New Generation of Osteoinductive and Antimicrobial Polycaprolactone-Based Scaffolds in Bone Tissue Engineering: A Review
Abstract
:1. Introduction
2. Development Processes of PCL-Based Biomaterials
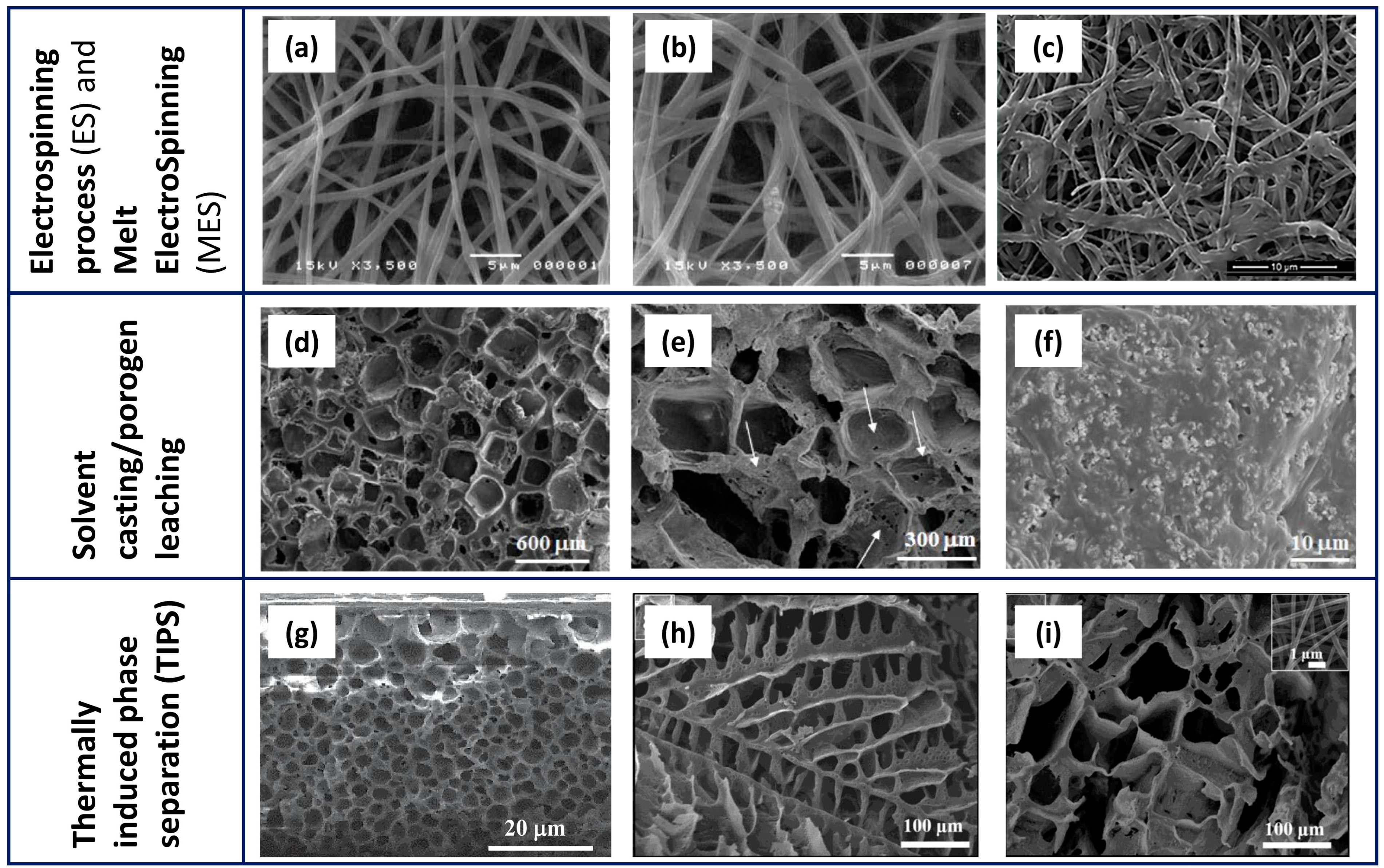
| Technology | Scaffold Main Features | Advantages | Open Challenges | References |
|---|---|---|---|---|
| ES and MES |
|
|
| [18,19,21,25,61,62,63] |
| SC/PL |
|
|
| [42,43,44,45,62,64] |
| TIPS and TIPS/PL |
|
|
| [46,51] |
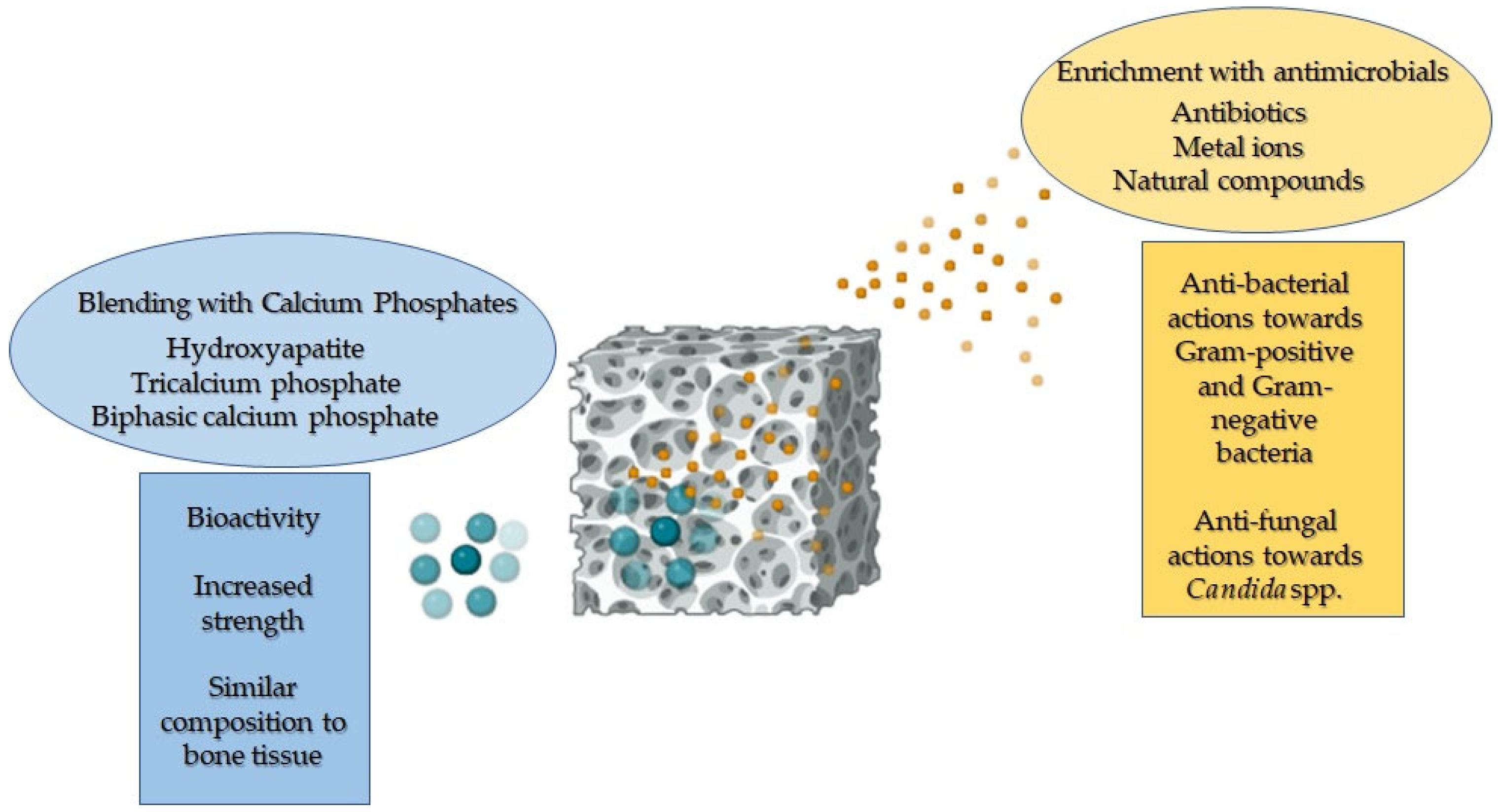
3. Blending of PCL-Based Biomaterials with Calcium Phosphates to Allow Osteogenesis
4. Antimicrobial Properties of PCL-Based Biomaterials Loaded with Antimicrobial Agents
5. Cytocompatibility of PCL-Based Biomaterials Functionalised with Both Antimicrobials and Calcium Phosphates
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-J.; Sarkar, S.K.; Kim, W.-J.; Kim, B.-R.; Park, J.-S.; Lee, B.-T. Bone Regeneration by Multichannel Cylindrical Granular Bone Substitute for Regeneration of Bone in Cases of Tumor, Fracture, and Arthroplasty. Int. J. Environ. Res. Public Health 2022, 19, 8228. [Google Scholar] [CrossRef] [PubMed]
- Yudoh, K.; Sugishita, Y.; Suzuki-Takahashi, Y. Bone Development and Regeneration 2.0. Int. J. Mol. Sci. 2023, 24, 8761. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Sharma, C.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Chou, C.-F.; Mishra, N.C. Gelatin-Polycaprolactone-Nanohydroxyapatite Electrospun Nanocomposite Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C 2021, 119, 111588. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Pinto, S.A.; de Nadai Dias, F.J.; Brasil Camargo Cardoso, G.; Dos Santos Junior, A.R.; de Aro, A.A.; Pino, D.S.; Meneghetti, D.H.; Vitti, R.P.; Dos Santos, G.M.T.; de Carvalho Zavaglia, C.A. Polycaprolactone/Beta-Tricalcium Phosphate Scaffolds Obtained via Rotary Jet-Spinning: In Vitro and in Vivo Evaluation. Cells Tissues Organs 2021, 211, 477–491. [Google Scholar] [CrossRef]
- Allizond, V.; Comini, S.; Cuffini, A.M.; Banche, G. Current Knowledge on Biomaterials for Orthopedic Applications Modified to Reduce Bacterial Adhesive Ability. Antibiotics 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Safari, B.; Davaran, S.; Aghanejad, A. Osteogenic Potential of the Growth Factors and Bioactive Molecules in Bone Regeneration. Int. J. Biol. Macromol. 2021, 175, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.F.; Neves, S.C.; Pereira, A.T.; Borges, I.; Granja, P.L.; Magalhães, F.D.; Gonçalves, I.C. Incorporation of Graphene Oxide into Poly(ε-Caprolactone) 3D Printed Fibrous Scaffolds Improves Their Antimicrobial Properties. Mater. Sci. Eng. C Mater. Biol. Appl 2020, 109, 110537. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bonilla, A.; Cerrada, M.L.; Fernández-García, M.; Kubacka, A.; Ferrer, M.; Fernández-García, M. Biodegradable Polycaprolactone-Titania Nanocomposites: Preparation, Characterization and Antimicrobial Properties. Int. J. Mol. Sci. 2013, 14, 9249. [Google Scholar] [CrossRef] [PubMed]
- Tardajos, M.G.; Cama, G.; Dash, M.; Misseeuw, L.; Gheysens, T.; Gorzelanny, C.; Coenye, T.; Dubruel, P. Chitosan Functionalized Poly-ε-Caprolactone Electrospun Fibers and 3D Printed Scaffolds as Antibacterial Materials for Tissue Engineering Applications. Carbohydr. Polym. 2018, 191, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Gurler, E.B.; Ergul, N.M.; Ozbek, B.; Ekren, N.; Oktar, F.N.; Haskoylu, M.E.; Oner, E.T.; Eroglu, M.S.; Ozbeyli, D.; Korkut, V.; et al. Encapsulated Melatonin in Polycaprolactone (PCL) Microparticles as a Promising Graft Material. Mater. Sci. Eng. C 2019, 100, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Holešová, S.; Čech Barabaszová, K.; Hundáková, M.; Ščuková, M.; Hrabovská, K.; Joszko, K.; Antonowicz, M.; Gzik-Zroska, B. Development of Novel Thin Polycaprolactone (PCL)/Clay Nanocomposite Films with Antimicrobial Activity Promoted by the Study of Mechanical, Thermal, and Surface Properties. Polymers 2021, 13, 3193. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Perez, M.A.; Guarino, V.; Cirillo, V.; Ambrosio, L. Influence of Gelatin Cues in PCL Electrospun Membranes on Nerve Outgrowth. Biomacromolecules 2010, 11, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Loyo, C.; Cordoba, A.; Palza, H.; Canales, D.; Melo, F.; Vivanco, J.F.; Baier, R.V.; Millán, C.; Corrales, T.; Zapata, P.A. Effect of Gelatin Coating and GO Incorporation on the Properties and Degradability of Electrospun PCL Scaffolds for Bone Tissue Regeneration. Polymers 2024, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Shirzaei Sani, I.; Rezaei, M.; Baradar Khoshfetrat, A.; Razzaghi, D. Preparation and Characterization of Polycaprolactone/Chitosan-g-Polycaprolactone/Hydroxyapatite Electrospun Nanocomposite Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2021, 182, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Wutticharoenmongkol, P.; Sanchavanakit, N.; Pavasant, P.; Supaphol, P. Preparation and Characterization of Novel Bone Scaffolds Based on Electrospun Polycaprolactone Fibers Filled with Nanoparticles. Macromol. Biosci. 2006, 6, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Kishori, B.; Rao, S.; Anjum, M.; Hemanth, V.; Das, S.; Jabbari, E. Electropsun Polycaprolactone Fibres in Bone Tissue Engineering: A Review. Mol. Biotechnol. 2021, 63, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent Advances in 3D-Printed Polylactide and Polycaprolactone-Based Biomaterials for Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, B.; Erdogan, B.; Ekren, N.; Oktar, F.N.; Akyol, S.; Ben-Nissan, B.; Sasmazel, H.T.; Kalkandelen, C.; Mergen, A.; Kuruca, S.E.; et al. Production of the Novel Fibrous Structure of Poly(ε-Caprolactone)/Tri-Calcium Phosphate/Hexagonal Boron Nitride Composites for Bone Tissue Engineering. J. Aust. Ceram. Soc. 2018, 54, 251–260. [Google Scholar] [CrossRef]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (ε-Caprolactone)-Based Electrospun Nano-Featured Substrate for Tissue Engineering Applications: A Review. Prog. Biomater. 2021, 10, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Fadaie, M.; Mirzaei, E.; Geramizadeh, B.; Asvar, Z. Incorporation of Nanofibrillated Chitosan into Electrospun PCL Nanofibers Makes Scaffolds with Enhanced Mechanical and Biological Properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Li, M.; Zhang, C.; Chen, D.; Zhou, Z. Cytocompatibility of Electrospun Nanofiber Tubular Scaffolds for Small Diameter Tissue Engineering Blood Vessels. Int. J. Biol. Macromol. 2011, 49, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Hiep, N.T.; Lee, B.-T. Electro-Spinning of PLGA/PCL Blends for Tissue Engineering and Their Biocompatibility. J. Mater. Sci. Mater. Med. 2010, 21, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Moghaddasi, M.; Özdemir, M.M.M.; Noshahr, A.T.; Özadenç, H.M.; Oktay, B.; Bingöl, A.B.; Arayıcı, P.P.; Eraslan, A.; Şenel, İ.; Chifiriuc, M.C.; et al. Blend Electrospinning of Nigella Sativa-Incorporating PCL/PLA/HA Fibers and Its Investigation for Bone Healing Applications. ACS Omega 2024, 9, 10267–10275. [Google Scholar] [CrossRef] [PubMed]
- Goreninskii, S.; Chernova, U.; Prosetskaya, E.; Laushkina, A.; Mishanin, A.; Golovkin, A.; Bolbasov, E. Single-Channel and Multi-Channel Electrospinning for the Fabrication of PLA/PCL Tissue Engineering Scaffolds: Comparative Study of the Materials Physicochemical and Biological Properties. arXiv 2024, arXiv:2403.00767. [Google Scholar] [CrossRef]
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided Bone Regeneration Membrane Made of Polycaprolactone/Calcium Carbonate Composite Nano-Fibers. Biomaterials 2005, 26, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Prado-Prone, G.; Silva-Bermudez, P.; Almaguer-Flores, A.; García-Macedo, J.A.; García, V.I.; Rodil, S.E.; Ibarra, C.; Velasquillo, C. Enhanced Antibacterial Nanocomposite Mats by Coaxial Electrospinning of Polycaprolactone Fibers Loaded with Zn-Based Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cui, Y.; Liu, H.; Tian, Y.; Wang, G.; Fan, Y.; Wang, J.; Wu, D.; Wang, Y. Application of Bioactive Metal Ions in the Treatment of Bone Defects. J. Mater. Chem. B 2022, 10, 9369–9388. [Google Scholar] [CrossRef] [PubMed]
- Abudhahir, M.; Saleem, A.; Paramita, P.; Kumar, S.D.; Tze-Wen, C.; Selvamurugan, N.; Moorthi, A. Polycaprolactone Fibrous Electrospun Scaffolds Reinforced with Copper Doped Wollastonite for Bone Tissue Engineering Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 654–664. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Gradys, A.; Sajkiewicz, P. Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers 2020, 12, 2636. [Google Scholar] [CrossRef] [PubMed]
- Goreninskii, S.; Yuriev, Y.; Runts, A.; Prosetskaya, E.; Sviridova, E.; Plotnikov, E.; Stankevich, K.; Bolbasov, E. Pulsed Vacuum Arc Deposition of Nitrogen-Doped Diamond-like Coatings for Long-Term Hydrophilicity of Electrospun Poly(ε-Caprolactone) Scaffolds. Membranes 2022, 12, 1080. [Google Scholar] [CrossRef] [PubMed]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/Hydroxyapatite Composite Scaffolds: Preparation, Characterization, and in Vitro and in Vivo Biological Responses of Human Primary Bone Cells. J. Biomed. Mater. Res. A 2010, 94, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Biscaia, S.; Branquinho, M.V.; Alvites, R.D.; Fonseca, R.; Sousa, A.C.; Pedrosa, S.S.; Caseiro, A.R.; Guedes, F.; Patrício, T.; Viana, T.; et al. 3D Printed Poly(ε-Caprolactone)/Hydroxyapatite Scaffolds for Bone Tissue Engineering: A Comparative Study on a Composite Preparation by Melt Blending or Solvent Casting Techniques and the Influence of Bioceramic Content on Scaffold Properties. Int. J. Mol. Sci. 2022, 23, 2318. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Yang, S.-S.; Lee, J. A Polycaprolactone/Cuttlefish Bone-Derived Hydroxyapatite Composite Porous Scaffold for Bone Tissue Engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Kim, S.E.; Hyun, Y.T.; Kim, D.H.; Lee, H.M.; Hwang, Y.M.; Park, S.A.; Shin, J.W. In Vitro Evaluation of Poly ε-Caprolactone/Hydroxyapatite Composite as Scaffolds for Bone Tissue Engineering with Human Bone Marrow Stromal Cells. Key Eng. Mater. 2007, 342–343, 369–372. [Google Scholar] [CrossRef]
- Thadavirul, N.; Pavasant, P.; Supaphol, P. Improvement of Dual-Leached Polycaprolactone Porous Scaffolds by Incorporating with Hydroxyapatite for Bone Tissue Regeneration. J. Biomater. Sci. Polym. Ed. 2014, 25, 1986–2008. [Google Scholar] [CrossRef] [PubMed]
- Thadavirul, N.; Pavasant, P.; Supaphol, P. Development of Polycaprolactone Porous Scaffolds by Combining Solvent Casting, Particulate Leaching, and Polymer Leaching Techniques for Bone Tissue Engineering. J. Biomed. Mater. Res. Part A 2014, 102, 3379–3392. [Google Scholar] [CrossRef]
- Balcucho, J.; Narváez, D.M.; Castro-Mayorga, J.L. Antimicrobial and Biocompatible Polycaprolactone and Copper Oxide Nanoparticle Wound Dressings against Methicillin-Resistant Staphylococcus Aureus. Nanomaterials 2020, 10, 1692. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Li, C.; Cao, J.; He, E.; Wu, X.; Wang, F.; Wang, L. Facile Preparation PCL/ Modified Nano ZnO Organic-Inorganic Composite and Its Application in Antibacterial Materials. J. Polym. Res. 2020, 27, 78. [Google Scholar] [CrossRef]
- de Menezes, B.R.C.; Montanheiro, T.L.D.A.; Sampaio, A.D.G.; Koga-Ito, C.Y.; Thim, G.P.; Montagna, L.S. PCL/β-AgVO3 Nanocomposites Obtained by Solvent Casting as Potential Antimicrobial Biomaterials. J. Appl. Polym. Sci. 2021, 138, 50130. [Google Scholar] [CrossRef]
- Comini, S.; Sparti, R.; Coppola, B.; Mohammadi, M.; Scutera, S.; Menotti, F.; Banche, G.; Cuffini, A.M.; Palmero, P.; Allizond, V. Novel Silver-Functionalized Poly(ε-Caprolactone)/Biphasic Calcium Phosphate Scaffolds Designed to Counteract Post-Surgical Infections in Orthopedic Applications. Int. J. Mol. Sci. 2021, 22, 10176. [Google Scholar] [CrossRef] [PubMed]
- Comini, S.; Scutera, S.; Sparti, R.; Banche, G.; Coppola, B.; Bertea, C.M.; Bianco, G.; Gatti, N.; Cuffini, A.M.; Palmero, P.; et al. Combination of Poly(ε-Caprolactone) Biomaterials and Essential Oils to Achieve Anti-Bacterial and Osteo-Proliferative Properties for 3D-Scaffolds in Regenerative Medicine. Pharmaceutics 2022, 14, 1873. [Google Scholar] [CrossRef]
- Menotti, F.; Scutera, S.; Coppola, B.; Longo, F.; Mandras, N.; Cavallo, L.; Comini, S.; Sparti, R.; Fiume, E.; Cuffini, A.M.; et al. Tuning of Silver Content on the Antibacterial and Biological Properties of Poly(ε-Caprolactone)/Biphasic Calcium Phosphate 3D-Scaffolds for Bone Tissue Engineering. Polymer 2023, 15, 3618. [Google Scholar] [CrossRef]
- Menotti, F.; Scutera, S.; Maniscalco, E.; Coppola, B.; Bondi, A.; Costa, C.; Longo, F.; Mandras, N.; Pagano, C.; Cavallo, L.; et al. Is Silver Addition to Scaffolds Based on Polycaprolactone Blended with Calcium Phosphates Able to Inhibit Candida Albicans and Candida Auris Adhesion and Biofilm Formation? Int. J. Mol. Sci. 2024, 25, 2784. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, R.; del Valle, L.J.; Torras, J.; Puiggalí, J. Recent Progress on Biodegradable Tissue Engineering Scaffolds Prepared by Thermally-Induced Phase Separation (TIPS). Int. J. Mol. Sci. 2021, 22, 3504. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Hu, J.; Wu, Z.; Chen, H. Fabrication and Characterization of Polylactic Acid/Polycaprolactone Composite Macroporous Micro-Nanofiber Scaffolds by Phase Separation. New J. Chem. 2020, 44, 17382–17390. [Google Scholar] [CrossRef]
- Sultana, N.; Hayat Khan, T. Polycaprolactone Scaffolds and Hydroxyapatite/Polycaprolactone Composite Scaffolds for Bone Tissue Engineering. J. Bionanosci. 2013, 7, 169–173. [Google Scholar] [CrossRef]
- Guan, J.; Fujimoto, K.L.; Sacks, M.S.; Wagner, W.R. Preparation and Characterization of Highly Porous, Biodegradable Polyurethane Scaffolds for Soft Tissue Applications. Biomaterials 2005, 26, 3961–3971. [Google Scholar] [CrossRef] [PubMed]
- Grandi, C.; Di Liddo, R.; Paganin, P.; Lora, S.; Dalzoppo, D.; Feltrin, G.; Giraudo, C.; Tommasini, M.; Conconi, M.T.; Parnigotto, P.P. Porous Alginate/Poly(ε-Caprolactone) Scaffolds: Preparation, Characterization and in Vitro Biological Activity. Int. J. Mol. Med. 2011, 27, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Montanheiro, T.L.D.A.; Montagna, L.S.; Patrulea, V.; Jordan, O.; Borchard, G.; Lobato, G.M.M.; Catalani, L.H.; Lemes, A.P. Evaluation of Cellulose Nanocrystal Addition on Morphology, Compression Modulus and Cytotoxicity of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Scaffolds. J. Mater. Sci. 2019, 54, 7198–7210. [Google Scholar] [CrossRef]
- Seyed Hakim, R.; Maghsoud, Z.; Halabian, R. Fabrication and Evaluation of Polycaprolactone/Olive Oil Scaffolds by Phase Inversion for Tissue Engineering. Eur. Polym. J. 2021, 150, 110394. [Google Scholar] [CrossRef]
- Samadian, H.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Bit, A.; Alam, M.; Goodarzi, A.; Darya, G.; Salehi, M. A Tailored Polylactic Acid/Polycaprolactone Biodegradable and Bioactive 3D Porous Scaffold Containing Gelatin Nanofibers and Taurine for Bone Regeneration. Sci. Rep. 2020, 10, 13366. [Google Scholar] [CrossRef] [PubMed]
- Kozehkonan, G.S.; Salehi, M.; Farzamfar, S.; Ghanbari, H.; Adabi, M.; Amani, A. Preparation and Characterization of PCL Polymeric Scaffolds Coated with Chitosan/ Bioactive Glass/Gelatin Nanoparticles Using the Tips Methodology for Bone Tissue Engineering. Nanomed. J. 2019, 6, 311–320. [Google Scholar] [CrossRef]
- Katsogiannis, K.; Vladisavljević, G.; Georgiadou, S. Porous Electrospun Polycaprolactone (PCL) Fibres by Phase Separation. Eur. Polym. J. 2015, 53, 284–295. [Google Scholar] [CrossRef]
- Salerno, A.; Domingo, C. A Novel Bio-Safe Phase Separation Process for Preparing Open-Pore Biodegradable Polycaprolactone Microparticles. Mater. Sci. Eng. C 2014, 42, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.-M.; Liu, J.; Sheppard, R.; Koo, S.; Silverstein, J.; Zhang, J.; James, P.F. I-Optimal Design of Hierarchical 3D Scaffolds Produced by Combining Additive Manufacturing and Thermally Induced Phase Separation. ACS Appl. Bio Mater. 2019, 2, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Gay, S.; Lefebvre, G.; Bonnin, M.; Nottelet, B.; Boury, F.; Gibaud, A.; Calvignac, B. PLA Scaffolds Production from Thermally Induced Phase Separation: Effect of Process Parameters and Development of an Environmentally Improved Route Assisted by Supercritical Carbon Dioxide. J. Supercrit. Fluids 2018, 136, 123–135. [Google Scholar] [CrossRef]
- Farzamfar, S.; Naseri-Nosar, M.; Sahrapeyma, H.; Ehterami, A.; Goodarzi, A.; Rahmati, M.; Ahmadi Lakalayeh, G.; Ghorbani, S.; Vaez, A.; Salehi, M. Tetracycline Hydrochloride-Containing Poly (ε-Caprolactone)/Poly Lactic Acid Scaffold for Bone Tissue Engineering Application: In Vitro and in Vivo Study. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 472–479. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, C.; Yan, L.; Lei, H.; Peng, C.; Liu, S.; Fan, L.; Chu, Y. Antibacterial and Osteoconductive Polycaprolactone/Polylactic Acid/Nano-Hydroxyapatite/Cu@ZIF-8 GBR Membrane with Asymmetric Porous Structure. Int. J. Biol. Macromol. 2023, 224, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Montanheiro, T.L.D.A.; Schatkoski, V.M.; de Menezes, B.R.C.; Pereira, R.M.; Ribas, R.G.; De Sousa, A.; Lemes, A.P.; Fernandes, M.H.F.V.; Thim, G.P. Recent Progress on Polymer Scaffolds Production: Methods, Main Results, Advantages and Disadvantages. Express Polym. Lett. 2022, 16, 197–219. [Google Scholar] [CrossRef]
- Ghofrani, A.; Taghavi, L.; Khalilivavdareh, B.; Rohani Shirvan, A.; Nouri, A. Additive Manufacturing and Advanced Functionalities of Cardiac Patches: A Review. Eur. Polym. J. 2022, 174, 111332. [Google Scholar] [CrossRef]
- Tay, B.Y.; Zhang, S.X.; Myint, M.H.; Ng, F.L.; Chandrasekaran, M.; Tan, L.K.A. Processing of Polycaprolactone Porous Structure for Scaffold Development. J. Mater. Process. Technol. 2007, 182, 117–121. [Google Scholar] [CrossRef]
- Backes, E.H.; Harb, S.V.; Beatrice, C.A.G.; Shimomura, K.M.B.; Passador, F.R.; Costa, L.C.; Pessan, L.A. Polycaprolactone Usage in Additive Manufacturing Strategies for Tissue Engineering Applications: A Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 1479–1503. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; James, J.; Grohens, Y.; Kalarikkal, N.; Thomas, S. Additive Manufacturing of Poly (ε-Caprolactone) for Tissue Engineering. JOM 2020, 72, 4127–4138. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kim, Y.-C.; Min, J.-C.; Park, H.-J.; Lee, E.-J.; Shim, J.-H.; Choi, J.-W. Clinical Application of 3D-Printed Patient-Specific Polycaprolactone/Beta Tricalcium Phosphate Scaffold for Complex Zygomatico-Maxillary Defects. Polymers 2022, 14, 740. [Google Scholar] [CrossRef] [PubMed]
- Bini, F.; Guachi, R.; Marconato, P.; Del Gaudio, C.; Marinozzi, F. Combining Additive Manufacturing and Computational Fluid Dynamics to Optimize Scaffold Design: A Preliminary Study. Mater. Today Proc. 2019, 7, 484–491. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef] [PubMed]
- Gharibshahian, M.; Salehi, M.; Beheshtizadeh, N.; Kamalabadi-Farahani, M.; Atashi, A.; Nourbakhsh, M.-S.; Alizadeh, M. Recent Advances on 3D-Printed PCL-Based Composite Scaffolds for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1168504. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone Tissue Engineering Using Polycaprolactone Scaffolds Fabricated via Selective Laser Sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Masood, S.H. 10.04—Advances in Fused Deposition Modeling. In Comprehensive Materials Processing; Hashmi, S., Batalha, G.F., Van Tyne, C.J., Yilbas, B., Eds.; Elsevier: Oxford, UK, 2014; pp. 69–91. ISBN 978-0-08-096533-8. [Google Scholar]
- Jiao, Z.; Luo, B.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D Printing of HA/PCL Composite Tissue Engineering Scaffolds. Adv. Ind. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Safiaghdam, H.; Nokhbatolfoghahaei, H.; Farzad-Mohajeri, S.; Dehghan, M.M.; Farajpour, H.; Aminianfar, H.; Bakhtiari, Z.; Jabbari Fakhr, M.; Hosseinzadeh, S.; Khojasteh, A. 3D-Printed MgO Nanoparticle Loaded Polycaprolactone β-Tricalcium Phosphate Composite Scaffold for Bone Tissue Engineering Applications: In-Vitro and in-Vivo Evaluation. J. Biomed. Mater. Res. Part A 2023, 111, 322–339. [Google Scholar] [CrossRef] [PubMed]
- Krobot, Š.; Melčová, V.; Menčík, P.; Kontárová, S.; Rampichová, M.; Hedvičáková, V.; Mojžišová, E.; Baco, A.; Přikryl, R. Poly(3-Hydroxybutyrate) (PHB) and Polycaprolactone (PCL) Based Blends for Tissue Engineering and Bone Medical Applications Processed by FDM 3D Printing. Polymers 2023, 15, 2404. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Kim, B.-H.; Kim, M.-S. Amine Plasma-Polymerization of 3D Polycaprolactone/β-Tricalcium Phosphate Scaffold to Improving Osteogenic Differentiation In Vitro. Materials 2022, 15, 366. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.A.; Song, S.J.; Susanto, E.; Chuan, P.; Lam, C.X.F.; Woodruff, M.A.; Hutmacher, D.W.; Cool, S.M. The Stimulation of Healing within a Rat Calvarial Defect by mPCL–TCP/Collagen Scaffolds Loaded with rhBMP-2. Biomaterials 2009, 30, 2479–2488. [Google Scholar] [CrossRef]
- Idaszek, J.; Bruinink, A.; Święszkowski, W. Ternary Composite Scaffolds with Tailorable Degradation Rate and Highly Improved Colonization by Human Bone Marrow Stromal Cells. J. Biomed. Mater. Res. Part A 2015, 103, 2394–2404. [Google Scholar] [CrossRef]
- Schantz, J.-T.; Brandwood, A.; Hutmacher, D.W.; Khor, H.L.; Bittner, K. Osteogenic Differentiation of Mesenchymal Progenitor Cells in Computer Designed Fibrin-Polymer-Ceramic Scaffolds Manufactured by Fused Deposition Modeling. J. Mater. Sci. Mater. Med. 2005, 16, 807–819. [Google Scholar] [CrossRef]
- Rezania, N.; Asadi-Eydivand, M.; Abolfathi, N.; Bonakdar, S.; Mehrjoo, M.; Solati-Hashjin, M. Three-Dimensional Printing of Polycaprolactone/Hydroxyapatite Bone Tissue Engineering Scaffolds Mechanical Properties and Biological Behavior. J. Mater. Sci. Mater. Med. 2022, 33, 31. [Google Scholar] [CrossRef] [PubMed]
- Oberdiek, F.; Vargas, C.I.; Rider, P.; Batinic, M.; Görke, O.; Radenković, M.; Najman, S.; Baena, J.M.; Jung, O.; Barbeck, M. Ex Vivo and In Vivo Analyses of Novel 3D-Printed Bone Substitute Scaffolds Incorporating Biphasic Calcium Phosphate Granules for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 3588. [Google Scholar] [CrossRef] [PubMed]
- Duymaz, B.T.; Erdiler, F.B.; Alan, T.; Aydogdu, M.O.; Inan, A.T.; Ekren, N.; Uzun, M.; Sahin, Y.M.; Bulus, E.; Oktar, F.N.; et al. 3D Bio-Printing of Levan/Polycaprolactone/Gelatin Blends for Bone Tissue Engineering: Characterization of the Cellular Behavior. Eur. Polym. J. 2019, 119, 426–437. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, J.-H.; Ahn, C.B.; Hong, J.H.; Son, K.H.; Lee, J.W. Development of a 3D-Printed Drug-Eluting Stent for Treating Obstructive Salivary Gland Disease. ACS Biomater. Sci. Eng. 2019, 5, 3572–3581. [Google Scholar] [CrossRef] [PubMed]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-Specific 3D Scanned and 3D Printed Antimicrobial Polycaprolactone Wound Dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Salmoria, G.V.; Paggi, R.A.; Kanis, L.A. Manufacturing of PCL/SAg Tubes by Melt-Extrusion for Nerve Regeneration: Structure and Mechanical Properties. Polym. Test. 2016, 55, 160–165. [Google Scholar] [CrossRef]
- Wang, S.; Gu, R.; Wang, F.; Zhao, X.; Yang, F.; Xu, Y.; Yan, F.; Zhu, Y.; Xia, D.; Liu, Y. 3D-Printed PCL/Zn Scaffolds for Bone Regeneration with a Dose-Dependent Effect on Osteogenesis and Osteoclastogenesis. Mater. Today Bio 2022, 13, 100202. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, Z.; Sun, Y.; Cheng, F.; Zhu, L.; Zhang, Y.; Zhang, Z.; Wu, J.; Wang, J. Surface Roughness and Biocompatibility of Polycaprolactone Bone Scaffolds: An Energy-Density-Guided Parameter Optimization for Selective Laser Sintering. Front. Bioeng. Biotechnol. 2022, 10, 888267. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, J.; Xiang, X.; Xie, L.; Chen, R.; Li, L.; Ma, K.; Sun, Q.; Yang, R.; Huang, T.; et al. Biodegradable BBG/PCL Composite Scaffolds Fabricated by Selective Laser Sintering for Directed Regeneration of Critical-Sized Bone Defects. Mater. Des. 2023, 225, 111543. [Google Scholar] [CrossRef]
- Partee, B.; Hollister, S.J.; Das, S. Selective Laser Sintering of Polycaprolactone Bone Tissue Engineering Scaffolds. MRS Online Proc. Libr. 2004, 845, 340–346. [Google Scholar] [CrossRef]
- Eosoly, S.; Brabazon, D.; Lohfeld, S.; Looney, L. Selective Laser Sintering of Hydroxyapatite/Poly-ε-Caprolactone Scaffolds. Acta Biomater. 2010, 6, 2511–2517. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhu, W.; Zhu, X.; Yi, X.; Yao, J.; Yuan, X.; Chen, F.; Han, X. Development of Hydroxyapatite/Polycaprolactone Composite Biomaterials for Laser Powder Bed Fusion: Evaluation of Powder Characteristics, Mechanical Properties and Biocompatibility. Polymer 2024, 16, 731. [Google Scholar] [CrossRef] [PubMed]
- Chartrain, N.A.; Williams, C.B.; Whittington, A.R. A Review on Fabricating Tissue Scaffolds Using Vat Photopolymerization. Acta Biomater. 2018, 74, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Ronca, A.; Ronca, S.; Forte, G.; Ambrosio, L. Synthesis of an UV-Curable Divinyl-Fumarate Poly-ε-Caprolactone for Stereolithography Applications. In Computer-Aided Tissue Engineering: Methods and Protocols; Rainer, A., Moroni, L., Eds.; Springer: New York, NY, USA, 2021; pp. 55–62. ISBN 978-1-07-160611-7. [Google Scholar]
- Green, B.J.; Worthington, K.S.; Thompson, J.R.; Bunn, S.J.; Rethwisch, M.; Kaalberg, E.E.; Jiao, C.; Wiley, L.A.; Mullins, R.F.; Stone, E.M.; et al. Effect of Molecular Weight and Functionality on Acrylated Poly(Caprolactone) for Stereolithography and Biomedical Applications. Biomacromolecules 2018, 19, 3682–3692. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Keshi, E.; Sauer, I.M.; Weinhart, M. Development of GelMA/PCL and dECM/PCL Resins for 3D Printing of Acellular in Vitro Tissue Scaffolds by Stereolithography. Mater. Sci. Eng. C 2020, 112, 110958. [Google Scholar] [CrossRef]
- Elomaa, L.; Teixeira, S.; Hakala, R.; Korhonen, H.; Grijpma, D.W.; Seppälä, J.V. Preparation of Poly(ε-Caprolactone)-Based Tissue Engineering Scaffolds by Stereolithography. Acta Biomater. 2011, 7, 3850–3856. [Google Scholar] [CrossRef]
- Cunniffe, G.M.; Gonzalez-Fernandez, T.; Daly, A.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. Three-Dimensional Bioprinting of Polycaprolactone Reinforced Gene Activated Bioinks for Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on Bone Substitutes through 3D Bioprinting. Int. J. Mol. Sci. 2020, 21, 7012. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Barrère, F.; Van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Biomimetic Hydroxyapatite Coating on Metal Implants. J. Am. Ceram. Soc. 2002, 85, 517–522. [Google Scholar] [CrossRef]
- Darimont, G.L.; Cloots, R.; Heinen, E.; Seidel, L.; Legrand, R. In Vivo Behaviour of Hydroxyapatite Coatings on Titanium Implants: A Quantitative Study in the Rabbit. Biomaterials 2002, 23, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Basgorenay, B.; Ulubayram, K.; Serbetci, K.; Onurhan, E.; Hasirci, N. Preparation, Modification, and Characterization of Acrylic Cements. J. Appl. Polym. Sci. 2006, 99, 3631–3637. [Google Scholar] [CrossRef]
- Yousefi, A.-M. A Review of Calcium Phosphate Cements and Acrylic Bone Cements as Injectable Materials for Bone Repair and Implant Fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019872594. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wu, S.; Li, J.; Li, X.; Yang, P.; Li, G. Chitosan/Calcium Phosphate Flower-like Microparticles as Carriers for Drug Delivery Platform. Int. J. Biol. Macromol. 2020, 155, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; El-Sakhawy, M. Preparation of Polyelectrolyte/Calcium Phosphate Hybrids for Drug Delivery Application. Carbohydr. Polym. 2014, 113, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Baker, B.A.; Mou, X.; Ren, N.; Qiu, J.; Boughton, R.I.; Liu, H. Biopolymer/Calcium Phosphate Scaffolds for Bone Tissue Engineering. Adv. Healthc. Mater. 2014, 3, 469–484. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, T.A.; Geurts, J.A.P.; Arts, J.J.; Lindfors, N.C. 3—Biomaterials in Treatment of Orthopedic Infections. In Management of Periprosthetic Joint Infections (PJIs); Arts, J.J.C., Geurts, J., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 41–68. ISBN 978-0-08-100205-6. [Google Scholar]
- Chai, Y.C.; Carlier, A.; Bolander, J.; Roberts, S.J.; Geris, L.; Schrooten, J.; Van Oosterwyck, H.; Luyten, F.P. Current Views on Calcium Phosphate Osteogenicity and the Translation into Effective Bone Regeneration Strategies. Acta Biomater. 2012, 8, 3876–3887. [Google Scholar] [CrossRef] [PubMed]
- Rather, H.A.; Varghese, J.F.; Dhimmar, B.; Yadav, U.C.S.; Vasita, R. Polycaprolactone-Collagen Nanofibers Loaded with Dexamethasone and Simvastatin as an Osteoinductive and Immunocompatible Scaffold for Bone Regeneration Applications. Biomater. Biosyst. 2022, 8, 100064. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, C.; Hermosilla, J.; Klein, C.; Chaparro, A.; Valdivia-Gandur, I.; Beltrán, V.; Acevedo, F. Osteoinductive Electrospun Scaffold Based on PCL-Col as a Regenerative Therapy for Peri-Implantitis. Pharmaceutics 2023, 15, 1939. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Byambaa, B.; Morshed, M.; Cheikh, M.I.; Shakoor, R.A.; Mustafy, T.; Marei, H.E. Advances in Osteobiologic Materials for Bone Substitutes. J. Tissue Eng. Regen. Med. 2018, 12, 1448–1468. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, S.; Mostafavi, A.; Ramesh, S.; Memic, A.; Rivero, I.V.; Rao, P.; Tamayol, A. Process–Structure–Quality Relationships of Three-Dimensional Printed Poly(Caprolactone)-Hydroxyapatite Scaffolds. Tissue Eng. Part A 2020, 26, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic Bone Graft Substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Tulliani, J.-M.; Tadier, S.; Meille, S.; Chevalier, J.; Palmero, P. Novel Calcium Phosphate/PCL Graded Samples: Design and Development in View of Biomedical Applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.; Ho, V.H.; Jung, H.-I.; Lee, B.-T. Physico-Mechanical and in-Vivo Evaluations of Tri-Layered Alginate-Gelatin/Polycaprolactone-Gelatin-β-TCP Membranes for Guided Bone Regeneration. J. Biomater. Sci. Polym. Ed. 2023, 34, 18–34. [Google Scholar] [CrossRef]
- Gómez-Lizárraga, K.K.; Flores-Morales, C.; Del Prado-Audelo, M.L.; Álvarez-Pérez, M.A.; Piña-Barba, M.C.; Escobedo, C. Polycaprolactone- and Polycaprolactone/Ceramic-Based 3D-Bioplotted Porous Scaffolds for Bone Regeneration: A Comparative Study. Mater. Sci. Eng. C 2017, 79, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Pascaud-Mathieu, P.; Allizond, V.; Tulliani, J.-M.; Coppola, B.; Banche, G.; Chaput, C.; Cuffini, A.M.; Rossignol, F.; Palmero, P. Robocasting of Single and Multi-Functional Calcium Phosphate Scaffolds and Its Hybridization with Conventional Techniques: Design, Fabrication and Characterization. Appl. Sci. 2020, 10, 8677. [Google Scholar] [CrossRef]
- Harikrishnan, P.; Islam, H.; Sivasamy, A. Biocompatibility Studies of Nanoengineered Polycaprolactone and Nanohydroxyapatite Scaffold for Craniomaxillofacial Bone Regeneration. J. Craniofacial Surg. 2019, 30, 265. [Google Scholar] [CrossRef] [PubMed]
- Machado-Paula, M.M.; Corat, M.A.F.; de Vasconcellos, L.M.R.; Araújo, J.C.R.; Mi, G.; Ghannadian, P.; Toniato, T.V.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Rotary Jet-Spun Polycaprolactone/Hydroxyapatite and Carbon Nanotube Scaffolds Seeded with Bone Marrow Mesenchymal Stem Cells Increase Bone Neoformation. ACS Appl. Bio Mater. 2022, 5, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Miszuk, J.; Liang, Z.; Hu, J.; Sanyour, H.; Hong, Z.; Fong, H.; Sun, H. An Elastic Mineralized 3D Electrospun PCL Nanofibrous Scaffold for Drug Release and Bone Tissue Engineering. ACS Appl. Bio Mater. 2021, 4, 3639–3648. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, K.; Kwaśniak, K.; Gnilitskyi, I.; Barylyak, A.; Zinchenko, V.; Fahmi, A.; Korchynskyi, O.; Bobitski, Y. Functionalization of Polycaprolactone Electrospun Osteoplastic Scaffolds with Fluorapatite and Hydroxyapatite Nanoparticles: Biocompatibility Comparison of Human Versus Mouse Mesenchymal Stem Cells. Materials 2021, 14, 1333. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, F.; Bagheri-Khoulenjani, S.; Olov, N.; Zeini, D.; Solouk, A.; Mirzadeh, H. Fabrication of Nanocomposite/Nanofibrous Functionally Graded Biomimetic Scaffolds for Osteochondral Tissue Regeneration. J. Biomed. Mater. Res. A 2021, 109, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt Electrowriting of PLA, PCL, and Composite PLA/PCL Scaffolds for Tissue Engineering Application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef]
- Thuaksuban, N.; Monmaturapoj, N.; Luntheng, T. Effects of Polycaprolactone-Biphasic Calcium Phosphate Scaffolds on Enhancing Growth and Differentiation of Osteoblasts. Bio-Med. Mater. Eng. 2018, 29, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, C.; Auriemma, G.; Sardo, C.; Alvarez-Lorenzo, C.; Garofalo, E.; Morello, S.; Falcone, G.; Aquino, R.P. 3D Printed Macroporous Scaffolds of PCL and Inulin-g-P(D,L)LA for Bone Tissue Engineering Applications. Int. J. Pharm. 2023, 641, 123093. [Google Scholar] [CrossRef] [PubMed]
- Tamjid, E.; Bohlouli, M.; Mohammadi, S.; Alipour, H.; Nikkhah, M. Sustainable Drug Release from Highly Porous and Architecturally Engineered Composite Scaffolds Prepared by 3D Printing. J. Biomed. Mater. Res. Part A 2020, 108, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Felice, B.; Sánchez, M.A.; Socci, M.C.; Sappia, L.D.; Gómez, M.I.; Cruz, M.K.; Felice, C.J.; Martí, M.; Pividori, M.I.; Simonelli, G.; et al. Controlled Degradability of PCL-ZnO Nanofibrous Scaffolds for Bone Tissue Engineering and Their Antibacterial Activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Benmassaoud, M.M.; Kohama, C.; Kim, T.W.B.; Kadlowec, J.A.; Foltiny, B.; Mercurio, T.; Ranganathan, S.I. Efficacy of Eluted Antibiotics through 3D Printed Femoral Implants. Biomed. Microdev. 2019, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Korelidou, A.; Domínguez-Robles, J.; Magill, E.R.; Eleftheriadou, M.; Cornelius, V.A.; Donnelly, R.F.; Margariti, A.; Larrañeta, E. 3D-Printed Reservoir-Type Implants Containing Poly(Lactic Acid)/Poly(Caprolactone) Porous Membranes for Sustained Drug Delivery. Biomater. Adv. 2022, 139, 213024. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yao, Q.; Li, L.; Zhang, X.; Wei, B.; Yuan, L.; Wang, L. Antimicrobial Activity of 3D-Printed Poly(ε-Caprolactone) (PCL) Composite Scaffolds Presenting Vancomycin-Loaded Polylactic Acid-Glycolic Acid (PLGA) Microspheres. Med. Sci. Monit. 2018, 24, 6934–6945. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yu, X.; Chen, L.; Shi, T.; Bou-Akl, T.; Markel, D.C. Osteoblastic Differentiation and Bactericidal Activity Are Enhanced by Erythromycin Released from PCL/PLGA-PVA Coaxial Nanofibers. J. Biomater. Appl. 2022, 37, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Allizond, V.; Banche, G.; Salvoni, M.; Malandrino, M.; Cecone, C.; Cuffini, A.M.; Bracco, P. Facile One-Step Electrospinning Process to Prepare AgNPs-Loaded PLA and PLA/PEO Mats with Antibacterial Activity. Polymers 2023, 15, 1470. [Google Scholar] [CrossRef] [PubMed]
- Afghah, F.; Ullah, M.; Seyyed Monfared Zanjani, J.; Akkus Sut, P.; Sen, O.; Emanet, M.; Saner Okan, B.; Culha, M.; Menceloglu, Y.; Yildiz, M.; et al. 3D Printing of Silver-Doped Polycaprolactone-Poly(Propylene Succinate) Composite Scaffolds for Skin Tissue Engineering. Biomed Mater. 2020, 15, 035015. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef] [PubMed]
- Seo, N.; Park, C.; Stahl, A.M.; Cho, H.; Park, S.-W.; Yim, S.-H.; Yun, K.-D.; Ji, M.-K.; Kim, H.; Yang, Y.P.; et al. Effect of Zinc Oxide Nanoparticle Addition to Polycaprolactone Periodontal Membranes on Antibacterial Activity and Cell Viability. J. Nanosci. Nanotechnol. 2021, 21, 3683–3688. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Homem, N.C.; Teixeira, M.A.; Ribeiro, A.R.M.; Antunes, J.C.; Amorim, M.T.P. Physical, Thermal, and Antibacterial Effects of Active Essential Oils with Potential for Biomedical Applications Loaded onto Cellulose Acetate/Polycaprolactone Wet-Spun Microfibers. Biomolecules 2020, 10, 1129. [Google Scholar] [CrossRef]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm Inhibition by Biocompatible Poly(ε-Caprolactone) Nanocapsules Loaded with Essential Oils and Their Cyto/Genotoxicity to Human Keratinocyte Cell Line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef] [PubMed]
- Motealleh, B.; Zahedi, P.; Rezaeian, I.; Moghimi, M.; Abdolghaffari, A.H.; Zarandi, M.A. Morphology, Drug Release, Antibacterial, Cell Proliferation, and Histology Studies of Chamomile-Loaded Wound Dressing Mats Based on Electrospun Nanofibrous Poly(ε-Caprolactone)/Polystyrene Blends. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 977–987. [Google Scholar] [CrossRef] [PubMed]
- da Silva, N.P.; Pereira, E.D.C.R.L.; Duarte, L.M.; de Oliveira Freitas, J.C.; de Almeida, C.G.; da Silva, T.P.; Melo, R.C.N.; Morais Apolônio, A.C.; de Oliveira, M.A.L.; de Mello Brandão, H.; et al. Improved Anti-Cutibacterium Acnes Activity of Tea Tree Oil-Loaded Chitosan-Poly(ε-Caprolactone) Core-Shell Nanocapsules. Colloids Surf. B Biointerfaces 2020, 196, 111371. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Mandras, N.; Allizond, V.; Nostro, A.; Roana, J.; Merlino, C.; Banche, G.; Scalas, D.; Cuffini, A.M. Positive Interaction of Thyme (Red) Essential Oil with Human Polymorphonuclear Granulocytes in Eradicating Intracellular Candida Albicans. Planta Med. 2012, 78, 1633–1635. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.H.; Graham, L.; Adukwu, E.C. In Vitro Antifungal Activity of Cinnamomum Zeylanicum Bark and Leaf Essential Oils against Candida Albicans and Candida Auris. Appl. Microbiol. Biotechnol. 2020, 104, 8911. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Pouya, B.; Aghazadeh, Z.; SamadiKafil, H.; Ghorbani, M.; Alizadeh, S.; Aghazadeh, M.; Dalir Abdolahinia, E. The Antimicrobial, Antioxidative, and Anti-Inflammatory Effects of Polycaprolactone/Gelatin Scaffolds Containing Chrysin for Regenerative Endodontic Purposes. Stem Cells Int. 2021, 2021, e3828777. [Google Scholar] [CrossRef]
- Polo, L.; Díaz de Greñu, B.; Della Bella, E.; Pagani, S.; Torricelli, P.; Vivancos, J.L.; Ruiz-Rico, M.; Barat, J.M.; Aznar, E.; Martínez-Máñez, R.; et al. Antimicrobial Activity of Commercial Calcium Phosphate Based Materials Functionalized with Vanillin. Acta Biomater. 2018, 81, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Sahal, G.; Nasseri, B.; Ebrahimi, A.; Bilkay, I.S. Electrospun Essential Oil-Polycaprolactone Nanofibers as Antibiofilm Surfaces against Clinical Candida Tropicalis Isolates. Biotechnol. Lett. 2019, 41, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A Review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Goh, C.; Shrestha, A. Biomaterial Properties Modulating Bone Regeneration. Macromol. Biosci. 2021, 21, e2000365. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Muslimov, A.R.; Zyuzin, M.V.; Peltek, O.O.; Karpov, T.E.; Sergeev, I.S.; Dotsenko, A.I.; Goncharenko, A.A.; Yolshin, N.D.; Sinelnik, A.; et al. Multifunctional Scaffolds with Improved Antimicrobial Properties and Osteogenicity Based on Piezoelectric Electrospun Fibers Decorated with Bioactive Composite Microcapsules. ACS Appl. Mater. Interfaces 2018, 10, 34849–34868. [Google Scholar] [CrossRef] [PubMed]
- Hee, C.K.; Nicoll, S.B. Induction of Osteoblast Differentiation Markers in Human Dermal Fibroblasts: Potential Application to Bone Tissue Engineering. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 2006, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, A.; Abudula, T.; Russell, C.S.; Mostafavi, E.; Williams, T.J.; Salah, N.; Alshahrie, A.; Harris, S.; Basri, S.M.M.; Mishra, Y.K.; et al. In Situ Printing of Scaffolds for Reconstruction of Bone Defects. Acta Biomater. 2021, 127, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Tettey, F.; Saudi, S.; Davies, D.; Shrestha, S.; Johnson, K.; Fialkova, S.; Subedi, K.; Bastakoti, B.P.; Sankar, J.; Desai, S.; et al. Fabrication and Characterization of Zn Particle Incorporated Fibrous Scaffolds for Potential Application in Tissue Healing and Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 48913–48929. [Google Scholar] [CrossRef] [PubMed]

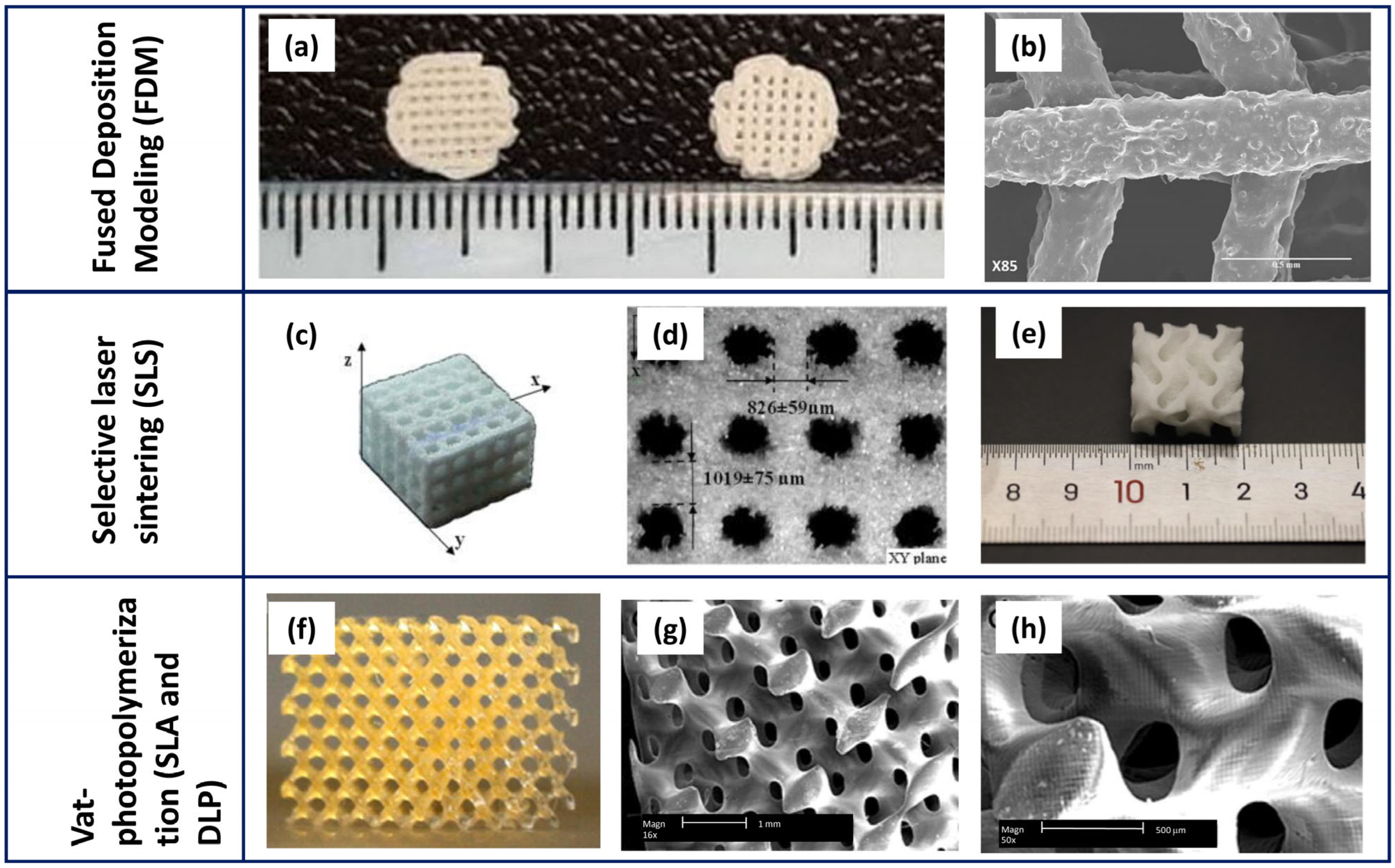


| Technology | Scaffold Main Features | Advantages | Open Challenges | References |
|---|---|---|---|---|
| Extrusion-based printing (EBP): FDM |
|
|
| [62,65,69] |
| Selective laser sintering (SLS) |
|
|
| [62,67,70,71] |
| Vat-photopolymerisation (SLA, DLP) |
|
|
| [70] |
| Scaffold Composition | Key Findings | References |
|---|---|---|
| Composite multi-layer scaffold made of PCL and BCP | It had a resistant bulk (ceramic core) and a porous surface (polymer external layer), and displayed the appropriate gradient of porosity and a degradation rate | [113] |
| Functionally graded 3D scaffold designed with a ceramic inner core and a PCL external layer | The different layers were closely linked and the degradation rate of the inner core revealed bioactivity | [116] |
| Scaffold based on PCL and HA | The pores were uniformly distributed and HA was dispersed in the constructs, with some agglomeration | [80] |
| 3D-printed scaffold made of PCL and enriched with HA | The composite material revealed small spaces among pores | [111] |
| PCL and HA blended in a 3D scaffold | HA in PCL-based scaffold was finely distributed with aggregates of different size | [115] |
| 3D scaffold based on PCL and blended with HA | The appearance of CaPs was revealed in the structure | [117] |
| PCL incorporated with 1% of HA into 3D scaffold | The addition of HA to PCL provoked an increase in roughness | [118] |
| Biphasic PCL/HA nanofibrous scaffold | The pores displayed a high degree of interconnection. Their diameter was about 2.4 μm | [119] |
| PCL enriched with HA and fluorapatite to obtain a 3D composite scaffold | The presence of apatite particles was detected in the surface | [120] |
| 3D functionally graded scaffold based on PCL, gelatin, and nanohydroxyapatite | The layers were interconnected to reach a suitable porosity. An initial high degradation rate within 2 days was recorded that, thereafter, slowed | [121] |
| 3D functionally graded scaffold based on PCL, gelatin, and nanohydroxyapatite | It had an average pore size of 4.7 ± 1.04 μm with the uniform, and adequate deposition of nanohydroxyapatite on its surface, but the degradation in aqueous medium determined a rupture of its structure | [4] |
| Scaffold prepared with PCL, HA, and chitosan | Specimen with an interconnected porosity and with the presence of HA. The deposition of an apatite layer was highlighted | [16] |
| PCL and chitosan cubic-shaped scaffold | The construct presented a squared porosity (average width of 440 ± 16 μm and a height of 120 ± 5 μm) | [10] |
| PCL-based scaffold with or without PLA | An increase in surface roughness was observed in pure PCL scaffold compared to that prepared with PLA or with PLA/PCL | [122] |
| Cylindrical-shaped multichannel bone substitutes prepared using BCP (60 HA + 40 β-TCP) | A high bulk macro-porosity was revealed (1, 2, and 3 mm of diameter) and the compressive strength increased with the pore’s diameter | [2] |
| Three types of 3D scaffolds: pure PCL, and PCL added with BCP at 20% or 30% | The scaffolds had large pore size and released calcium and phosphates over time. BCP at 30% produced fractures in the construct | [123] |
| PCL-based scaffold blended with BCP (70 HA + 30 β-TCP), and added with 1.67% of silver | The 3D scaffold was featured by a highly interconnected and homogeneously distributed porosity, a homogeneous and fine dispersion of BCP, and an increased stiffness | [42] |
| PCL-based scaffold blended with BCP (70 HA + 30 β-TCP), and added with essential oils | The salt-leaching process formed two types of pores (about 234–208 µm): NaCl determined squared regular pores, whereas NaNO3 produced pores with a less defined geometry. The pure PCL specimens slowly lost weight during the immersion | [43] |
| PCL-based scaffold blended with BCP (70 HA + 30 β-TCP), and added with ~1% of silver | The blending of PCL with BCP provokes a faster weight loss respective to pure PCL | [44] |
| PCL scaffold with deposition of β-TCP nanoparticle | FESEM analysis demonstrated the random distribution of β-TCP nanoparticles on the surface and the pores of about 300 μm. The XRD revealed the peaks related to PCL and β-TCP | [76] |
| 3D scaffold of PCL and β-TCP, and added or not with MgO nanoparticles | A well-defined microstructure with the pore size of ~500 μm, and the dispersion of the MgO nanoparticles was revealed | [74] |
| Composite scaffold based on PCL and HA, and incorporated with tetracycline | FESEM images showed the homogeneous distribution of tetracycline on the PCL surface. A high efficiency of its encapsulation was revealed as well as a sustained release over time | [125] |
| 3D scaffold made of PCL and HA, and loaded with ZnO | The higher content of HA makes the constructs more fragile, whereas ZnO was presented in agglomerates | [126] |
| Scaffold Composition | Microorganisms | Methods | Key Findings | References |
|---|---|---|---|---|
| PCL loaded with doxycycline | Escherichia coli K-12 | Agar diffusion test | The drug was successfully loaded in the scaffold and its release produced an inhibition halo of 1 cm | [127] |
| PCL and HA incorporated with tetracycline | Escherichia coli (ATCC® 25922) and Staphylococcus aureus (ATCC® 25923) | Agar diffusion test | Despite the drug concentration, the inhibition halo was revealed, but it was more pronounced for S. aureus respective to E. coli | [125] |
| Porous membranes of PCL and PLA added with tetracycline | Staphylococcus aureus (NCTC 10788) and Escherichia coli (NSM59) | Agar diffusion test | A pronounced antibacterial activity of the constructs up to 21 days of incubation, and a larger inhibition halo against E. coli | [128] |
| PCL and β-TCP loaded with microspheres of ceftriaxone | Escherichia coli | Agar diffusion test | A sustained drug release was demonstrated with an inhibition halo of 3 cm, after 24 h of incubation | [131] |
| PCL coated with PLA vancomycin-loaded microspheres | Staphylococcus aureus (ATCC® 29213) | Agar diffusion test | A relevant anti-S. aureus action was demonstrated over 28 days of incubation | [129] |
| Coaxial structure based on PCL/PLGA-PVA loaded with erythromycin | Staphylococcus aureus (ATCC® 49230) | Agar diffusion test | Higher diameter of inhibition in the growth of S. aureus was revealed in relation to erythromycin concentration in the construct | [130] |
| In situ-added silver nanoparticles on PLGA/PCL | Staphylococcus aureus and Streptococcus mutans | Agar diffusion test, FESEM images | A wider diameter of inhibition halo for S. aureus respective to S. mutans was registered, whereas both bacteria attached to the scaffold | [134] |
| PCL loaded with silver | Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans | Agar diffusion test | The antimicrobial effect was different depending on the microorganisms: C. albicans was the most susceptible to silver followed by E. coli, S. aureus, and Ps. aeruginosa | [133] |
| Composites of PCL and BCP enriched with 1.67% of silver | Staphylococcus aureus (ATCC® 29213) | Agar diffusion test, adhesion assay | An inhibition halo around the specimen was shown, as well as a reduction in both adhered and planktonic staphylococci | [42] |
| Composites of PCL and BCP enriched with ~1% of silver | Staphylococcus aureus (ATCC® 29213), Staphylococcus epidermidis (ATCC® 35984), and Escherichia coli (ATCC® 25922) | Agar diffusion test, adhesion assay, FESEM images | An inhibition halo around the silver-enriched sample was shown. A reduction in adherent and planktonic bacteria, and an alteration in their morphology, was revealed. No biofilm formation was shown on the enriched scaffold | [44] |
| Composites of PCL and BCP enriched with ~1% of silver | Candida albicans (ATCC® 10231) and C. auris (clinical isolate) | Agar diffusion test, adhesion assay, FESEM images | An inhibition halo around the silver-enriched sample was shown for both strains. A reduction in adherent and planktonic yeasts and a filamentous morphology were revealed. No biofilm formation was shown on the enriched scaffold | [45] |
| PCL and HA loaded with ZnO | Staphylococcus aureus (ATCC® 25923) | Contact with the scaffolds | The release of Zn reduced S. aureus load when placed in contact with the scaffold | [126] |
| Composite 3D membrane of PCL blended with ZnO (from 1% to 7%) | Staphylococcus aureus (ATCC® 29923) and Escherichia coli (ATCC® 25922) | Agar diffusion test, adhesion assay, FESEM images | Good antibacterial activity on S. aureus and E. coli, and a reduction in their adhesion to the construct especially at 7% of ZnO | [40] |
| ZnO nanoparticles added in PCL | Streptococcus mutans (KCOM 1504) and Porphyromonas gingivalis (KCOM 2804) | Contact with the scaffolds | No significant differences in the bacterial load were obtained by varying the construct composition | [135] |
| PCL reinforced with copper | Staphylococcus aureus and Escherichia coli | Agar diffusion test | S. aureus (Gram-positive) was more susceptible to copper activity compared to E. coli (Gram-negative) | [30] |
| PCL and gelatin supplemented with chrysin | Acinetobacter baumannii (ATCC® BAA-747), Pseudomonas aeruginosa (ATCC® 27853), Staphylococcus aureus (ATCC® 6538), and Enterococcus faecalis (ATCC® 13048) | Agar diffusion test and live/death assay | The scaffold inhibited A. baumannii, Ps. aeruginosa, S. aureus, and E. faecalis growth | [142] |
| CaPs enriched with vanillin | Escherichia coli (DH5α) | CFU count after contact with the scaffolds | A reduction in the CFU of E. coli only in vanillin presence, as well as an altered morphology of the bacterium | [143] |
| PCL enriched with 0%, 2%, 4%, and 8% of clove and red thyme | Candida tropicalis clinical isolates | Biofilm quantification by crystal violet | The biofilm formation of C. tropicalis clinical strains was inhibited when the concentration of the EOs was at 4% | [144] |
| PCL with cinnamon or thyme at 30%, 40%, and 50% | Staphylococcus aureus (ATCC® 29213), Staphylococcus epidermidis (ATCC® 35984), and Escherichia coli (ATCC® 25922) | Agar diffusion test, adhesion assay, FESEM images | All the concentrations of EOs were able to inhibit the bacteria in growth, adhesion, and biofilm formation. The EO presence modified the bacterial morphology as well | [43] |
| PCL/PLA enriched with HA and Nigella sativa oil at 15, 18, and 20 wt% | Staphylococcus aureus and Escherichia coli | Agar diffusion test | When Nigella sativa was added, the antibacterial activity was obtained only towards S. aureus since E. coli displayed a natural resistance to this compound | [25] |
| PCL with graphene oxide at 5% and 7.5% | Staphylococcus epidermidis (ATCC® 35984) and Escherichia coli (ATCC® 25922) | Live/death assay | The presence of GO increased the number of S. epidermidis and E. coli dead cells, which was more pronounced at 7.5% of GO | [8] |
| PCL with chitosan with different molecular weight | Staphylococcus aureus (ATCC® 6538) and S. epidermidis (ET13) | Adhesion assay and biofilm formation | The addition of chitosan reduced adhesion and biofilm formation of both staphylococci | [10] |
| Scaffold Composition | Cells | Key Findings | References |
|---|---|---|---|
| PCL with CaPs and gelatin | Mesenchymal stem cells | Cells were not impaired in their viability and proliferation, and non-toxic products were released by the scaffold | [121] |
| PCL enriched with HA and ZnO (1% w/w) | Mesenchymal stem cells | Cells expressed osteodifferentiation markers and a high calcium deposition was detected in HA presence. Cells colonised the scaffold and differentiated in osteoblasts | [149] |
| PCL enriched with HA | Mesenchymal stem cells | Cells were anchored and proliferated into the scaffold | [111] |
| PCL with silica microcapsules | Mesenchymal stem cells | Cells lived in, adhered to, and proliferated into the construct | [147] |
| PCL functionalised with different concentration of HA | Mesenchymal stem cells | Cells were not hampered in their viability. The greater HA concentration (7%) promoted a superior cell attachment. The cells produced high early-stage differentiation marker on PCL with HA | [120] |
| PCL/β-TCP or PCL/β-TCP with nano-MgO | Bone marrow mesenchymal stromal cells | Alizarin red S staining revealed the osteoinduction of cells. These cells displayed a long-term viability in MgO presence, as well as an increase in their ALP activity | [74] |
| PCL coated with PLA vancomycin-loaded microspheres | Rabbit bone marrow-derived mesenchymal stem cells | Eukaryotic cells increased in their amount over time, and some of them, after the attachment, secreted matrix | [129] |
| Coaxial structure based on PCL/PLGA-PVA loaded with erythromycin | Rat bone marrow stromal cells | Cell growth augmented at erythromycin concentration of 100 μg/mL, but ALP activity decreased at a drug concentration of 500 and 1000 μg/mL | [130] |
| PCL enriched with different percentages of graphene oxide | Human foreskin fibroblast (HFF-1) cells | Cells adhered to and spread into the construct, for up to 14 days of incubation | [8] |
| PCL with gelatin and graphene oxide (1% or 2%) | Human gingival mesenchymal stem cells | The scaffold promoted cell adhesion and proliferation | [15] |
| PCL coated with chitosan, gelatin, and bioactive glass | Fibroblast cells (MG-63) | Human cells were not impaired in viability and proliferation, and the coating enhanced their calcium deposition | [54] |
| PCL/PLA enriched with gelatin and taurine | Fibroblast cells (MG-63) | The cells were not compromised in their viability and proliferation, after 24 and 72 h | [53] |
| PCL/PLA and HA, enriched with black curcumin essential oil at increasing concentrations | Human fibroblast cells (CCD-1072-SK) | The oil presence reduced the viability of cells after 24 h of incubation; a lower effect was revealed after 48 h | [25] |
| Cubic-shaped PCL and chitosan | Mouse murine fibroblast cells (L929) | Cells displayed a higher viability and maintained their phenotype | [10] |
| Composites of PCL and HA incorporated with tetracycline | Fibroblast cells (L929) | Cells were viable and the tetracycline concentration did not affect their viability | [125] |
| PCL | Mouse murine fibroblast cells (L929) | Cells were not impaired in their viability and morphology, and they preserved the spherical shape | [122] |
| CaPs enriched with vanillin | Fibroblast-like cells (ATCC® L929) and human osteoblast-like cells (ATCC® MG-63) | Fibroblasts were not impaired in viability when vanillin was present and were uniformly distributed. Also, the viability of osteoblasts was promoted within a short time of incubation | [143] |
| PCL with β-TCP nanoparticle deposition | Pre-osteoblasts (MC3T3-E1) | Cells showed high viability, proliferation, and adhesion as well as an increase in ALP activity and mineralisation | [76] |
| Composites based on PCL and Zn (at 1, 2, or 3 wt%) | Pre-osteoblasts (MC3T3-E1) | A greater number of live cells was recorded at 2–3 wt% of Zn respective to pure PCL or the one with 1 wt% of Zn | [86] |
| PCL with the copolymer Inulin-g-poly(D,L)lactide | Human fibroblasts (ATCC® MRC-5-CCL-171) and human adipose-derived mesenchymal stem cells | An adequate cytocompatibility towards fibroblasts was proved. Additionally, a 100% viability of human adipose-derived mesenchymal stem cells and their attachment to the biomaterial surface was revealed, as well as their high production of differentiation markers | [124] |
| PCL loaded with silver | Human dermal fibroblasts | High cytocompatibility of PCL added with silver but only at low concentrations (from 2.5% to 1%) | [133] |
| Pure PCL or PCL blended with BCP at 20% or 30% | Osteoblast cell lines (MC3T3-E1, subclone 4) | Cells attached and proliferated with multi-layers but also differentiated as a result of an increase in ALP activity and in OCN gene expression | [123] |
| Composites of PLA and PCL | Osteoblast cell lines (MC3T3-E1, subclone 4) | Cells proliferated in the scaffold, while displaying a good cytocompatibility, at 2 and 3 days of incubation | [47] |
| ZnO nanoparticles added in PCL constructs | Osteoblast cell lines (MC3T3-E1, subclone 4) | No significant difference in the viability of cells was observed for pure PCL compared to the modified one | [135] |
| In situ-added silver nanoparticles on PLGA/PCL scaffolds | Osteoblast cell lines (MC3T3-E1, subclone 14) | Cells cultured with the scaffolds reported higher proliferative capability when silver was at the lowest concentration. FESEM images showed cell attachment to the scaffolds as well as the presence of extended filopodia. Furthermore, an increase in ALP and mineralisation was demonstrated | [134] |
| PCL reinforced with copper | Human osteoblastic-like cells (MG63) and mouse mesenchymal stem cells | Mesenchymal stem cells were not impaired in either viability or migratory capability. Also, they showed increased expression of osteoblast differentiation markers, as well as calcium deposition | [30] |
| PCL and HA loaded with ZnO | Human foetal osteoblast cell line (HFOb 1.19) | Zn presence enhanced ALP activity and promoted cells’ calcium deposition. However, it reduced their viability | [126] |
| PCL–chitosan enriched with Zn at increasing concentrations | Mouse fibroblast cell (NIH-3T3 lines) | Only the lower Zn concentrations (10 and 20) allowed a 100% viability of cells with an increase in cellular attachment and proliferation | [150] |
| PCL blended with BCP and enriched with silver at 1.67% or essential oils | Human osteosarcoma cell—Saos-2 | Specimens were not toxic for osteoblasts and the addition of BCP did not impair their viability. Cells adhered, proliferated, and did not alter their morphology. Silver at 1.67% impaired cell viability, as well as essential oils at 40–50% | [42,43] |
| PCL and β-TCP loaded with ceftriaxone microspheres | Human osteosarcoma cell—Saos-2 | An increased number of viable cells was determined within 28 days of incubation, with the attachment and spreading of osteoblasts | [131] |
| PCL blended with BCP and enriched with silver at ~1% | Human osteosarcoma cell—Saos-2 | Pure PCL was not toxic for Saos-2 cells, whereas only the lowest silver concentration allowed cellular survival and proliferation | [44] |
| PCL and blended with BCP, and enriched with silver at ~1% | Human osteosarcoma cell—Saos-2 | The scaffolds were able to promote calcium deposition by SaoS-2 cells | [45] |
| PCL and HA at different percentages | Human osteoblast cell line (MG-63) | The biomaterial was non-toxic and permitted the colonisation by the cells, which maintained their spherical shape. Their calcium deposition was also demonstrated | [80] |
| PCL and HA at increasing percentages | Human osteoblasts | Cells proliferated into, attached to, and covered the surface, while displaying a flat poliedric morphology | [115] |
| PCL blended with HA | Human osteoblast cell line (MG-63) | Cells successfully proliferated, deposed calcium, and upregulated the expression of genes involved in differentiation, mainly in HA presence | [117] |
| PCL blended with 1% of HA | Human osteoblasts (HOB-Promocell C-12720) | HA addition increased the viability, proliferation, and calcium deposition of human osteoblasts, which were featured by filopodia | [118] |
| PCL, HA, and chitosan | Human osteosarcoma cells (MG-63) | Osteoblasts were viable and their proliferation increased proportionally to HA concentration. Cells expressed different genes involved in osteogenesis | [16] |
| PCL, gelatin, and nanohydroxyapatite | Human osteoblasts | Cells increased their viability and proliferation and displayed a polygonal-shaped morphology | [4] |
| Biphasic PCL/HA nanofibrous scaffold | Immortalised myoblast cell line (C2C12) | Cells attached, spread, and increased ALP activity mainly when HA was added | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, B.; Menotti, F.; Longo, F.; Banche, G.; Mandras, N.; Palmero, P.; Allizond, V. New Generation of Osteoinductive and Antimicrobial Polycaprolactone-Based Scaffolds in Bone Tissue Engineering: A Review. Polymers 2024, 16, 1668. https://doi.org/10.3390/polym16121668
Coppola B, Menotti F, Longo F, Banche G, Mandras N, Palmero P, Allizond V. New Generation of Osteoinductive and Antimicrobial Polycaprolactone-Based Scaffolds in Bone Tissue Engineering: A Review. Polymers. 2024; 16(12):1668. https://doi.org/10.3390/polym16121668
Chicago/Turabian StyleCoppola, Bartolomeo, Francesca Menotti, Fabio Longo, Giuliana Banche, Narcisa Mandras, Paola Palmero, and Valeria Allizond. 2024. "New Generation of Osteoinductive and Antimicrobial Polycaprolactone-Based Scaffolds in Bone Tissue Engineering: A Review" Polymers 16, no. 12: 1668. https://doi.org/10.3390/polym16121668
APA StyleCoppola, B., Menotti, F., Longo, F., Banche, G., Mandras, N., Palmero, P., & Allizond, V. (2024). New Generation of Osteoinductive and Antimicrobial Polycaprolactone-Based Scaffolds in Bone Tissue Engineering: A Review. Polymers, 16(12), 1668. https://doi.org/10.3390/polym16121668










