Chemical Synthesis of Atactic Poly-3-hydroxybutyrate (a-P3HB) by Self-Polycondensation: Catalyst Screening and Characterization
Abstract
1. Introduction
2. Materials and Methods
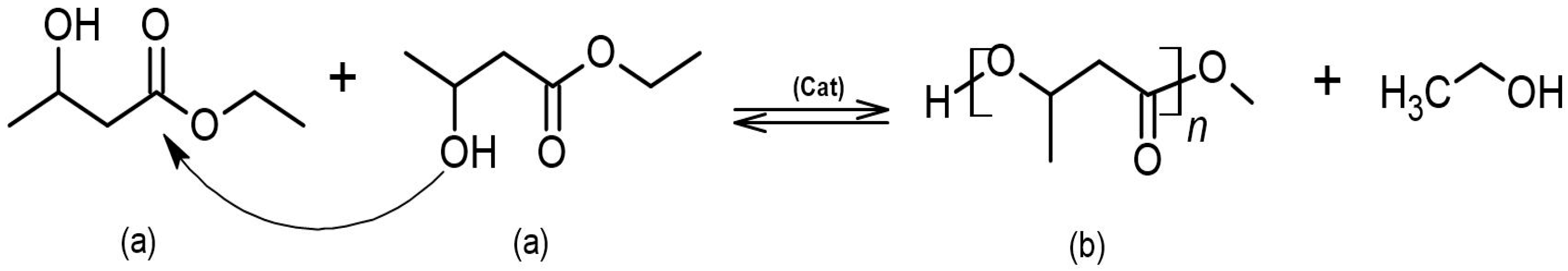
2.1. Synthesis of Racemic Ethyl-3-hydroxybutyrate
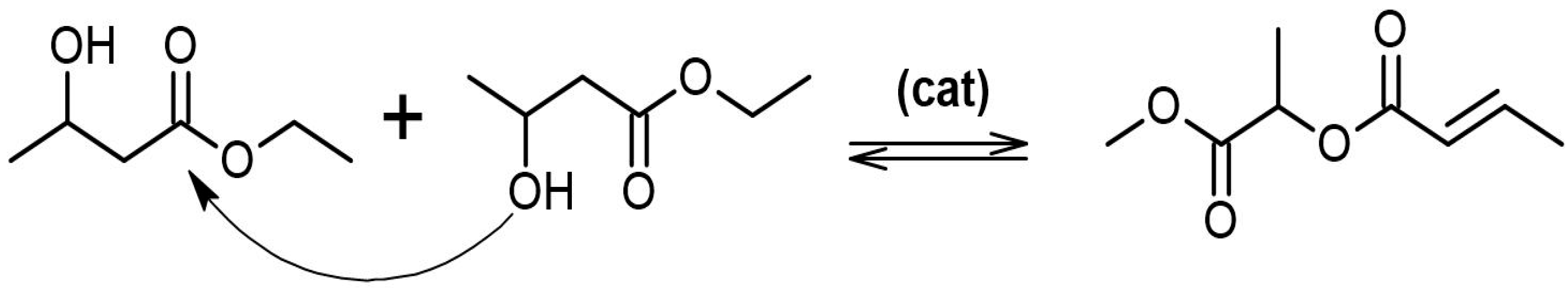
2.2. Synthesis of Atactic P3HB by Self-Polycondensation from Racemic Precursor and by Ring-Opening Polymerization
2.3. Fourier Transform Infrared Spectroscopy
2.4. Gel Permeation Chromatography
2.5. UV–VIS Spectroscopy
3. Results and Discussion
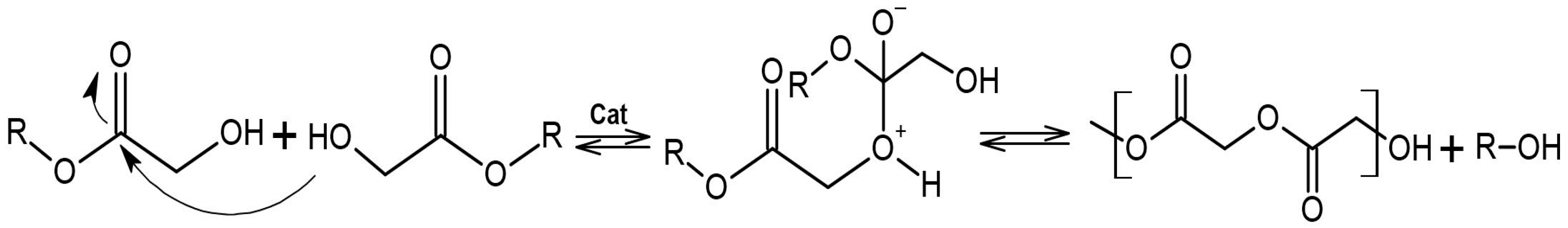
3.1. Synthesis of a-P3HB by Self-Polycondensation and ROP
3.2. Screening Tests with Different Catalysts
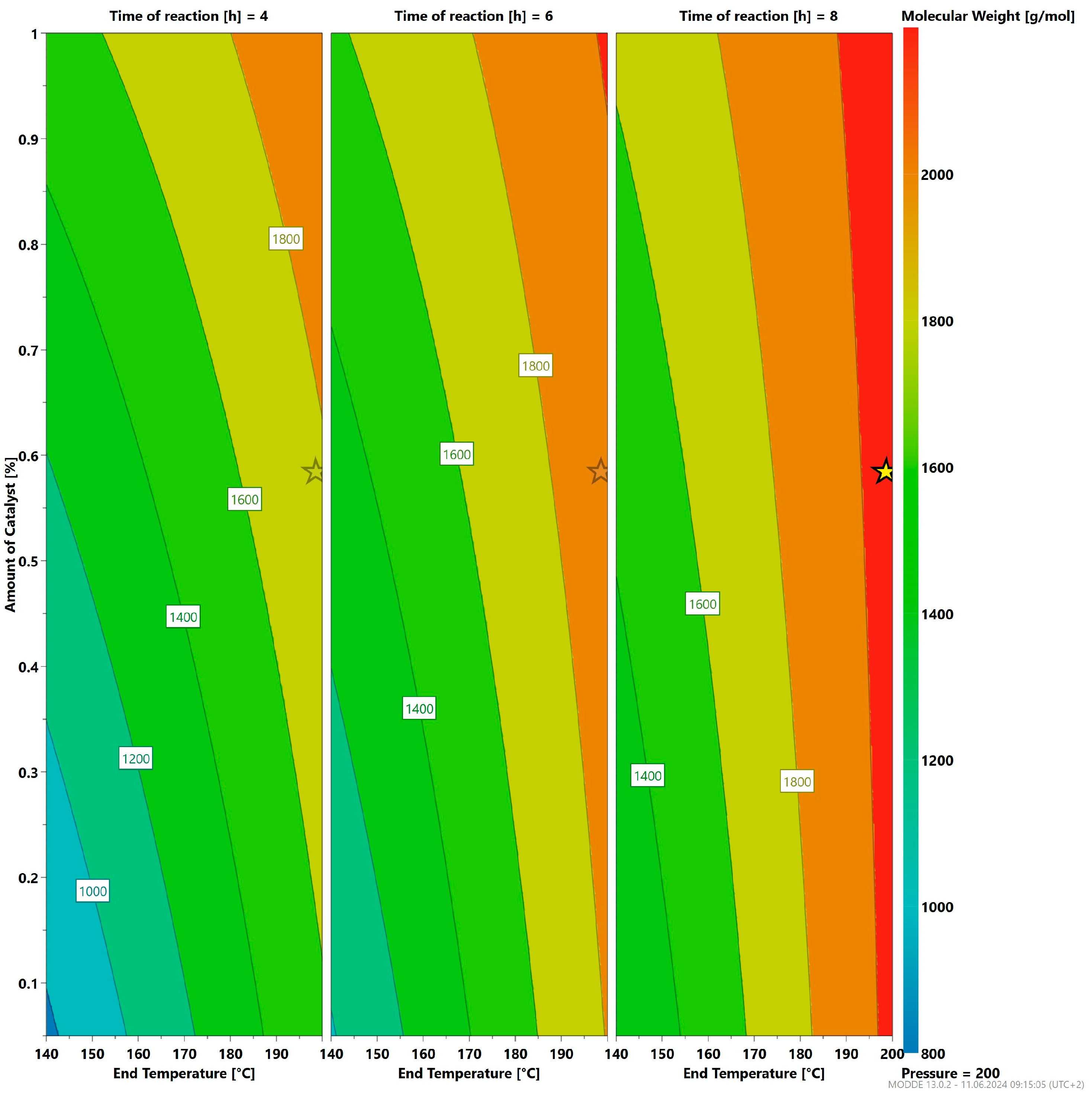
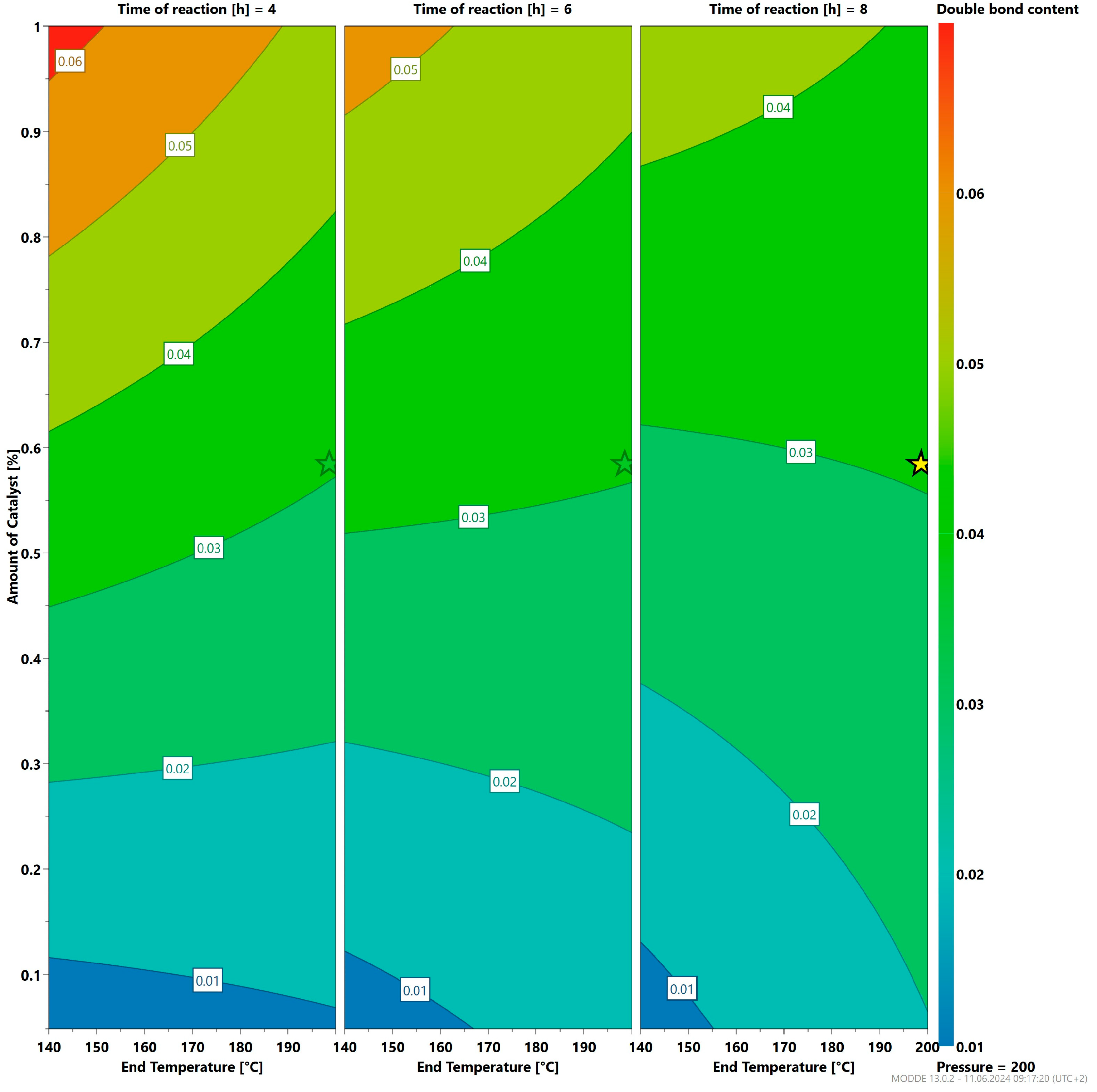
3.3. Optimizing the Reaction Parameters
3.4. LCA Analysis of a-P3HB Chemical Synthesis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ke, Y.; Zhang, X.Y.; Ramakrishna, S.; He, L.M.; Wu, G. Synthetic routes to degradable copolymers deriving from the biosynthesized polyhydroxyalkanoates: A mini review. Express Polym. Lett. 2016, 10, 36–53. [Google Scholar] [CrossRef]
- Lemoigne, M. Products of dehydration and of polymerization of β-hydroxybutyric acid. Bull. Soc. Chem. Biol. 1926, 8, 770–782. [Google Scholar]
- Winnacker, M. Polyhydroxyalkanoates: Recent Advances in Their Synthesis and Applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101. [Google Scholar] [CrossRef]
- Chodak, I. Polyhydroxyalkanoates: Properties and Modification for High Volume Applications. In Degradable Polymers; Scott, G., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 295–319. ISBN 978-90-481-6091-4. [Google Scholar]
- Luo, Z.; Wu, Y.-L.; Li, Z.; Loh, X.J. Recent Progress in Polyhydroxyalkanoates-Based Copolymers for Biomedical Applications. Biotechnol. J. 2019, 14, e1900283. [Google Scholar] [CrossRef]
- Kenny, S.T.; Runic, J.N.; Kaminsky, W.; Woods, T.; Babu, R.P.; O’Connor, K.E. Development of a bioprocess to convert PET derived terephthalic acid and biodiesel derived glycerol to medium chain length polyhydroxyalkanoate. Appl. Microbiol. Biotechnol. 2012, 95, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Gabirondo, E.; Sangroniz, A.; Etxeberria, A.; Torres-Giner, S.; Sardon, H. Poly(hydroxy acids) derived from the self-condensation of hydroxy acids: From polymerization to end-of-life options. Polym. Chem. 2020, 11, 4861–4874. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1954. [Google Scholar]
- Odian, G. Principles of Polymerization; John Willey & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Rogers, S.R.; Long, M.E.; Turner, T.E. Introduction to Synthetic Methods in Step-Growth Polymers; John Willey & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Gupta, S.K.; Kumar, A. Reaction Engineering of Step Growth Polymerization; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Ajioka, M.; Enomoto, K.; Suzuki, K.; Yamaguchi, A. Basic properties of polylactic acid produced by the direct condensation polymerization of lactic acid. Chem. Soc. Jpn. 1995, 68, 2125–2131. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hayashi, Y.; Yamamoto, A. A novel type of polycondensation utilizing transition metal-catalyzed C–C coupling. I. Preparation of thermostable polyphenylene type polymers. Bull. Chem. Soc. Jpn. 1978, 51, 2091–2097. [Google Scholar] [CrossRef]
- Takahashi, K.; Taniguchi, I.; Miyamoto, M.; Kimura, Y. Melt/solid polycondensation of glycolic acid to obtain high-molecular-weight poly(glycolic acid). Polymer 2000, 41, 8725–8728. [Google Scholar] [CrossRef]
- Okada, M. Chemical syntheses of biodegradable polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Okada, M.; Aoi, K.; Mizuno, W.; Ito, S. Synthesis and properties of new polyesters having tetrahydrofuran rings in their main chains. J. Polym. Sci. 1993, 31, 1135–1440. [Google Scholar] [CrossRef]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry Part A: Structure and Mechanisms, 5th ed.; Springer: New York, NY, USA, 2007; ISBN 9780387683461. [Google Scholar]
- Mozingo, R.; Spencer, C.; Folkers, K. Hydrogenation by raney nickel catalyst without gaseous hydrogen. J. Am. Chem. Soc. 1944, 66, 1859–1860. [Google Scholar] [CrossRef]
- Wipf, B.; Kupfer, E.; Bertazzi, R.; Leuenberger, H.G.W. Production of (+)-(S)-Ethyl 3-Hydroxybutyrate and (-)-(R)-Ethyl 3-Hydroxybutyrate by Microbial Reduction of Ethyl Acetoacetate. Helv. Chim. Acta 1983, 66, 485–488. [Google Scholar] [CrossRef]
- Pohl, N.; Clague, A.; Schwarz, K. Chiral Compounds and Green Chemistry in Undergraduate Organic Laboratories: Reduction of a Ketone by Sodium Borohydride and Baker’s Yeast. J. Chem. Educ. 2002, 79, 727. [Google Scholar] [CrossRef]
- Jedliński, Z.; Kowalczuk, M.; Kurcok, P.; Adamus, G.; Matuszowicz, A.; Sikorska, W.; Gross, R.A.; Xu, J.; Lenz, R.W. Stereochemical Control in the Anionic Polymerization of β-Butyrolactone Initiated with Alkali-Metal Alkoxides. Macromolecules 1996, 29, 3773–3777. [Google Scholar] [CrossRef]
- Hori, Y.; Suzuki, M.; Yamaguchi, A.; Nishishita, T. Ring-opening polymerization of optically active.beta.-butyrolactone using distannoxane catalysts: Synthesis of high-molecular-weight poly(3-hydroxybutyrate). Macromolecules 1993, 26, 5533–5534. [Google Scholar] [CrossRef]
- Harding, K.G.; Dennis, J.S.; von Blottnitz, H.; Harrison, S.T.L. Environmental analysis of plastic production processes: Comparing petroleum-based polypropylene and polyethylene with biologically-based poly-beta-hydroxybutyric acid using life cycle analysis. J. Biotechnol. 2007, 130, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Frischknecht, R.; Suter, P. Ökoinventare von Energiesystemen; ESU-services: Schaffhausen, Switzerland, 1996. [Google Scholar]

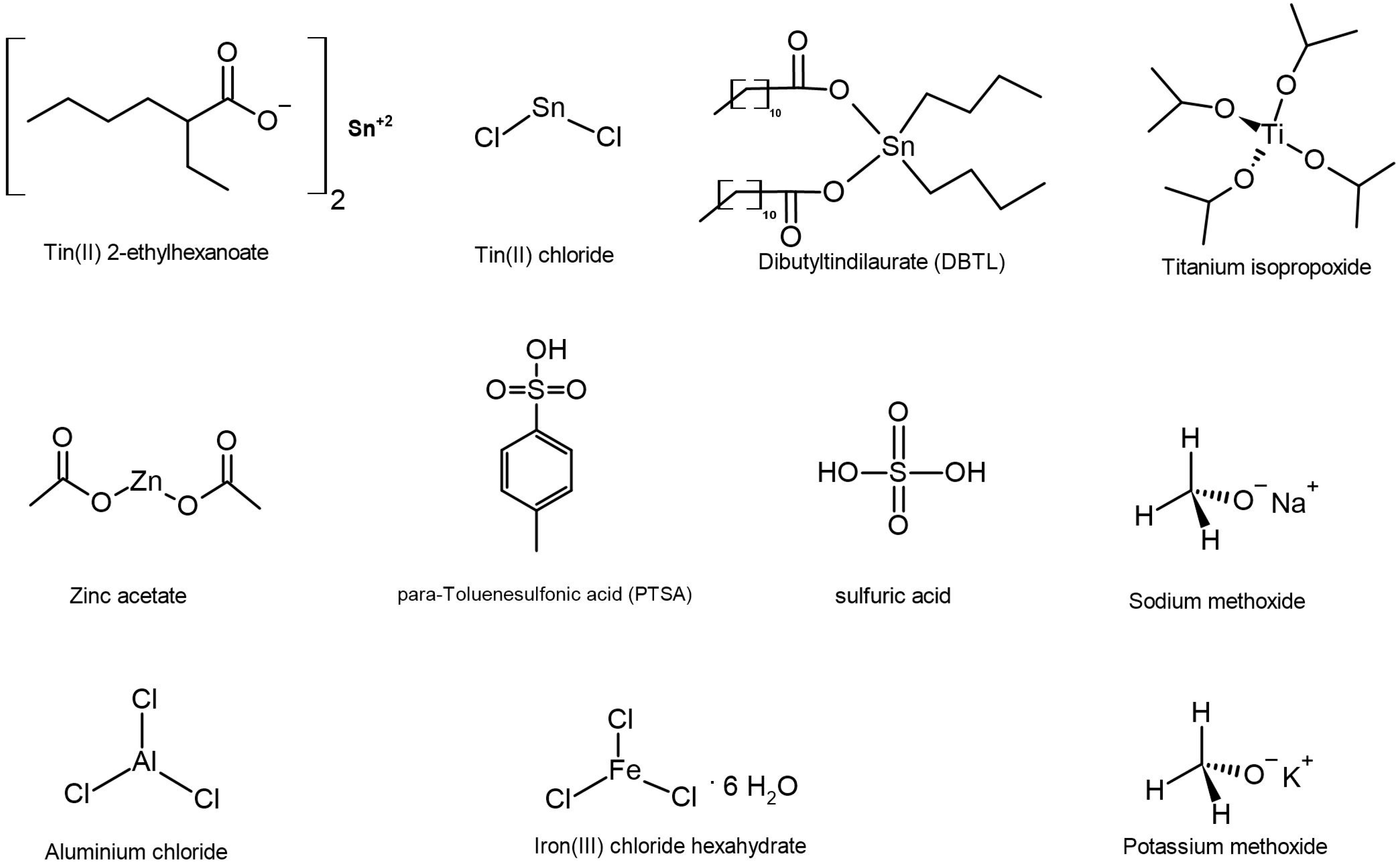




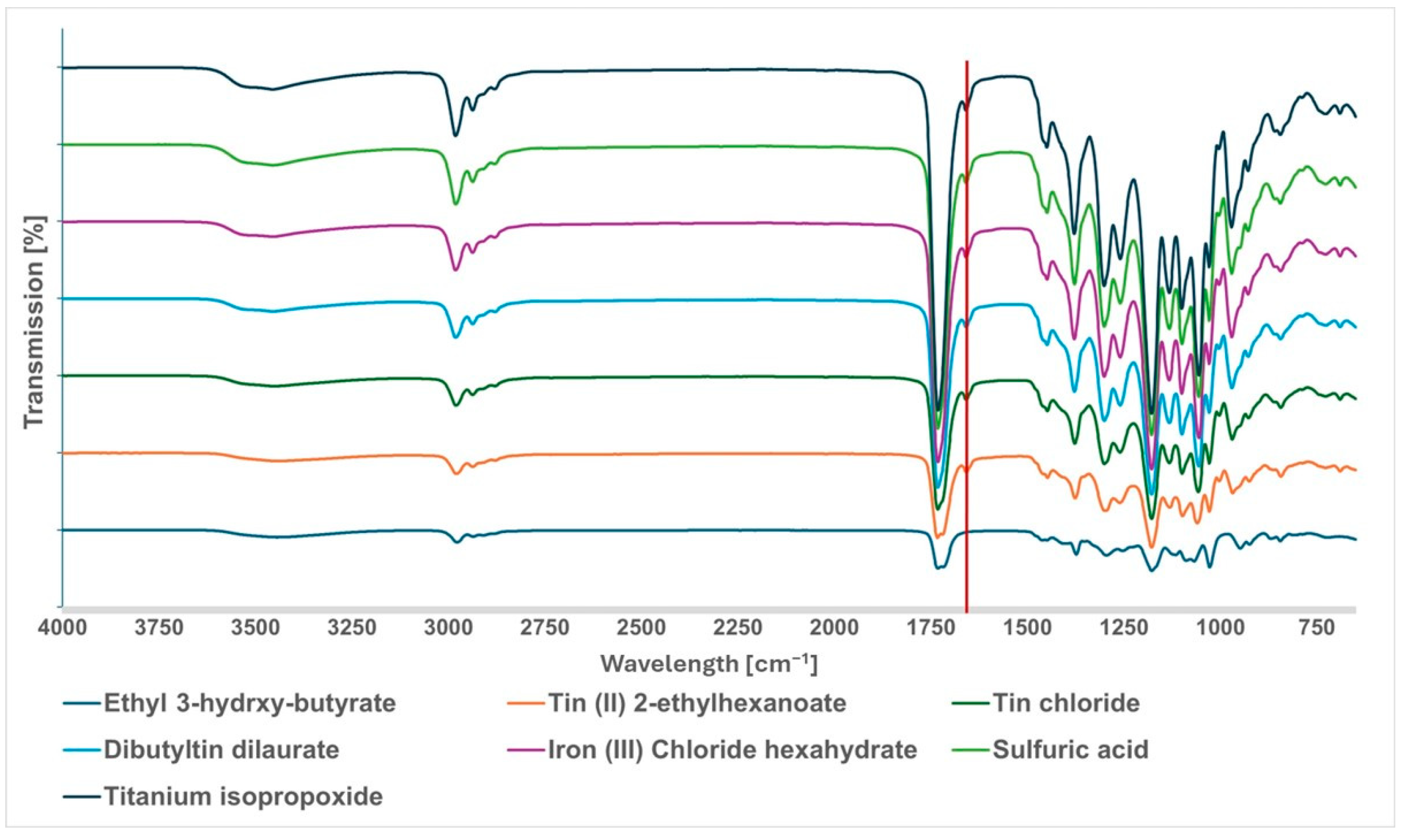
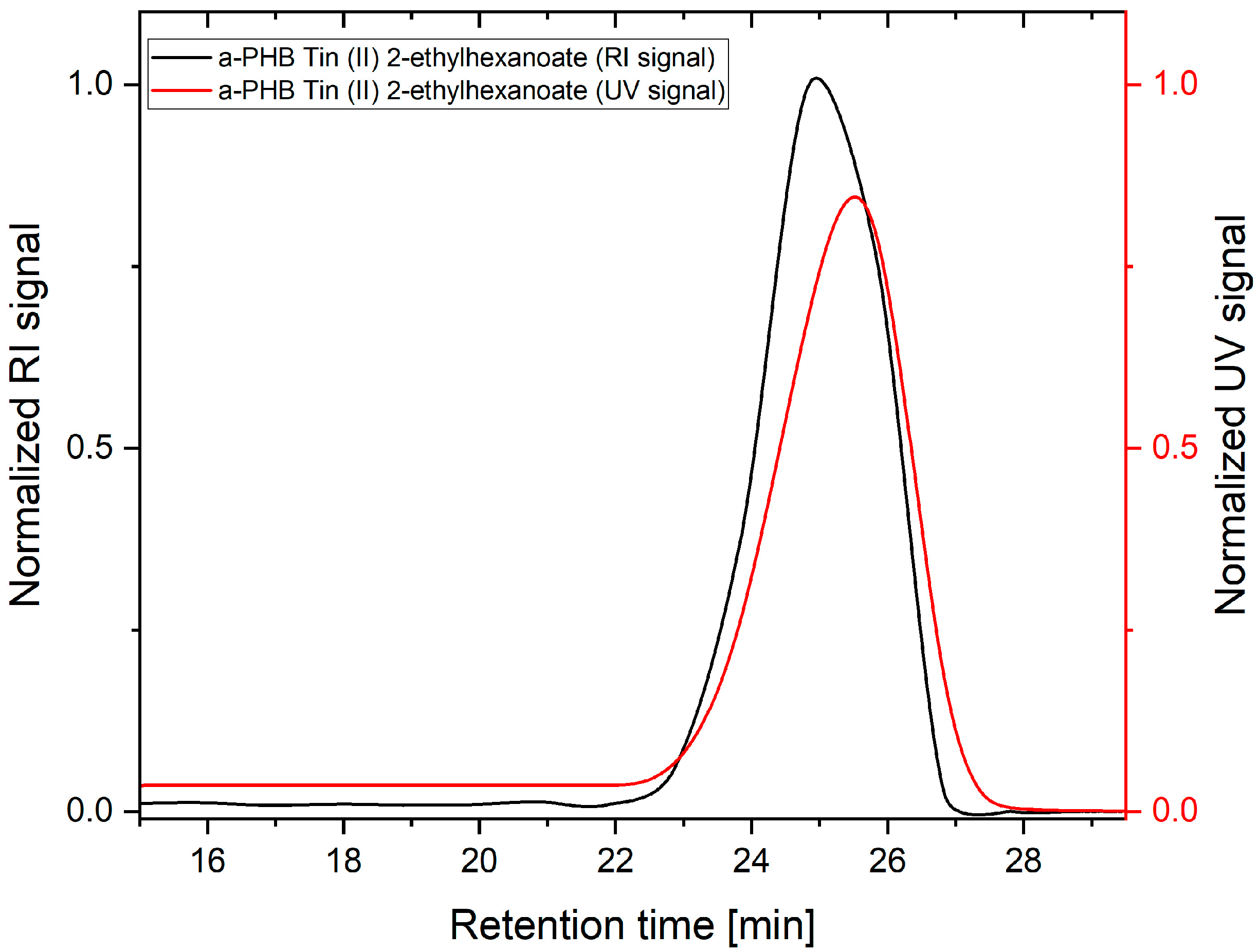
| Catalyst | Result | Mw (g/mol) | Mw Range (g/mol) | PDI | DBC (%) |
|---|---|---|---|---|---|
| Tin (II) 2-ethylhexanoate | successful | 906 | (3268–268) | 1.2 | 0.0197 (±0.0002) |
| Tin chloride | successful | 696 | (2499–263) | 1.1 | 0.0436 (±0.0012) |
| Dibutyltin dilaurate | successful | 1067 | (6131–263) | 1.3 | 0.0198 (±0.0002) |
| Zn acetate | unsuccessful | - | |||
| Sodium methoxide | unsuccessful | - | |||
| Aluminum (III) chloride | unsuccessful | - | |||
| Iron (III) Chloride hexahydrate | successful | 908 | (6908–238) | 1.3 | 0.1008 (±0.0023) |
| p-toluene sulfonic acid | unsuccessful | - | |||
| Sulfuric acid 1 | successful | 1253 | (9102–343) | 1.8 | 0.0036 (±0.0002) |
| Titanium isopropoxide | successful | 2352 | (31,021–694) | 1.7 | 0.0674 (±0.0012) |
| KOCH3/18-crown-6 | successful | 3443 2 | (10,061–147) | 1.5 | 0.0343 (±0.0007) |
| Polymer | CO2 Emissions | Energy Consumption | References | |
|---|---|---|---|---|
| kg CO2/kg Polymer | MJ/kg Polymer | |||
| PHA | P3HB (fermentation) | 2.6 | 44.7 | [23] |
| a-P3HB (self-polycondensation) | 1.7 | 12.7 | ||
| a-PHB (ROP) | 13.7 | 22.5 | ||
| Polyolefins | Polypropylene (PP) | 3.4 | 85.9 | [24] |
| High density polyethylene (HDPE) | 2.5 | 73.7 | [24] | |
| Low density polyethylene (LDPE) | 3.0 | 81.8 | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almustafa, W.; Schubert, D.W.; Grishchuk, S.; Sebastian, J.; Grun, G. Chemical Synthesis of Atactic Poly-3-hydroxybutyrate (a-P3HB) by Self-Polycondensation: Catalyst Screening and Characterization. Polymers 2024, 16, 1655. https://doi.org/10.3390/polym16121655
Almustafa W, Schubert DW, Grishchuk S, Sebastian J, Grun G. Chemical Synthesis of Atactic Poly-3-hydroxybutyrate (a-P3HB) by Self-Polycondensation: Catalyst Screening and Characterization. Polymers. 2024; 16(12):1655. https://doi.org/10.3390/polym16121655
Chicago/Turabian StyleAlmustafa, Wael, Dirk W. Schubert, Sergiy Grishchuk, Jörg Sebastian, and Gregor Grun. 2024. "Chemical Synthesis of Atactic Poly-3-hydroxybutyrate (a-P3HB) by Self-Polycondensation: Catalyst Screening and Characterization" Polymers 16, no. 12: 1655. https://doi.org/10.3390/polym16121655
APA StyleAlmustafa, W., Schubert, D. W., Grishchuk, S., Sebastian, J., & Grun, G. (2024). Chemical Synthesis of Atactic Poly-3-hydroxybutyrate (a-P3HB) by Self-Polycondensation: Catalyst Screening and Characterization. Polymers, 16(12), 1655. https://doi.org/10.3390/polym16121655









