Nature’s Plastic Predators: A Comprehensive and Bibliometric Review of Plastivore Insects
Abstract
:1. Introduction
2. Literature Analysis
2.1. The Plastic Waste Problem: Statistical Magnitude and Threats
2.2. Bugging out Plastic: History of Plastic Consumption by Insects
2.3. Exaptation in Plastic Biodegradation: Mechanisms and Polymer Similarities
2.3.1. Natural and Synthetic Polymers Similarities: Preconditioning Substrates
2.3.2. Wood to Plastic: Termites and Ants’ Appetite for Lignocellulose-like Polymers
2.3.3. Plastivorous Moths and Beetles: Beeswax, Chitin, and Keratin
2.3.4. Survival and Developmental Impact of Plastic Consumption on Insects
2.3.5. Mechanisms of Insect-Mediated Plastic Degradation
2.3.6. Factors Affecting Plastic Biodegradation of Plastics
3. Bibliometric Materials and Methods
3.1. Data Acquisition
3.2. Search Strategy
- TI = (((“biodegradation” OR “*degradation” OR “ mineralisation” OR “*degrad*” OR “depolymerization” OR “biodeterioration”) AND (“*plastic*” OR “polymer” OR “*polyethylene” OR “polystyrene” OR “polypropylene” OR “polyethylene terephthalate”) AND (“insect” OR “moth” OR “larva*” OR “termite*” OR “ant” OR “beetle*” OR “*worm*” OR “mellonella” OR “molitor” OR “castaneum” OR “interpunctella” OR “grisella” OR “obscurus” OR “cephalonica” OR “confusum” OR “atratus” OR “davidis” OR “dominica” OR “postvittana” OR “nymphaeata” OR “pseudospretella” OR “destructor” OR “brevis”))). The query allows for three mandatory main keywords covering the topic and ensuring refinement based on alternative keywords for the main ones.
3.3. Data Analysis
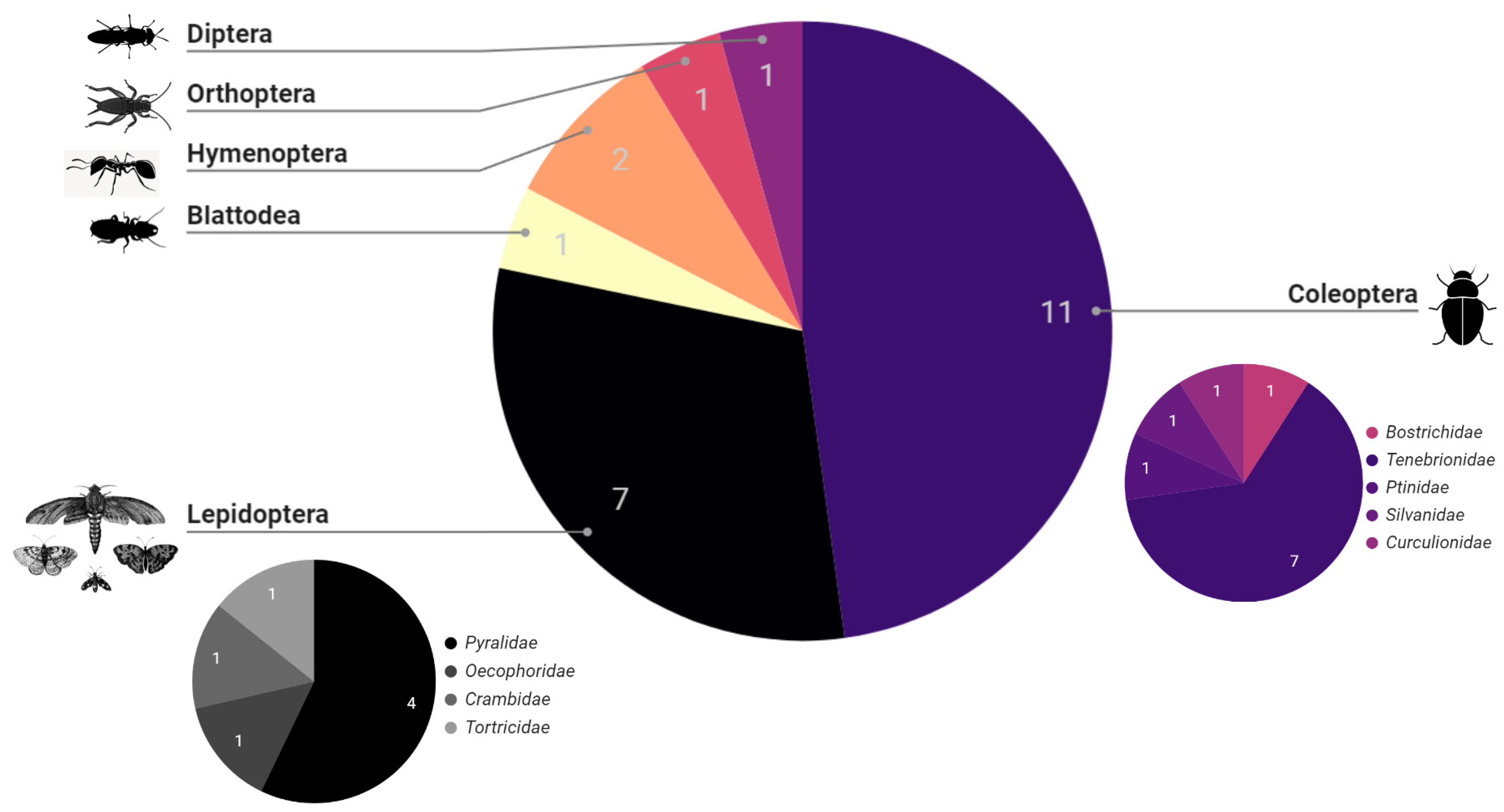
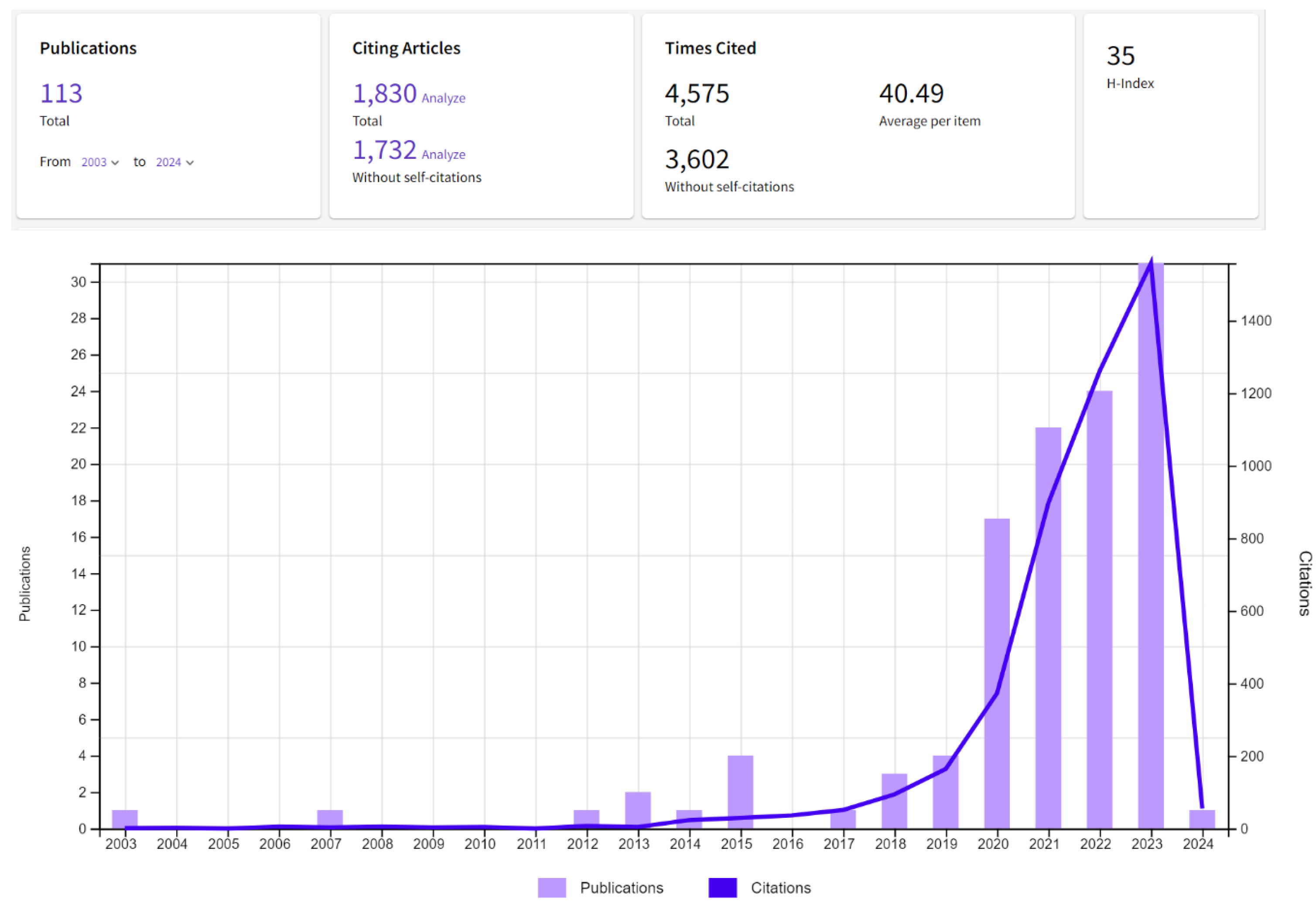
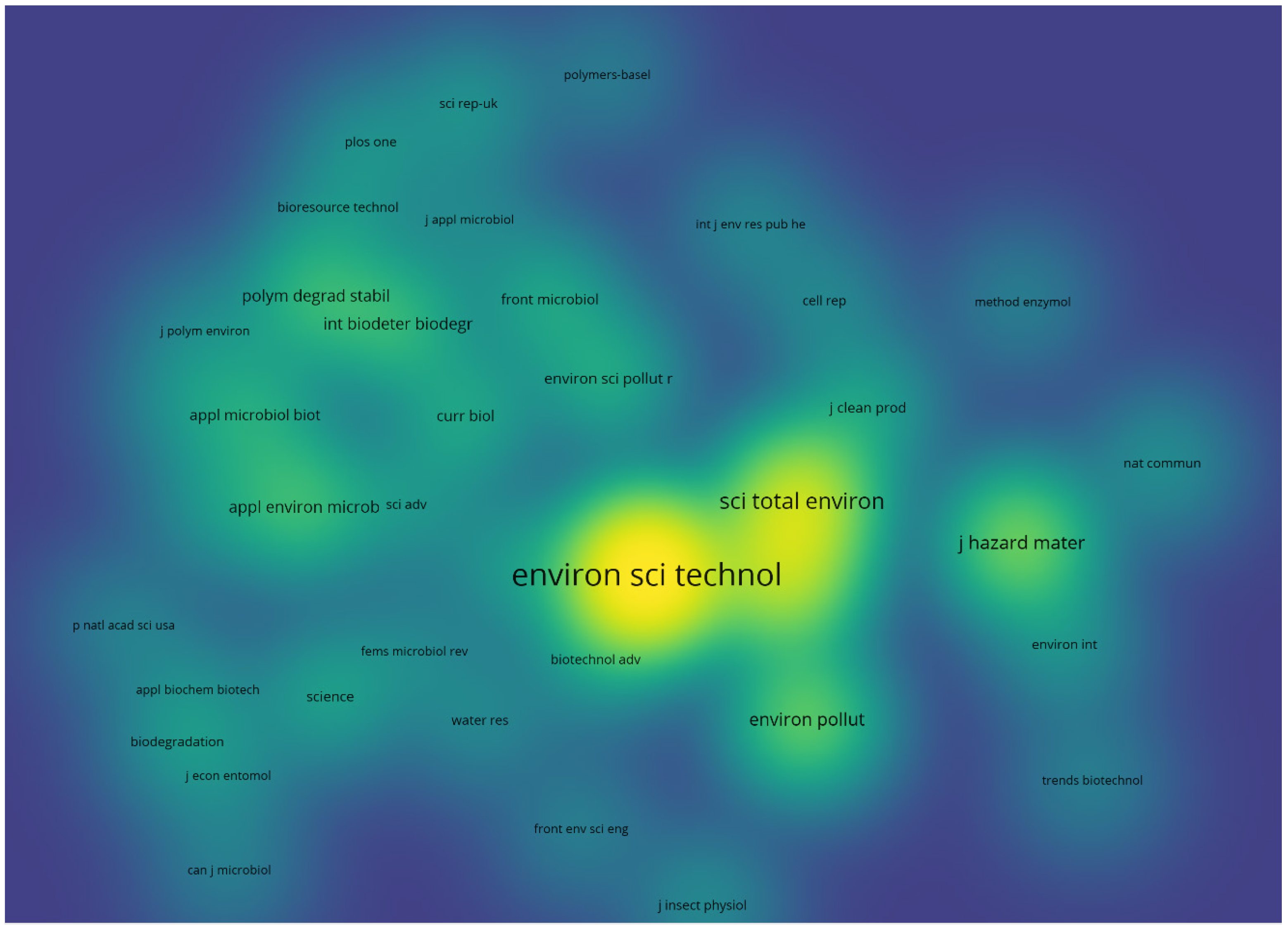
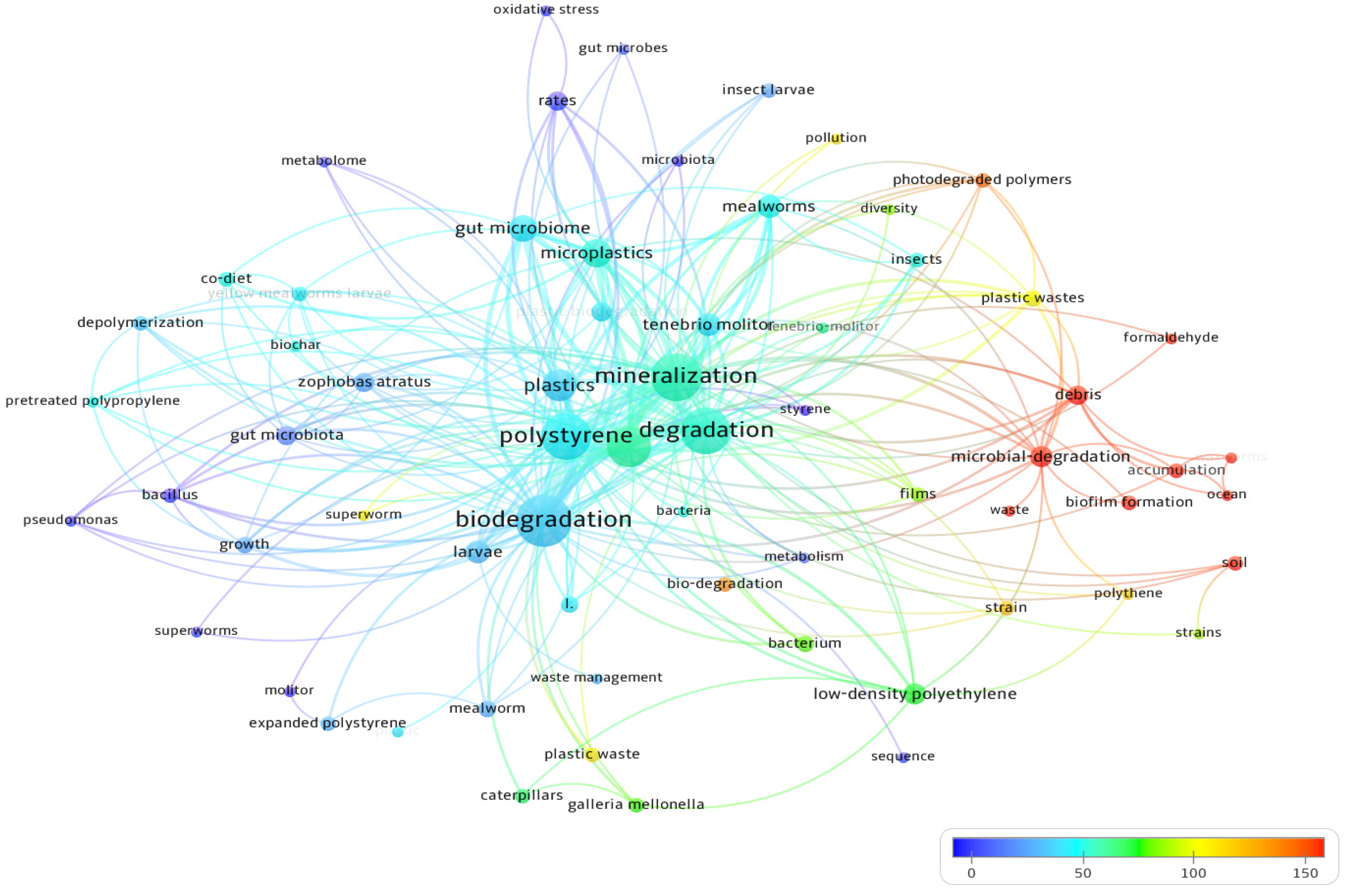
4. Bibliometric Results
5. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bernardini, F.; Tuniz, C.; Coppa, A.; Mancini, L.; Dreossi, D.; Eichert, D.; Turco, G.; Biasotto, M.; Terrasi, F.; De Cesare, N.; et al. Beeswax as dental filling on a neolithic human tooth. PLoS ONE 2012, 7, e44904. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, L.; Gong, D.; Yin, H.; Zhang, J. Biomolecular Evidence of Silk from 8500 Years Ago. PLoS ONE 2016, 11, e0168042. [Google Scholar] [CrossRef] [PubMed]
- Friedel, R. Beyond Bakelite: Leo Baekeland and the Business of Science and Invention by Joris Mercelis. Technol. Cult. 2022, 63, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.M.; Hester, R.E. Plastics and the Environment. In Google Books; Royal Society of Chemistry: London, UK, 2018; Available online: https://books.google.com.au/books?hl=en&lr=&id=aC97DwAAQBAJ&oi=fnd&pg=PA1&dq=plexiglas+invention+to+replace+glass+windows&ots=s6jhfrhRWn&sig=Zuzm40t0tvmNmXqEwHYgJg8pqn8#v=onepage&q&f=false (accessed on 10 February 2022).
- Savoca, M.S.; Kühn, S.; Sun, C.; Avery-Gomm, S.; Choy, C.A.; Dudas, S.; Hong, S.H.; Hyrenbach, K.D.; Li, T.-H.; Ng, C.K.; et al. Towards a North Pacific Ocean long-term monitoring program for plastic pollution: A review and recommendations for plastic ingestion bioindicators. Environ. Pollut. 2022, 310, 119861. [Google Scholar] [CrossRef] [PubMed]
- Porta, R. Anthropocene, the plastic age and future perspectives. FEBS Open Bio 2021, 11, 948–953. [Google Scholar] [CrossRef] [PubMed]
- OECD. Plastic Pollution Is Growing Relentlessly as Waste Management and Recycling Fall Short, Says OECD. Available online: https://www.oecd.org/newsroom/plastic-pollution-is-growing-relentlessly-as-waste-management-and-recycling-fall-short.htm (accessed on 22 February 2022).
- Ritchie, H.; Roser, M. Plastic Pollution; Our World in Data: Oxford, UK, 2018; Available online: https://ourworldindata.org/plastic-pollution (accessed on 20 February 2023).
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Greenspoon, L.; Krieger, E.; Sender, R.; Rosenberg, Y.; Bar-On, Y.M.; Moran, U.; Antman, T.; Meiri, S.; Roll, U.; Noor, E.; et al. The global biomass of wild mammals. Proc. Natl. Acad. Sci. USA 2023, 120, e2204892120. [Google Scholar] [CrossRef]
- Roman, L.; Schuyler, Q.; Wilcox, C.; Hardesty, B.D. Plastic pollution is killing marine megafauna, but how do we prioritize policies to reduce mortality? Conserv. Lett. 2020, 14, e12781. [Google Scholar] [CrossRef]
- Rios, L.M.; Jones, P.R.; Moore, C.; Narayan, U.V. Quantitation of persistent organic pollutants adsorbed on plastic debris from the Northern Pacific Gyre’s “eastern garbage patch”. J. Environ. Monit. 2010, 12, 2226. [Google Scholar] [CrossRef]
- Leslie, H.A.; JM van Velzen, M.; Brandsma, S.H.; Vethaak, D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Danopoulos, E.; Twiddy, M.; West, R.; Rotchell, J.M. A rapid review and meta-regression analyses of the toxicological impacts of microplastic exposure in human cells. J. Hazard. Mater. 2021, 427, 127861. [Google Scholar] [CrossRef]
- Callaghan, M.A.; Alatorre-Hinojosa, S.; Connors, L.T.; Singh, R.D.; Thompson, J.A. Plasticizers and Cardiovascular Health: Role of Adipose Tissue Dysfunction. Front. Pharmacol. 2020, 11, 626448. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, G.N. Factors in natural resistance of woods. Caribb. For. 1946, 7, 121–134. [Google Scholar]
- Abdulhay, H.S. Biodegradation of plastic wastes by confused flour beetle Tribolium confusum Jacquelin du Val larvae. Asian J. Agric. Biol. 2020, 8, 201–206. [Google Scholar] [CrossRef]
- Yang, X.-G.; Wen, P.-P.; Yang, Y.-F.; Jia, P.-P.; Li, W.-G.; Pei, D.-S. Plastic biodegradation by in vitro environmental microorganisms and in vivo gut microorganisms of insects. Front. Microbiol. 2023, 13, 1001750. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, J.; Liu, Y.; Zhuang, G.; Peng, X.; Wu, W.-M.; Zhuang, X. Biodegradation of expanded polystyrene and low-density polyethylene foams in larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae): Broad versus limited extent depolymerization and microbe-dependence versus independence. Chemosphere 2021, 262, 127818. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.G.; Kim, H.H.; Chung, J.; Jun, J.; Lee, S.; Kim, H.-M.; Jeon, S.; Park, S.G.; Bhak, J.; Ryu, C.-M. The Galleria mellonella Hologenome Supports Microbiota-Independent Metabolism of Long-Chain Hydrocarbon Beeswax. Cell Rep. 2019, 26, 2451–2464. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Acosta, D.J.; Alexander, B.R.; Cole, H.O.; Zhang, Y.; et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Young, R.; Khandaker Asif Ahmed Court, L.; Castro-Vargas, C.; Marcora, A.; Boctor, J.; Paull, C.; Wijffels, G.; Rane, R.; Edwards, O.; Walsh, T.; et al. Improved, reference quality genome sequence of the plastic-degrading greater wax moth, Galleria mellonella. G3 Genes Genomes Genet. 2024, 141, jkae070. [Google Scholar] [CrossRef]
- Gibb, B.C. Plastics are forever. Nat. Chem. 2019, 11, 394–395. [Google Scholar] [CrossRef]
- Wu, H.-H. A study on transnational regulatory governance for marine plastic debris: Trends, challenges, and prospect. Mar. Policy 2020, 136, 103988. [Google Scholar] [CrossRef]
- Tuladhar, R.; Yin, S. Sustainability of Using Recycled Plastic Fiber in Concrete; Pacheco-Torgal, F., Khatib, J., Colangelo, F., Tuladhar, R., Eds.; ScienceDirect; Woodhead Publishing: Sawston, UK, 2019; Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780081026762000219 (accessed on 1 January 2019).
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro)plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton LC, M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, I.F.; Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Worldwide contamination of fish with microplastics: A brief global overview. Mar. Pollut. Bull. 2020, 160, 111681. [Google Scholar] [CrossRef] [PubMed]
- Meijer LJ, J.; van Emmerik, T.; van der Ent, R.; Schmidt, C.; Lebreton, L. More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Sci. Adv. 2021, 7, eaaz5803. [Google Scholar] [CrossRef]
- Population—The World Factbook. 2021. Available online: https://www.cia.gov/the-world-factbook/about/archives/2021/field/population/country-comparison (accessed on 10 February 2023).
- Waldrop, M.M. Core Concept: Bioplastics offer carbon-cutting advantages but are no panacea. Proc. Natl. Acad. Sci. USA 2021, 118, e2103183118. [Google Scholar] [CrossRef] [PubMed]
- Eiamthong, B.; Meesawat, P.; Wongsatit, T.; Jitdee, J.; Sangsri, R.; Patchsung, M.; Aphicho, K.; Suraritdechachai, S.; Huguenin-Dezot, N.; Tang, S.; et al. Discovery and Genetic Code Expansion of a Polyethylene Terephthalate (PET) Hydrolase from the Human Saliva Metagenome for the Degradation and Bio-Functionalization of PET. Angew. Chem. Int. Ed. 2022, 61, e202203061. [Google Scholar] [CrossRef]
- Mills, J.; Klausmeier, R.E. The biodeterioration of synthetic polymers and plasticizers. Crit. Rev. Environ. Sci. Technol. 1974, 4, 341–351. [Google Scholar] [CrossRef]
- Mitra, B.; Das, A. The Ability of Insects to Degrade Complex Synthetic Polymers; IntechOpen: London, UK, 2023; Available online: https://www.intechopen.com/chapters/84197 (accessed on 6 February 2023).
- Kjer, K.M.; Simon, C.; Yavorskaya, M.; Beutel, R.G. Progress, pitfalls and parallel universes: A history of insect phylogenetics. J. R. Soc. Interface 2016, 13, 20160363. [Google Scholar] [CrossRef]
- Snyder, T.E. Termite attack on plastics and fabrics. Pest Control 1955, 23, 48. [Google Scholar]
- Whalley, P.E.S. Damage to Polythene by Aquatic Moths. Nature 1965, 207, 104. [Google Scholar] [CrossRef]
- Singh, P.; Jerram, E.M. Plastic Damage by Insects. N. Z. Entomol. 1976, 6, 188. [Google Scholar] [CrossRef]
- Highland, H.A.; Cline, L.D. Resistance to Insect Penetration of Food Pouches Made of Untreated Polyester or Permethrin-treated Polypropylene Film. J. Econ. Entomol. 1986, 79, 527–529. [Google Scholar] [CrossRef]
- Mueller, D.K. Stored Product Protection—A Period of Transition; Insects Limited, Inc.: Indianapolis, IN, USA, 1998. [Google Scholar]
- Yang, J.; Yang, Y.; Wu, W.-M.; Zhao, J.; Jiang, L. Evidence of Polyethylene Biodegradation by Bacterial Strains from the Guts of Plastic-Eating Waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, J.; Wu, W.-M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralisation of Polystyrene by Plastic-Eating Mealworms: Part 1. Chemical and Physical Characterization and Isotopic Tests. Environ. Sci. Technol. 2015, 49, 12080–12086. [Google Scholar] [CrossRef] [PubMed]
- Bombelli, P.; Howe, C.J.; Bertocchini, F. Polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella. Curr. Biol. 2017, 27, R292–R293. [Google Scholar] [CrossRef] [PubMed]
- Kundungal, H.; Gangarapu, M.; Sarangapani, S.; Patchaiyappan, A.; Devipriya, S.P. Efficient biodegradation of polyethylene (HDPE) waste by the plastic-eating lesser waxworm (Achroia grisella). Environ. Sci. Pollut. Res. 2019, 26, 18509–18519. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.-Y.; Su, Y.; Chen, Z.; Chen, J.; Zhou, X.; Benbow, M.E.; Criddle, C.S.; Wu, W.-M.; Zhang, Y. Biodegradation of Polystyrene by Dark (Tenebrio obscurus) and Yellow (Tenebrio molitor) Mealworms (Coleoptera: Tenebrionidae). Environ. Sci. Technol. 2019, 53, 5256–5265. [Google Scholar] [CrossRef]
- Kesti, S.S.; Thimmappa, S.C. First report on biodegradation of low density polyethylene by rice moth larvae, Corcyra cephalonica (stainton). Holist. Approach Environ. 2019, 9, 79–83. [Google Scholar] [CrossRef]
- Wang, Z.; Xin, X.; Shi, X.; Zhang, Y. A polystyrene-degrading Acinetobacter bacterium isolated from the larvae of Tribolium castaneum. Sci. Total Environ. 2020, 726, 138564. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Xia, M. Biodegradation and mineralization of polystyrene by plastic-eating superworms Zophobas atratus. Sci. Total Environ. 2020, 708, 135233. [Google Scholar] [CrossRef] [PubMed]
- Sanluis-Verdes, A.; Colomer-Vidal, P.; Rodriguez-Ventura, F.; Bello-Villarino, M.; Spinola-Amilibia, M.; Ruiz-Lopez, E.; Illanes-Vicioso, R.; Castroviejo, P.; Cigliano, R.A.; Montoya, M.; et al. Wax worm saliva and the enzymes therein are the key to polyethylene degradation by Galleria mellonella. Nat. Commun. 2022, 13, 5568. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, L.; Li, X.; Wang, J.; Wang, H.; Chen, C.; Guo, H.; Han, T.; Zhou, A.; Zhao, X. Different plastics ingestion preferences and efficiencies of superworm (Zophobas atratus Fab.) and yellow mealworm (Tenebrio molitor Linn.) associated with distinct gut microbiome changes. Sci. Total Environ. 2022, 837, 155719. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.-Y.; Li, Y.; Fan, R.; Chen, Z.; Chen, J.; Brandon, A.M.; Criddle, C.S.; Zhang, Y.; Wu, W.-M. Biodegradation of low-density polyethylene and polystyrene in superworms, larvae of Zophobas atratus (Coleoptera: Tenebrionidae): Broad and limited extent depolymerization. Environ. Pollut. 2020, 266, 115206. [Google Scholar] [CrossRef]
- De Filippis, F.; Bonelli, M.; Bruno, D.; Sequino, G.; Montali, A.; Reguzzoni, M.; Pasolli, E.; Savy, D.; Cangemi, S.; Cozzolino, V.; et al. Plastics shape the black soldier fly larvae gut microbiome and select for biodegrading functions. Microbiome 2023, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Dong, Y.; Nadir, S.; Schaefer, D.A.; Mortimer, P.E.; Xu, J.; Ye, L.; Gui, H.; Wanasinghe, D.N.; Dossa GG, O.; et al. Valorizing plastic waste by insect consumption. Circ. Agric. Syst. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Woo, S.; Song, I.; Cha, H.J. Fast and Facile Biodegradation of Polystyrene by the Gut Microbial Flora of Plesiophthalmus davidis Larvae. Appl. Environ. Microbiol. 2020, 86, e01361-20. [Google Scholar] [CrossRef]
- An, R.; Liu, C.; Wang, J.; Jia, P. Recent Advances in Degradation of Polymer Plastics by Insects Inhabiting Microorganisms. Polymers 2023, 15, 1307. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P.D. Natural and synthetic polymers. Nature 1975, 253, 504. [Google Scholar] [CrossRef]
- Sethi, A.; Slack, J.M.; Kovaleva, E.S.; Buchman, G.W.; Scharf, M.E. Lignin-associated metagene expression in a lignocellulose-digesting termite. Insect Biochem. Mol. Biol. 2013, 43, 91–101. [Google Scholar] [CrossRef]
- Fitzgerald, M.P.; Donnelly, M.; Vala, L.; Allen-Napoli, L.; Abend, N.S. Collodion Remover Can Degrade Plastic-Containing Medical Devices Commonly Used in the Intensive Care Unit. Neurodiagnostic J. 2019, 59, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, D.; Chirsteen, J.; Doble, M. Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour. Technol. 2013, 148, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Elsamahy, T.; Eltohamy, R.; Sun, J. Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 2021, 780, 146590. [Google Scholar] [CrossRef] [PubMed]
- Hanstveit, A.O. Biodegradability of petroleum waxes and beeswax in an adapted CO2 evolution test. Chemosphere 1992, 25, 605–620. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Suan, M.S.M. Thermoplastic starch/beeswax blend: Characterization on thermal mechanical and moisture absorption properties. Int. J. Biol. Macromol. 2021, 190, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Dobrosielska, M.; Dobrucka, R.; Kozera, P.; Brząkalski, D.; Gabriel, E.; Głowacka, J.; Jałbrzykowski, M.; Kurzydłowski, K.J.; Przekop, R.E. Beeswax as a natural alternative to synthetic waxes for fabrication of PLA/diatomaceous earth composites. Sci. Rep. 2023, 13, 1161. [Google Scholar] [CrossRef] [PubMed]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Yanat, M.; Schroën, K. Advances in chitin-based nanoparticle use in biodegradable polymers: A review. Carbohydr. Polym. 2023, 312, 120789. [Google Scholar] [CrossRef]
- Ramakrishnan, N.; Sharma, S.; Gupta, A.; Alashwal, B.Y. Keratin based bioplastic film from chicken feathers and its characterization. Int. J. Biol. Macromol. 2018, 111, 352–358. [Google Scholar] [CrossRef]
- Cai, C.; Chen, J.; Qi, J.; Yin, Y.; Zheng, X. Purification and characterization of keratinase from a new Bacillus subtilis strain. J. Zhejiang Univ. Sci. B 2008, 9, 713–720. [Google Scholar] [CrossRef] [PubMed]
- O’brien, R.; Slay, M.; Bioi, A. Role of Microorganisms in the Metabolism of Termites. Aust. J. Biol. Sci. 1982, 35, 239–262. Available online: https://www.publish.csiro.au/bi/pdf/bi9820239 (accessed on 20 January 2024). [CrossRef]
- Ali, S.S.; Wu, J.; Xie, R.; Zhou, F.; Sun, J.; Huang, M. Screening and characterizing of xylanolytic and xylose-fermenting yeasts isolated from the wood-feeding termite, Reticulitermes chinensis. PLoS ONE 2017, 12, e0181141. [Google Scholar] [CrossRef] [PubMed]
- Chandra, M.R.G.S.; Madakka, M. Chapter 11—Comparative Biochemistry and Kinetics of Microbial Lignocellulolytic Enzymes; Buddolla, V., Ed.; ScienceDirect; Academic Press: Cambridge, MA, USA, 2019; Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780128163283000118 (accessed on 1 January 2019).
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of lignocellulose using ligninolytic enzymes from white-rot fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef] [PubMed]
- Schiøtt, M.; Boomsma, J.J. Proteomics reveals synergy between biomass degrading enzymes and inorganic Fenton chemistry in leaf-cutting ant colonies. eLife 2021, 10, e61816. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Zhang, M.; Elsamahy, T.; Abdelkarim, E.A.; Jiao, H.; Sun, S.; Sun, J. Environmental and Human Health Impact of Disposable Face Masks During the COVID-19 Pandemic: Wood-Feeding Termites as a Model for Plastic Biodegradation. Appl. Biochem. Biotechnol. 2022, 195, 2093–2113. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ding, S.-Y.; Peterson, J. Biological Conversion of Biomass for Fuels and Chemicals; Sun, J., Ding, S.-Y., Peterson, D., Eds.; Energy and Environment Series; Royal Society of Chemistry: London, UK, 2013. [Google Scholar] [CrossRef]
- Sanchez-Hernandez, J.C. A toxicological perspective of plastic biodegradation by insect larvae. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2021, 248, 109117. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.L.; Attwood, G.; Hopcroft, D.H.; Christeller, J.T. Characterization of lactic acid bacteria in the larval midgut of the keratinophagous lepidopteran, Hofmannophila pseudospretella. Lett. Appl. Microbiol. 2008, 32, 36–41. [Google Scholar] [CrossRef]
- Downs, C.T.; van Dyk, R.J.; Iji, P. Wax digestion by the lesser honeyguide Indicator minor. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2002, 133, 125–134. [Google Scholar] [CrossRef]
- Micocci, K.C.; Moreira, A.C.; Sanchez, A.D.; Pettinatti, J.L.; Rocha, M.C.; Dionizio, B.S.; Correa KC, S.; Malavazi, I.; Wouters, F.C.; Bueno, O.C.; et al. Identification, cloning, and characterization of a novel chitinase from leaf-cutting ant Atta sexdens: An enzyme with antifungal and insecticidal activity. Biochimica et Biophysica Acta. Gen. Subj. 2023, 1867, 130249. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Arroyo, M.; de la Mata, I.; Mirończuk, A.M. Identification of novel extracellular putative chitinase and hydrolase from Geomyces sp. B10I with the biodegradation activity towards polyesters. AMB Express 2022, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.; Černoša, A.; Fernández-Sanmartín, P.; Cortizas, A.M.; Aranda, E.; Luo, Y.; Zalar, P.; Podlogar, M.; Gunde-Cimerman, N.; Gostinčar, C. Degradation of polypropylene by fungi Coniochaeta hoffmannii and Pleurostoma richardsiae. Microbiol. Res. 2023, 277, 127507. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Vogler, A.P. Gene expression in the gut of keratin-feeding clothes moths (Tineola) and keratin beetles (Trox) revealed by subtracted cDNA libraries. Insect Biochem. Mol. Biol. 2006, 36, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Aqueel, M.A.; Ellis, J.D.; Raza, A.M.; Ullah, S. Consumption, digestion, and utilization of beeswax by greater wax moths (Galleria mellonella L.). J. Apic. Res. 2020, 59, 876–882. [Google Scholar] [CrossRef]
- Hickin, M.; Nadel, H.; Schal, C.; Cohen, A.C. Optimization of a Diet for the Greater Wax Moth (Lepidoptera: Pyralidae) Using Full Factorial and Mixture Design. J. Econ. Entomol. 2021, 114, 1091–1103. [Google Scholar] [CrossRef]
- Lou, Y.; Ekaterina, P.; Yang, S.-S.; Lu, B.; Liu, B.; Ren, N.; Corvini, P.F.-X.; Xing, D. Biodegradation of Polyethylene and Polystyrene by Greater Wax Moth Larvae (Galleria mellonella L.) and the Effect of Co-diet Supplementation on the Core Gut Microbiome. Environ. Sci. Technol. 2020, 54, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. PlasticDB: A database of microorganisms and proteins linked to plastic biodegradation. Database 2022, 2022, baac008. [Google Scholar] [CrossRef]
- Sarkar, A.; Sharma, B.; Shekhar, S. Biodegradability of Conventional Plastics; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Akinpelu, E.A.; Nchu, F. A Bibliometric Analysis of Research Trends in Biodegradation of Plastics. Polymers 2022, 14, 2642. [Google Scholar] [CrossRef]
- Can-Güven, E. Microplastics as emerging atmospheric pollutants: A review and bibliometric analysis. Air Qual. Atmosphere Health 2020, 14, 203–215. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, H.; Yang, D.; Zhang, G.; Zhang, J.; Ju, F. Polyvinyl chloride degradation by a bacterium isolated from the gut of insect larvae. Nat. Commun. 2022, 13, 5360. [Google Scholar] [CrossRef]
- DeAngelis, K.M.; Sharma, D.; Varney, R.M.; Simmons, B.A.; Isern, N.G.; Lye Meng Markilllie Nicora, C.D.; Norbeck, A.D.; Taylor, R.P.; Aldrich, J.T.; Robinson, E.W. Evidence supporting dissimilatory and assimilatory lignin degradation in Enterobacter lignolyticus SCF1. Front. Microbiol. 2013, 4, 280. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, V.G.; Sridharan, R.; Kumar, P.S.; Fathima, M.J. Cellulase enzyme catalyst producing bacterial strains from vermicompost and its application in low-density polyethylene degradation. Chemosphere 2022, 288, 132552. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Avargani, M.; Bazooyar, F.; Biria, D.; Zamani, A.; Skrifvars, M. The promiscuous potential of cellulase in degradation of polylactic acid and its jute composite. Chemosphere 2021, 278, 130443. [Google Scholar] [CrossRef] [PubMed]
- Temporiti ME, E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms 2022, 10, 1180. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Zamudio, P.A.; Flórez-Restrepo, M.A.; López-Legarda, X.; Monroy-Giraldo, L.C.; Segura-Sánchez, F. Biodegradation of plastics by white-rot fungi: A review. Sci. Total Environ. 2023, 901, 165950. [Google Scholar] [CrossRef]
- Watanabe, H.; Tokuda, G. Cellulolytic Systems in Insects. Annu. Rev. Entomol. 2010, 55, 609–632. [Google Scholar] [CrossRef]

| Species | Report | Polymer Type/s | Reference |
|---|---|---|---|
| Cryptotermes brevis | Damage | 5% Polymethyl methacrylate (PMMA) | [16] |
| Unspecified (Coptotermes, Heterotermes and Reticulitermes) | Damage | Polyethylene (PE), Polyvinyl chloride (PVC), Neoprene and Rubber | [36] |
| Monomorium destructor & Camponotus spp. | Consumption | PE, PVC PE | [33] |
| Hofmannophila pseudospretella Stainton & Elophila nymphaeata | Consumption | PE, Polystyrene (PS) and Nylon PE | [37] |
| Galleria mellonella and Epiphyas postvittana | Damage | Unspecified Plastic Containers | [38] |
| Rhyzopertha dominica & Tribolium castaneum | Penetration | PE, Polypropylene (PP) and Polyethylene Terephthalate (PET) PE | [39] |
| Plodia interpunctella | Penetration | Unspecified Plastic Packaging | [40] |
| Plodia interpunctella | Breakdown by gut bacteria isolate | PE | [41] |
| Tenebrio molitor | Breakdown (Dependency not assessed) | PS | [42] |
| Galleria mellonella | Breakdown (Dependency not assessed) | PE | [43] |
| Achroia grisella | Breakdown (Dependency not assessed) | High-Density Polyethylene (HDPE) | [44] |
| Tenebrio molitor & Tenebrio obscurus | Breakdown (Microbially dependent) | PS | [45] |
| Corcyra cephalonica | Breakdown (Microbially independent) | Low-Density Polyethylene (LDPE) | [46] |
| Tribolium castaneum | Breakdown (Microbially dependent) | PS | [47] |
| Tribolium confusum | Breakdown (Dependency not assessed) | PS, PE and Ethylene-Vinyl Acetate (EVA) | [17] |
| Zophobas atratus | Breakdown (Dependency not assessed) | PS | [48] |
| Plesiophthalmus davidis | Breakdown (Microbially dependent) | PS | [36] |
| Tenebrio molitor | Breakdown (Microbially independent) | LDPE | [19] |
| Galleria mellonella | Breakdown (Microbially independent salivary enzymes characterized) | PE | [49] |
| Species | Study Overview | References |
|---|---|---|
| Waxworm (Plodia interpunctella) | Waxworms depend on gut microbes to break down polyethylene (PE) as their sole carbon source | [50] |
| Mealworm (Tenebrio molitor) | Mealworms, capable of degrading polystyrene (PS) and other fossil-based polymers, convert 47.7% of ingested PS into carbon dioxide, with gut microbes playing a crucial role | [42,51] |
| Greater Wax Moth (Galleria mellonella) | Extensively studied, greater wax moths demonstrate polyethylene (PE) degradation and microbially independent plastic breakdown potential | [20,43] |
| Rice Moth (Corcyra cephalonica) | Rice moths exhibit microbially independent degradation of low-density polyethylene (LDPE), resulting in a weight loss of 21%, indicating their potential role in plastic waste management | [46] |
| Lesser Wax Moths (Achroia grisella) | Investigated for their potential to degrade high-density polyethylene (HDPE), lesser wax moths contribute to understanding plastic degradation mechanisms | [44] |
| Tenebrio molitor and Tenebrio obscurus | Both species are involved in microbially mediated polystyrene (PS) degradation, with reported rates of conversion to carbon dioxide, expanding our knowledge of plastic degradation pathways | [46] |
| Tribolium castaneum and Tribolium confusum | Investigation reveals insights into plastic degradation pathways, albeit without fully characterizing responsible enzymes | [17,47] |
| Black Soldier Fly (Hermetia illucens) | The black soldier fly contributes to polyethylene (PE) and polystyrene (PS) degradation, emphasizing the role of gut microbes in plastic breakdown processes | [52] |
| Cricket species (Gryllus bimaculatus) | Actively participate in plastic waste degradation, with studies highlighting their ability to degrade polyurethane (PU) foam at a rate of 0.28 consumption (mg)/live weight (g) per day | [53] |
| Beetle Species (Zophobas atratus and Plesiophthalmus davidis) | Beetle species showed promise in polystyrene (PS) degradation, with microbial involvement highlighted | [48,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boctor, J.; Pandey, G.; Xu, W.; Murphy, D.V.; Hoyle, F.C. Nature’s Plastic Predators: A Comprehensive and Bibliometric Review of Plastivore Insects. Polymers 2024, 16, 1671. https://doi.org/10.3390/polym16121671
Boctor J, Pandey G, Xu W, Murphy DV, Hoyle FC. Nature’s Plastic Predators: A Comprehensive and Bibliometric Review of Plastivore Insects. Polymers. 2024; 16(12):1671. https://doi.org/10.3390/polym16121671
Chicago/Turabian StyleBoctor, Joseph, Gunjan Pandey, Wei Xu, Daniel V. Murphy, and Frances C. Hoyle. 2024. "Nature’s Plastic Predators: A Comprehensive and Bibliometric Review of Plastivore Insects" Polymers 16, no. 12: 1671. https://doi.org/10.3390/polym16121671






