Biodegradation Mechanism of Polystyrene by Mealworms (Tenebrio molitor) and Nutrients Influencing Their Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mealworm Maintenance
2.3. Biodegradations of Expanded Polystyrene (EPS), EPS/Bran, Bran and EPS/Urethane Foam (PU)
2.4. Method of Polystyrene (PS) Recovery from Frass
2.5. Degradation Using Sulfate Ion Radicals in Seawater (Enhanced Degradation Method)
2.6. Characterization and Analysis
3. Results and Discussion
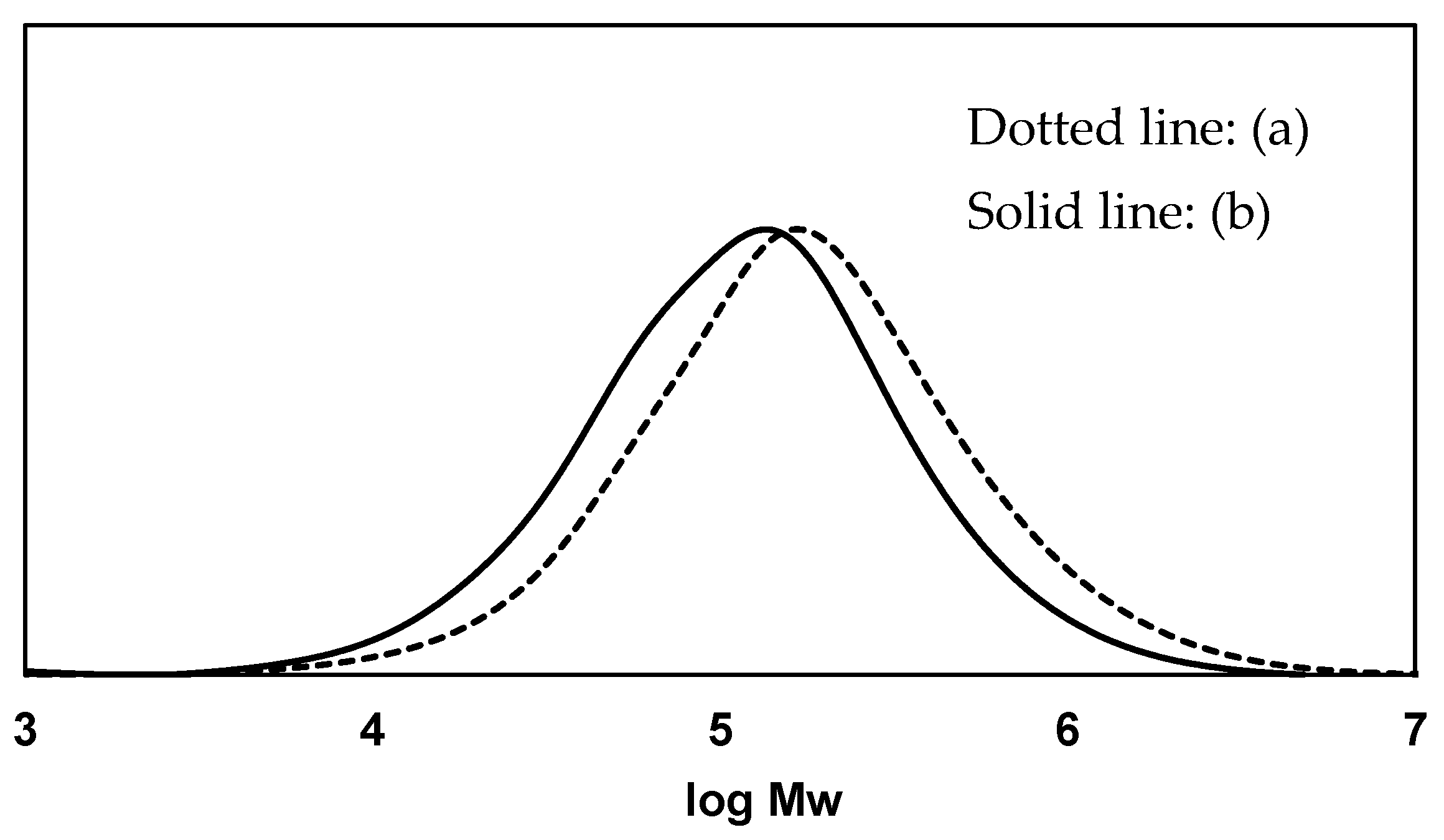
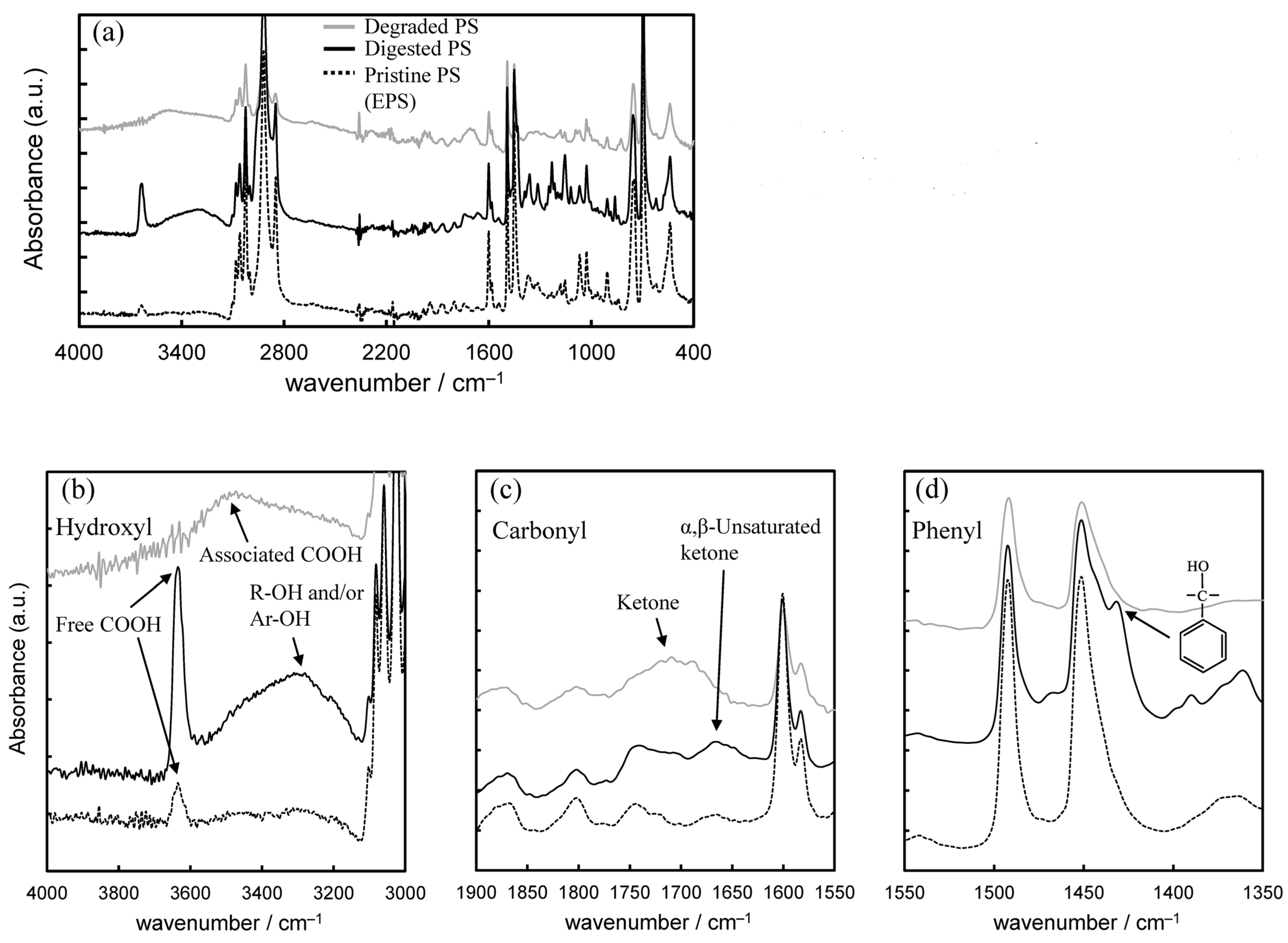
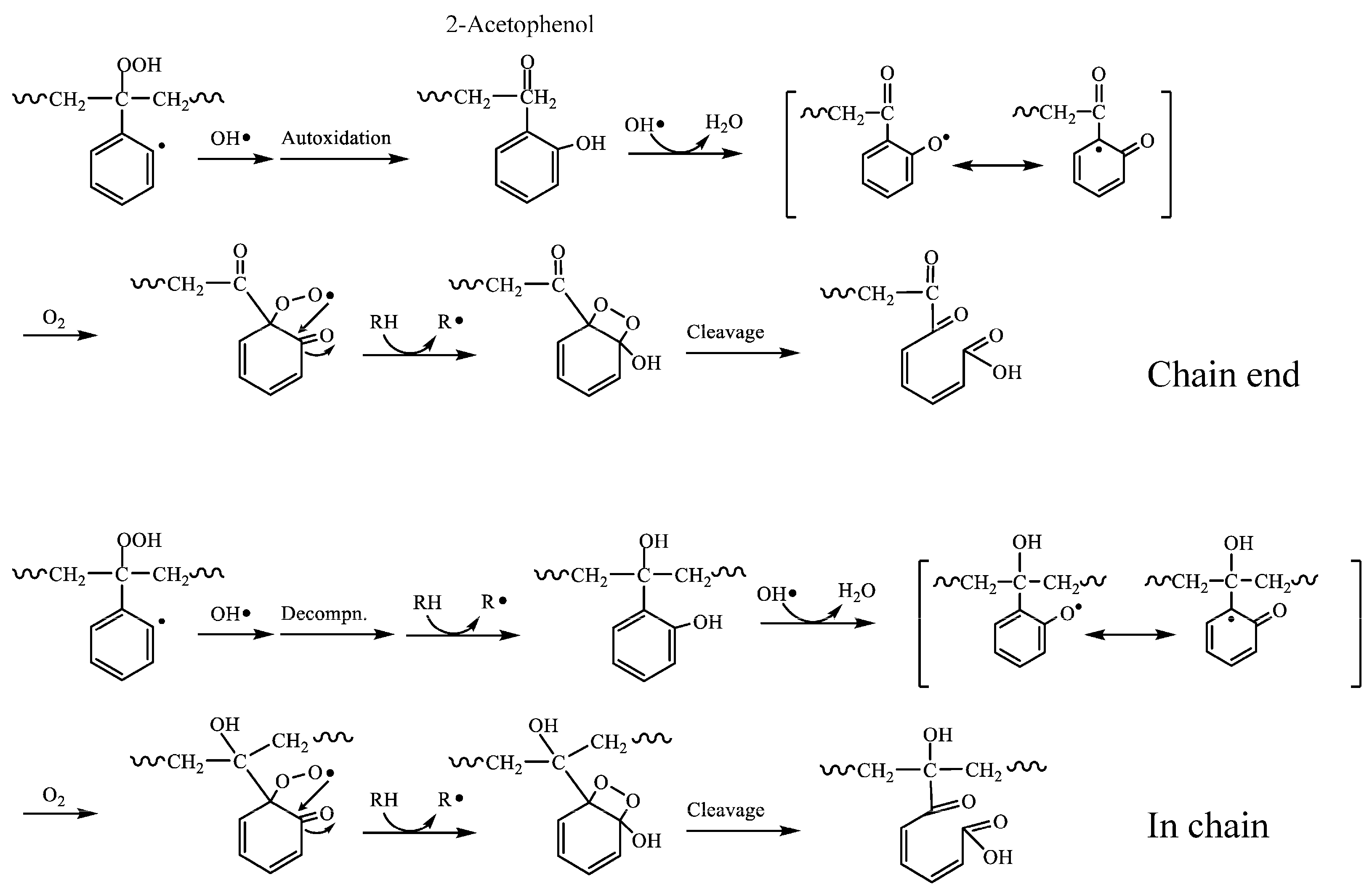
3.1. Biodegradation Mechanism of Polystyrene (PS)
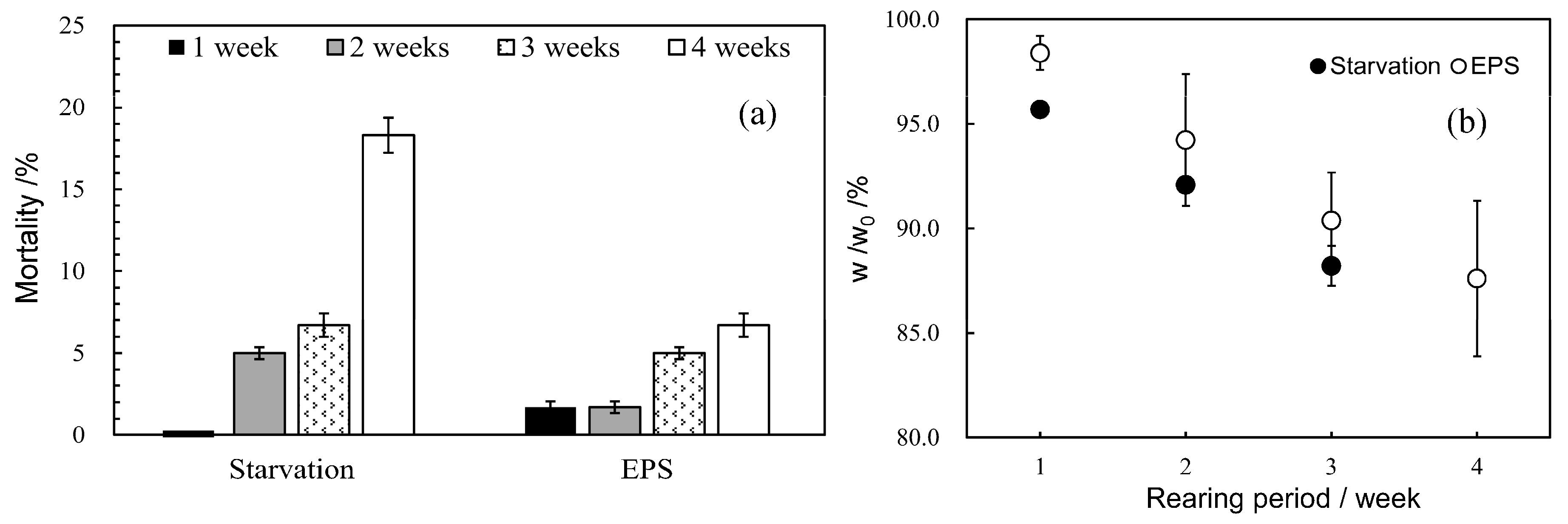
3.2. Effect of Bran Addition on Mealworm Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Law, K.L. Plastics in the Marine Environment. Annu. Rev. Mar. Sci. 2017, 9, 205–229. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Fauser, P.; Vorkamp, K.; Strand, J. Residual Additives in Marine Microplastics and Their Risk Assessment—A critical review. Mar. Pollut. Bull. 2022, 177, 113467. [Google Scholar] [CrossRef]
- Rillig, M.C.; Kim, S.W.; Kim, T.Y.; Waldman, W.R. The Global Plastic Toxicity Debt. Environ. Sci. Technol. 2021, 55, 2717–2719. [Google Scholar] [CrossRef]
- Lobelle, D.; Cunliffe, M. Early Microbial Biofilm Formation on Marine Plastic Debris. Mar. Pollut. Bull. 2011, 62, 197–200. [Google Scholar] [CrossRef]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the ‘Plastisphere’: Microbial Communities on Plastic Marine Debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Michels, J.; Stippkugel, A.; Lenz, M.; Wirtz, K.; Engel, A. Rapid Aggregation of Biofilm-covered Microplastics with Marine biogenic particles. Proc. R. Soc. B 2018, 285, 1203–1211. [Google Scholar] [CrossRef]
- Devriese, L.I.; Witte, B.D.; Vethaak, A.D.; Hostens, K.; Leslie, H.A. Bioaccumulation of PCBs from Microplastics in Norway Lobster (Nephrops norvegicus): An Experimental Study. Chemosphere 2017, 186, 10–16. [Google Scholar] [CrossRef]
- Nor, N.H.M.; Koelmans, A.A. Transfer of PCBs from Microplastics under Simulated Gut Fluid Conditions is Biphasic and Rreversible. Environ. Sci. Technol. 2019, 53, 1874–1883. [Google Scholar]
- Achilias, D.S.; Kanellopoulou, I.; Megalokonomos, P.; Antonakou, E.; Lappas, A.A. Chemical Recycling of Polystyrene by Pyrolysis: Potential Use of the Liquid Product for the Reproduction of Polymer. Macromol. Mater. Eng. 2007, 292, 923–934. [Google Scholar] [CrossRef]
- Mistry, C.R.; Pandian, S.; Choksi, H. Investigating the Impact of Microwave and Conventional Heating Sources on the Co-pyrolysis of Plastics with Non–biomass Materials. Anal. Appl. Pyrol. 2024, 177, 106364. [Google Scholar] [CrossRef]
- Suzuki, G.; Uchida, N.; Tuyen, L.H.; Tanaka, K.; Matsukami, H.; Kunisue, T.; Takahashi, S.; Viet, P.H.; Kuramochi, H.; Osako, M. Mechanical Recycling of Plastic Waste as A Point Source of Microplastic Pollution. Environ. Pollut. 2022, 303, 119114. [Google Scholar] [CrossRef]
- Brown, E.; MacDonald, A.; Allen, S.; Allen, D. The Potential for A Plastic Recycling Facility to Release Microplastic Pollution and Possible Filtration Remediation Effectiveness. J. Hazard. Mater. Adv. 2023, 10, 100309. [Google Scholar] [CrossRef]
- Gijsman, P.; Fiorio, R. Long Term Thermo–oxidative Degradation and Stabilization of Polypropylene (PP) and the Implications for its Recyclability. Polym. Degad. Stab. 2023, 208, 110260. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, J.; Wu, W.M.; Zhao, J.; Son, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic–eating Mealworms: Part 1. Chemical and Physical Characterization and Isotopic Tests. Environ. Sci. Technol. 2015, 49, 12080–12086. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, J.; Wu, W.M.; Zhao, J.; Son, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic–eating Mealworms: Part 2. Role of Gut Microorganisms. Environ. Sci. Technol. 2015, 49, 12087–12093. [Google Scholar] [CrossRef]
- Brandon, A.M.; Gao, S.H.; Tian, R.; Ning, D.; Yang, S.S.; Zhou, J.; Wu, W.M.; Criddle, C.S. Biodegradation of Polyethylene and Plastic Mixtures in Mealworms (Larvae of Tenebrio molitor) and Effects on the Gut Microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef]
- Yang, S.S.; Brandon, A.M.; Flanagan, J.C.A.; Yang, J.; Ning, D.; Cai, S.Y.; Fan, H.Q.; Wang, Z.Y.; Ren, J.; Benbow, E.; et al. Biodegradation of Polystyrene Wastes in Yellow Mealworms (larvae of Tenebrio molitor Linnaeus): Factors Affecting Biodegradation Rates and the Ability of Polystyrene–fed Larvae to Complete their Life Cycle. Chemosphere 2018, 191, 979–989. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of Various Commodities for the Development of the Yellow Mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef]
- Tsochatzi, E.D.; Berggreen, I.E.; Nørgaar, J.V.; Theodoridi, G.; Dalsgaard, T.K. Biodegradation of Expanded Polystyrene by Mealworm Larvae under Different Feeding Strategies Evaluated by Metabolic Profiling using GC-TOF-MS. Chemosphere 2021, 281, 130840. [Google Scholar] [CrossRef]
- Lou, Y.; Li, Y.; Lu, B.; Liu, Q.; Yang, S.S.; Liu, B.; Ren, N.; Wu, W.M.; Xing, D. Response of the Yellow Mealworm (Tenebrio molitor) Gut Microbiome to Diet Shifts during Polystyrene and Polyethylene Biodegradation. J. Hazard. Mater. 2021, 416, 126222. [Google Scholar] [CrossRef]
- Pham, T.Q.; Longing, S.; Siebecker, M.G. Consumption and Degradation of Different Consumer Plastics by Mealworms (Tenebrio molitor): Effects of Plastic Type, Time, and Mealworm Origin. J. Clean. Prod. 2021, 403, 136842. [Google Scholar] [CrossRef]
- Bae, J.; Cho, H.W.; Jung, H.; Park, J.; Yun, S.; Ha, S.; Lee, Y.; Kim, T.J. Changes in Intestinal Microbiota Due to the Expanded Polystyrene Diet of Mealworms (Tenebrio molitor). Indian J. Microbiol. 2021, 61, 130–136. [Google Scholar] [CrossRef]
- Zhong, Z.; Nong, W.; Xie, Y.; Hui, J.H.L.; Chu, L.M. Long–term Effect of Plastic Feeding on Growth and Transcriptomic Response of Mealworms (Tenebrio molitor L.). Chemosphere 2022, 287, 130840. [Google Scholar] [CrossRef]
- Yang, S.S.; Ding, M.Q.; Ren, X.R.; Zhang, Z.R.; Li, M.X.; Zhang, L.L.; Pang, J.W.; Chen, C.X.; Zhao, L.; Xing, D.F.; et al. Impacts of Physical–chemical Property of Polyethylene on Depolymerization and Biodegradation in Yellow and Dark Mealworms with High Purity Microplastics. Sci. Total Environ. 2022, 828, 154458. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, L.; Li, X.; Wang, J.; Wang, H.; Chen, C.; Guo, H.; Han, T.; Zhou, A.; Zhao, X. Different Plastics Ingestion Preferences and Efficiencies of Superworm (Zophobas atratus Fab.) and Yellow Mealworm (Tenebrio molitor Linn.) Associated with Ddistinct Gut Microbiome Changes. Sci. Total Environ. 2022, 837, 155719. [Google Scholar] [CrossRef]
- Peng, B.Y.; Sun, Y.; Xiao, S.; Chen, J.; Zhou, X.; Wu, W.M.; Zhang, Y. Influence of Polymer Size on Polystyrene Biodegradation in Mealworms (Tenebrio molitor): Responses of Depolymerization Pattern, Gut Microbiome, and Metabolome to Polymers with Low to Ultrahigh Molecular Weight. Environ. Sci. Technol. 2022, 56, 17310–17320. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, H.W.W.; Lu, Y.; Zhang, H. Cooperation between Tenebrio Molitor (Mealworm larvae) and Their Symbiotic Microorganisms Improves the Bioavailability of Polyethylene. J. Polym. Environ. 2023, 31, 3925–3936. [Google Scholar] [CrossRef]
- Brandon, A.M.; El Abbadi, S.H.; Ibekwe, U.A.; Cho, Y.M.; Wu, W.M.; Criddle, C.S. Fate of Hexabromocyclododecane (HBCD), A Common Flame Retardant, in Polystyrene-degrading Mealworms: Elevated HBCD Levels in Egested Polymer but No Bioaccumulation. Environ. Sci. Technol. 2020, 54, 364–371. [Google Scholar] [CrossRef]
- Watanabe, T.; Katayama, S.; Enoki, M.; Honda, Y.; Kuwahara, M. Formation of Acyl radical in Lipid Peroxidation of Linoleic Acid by Manganese-Dependent Peroxidase from Ceriporiopsis subvermispora and Bjerkandera adusta. Eur. J. Biochem. 2000, 267, 4222–4231. [Google Scholar] [CrossRef]
- Rnby, B.; Lucki, J. New Aspects of Photodegradation and Photooxidation of PS. Pure Appl. Chem. 1980, 52, 295–303. [Google Scholar] [CrossRef]
- Nakatani, H.; Uchiyama, T.; Motokucho, S.; Dao, A.T.N.; Kim, H.J.; Yagi, M.; Kyozuka, Y. Differences in the Residual Behavior of A Bumetrizole–type Ultraviolet Light Absorber during the Degradation of Various Polymers. Polymers 2024, 16, 293. [Google Scholar] [CrossRef]
- Gugumus, F. Re-examination of the Role of Hydroperoxides in Polyethylene and Polypropylene: Chemical and Physical Aspects of Hydroperoxides in Polyethylene. Polym. Degrad. Stab. 1995, 49, 29–50. [Google Scholar] [CrossRef]
- Rajesh, K.T.; Praveen, K.; Parma, N.S. Magnesium Deficiency Induced Oxidative Stress and Antioxidant Responses in Mulberry Plants. Sci. Hortic. 2006, 108, 7–14. [Google Scholar]
- Hwang, T.; Tsai, C.C.; Chen, J.; Changchien, T.T.; Wang, C.; Wu, C. Magnesium Ascorbyl Phosphate and Coenzyme Q10 Protect Keratinocytes against UVA Irradiation by Suppressing Glutathione Depletion. Mol. Med. Rep. 2012, 6, 375–378. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakatani, H.; Yamaura, Y.; Mizuno, Y.; Motokucho, S.; Dao, A.T.N.; Nakahara, H. Biodegradation Mechanism of Polystyrene by Mealworms (Tenebrio molitor) and Nutrients Influencing Their Growth. Polymers 2024, 16, 1632. https://doi.org/10.3390/polym16121632
Nakatani H, Yamaura Y, Mizuno Y, Motokucho S, Dao ATN, Nakahara H. Biodegradation Mechanism of Polystyrene by Mealworms (Tenebrio molitor) and Nutrients Influencing Their Growth. Polymers. 2024; 16(12):1632. https://doi.org/10.3390/polym16121632
Chicago/Turabian StyleNakatani, Hisayuki, Yuto Yamaura, Yuma Mizuno, Suguru Motokucho, Anh Thi Ngoc Dao, and Hiroyuki Nakahara. 2024. "Biodegradation Mechanism of Polystyrene by Mealworms (Tenebrio molitor) and Nutrients Influencing Their Growth" Polymers 16, no. 12: 1632. https://doi.org/10.3390/polym16121632




