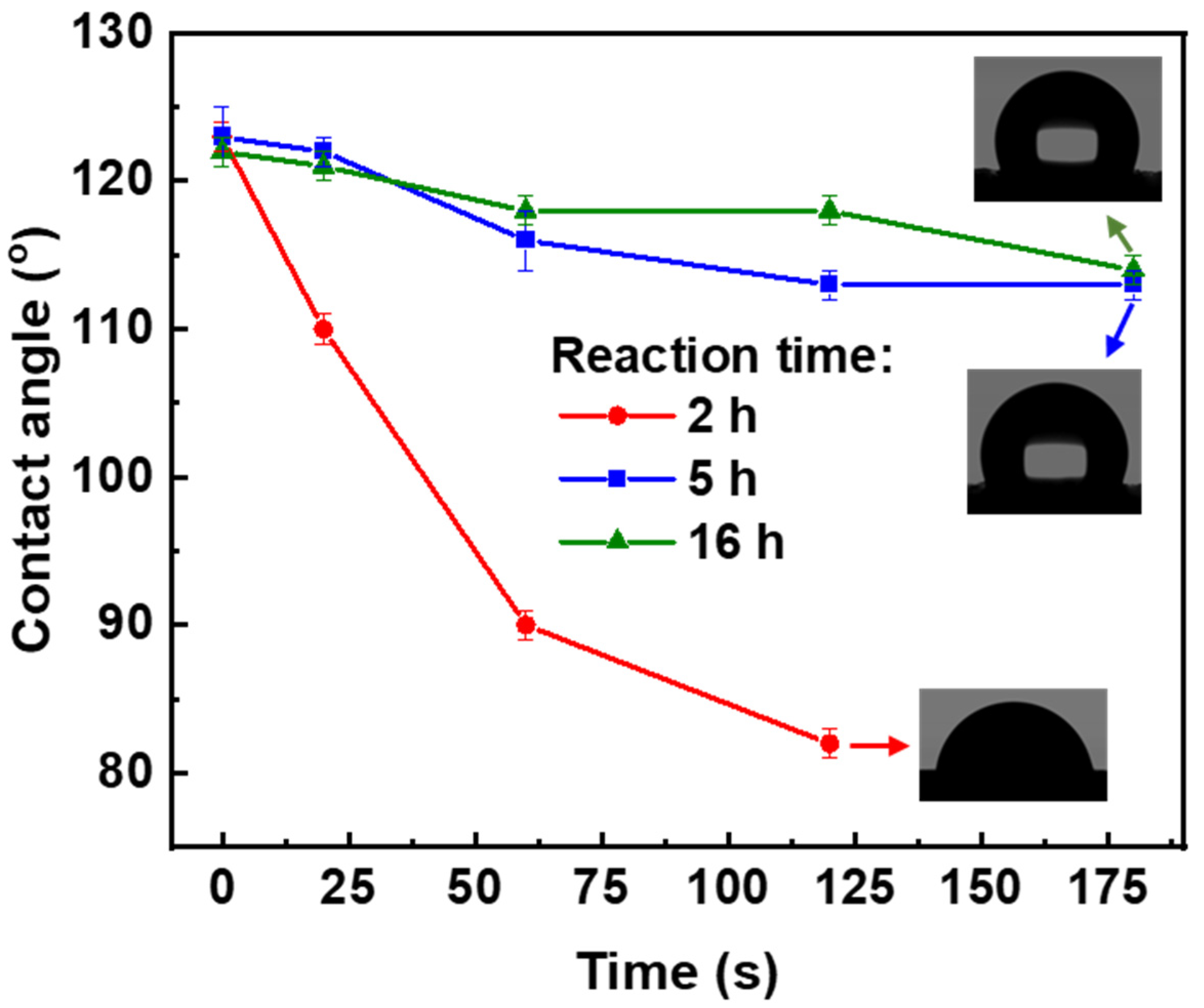
3.3. Influence of Reaction Time of MTMS Chemical Vapor Deposition on Composite Aerogel Properties
3.4. Aging Studies of Neat Pectin and Composite Aerogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Springer Handbook of Aerogels; Aegerter, M.A.; Leventis, N.; Koebel, M.; Steiner Iii, S.A. (Eds.) Springer Handbooks; Springer International Publishing: Cham, Switzerland, 2023; ISBN 978-3-030-27321-7. [Google Scholar]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica Aerogel; Synthesis, Properties and Characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Neugebauer, A.; Chen, K.; Tang, A.; Allgeier, A.; Glicksman, L.R.; Gibson, L.J. Thermal Conductivity and Characterization of Compacted, Granular Silica Aerogel. Energy Build. 2014, 79, 47–57. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel Insulation for Building Applications: A State-of-the-Art Review. Energy Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Linhares, T.; de Amorim, M.T.P.; Durães, L. Silica Aerogel Composites with Embedded Fibres: A Review on Their Preparation, Properties and Applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Lu, X.; Arduini-Schuster, M.C.; Kuhn, J.; Nilsson, O.; Fricke, J.; Pekala, R.W. Thermal Conductivity of Monolithic Organic Aerogels. Science 1992, 255, 971–972. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, A.; Marechal, J.C.; Repoux, M.; Moreno, M.; Achard, P. Preparation of Polyurethane-Based Aerogels and Xerogels for Thermal Superinsulation. J. Non-Cryst. Solids 2004, 350, 372–378. [Google Scholar] [CrossRef]
- Demilecamps, A.; Alves, M.; Rigacci, A.; Reichenauer, G.; Budtova, T. Nanostructured Interpenetrated Organic-Inorganic Aerogels with Thermal Superinsulating Properties. J. Non-Cryst. Solids 2016, 452, 259–265. [Google Scholar] [CrossRef]
- Markevicius, G.; Ladj, R.; Niemeyer, P.; Budtova, T.; Rigacci, A. Ambient-Dried Thermal Superinsulating Monolithic Silica-Based Aerogels with Short Cellulosic Fibers. J. Mater. Sci. 2017, 52, 2210–2221. [Google Scholar] [CrossRef]
- Karamikamkar, S.; Abidli, A.; Aghababaei Tafreshi, O.; Ghaffari-Mosanenzadeh, S.; Buahom, P.; Naguib, H.E.; Park, C.B. Nanocomposite Aerogel Network Featuring High Surface Area and Superinsulation Properties. Chem. Mater. 2024, 36, 642–656. [Google Scholar] [CrossRef]
- Gavillon, R.; Budtova, T. Aerocellulose: New Highly Porous Cellulose Prepared from Cellulose−NaOH Aqueous Solutions. Biomacromolecules 2008, 9, 269–277. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II Aerogels: A Review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Yoda, S. Chitosan Aerogels: Transparent, Flexible Thermal Insulators. Chem. Mater. 2015, 27, 7569–7572. [Google Scholar] [CrossRef]
- Baudron, V.; Taboada, M.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Production of Starch Aerogel in Form of Monoliths and Microparticles. Colloid Polym. Sci. 2020, 298, 477–494. [Google Scholar] [CrossRef]
- Druel, L.; Bardl, R.; Vorwerg, W.; Budtova, T. Starch Aerogels: A Member of the Family of Thermal Superinsulating Materials. Biomacromolecules 2017, 18, 4232–4239. [Google Scholar] [CrossRef] [PubMed]
- Alnaief, M.; Alzaitoun, M.A.; García-González, C.A.; Smirnova, I. Preparation of Biodegradable Nanoporous Microspherical Aerogel Based on Alginate. Carbohydr. Polym. 2011, 84, 1011–1018. [Google Scholar] [CrossRef]
- Rodríguez-Dorado, R.; López-Iglesias, C.; García-González, C.A.; Auriemma, G.; Aquino, R.P.; Del Gaudio, P. Design of Aerogels, Cryogels and Xerogels of Alginate: Effect of Molecular Weight, Gelation Conditions and Drying Method on Particles’ Micromeritics. Molecules 2019, 24, 1049. [Google Scholar] [CrossRef] [PubMed]
- Rudaz, C.; Courson, R.; Bonnet, L.; Calas-Etienne, S.; Sallée, H.; Budtova, T. Aeropectin: Fully Biomass-Based Mechanically Strong and Thermal Superinsulating Aerogel. Biomacromolecules 2014, 15, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Groult, S.; Budtova, T. Tuning Structure and Properties of Pectin Aerogels. Eur. Polym. J. 2018, 108, 250–261. [Google Scholar] [CrossRef]
- Groult, S.; Budtova, T. Thermal Conductivity/Structure Correlations in Thermal Super-Insulating Pectin Aerogels. Carbohydr. Polym. 2018, 196, 73–81. [Google Scholar] [CrossRef]
- Effraimopoulou, E.; Kalmár, J.; Paul, G.; Marchese, L.; Ioannou, D.; Paraskevopoulou, P.; Gurikov, P. Whey Protein Isolate-Based Aerogels with Improved Hydration Properties for Food Packaging Applications. ACS Appl. Nano Mater. 2024, 7, 618–627. [Google Scholar] [CrossRef]
- Betz, M.; García-González, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of Novel Whey Protein-Based Aerogels as Drug Carriers for Life Science Applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Cen, Q.; Chen, S.; Yang, D.; Zheng, D.; Qiu, X. Full Bio-Based Aerogel Incorporating Lignin for Excellent Flame Retardancy, Mechanical Resistance, and Thermal Insulation. ACS Sustain. Chem. Eng. 2023, 11, 4473–4484. [Google Scholar] [CrossRef]
- Zou, F.; Budtova, T. Polysaccharide-Based Aerogels for Thermal Insulation and Superinsulation: An Overview. Carbohydr. Polym. 2021, 266, 118130. [Google Scholar] [CrossRef] [PubMed]
- Budtova, T.; Lokki, T.; Malakooti, S.; Rege, A.; Lu, H.; Milow, B.; Vapaavuori, J.; Vivod, S.L. Acoustic Properties of Aerogels: Current Status and Prospects. Adv. Eng. Mater. 2023, 25, 2201137. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, W.; Wang, L.; Lu, H.; Nie, S.; Xu, L.; Yang, W.; Wei, C. Biomass Carbon Materials for High-Performance Secondary Battery Electrodes: A Review. Resour. Chem. Mater. 2024, 3, 123–145. [Google Scholar] [CrossRef]
- Nargatti, K.I.; Subhedar, A.R.; Ahankari, S.S.; Grace, A.N.; Dufresne, A. Nanocellulose-Based Aerogel Electrodes for Supercapacitors: A Review. Carbohydr. Polym. 2022, 297, 120039. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, R.; Qu, M.; Zheng, R.; Zhao, Q.; Tang, P.; Bin, Y.; Wang, H. Chitosan Based Aerogel Fibers for Piezoelectric and Moisture Electric Energy Harvesting. React. Funct. Polym. 2024, 195, 105806. [Google Scholar] [CrossRef]
- Verma, A.; Thakur, S.; Goel, G.; Raj, J.; Gupta, V.K.; Roberts, D.; Thakur, V.K. Bio-Based Sustainable Aerogels: New Sensation in CO2 Capture. Curr. Res. Green Sustain. Chem. 2020, 3, 100027. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Anastopoulos, I.; Giannakoudakis, D.A.; Arkas, M.; Paraskevopoulou, P.; Pashalidis, I. Uranium Removal from Aqueous Solutions by Aerogel-Based Adsorbents—A Critical Review. Nanomaterials 2023, 13, 363. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in Drug Delivery: From Design to Application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef] [PubMed]
- Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Functionalized Chitosan as a Green, Recyclable, Biopolymer-Supported Catalyst for the [3+2] Huisgen Cycloaddition. Angew. Chem. 2009, 121, 6030–6034. [Google Scholar] [CrossRef]
- Sivaraman, D.; Siqueira, G.; Maurya, A.K.; Zhao, S.; Koebel, M.M.; Nyström, G.; Lattuada, M.; Malfait, W.J. Superinsulating Nanocellulose Aerogels: Effect of Density and Nanofiber Alignment. Carbohydr. Polym. 2022, 292, 119675. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Saito, T.; Isogai, A. Aerogels with 3D Ordered Nanofiber Skeletons of Liquid-Crystalline Nanocellulose Derivatives as Tough and Transparent Insulators. Angew. Chem. Int. Ed. 2014, 53, 10394–10397. [Google Scholar] [CrossRef] [PubMed]
- Sundar Raj, A.A.; Rubila, S.; Jayabalan, R.; Ranganathan, T.V. A Review on Pectin: Chemistry Due to General Properties of Pectin and Its Pharmaceutical Uses. Sci. Rep. 2012, 1, 550–553. [Google Scholar] [CrossRef]
- Chemistry and Function of Pectins; Fishman, M.L.; Jen, J.J. (Eds.) ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1986; Volume 310, ISBN 978-0-8412-0974-9. [Google Scholar]
- Capel, F.; Nicolai, T.; Durand, D.; Boulenguer, P.; Langendorff, V. Calcium and Acid Induced Gelation of (Amidated) Low Methoxyl Pectin. Food Hydrocoll. 2006, 20, 901–907. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou-Kalkavoura, V.; Gordeyeva, K.; Lavoine, N.; Bergström, L. Thermal Conductivity of Hygroscopic Foams Based on Cellulose Nanofibrils and a Nonionic Polyoxamer. Cellulose 2018, 25, 1117–1126. [Google Scholar] [CrossRef]
- Shi, J.; Lu, L.; Guo, W.; Sun, Y.; Cao, Y. An Environment-Friendly Thermal Insulation Material from Cellulose and Plasma Modification. Journal of Applied Polymer Science 2013, 130, 3652–3658. [Google Scholar] [CrossRef]
- Rodríguez-Fabià, S.; Torstensen, J.; Johansson, L.; Syverud, K. Hydrophobisation of Lignocellulosic Materials Part I: Physical Modification. Cellulose 2022, 29, 5375–5393. [Google Scholar] [CrossRef]
- Rodríguez-Fabià, S.; Torstensen, J.; Johansson, L.; Syverud, K. Hydrophobization of Lignocellulosic Materials Part II: Chemical Modification. Cellulose 2022, 29, 8957–8995. [Google Scholar] [CrossRef]
- Rodríguez-Fabià, S.; Torstensen, J.; Johansson, L.; Syverud, K. Hydrophobization of Lignocellulosic Materials Part III: Modification with Polymers. Cellulose 2022, 29, 5943–5977. [Google Scholar] [CrossRef]
- Cunha, A.G.; Gandini, A. Turning Polysaccharides into Hydrophobic Materials: A Critical Review. Part 1. Cellulose. Cellulose 2010, 17, 875–889. [Google Scholar] [CrossRef]
- Cunha, A.G.; Gandini, A. Turning Polysaccharides into Hydrophobic Materials: A Critical Review. Part 2. Hemicelluloses, Chitin/Chitosan, Starch, Pectin and Alginates. Cellulose 2010, 17, 1045–1065. [Google Scholar] [CrossRef]
- Schroeter, B.; Jung, I.; Bauer, K.; Gurikov, P.; Smirnova, I. Hydrophobic Modification of Biopolymer Aerogels by Cold Plasma Coating. Polymers 2021, 13, 3000. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Li, A.; Zheng, T.; Lu, L.; Cao, Y. Hydrophobic and Flexible Cellulose Aerogel as an Efficient, Green and Reusable Oil Sorbent. RSC Adv. 2015, 5, 82027–82033. [Google Scholar] [CrossRef]
- Plappert, S.F.; Quraishi, S.; Nedelec, J.-M.; Konnerth, J.; Rennhofer, H.; Lichtenegger, H.C.; Liebner, F.W. Conformal Ultrathin Coating by scCO2-Mediated PMMA Deposition: A Facile Approach To Add Moisture Resistance to Lightweight Ordered Nanocellulose Aerogels. Chem. Mater. 2018, 30, 2322–2330. [Google Scholar] [CrossRef]
- Montheil, T.; Raimond, L.; Valot, L.; Maumus, M.; Simon, M.; Martinez, J.; Amblard, M.; Noël, D.; Mehdi, A.; Subra, G. Controlled Silylation of Polysaccharides: Attractive Building Blocks for Biocompatible Foams and Cell-Laden Hydrogels. ACS Appl. Polym. Mater. 2022, 4, 4087–4097. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Z.; Tang, H.; Zhou, J. Fabrication of Hydrophobic Cellulosic Materials via Gas–Solid Silylation Reaction for Oil/Water Separation. Cellulose 2019, 26, 4021–4037. [Google Scholar] [CrossRef]
- Beaumont, M.; Bacher, M.; Opietnik, M.; Gindl-Altmutter, W.; Potthast, A.; Rosenau, T. A General Aqueous Silanization Protocol to Introduce Vinyl, Mercapto or Azido Functionalities onto Cellulose Fibers and Nanocelluloses. Molecules 2018, 23, 1427. [Google Scholar] [CrossRef]
- Chhajed, M.; Yadav, C.; Agrawal, A.K.; Maji, P.K. Esterified Superhydrophobic Nanofibrillated Cellulose Based Aerogel for Oil Spill Treatment. Carbohydr. Polym. 2019, 226, 115286. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Winklehner, S.; Veigel, S.; Mundigler, N.; Gindl-Altmutter, W.; Potthast, A.; Rosenau, T. Wet Esterification of Never-Dried Cellulose: A Simple Process to Surface-Acetylated Cellulose Nanofibers. Green Chem. 2020, 22, 5605–5609. [Google Scholar] [CrossRef]
- Fox, S.C.; Li, B.; Xu, D.; Edgar, K.J. Regioselective Esterification and Etherification of Cellulose: A Review. Biomacromolecules 2011, 12, 1956–1972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Xu, Y.; Lu, Z.; Chen, L.; Huang, L.; Fan, M. Versatile Fabrication of a Superhydrophobic and Ultralight Cellulose-Based Aerogel for Oil Spillage Clean-Up. Phys. Chem. Chem. Phys. 2016, 18, 28297–28306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Z.; Sèbe, G.; Wu, R.; Rivera Virtudazo, R.V.; Tingaut, P.; Koebel, M.M. Multiscale Assembly of Superinsulating Silica Aerogels Within Silylated Nanocellulosic Scaffolds: Improved Mechanical Properties Promoted by Nanoscale Chemical Compatibilization. Adv. Funct. Mater. 2015, 25, 2326–2334. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Liu, X.; Liu, Y.; Ji, L.; Zhou, Y.; Sun, L. Comparison of Analytical Methods for Determining Methylesterification and Acetylation of Pectin. Appl. Sci. 2021, 11, 4461. [Google Scholar] [CrossRef]
- Masuelli, M.A. Mark-Houwink Parameters for Aqueous-Soluble Polymers and Biopolymers at Various Temperatures. J. Polym. Biopolym. Phys. Chem. 2014, 2, 37–43. [Google Scholar] [CrossRef]
- Cai, Z.; Lin, J.; Hong, X. Transparent Superhydrophobic Hollow Films (TSHFs) with Superior Thermal Stability and Moisture Resistance. RSC Adv. 2018, 8, 491–498. [Google Scholar] [CrossRef]
- Parakkulam Ramaswamy, A.; Rigacci, A. Superinsulating Composite Aerogels from Polymethylsilsesquioxane and Kapok Fibers. Mater. Chem. Phys. 2021, 261, 124252. [Google Scholar] [CrossRef]
- EN 12667; Thermal Performance of Building Materials and Products—Determination of Thermal Resistance by Means of Guarded Hot Plate and Heat Flow Meter Methods—Products of High and Medium Thermal Resistance. AFNOR: Paris, France, 2001.
- Kozioł, A.; Środa-Pomianek, K.; Górniak, A.; Wikiera, A.; Cyprych, K.; Malik, M. Structural Determination of Pectins by Spectroscopy Methods. Coatings 2022, 12, 546. [Google Scholar] [CrossRef]
- Wei, T.-Y.; Chang, T.-F.; Lu, S.-Y.; Chang, Y.-C. Preparation of Monolithic Silica Aerogel of Low Thermal Conductivity by Ambient Pressure Drying. J. Am. Ceram. Soc. 2007, 90, 2003–2007. [Google Scholar] [CrossRef]
- Jaxel, J.; Merio, A.; Kohlhuber, N.; Beaumont, M.; Wimmer, R.; Rosenau, T.; Hansmann, C.; Liebner, F.; Böhmdorfer, S. Facile Chemical Hydrophobization of Thin-Layer Plates by Vapor Deposition of Methyltrimethoxysilane for Reversed-Phase Chromatography. JPC-J. Planar Chromat. 2023, 36, 455–463. [Google Scholar] [CrossRef]
- Jabli, M.; Sebeia, N.; Bchetnia, A. Synthesis and Characterization of Pectin-Manganese Oxide and Pectin-Tin Oxide Nanocomposites: Application to the Degradation of Calmagite in Water. J. Polym. Environ. 2023, 31, 4326–4337. [Google Scholar] [CrossRef]

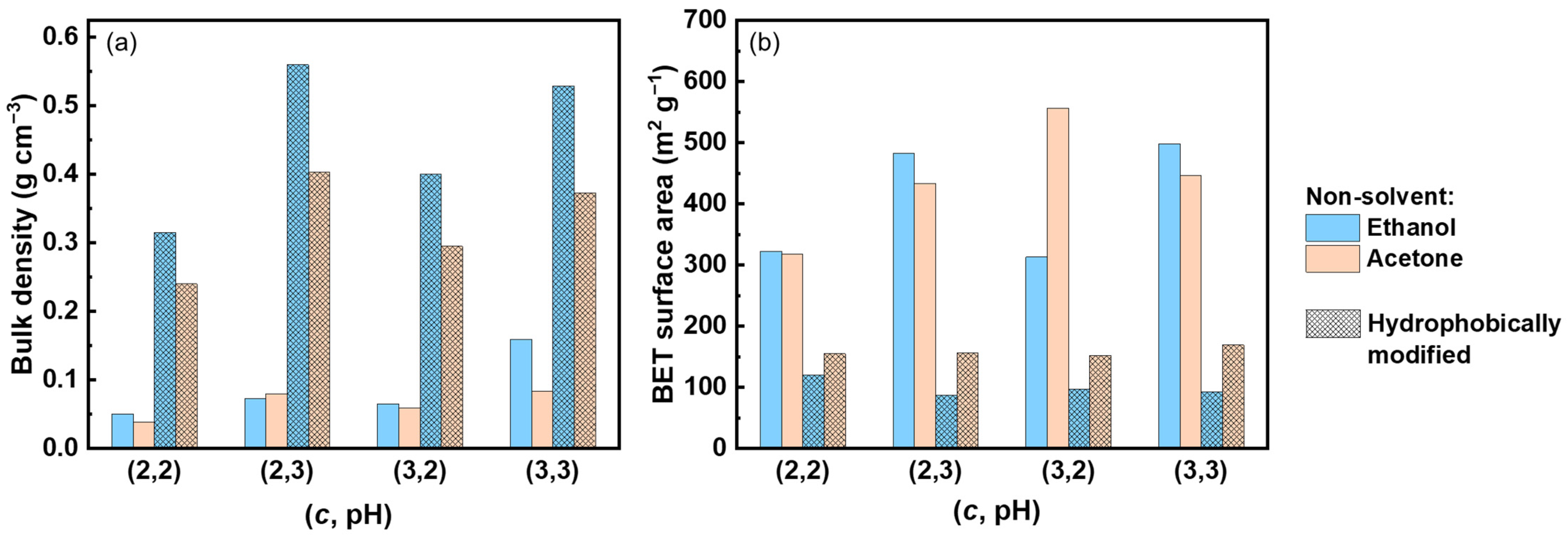






| Cpectin (wt%) | pH | Non-Solvent | Nomenclature | Bulk Density, ρb (g cm−3) | Porosity, ∏ (%) | BET Specific Surface Area (m2 g−1) | Thermal Conductivity, λ (W m−1 K−1) |
|---|---|---|---|---|---|---|---|
| 2 | 2 | ethanol | (2, 2, E) | 0.050 ± 0.001 | 97 ± 1 | 322 ± 10 | 0.0216 |
| 2 | 2 | acetone | (2, 2, A) | 0.038 ± 0.002 | 98 ± 1 | 308 ± 16 | 0.0228 |
| 2 | 3 | ethanol | (2, 3, E) | 0.073 ± 0.001 | 91 ± 1 | 462 ± 28 | 0.0169 |
| 2 | 3 | acetone | (2, 3, A) | 0.080 ± 0.001 | 94 ± 1 | 433 ± 25 | 0.0148 |
| 3 | 2 | ethanol | (3, 2, E) | 0.065 ± 0.003 | 96 ± 1 | 567 ± 11 | 0.0212 |
| 3 | 2 | acetone | (3, 2, A) | 0.059 ± 0.001 | 93 ± 1 | 556 + 20 | 0.0202 |
| 3 | 3 | ethanol | (3, 3, E) | 0.11 ± 0.01 | 93 ± 1 | 475 ± 32 | 0.0234 |
| 3 | 3 | acetone | (3, 3, A) | 0.083 ± 0.001 | 96 ± 1 | 439 ± 8 | 0.0152 |
| Element | Neat Pectin Aerogel | Composite Pectin Aerogel | ||||
|---|---|---|---|---|---|---|
| Mass (%) | Normalized Mass (%) | Atom (%) | Mass (%) | Normalized Mass (%) | Atom (%) | |
| C | 47.67 | 47.67 | 55.34 | 11.74 | 30.44 | 42.52 |
| O | 50.08 | 50.08 | 43.65 | 11.64 | 32.41 | 32.41 |
| Si | 0.56 | 0.56 | 0.28 | 15.18 | 24.07 | 24.07 |
| Na | 0.54 | 0.54 | 0.33 | 0 | 0 | 0 |
| K | 0.62 | 0.62 | 0.22 | 0 | 0 | 0 |
| Ca | 0.53 | 0.53 | 0.18 | 0 | 0 | 0 |
| Total | 100 | 100 | 100 | 37.57 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Effraimopoulou, E.; Jaxel, J.; Budtova, T.; Rigacci, A. Hydrophobic Modification of Pectin Aerogels via Chemical Vapor Deposition. Polymers 2024, 16, 1628. https://doi.org/10.3390/polym16121628
Effraimopoulou E, Jaxel J, Budtova T, Rigacci A. Hydrophobic Modification of Pectin Aerogels via Chemical Vapor Deposition. Polymers. 2024; 16(12):1628. https://doi.org/10.3390/polym16121628
Chicago/Turabian StyleEffraimopoulou, Eleni, Julien Jaxel, Tatiana Budtova, and Arnaud Rigacci. 2024. "Hydrophobic Modification of Pectin Aerogels via Chemical Vapor Deposition" Polymers 16, no. 12: 1628. https://doi.org/10.3390/polym16121628





