Mechanism Analysis and Potential Applications of Atomic Oxygen Erosion Protection for Kapton-Type Polyimide Based on Molecular Dynamics Simulations
Abstract
:1. Introduction
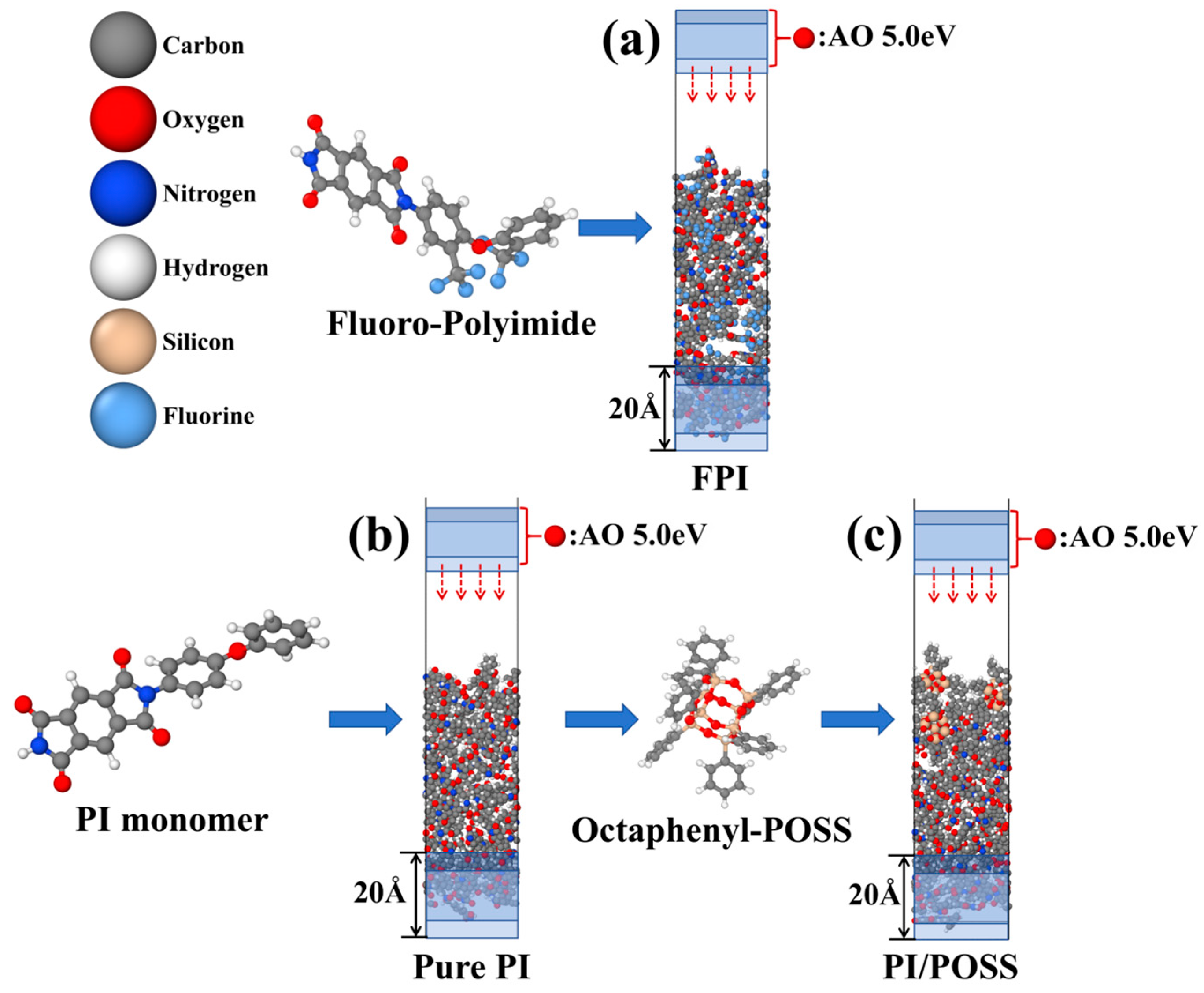
2. Methods and Models
3. Results and Discussion
3.1. Erosion Kinetics of Pure PI, FPI, and PI/POSS Nanocomposite Materials
3.2. Temperature Evolution during AO Erosion
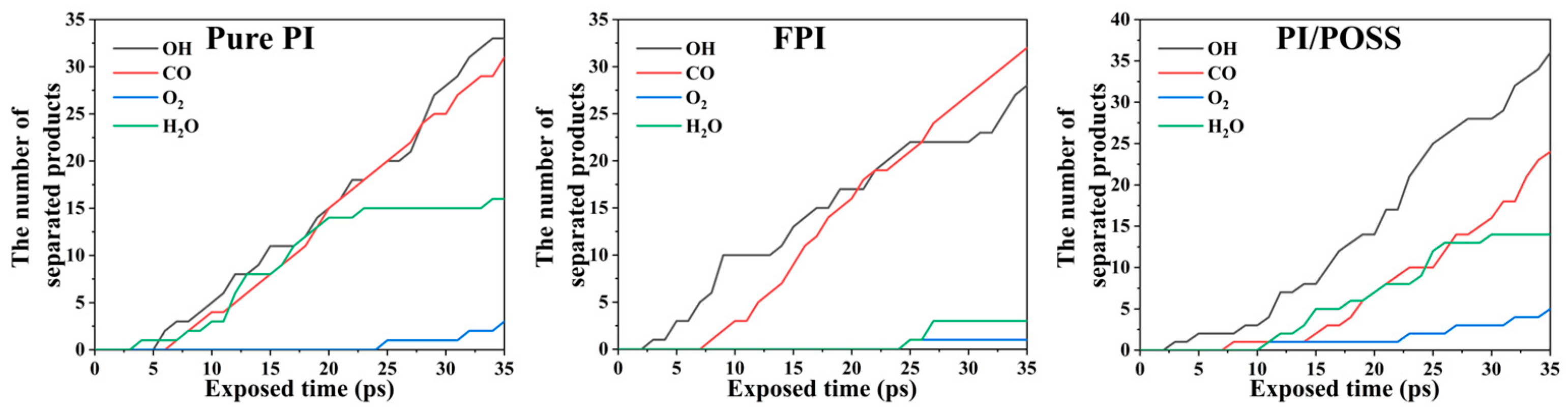
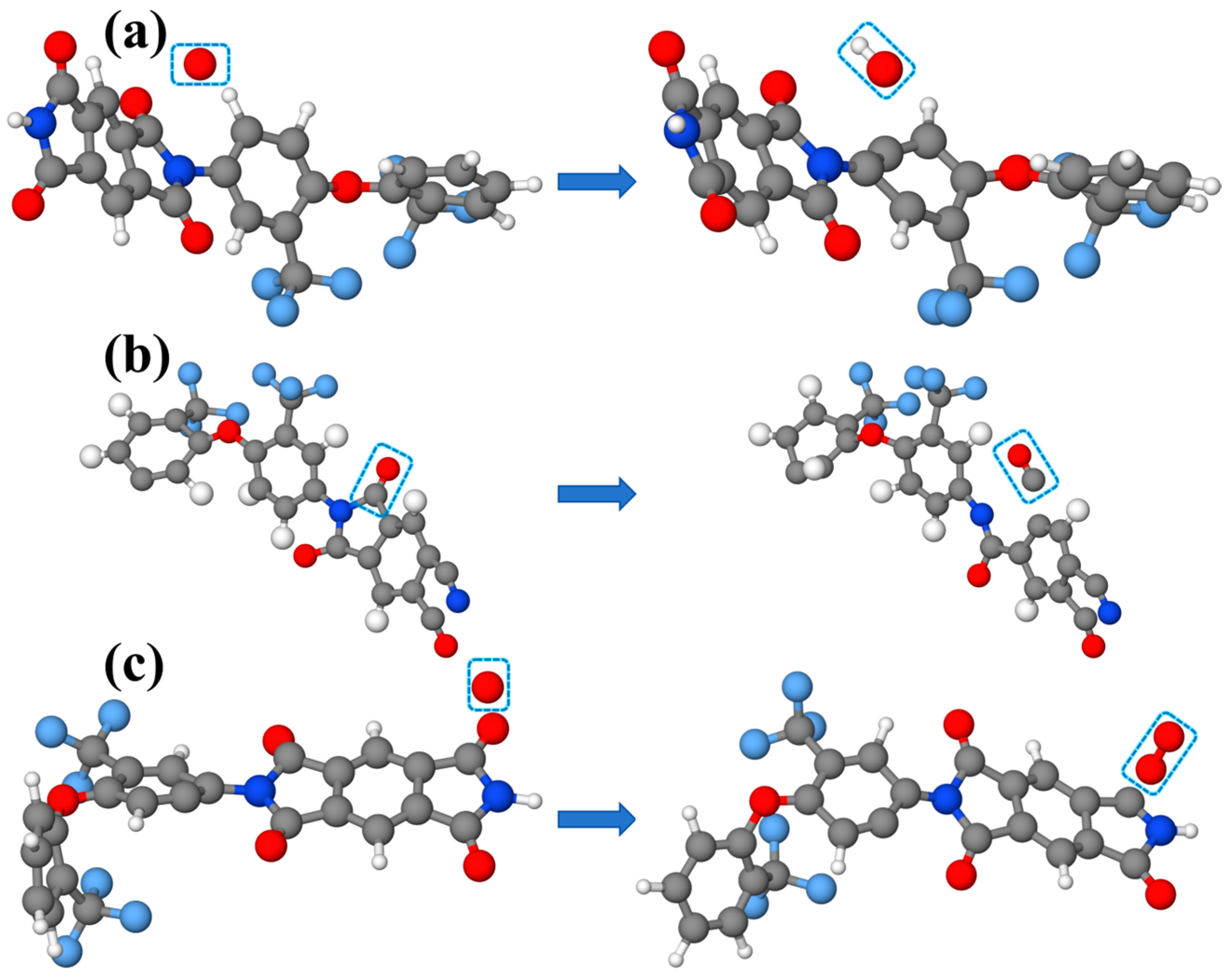
3.3. Analysis of AO Erosion Byproducts
4. Conclusions
- (1)
- The simulation of AO erosion on pure PI, FPI, and PI/POSS showed that AO erosion of polymers manifests in the two following aspects: thermal decomposition and oxidative erosion caused by the temperature rise induced by AO. PI/POSS exhibited the best protective effect, with POSS converting into a significant amount of SiOX under AO erosion, preventing further AO penetration into the PI matrix. FPI’s protective effect was second, with the introduction of -CF3 groups enhancing the polymer’s stability and thus showing better AO resistance.
- (2)
- The temperature rise in FPI was similar to that in the pure PI system. However, adding POSS and converting it into SiOX products under AO erosion effectively prevented heat transfer to the PI matrix. The temperature rise of the polyimide molecules beneath the POSS coating was effectively suppressed, preventing extensive thermal decomposition of the polyimide molecules and thus reducing the overall mass loss.
- (3)
- Under AO erosion, all three systems produced a significant amount of gaseous small molecular products, mainly CO and OH, related to the abundant carbon chains in polyimide and phenyl groups in POSS.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, S.; Wang, Y.; Zhang, X.; Cao, K.; Su, J.; Shen, J.; Wang, X. Fire-resistant polyimide-silica aerogel composite aerogels with low shrinkage, low density, and high hydrophobicity for aerospace applications. Polym. Test. 2023, 129, 108259. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Ouyang, C.; Yi, S.; Liu, S. Porous highly fluorinated polyimide/polydopamine nanocomposite films with simultaneously enhanced toughness, UV-shielding, and photostability for aerospace applications. Polym. Test. 2023, 118, 107899. [Google Scholar] [CrossRef]
- Jia, T.; Chen, H.; Fan, Z.; Xu, H.; Huang, J.; Wang, P.; Xing, H.; Jia, H.; Fan, X.; Zhou, H.; et al. High-strength and high-temperature-resistant multilayer interconnected polyimide paper derived from anisotropic aerogel via a hot-extrusion strategy for aerospace applications. Appl. Surf. Sci. 2023, 611, 155592. [Google Scholar] [CrossRef]
- Luo, H.; Liu, J.; Yang, Z.; Zhang, Q.; Ao, H.; Wan, Y. Manipulating thermal conductivity of polyimide composites by hybridizing micro- and nano-sized aluminum nitride for potential aerospace usage. J. Thermoplast. Compos. Mater. 2020, 33, 1017–1029. [Google Scholar] [CrossRef]
- Srinivasan, S.G.; van Duin, A.C.T. Molecular-dynamics-based study of the collisions of hyperthermal atomic oxygen with graphene using the ReaxFF reactive force field. J. Phys. Chem. A. 2011, 115, 13269–13280. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, J.; Niu, B.; Xing, Y.; Liang, X.; Zhang, Y.; Long, D. Atomic Oxygen-Induced Surface Erosion Behavior and Mechanical Degradation of Polyether Ether Ketone via Reactive Molecular Dynamics Simulations. J. Phys. Chem. B 2023, 127, 24. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Vashisth, A.; Bakis, C.E.; van Duin, A.C.T. Reactive Molecular Dynamics Simulations of the Atomic Oxygen Impact on Epoxies with Different Chemistries. J. Phys. Chem. C 2019, 123, 15145–15156. [Google Scholar]
- Suliga, A.; Jakubczyk, M.E.; Hamerton, I.; Viquerat, A. Analysis of atomic oxygen and ultraviolet exposure effects on cycloaliphatic epoxy resins reinforced with octa-functional POSS. Acta Astronaut. 2018, 142, 103–111. [Google Scholar] [CrossRef]
- Clausi, M.; Santonicola, G.M.; Schirone, L.; Laurenzi, S. Analysis of ultraviolet exposure effects on the surface properties of epoxy/graphene nanocomposite films on Mylar substrate. Acta Astronaut. 2017, 134, 307–313. [Google Scholar] [CrossRef]
- Andropova, U.S.; Chernik, V.N.; Novikov, L.S.; Sapozhnikov, D.A.; Tebeneva, N.A.; Aysin, R.R.; Serenko, O.A. Effect of nanoparticles and siloxane groups on the atomic oxygen erosion resistance of copolyimides. Polym. Degrad. Stab. 2024, 221, 110659. [Google Scholar] [CrossRef]
- Chen, L.; Li, Z.; Lee, C.; Wang, X.; Zhang, Y.; Liu, M.; Brown, T.; Smith, J.; Patel, R.; Kim, H.; et al. Unified model for low-Earth-orbital atomic-oxygen and atomic-oxygen/ultraviolet induced erosion of polymeric materials. Aerosp. Sci. Technol. 2016, 53, 194–206. [Google Scholar] [CrossRef]
- Yizhi, Z.; Zhigang, S.; Xiaojing, Z. Exploring graphene and graphene/nanoparticles as fillers to enhance atomic oxygen corrosion resistance of polyimide films. Colloids Surf. A. 2021, 629, 127431. [Google Scholar]
- Liu, L.; Shen, Z.; Liang, S.; Yi, M.; Zhang, X.; Ma, S.; Wang, P.; Zhou, Q.; Lin, J.; Gao, R.; et al. Enhanced atomic oxygen erosion resistance and mechanical properties of graphene/cellulose acetate composite films. J. Appl. Polym. 2013, 131, 40123. [Google Scholar]
- Young, J.N.; Bo, S.J.; Gon, C.K. Characteristics of Silane Treated Graphene Filled Nanocomposites Exposed to Low Earth Orbit Space Environment. Compos. Res. 2015, 28, 130–135. [Google Scholar]
- Wang, D.S.; Mi, S.M.; Liu, T.Z.; Li, M.S. SiO2 Coatings Prepared by Sol-Gel Process Protecting Silver from Atomic Oxygen Erosion. Appl. Mech. Mater. 2011, 121–126, 3044–3047. [Google Scholar]
- Minton, T.K.; Wright, M.E.; Tomczak, S.J.; Marquez, S.A.; Shen, L.; Brunsvold, A.L.; Petteys, B.J.; Doe, J.; Roe, J.; Smith, A.; et al. Atomic oxygen effects on POSS polyimides in low earth orbit. ACS. Appl. Mater. Interfaces 2012, 4, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, X.; Zhang, C.; Yang, Z.; Cheng, G.; Yang, Z.; Liu, W.; Smith, A.; Johnson, B.; Davis, C.; et al. Resistance to space atomic oxygen radiation of MAC-based supramolecular gel lubricant containing POSS. Tribol. Int. 2023, 177, 106987. [Google Scholar] [CrossRef]
- Duo, S.; Chang, Y.; Liu, T.; Zhang, H. Atomic Oxygen Erosion Resistance of Polysiloxane/POSS Hybrid Coatings on Kapton. Phys. Procedia 2013, 50, 337–342. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Feng, L. Preparation and anti-atomic oxygen erosion properties of OPPOSS/PI composites. Int. J. Miner. Metall. Mater. 2013, 20, 994–1000. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Zeng, F.; Wang, G.; Zhao, H.; Liu, M.; Chen, X.; Li, J.; Xu, K.; Huang, L.; et al. Characterization and analysis on atomic oxygen resistance of POSS/PVDF composites. Appl. Surf. Sci. 2014, 320, 908–913. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Liu, J.; Ni, H.; Yang, H.; Yang, S. Intrinsically Atomic-oxygen-resistant POSS-containing Polyimide Aerogels: Synthesis and Characterization. Chem. Lett. 2015, 44, 1083–1085. [Google Scholar] [CrossRef]
- Lei, X.; Qiao, M.; Tian, L.; Chen, Y.; Zhang, Q.; Wang, L.; Zhao, P.; Li, Z.; Zhang, R.; Sun, J.; et al. Evolution of surface chemistry and morphology of hyperbranched polysiloxane polyimides in simulated atomic oxygen environment. Corros. Sci. 2015, 98, 560–572. [Google Scholar] [CrossRef]
- Yuan, G.; Yang, B.; Chen, Y.; Jia, Y. Synthesis of a novel multi-structure synergistic POSS-GO-DOPO ternary graft flame retardant and its application in polypropylene. Compos. Part A 2019, 117, 345–356. [Google Scholar] [CrossRef]
- Atar, N.; Grossman, E.; Gouzman, I.; Bolker, A.; Murray, V.J.; Marshall, B.C.; Smith, J.; Brown, T.; Taylor, R.; Hanein, Y.; et al. Atomic-Oxygen-Durable and Electrically-Conductive CNT-POSS-Polyimide Flexible Films for Space Applications. ACS Appl. Mater. Interfaces 2015, 7, 12047–12056. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Qian, Y.; Qi, H.; Li, J.; Sun, J. Mechanically Robust Atomic Oxygen-Resistant Coatings Capable of Autonomously Healing Damage in Low Earth Orbit Space Environment. Adv. Mater. 2018, 30, e1803854. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cao, X.; Shi, Y.; Cong, T.; Liu, H.; Gao, Y. In situ formation of POSS layer on the surface of polyimide film and anti-atomic oxygen of SiO2/POSS coatings. Prog. Org. Coat. 2023, 182, 106208. [Google Scholar] [CrossRef]
- Minton, T.K.; Schwartzentruber, T.E.; Xu, C. On the Utility of Coated POSS-Polyimides for Vehicles in Very Low Earth Orbit. ACS Appl. Mater. Interfaces 2021, 13, 51673–51684. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Tian, H.; Niu, B.; Xing, Y.; Liang, X.; Zhang, Y.; Long, D. Molecular Design of Polyimide Films for Combating Atomic Oxygen Erosion Through Combining Experiments with Simulations: A State-of-the-Art Review. Polym. Degrad. Stab. 2024, 220, 110645. [Google Scholar] [CrossRef]
- Chen, S.; Wu, X.; Zhong, M.; Yan, D.; Huang, W. The atomic oxygen resistant study of a transparent polyimide film containing phosphorus and fluorine. Appl. Surf. Sci. 2023, 631, 127431. [Google Scholar]
- Zhang, Y.; Liao, B. Atomic oxygen degradation of a fluorine-containing colorless polyimide. Polym. Degrad. Stab. 2023, 215, 109482. [Google Scholar] [CrossRef]
- Dworak, D.P.; Banks, B.A.; Karniotis, C.A.; Soucek, M.D. Evaluation of Protective Silicone/Siloxane Coatings in Simulated Low-Earth-Orbit Environment. J. Spacecr. Rocket. 2006, 43, 393–401. [Google Scholar] [CrossRef]
- Cooper, R.; Upadhyaya, H.P.; Minton, T.K.; Berman, M.R.; Du, X.; George, S.M. Protection of polymer from atomic-oxygen erosion using Al2O3 atomic layer deposition coatings. Thin Solid Films 2007, 516, 4036–4039. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, X.; Zhang, Y.; Yang, C.; Han, S.; Qi, Y.; Liu, J. Preparation and Properties of Atomic-Oxygen Resistant Polyimide Films Based on Multi-Ring Fluoro-Containing Dianhydride and Phosphorus-Containing Diamine. Polymers 2024, 16, 343. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Li, X.; Jiang, Q.; Mu, J.; Jiang, Z. A Novel Structural Polyimide Material with Synergistic Phosphorus and POSS for Atomic Oxygen Resistance. RSC Adv. 2015, 5, 11980–11988. [Google Scholar] [CrossRef]
- Banks, B.A.; Backus, J.A.; Manno, M.V.; Waters, D.L.; Cameron, K.C.; de Groh, K.K. Prediction of Atomic Oxygen Erosion Yield for Spacecraft Polymers. J. Spacecr. Rocket. 2011, 1, 14–22. [Google Scholar] [CrossRef]
- Gotlib-Vainstein, K.; Gouzman, I.; Girshevitz, O.; Bolker, A.; Atar, N.; Grossman, E.; Sukenik, C.N. Liquid Phase Deposition of a Space-Durable, Antistatic SnO2 Coating on Kapton. ACS Appl. Mater. Interfaces 2015, 6, 3539–3546. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, F.; Nouranian, S.; Li, X.; Al-Ostaz, A. Reactive Molecular Simulation of the Damage Mitigation Efficacy of POSS-, Graphene-, and Carbon Nanotube-Loaded Polyimide Coatings Exposed to Atomic Oxygen Bombardment. ACS Appl. Mater. Interfaces 2017, 9, 12802–12811. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Peng, C.; Liu, Y.; Qu, J. Reactive Molecular Dynamics Simulations on the Disintegration of PVDF, FP-POSS, and Their Composite during Atomic Oxygen Impact. J. Phys. Chem. A 2015, 119, 8359–8368. [Google Scholar]
- Tersoff, J. Empirical interatomic potential for silicon with improved elastic properties. Phys. Rev. B 1988, 38, 9902–9905. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.W. Empirical potential for hydrocarbons for use in simulating the chemical vapor deposition of diamond films. Phys. Rev. B 1990, 42, 9458–9471. [Google Scholar] [CrossRef] [PubMed]
- Rahnamoun, A.; van Duin, A.C.T. Reactive molecular dynamics simulation on the disintegration of Kapton, POSS polyimide, amorphous silica, and teflon during atomic oxygen impact using the ReaxFF reactive force-field method. J. Phys. Chem. A 2014, 118, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide-Based Materials for Space Applications. Adv. Mater. 2019, 31, e1807738. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, G. Numerical simulation on atomic oxygen undercutting of Kapton film in low earth orbit. Acta Astronaut. 2010, 67, 388–395. [Google Scholar] [CrossRef]
- Banks, A.B.; Groh, D.K.K.; Miller, K.S. Low Earth Orbital Atomic Oxygen Interactions with Spacecraft Materials. MRS Proc. 2004, 851, NN8.1. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zhang, L.; Zou, L.; Ayubi, B.I.; Wang, Y. Mechanism Analysis and Potential Applications of Atomic Oxygen Erosion Protection for Kapton-Type Polyimide Based on Molecular Dynamics Simulations. Polymers 2024, 16, 1687. https://doi.org/10.3390/polym16121687
Zhou S, Zhang L, Zou L, Ayubi BI, Wang Y. Mechanism Analysis and Potential Applications of Atomic Oxygen Erosion Protection for Kapton-Type Polyimide Based on Molecular Dynamics Simulations. Polymers. 2024; 16(12):1687. https://doi.org/10.3390/polym16121687
Chicago/Turabian StyleZhou, Shengrui, Li Zhang, Liang Zou, Bilal Iqbal Ayubi, and Yiwei Wang. 2024. "Mechanism Analysis and Potential Applications of Atomic Oxygen Erosion Protection for Kapton-Type Polyimide Based on Molecular Dynamics Simulations" Polymers 16, no. 12: 1687. https://doi.org/10.3390/polym16121687





