Effect of Inorganic Fillers on Electrical and Mechanical Properties of Ceramizable Silicone Rubber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
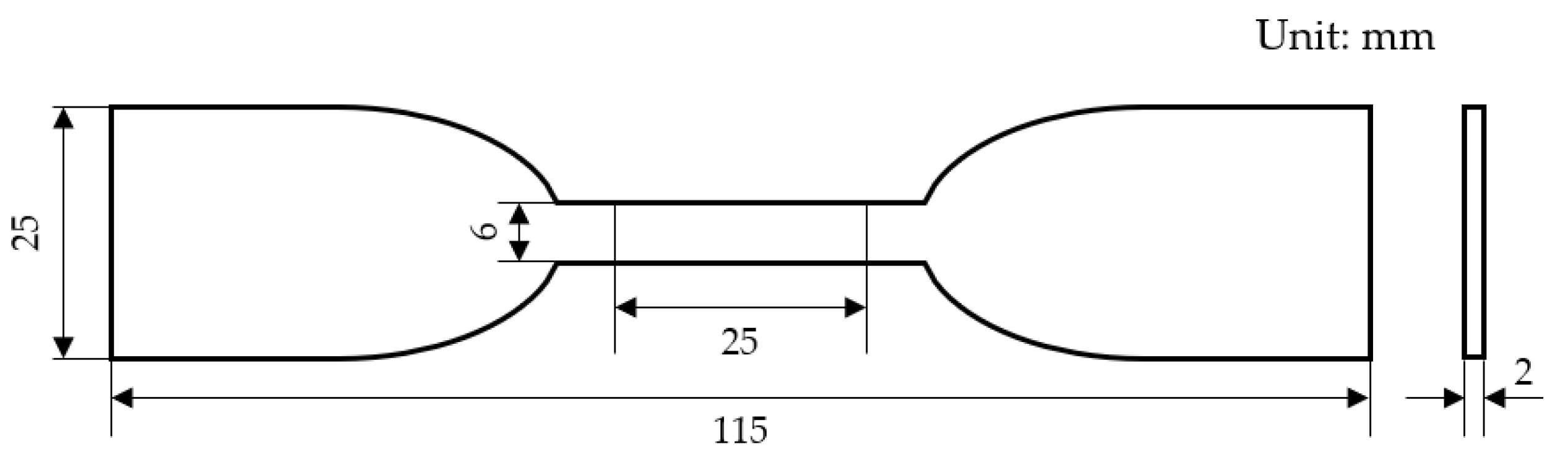
2.2. Mechanical Performance Test
2.3. Electrical Performance Test
2.4. Calculation of Shrinkage Rate
2.5. Density of Ceramic Samples
2.6. Microstructure Observation
2.7. Error Analysis
3. Results and Discussion
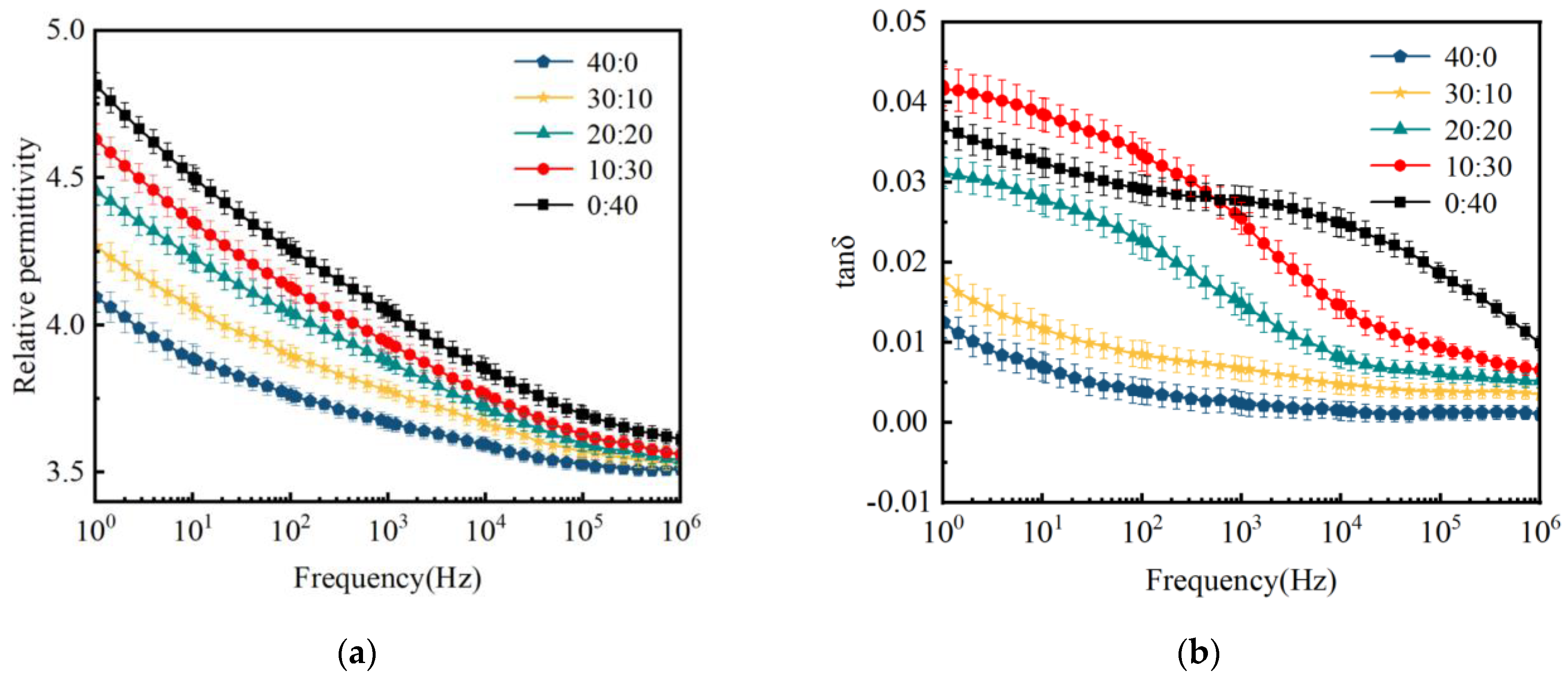
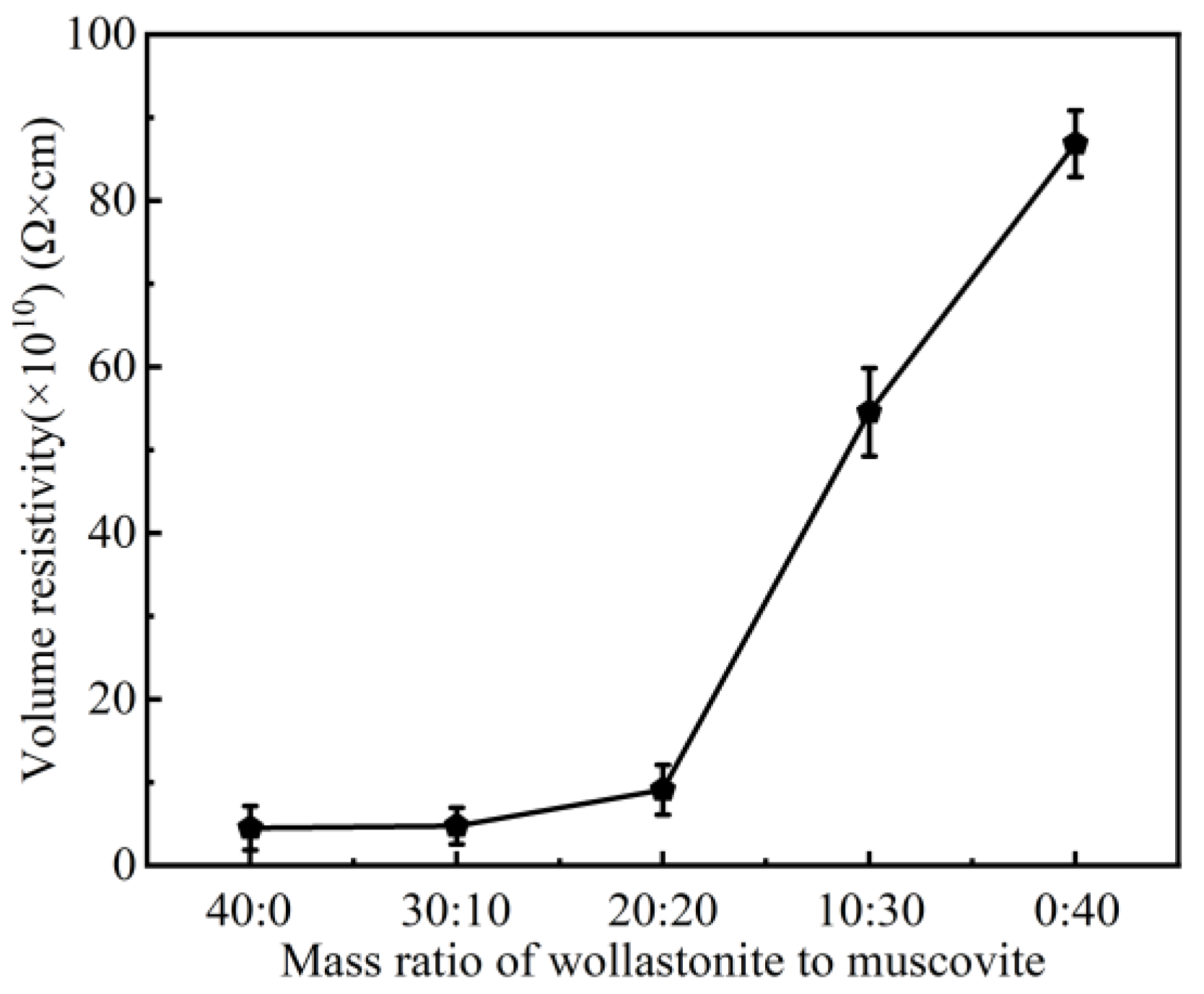
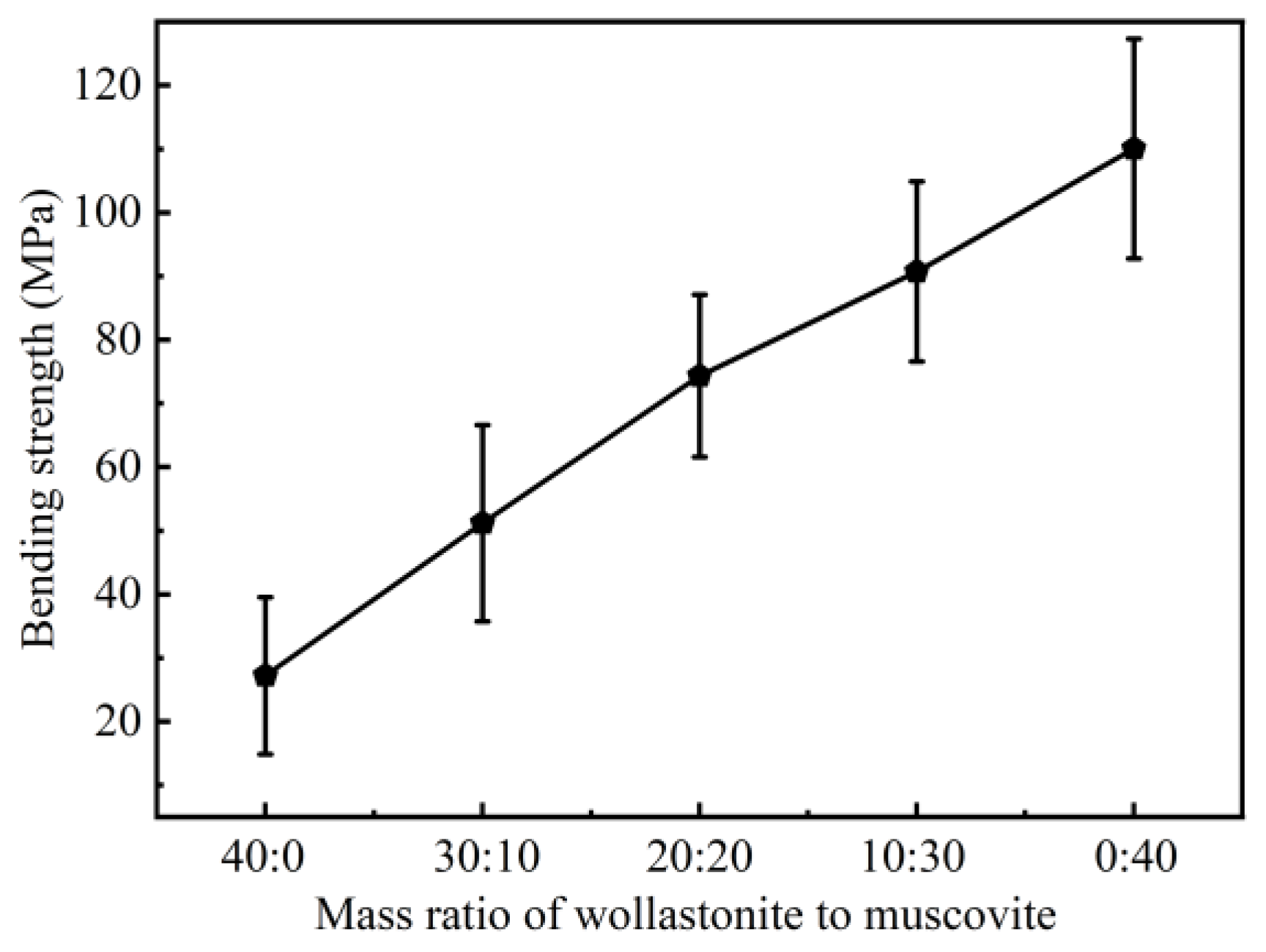
3.1. Effect of Inorganic Fillers on the Properties of CSR
3.2. Effect of Inorganic Fillers on the Morphology of Ceramic Samples
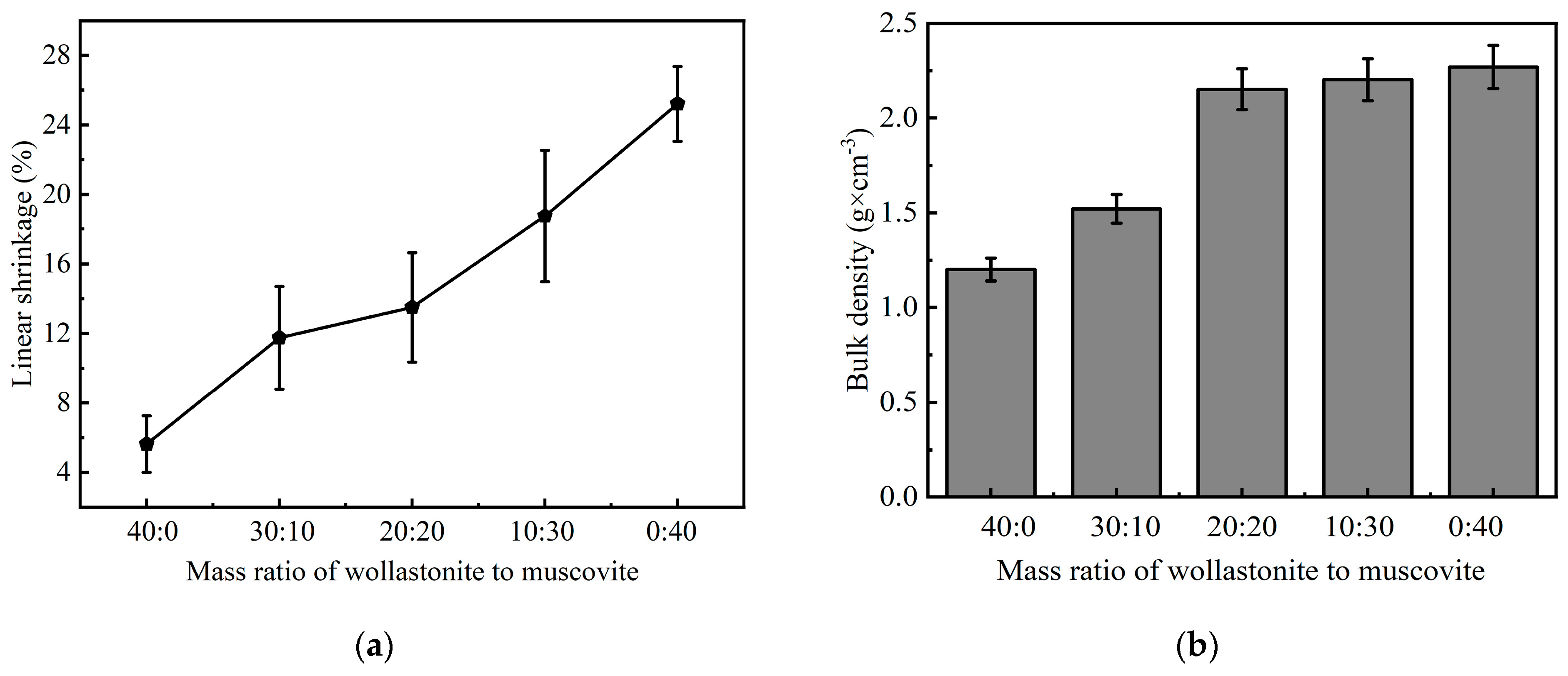
3.3. Effect of Inorganic Fillers on Ceramic Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, X.C. Preliminary Analysis of Application State and Problem of Domestic Fire Resistance Cable. Electr. Wire Cable 2016, 1, 10–12. [Google Scholar]
- Hsieha, C.Y.; Su, W.C.; Wu, C.S.; Lin, L.K.; Hsua, K.Y.; Liu, Y.L. Benzoxazine-containing Branched Polysiloxanes: Highly Efficient Reactive-type Flame Retardants and Property Enhancement Agents for Polymers. Polymers 2013, 54, 2945–2951. [Google Scholar] [CrossRef]
- Wawrzyn, E.; Schartel, B.; Seefeldt, H. What Reacts with What in Bisphenol A Polycarbonate/Silicon Rubber/Bisphenol A Bis (diphenyl phosphate) during Pyrolysis and Fire Behavior. Ind. Eng. Chem. Res. 2012, 51, 1244–1255. [Google Scholar] [CrossRef]
- Zhu, C.; Deng, C.; Cao, J.Y.; Wang, Y.Z. An Efficient Flame Retardant for Silicone Rubber: Preparation and Application. Polym. Degrad. Stabil. 2015, 121, 42–50. [Google Scholar] [CrossRef]
- Song, S.Q.; Ma, J.J.; Cao, K.; Chang, G.J.; Huang, Y.W.; Yang, J.X. Synthesis of A Novel Dicyclic Silicon-/Phosphorus Hybrid and Its Performance on Flame Retardancy of Epoxy Resin. Polym. Degrad. Stab. 2014, 99, 43–52. [Google Scholar] [CrossRef]
- Yu, X.H.; Wu, F. Research Progress in Inorganic Phosphorus Halogen-free Flame Retardants. Insul. Mater. 2014, 47, 6–11. [Google Scholar]
- Guan, J.; Yu, X.H. Research Status of Halogen-free Flame Retardant Cable Material. Insul. Mater. 2014, 47, 1–3. [Google Scholar]
- Zhao, H.; Huang, D.; Zhang, Q.C.; Hao, T.H.; Jiang, T.; Huang, S.Q. Research Progress on Additives in Flame Retardant for Silicone Rubber. Silicone Mater. 2017, 31, 189–192. [Google Scholar]
- Gao, S.; Gao, C.; Si, J.; Shi, J.B.; Zhao, Y.J.; Wang, L.M. The Preparation and Performance of Silicone Rubber Clean-repairing Agent for Power External Insulation. Electr. Power Eng. Technol. 2022, 41, 239–243. [Google Scholar]
- Zhang, J.H.; Shan, Y.D.; Li, J.X. Preparation of Sodium Based Low Temperature Ceramic and Its Application in Ceramic Polyolefin. Insul. Mater. 2017, 50, 11–15. [Google Scholar]
- Imiela, M.; Bieliński, D.M.; Lipińska, M.; Rybiński, P. Effect of Silicone Oil on Properties and Performance of Ceramizable Styrene-Butadiene Rubber-Based Composites. Polymers 2023, 15, 3204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Kong, L.C.; Wang, J.X.; Jiang, G.D.; Zhang, J.; Wang, T.W. Ceramifiable Silicone Rubber Composites with Enhanced Self-supporting and Ceramifiable Properties. Polymers 2022, 14, 1944. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Guo, J.H.; Zeng, X.R. Research Advancement on Ceramifying Composites. Spec. Purp. Rubber Prod. 2015, 36, 67–72. [Google Scholar]
- Wu, Q.; Feng, Y.J.; Pei, Y.B.; Xiao, W.J.; Wu, L.B. Research Progress on Application of Silicone Resin in Flame Retardant Materials. Mater. China 2018, 37, 185–190. [Google Scholar]
- Yan, W.; Qian, Z.M.; Zhang, W.J.; Liu, Y.X.; Lou, F.P.; Guo, W.H. Preparation and Properties of Ceramic Silicone Rubber Composites Improved by Lithium Porcelain or Borax. J. Funct. Polym. 2017, 30, 464–470. [Google Scholar]
- Zeng, H.; Wang, T.W.; Cai, H.B.; Gong, X.H. Preparation and Properties of Fire Retardant and Ceramifying Silicone Rubber. China Synth. Rubber Ind. 2015, 38, 304–307. [Google Scholar]
- Shao, H.B.; Gu, X.C.; Wang, T.W.; Zhang, E.M.; Wang, C.L. Influence of Addition of Low-Melting Point Filler on Properties of Ceramifying Polyolefine Composites. Wire Cable 2015, 5, 20–23. [Google Scholar]
- Hanu, L.G.; Simon, G.P.; Mansouri, J.; Burford, R.P.; Cheng, Y.B. Development of Polymer-ceramic Composites for Improved Fire Resistance. J. Mater. Process. Technol. 2004, 153, 401–407. [Google Scholar] [CrossRef]
- Pędzich, Z.; Dul, J. Optimization of the Ceramic Phase for Ceramizable Silicone Rubber Based Composites. Adv. Sci. Technol. 2010, 66, 162–167. [Google Scholar]
- Mansouri, J.; Burford, R.P.; Cheng, Y.B.; Hanu, L.G. Formation of Strong Ceramified Ash From Silicone-based Compositions. J. Mater. Sci. 2005, 40, 5741–5749. [Google Scholar] [CrossRef]
- Mansouri, J.; Burford, R.P.; Cheng, Y.B. Pyrolysis Behaviour of Silicone-based Ceramifying Composites. Mater. Sci. Eng. A 2006, 425, 7–14. [Google Scholar] [CrossRef]
- Mansouri, J.; Wood, A.C.; Roberts, K.; Cheng, Y.B.; Burford, R.P. Investigation of the Ceramifying Process of Modified Silicone-silicate Compositions. J. Mater. Sci. 2007, 42, 6046–6055. [Google Scholar] [CrossRef]
- Xu, Y.L. The Application of Fire-resistant Mica Tape in Wire and Cable. Electr. Wire Cable 2001, 5, 3–5. [Google Scholar]
- Chen, Y.; Wang, K.; Zhang, C.; Yang, W.; Qiao, B.; Yin, L. The Effect of Various Fillers on the Properties of Methyl Vinyl Silicone Rubber. Polymers 2023, 15, 1584. [Google Scholar] [CrossRef]
- Alexandrina, T.; Iosif, M.; Paula, S.; Nicolae, M.C. Electric and Dielectric Properties in Low-Frequency Fields of Composites Consisting of Silicone Rubber and Al Particles for Flexible Electronic Devices. Materials 2022, 15, 2309. [Google Scholar] [CrossRef]
- Tang, H.C.; Li, P.H.; Kuang, G.W.; Liu, H.D.; Jin, H.Y.; Gao, N.K. Effect of Glass Powder Content on Properties of Ceramizable Silicone Rubber. J. Chin. Ceram. Soc. 2022, 48, 870–876. [Google Scholar]
- Tariq, M.N.; Arslan, K.; Imrana, K.; Cheng, W.; Carlos, B.J.; Shakeel, A.; Shoaib, B.M.; Ghulam, Y.; Toan, P.B.; Heng, Y.G. Flame Retardancy and Excellent Electrical Insulation Performance of RTV Silicone Rubber. Polymers 2021, 13, 2854. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.Q.; Luo, K.; Guan, S.Y.; Ge, W.; Zhang, J.Y. Preliminary Study on the Test Method of Mineral Powder Resistivity—Taking Muscovite as an Example. Acta Mineral. Sin. 2010, 30, 113–115. [Google Scholar]
- Xie, Y.; Wang, L. Current Application Status and Development Suggestions of Muscovite Minerals in Insulating Materials. China Non-Metal. Miner. Ind. 2004, 6, 10–14. [Google Scholar]
- Wang, S.W.; Zhao, F.; Zhao, S.G. Influence of Interaction Between Crosslinked Network and Filler Network on the Properties of SSBR and BR Blend. Elastomers 2012, 22, 11–14. [Google Scholar]
- Abdullaziz, A.; Mohd, S.I.N.; Odiljon, M.; Mohd, A.A.K. Influence of Charge Carrier Density, Mobility and Diffusivity on Conductivity–temperature Dependence in Polyethylene Oxide–based Gel Polymer Electrolytes. High Perform. Polym. 2022, 34, 232–241. [Google Scholar]
- Zhao, L.H.; Li, Y.S.; Qiu, J.H.; Ren, J.W.; Jia, L.C.; Wang, Z. Absorption Characteristics and Key Electrical Properties of Silicone Rubber in Silicone Grease Environment. Electr. Power Eng. Technol. 2021, 5, 41–46. [Google Scholar]
- Li, Z.W.; Zhao, W.Y. New Electrical Engineering Handbook; Science and Technology Press: Hefei, China, 2002; p. 1792. [Google Scholar]
- Liu, Y.B.; Gao, J.H.; Zhong, L.S.; Mei, W.J.; Wang, J.R.; Pan, W.L. Breakdown Property of ±320 kV HVDC Cable System XLPE/EPDM Accessories. Electr. Power Eng. Technol. 2023, 42, 72–80. [Google Scholar]
- Li, Y.G.; Ca, L.; Liu, X.C.; Liu, Y.B.; Gao, J.H.; Zhong, L.S. Mechanical and DC Electrical Properties of Low Crosslinking Degree LLDPE for XLPE Insulation. Electr. Power Eng. Technol. 2023, 42, 154–160. [Google Scholar]
- Huang, X.Y.; Ke, Q.Q.; Jiang, P.K.; Wei, P.; Wang, G.L. Particle-filled Polymer Composites with High Dielectric Constant. Polym. Bull. 2006, 12, 39–45. [Google Scholar]
- Anada, Y. Effect of Motion of Impurity Ions on Electrical Properties of Polymer Materials. Energy Procedia 2013, 34, 17–25. [Google Scholar] [CrossRef]
- Zhou, Y.X.; Zhang, Z.H.; Zhang, Y.X.; Zhu, X.Q.; Huang, M. The Effect of Combined Thermal-mechanical Aging on the Cross-linking Network and Mechanical and Electrical Properties of Silicone Rubber. J. Electr. Eng. Technol. 2022, 37, 4474–4486. [Google Scholar]
- Xiao, Y.; Yan, L.Z.; Chang, T.H.; Hao, T.J.; Sui, Y.J.; Bian, H.G.; Wang, G.H.; Wang, X.M. Preparation of High-quality Wollastonite/Natural Rubber Composites by Atomization Sputtering Rapid Drying Process. Polym. Bull. 2022, 80, 6263–6284. [Google Scholar] [CrossRef]
- Park, E.S. Mechanical Properties and Processibilty of Glass-fiber-, Wollastonite-, and Fluoro-rubber-reinforced Silicone Rubber Composites. J. Appl. Polym. Sci. 2007, 105, 460–468. [Google Scholar] [CrossRef]
- Chatterjee, A.; Khobragade, P.S.; Mishra, S. Physicomechanical Properties of Wollastonite (CaSiO3)/Styrene Butadiene Rubber (SBR) Nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42811. [Google Scholar] [CrossRef]
- Ruan, K.J.; Ma, H.B.; Chen, F.D.; Zhao, S.C.; Li, J. Effect of Modified Wollastonite on the Properties of Ceramic Silicone Rubber Composites. J. Southwest Univ. Sci. Technol. 2020, 35, 7–12. [Google Scholar]
- Hao, B.H.; Qi, S.C.; Zhang, X.A.; Jiang, S.L.; Lyu, Y.F.; Luan, Y.G. Effect of Wollastonite on Properties of Ceramic Silicone Rubber. China Rubber Ind. 2018, 65, 289–293. [Google Scholar]
- Zha, J.W.; Dang, Z.M.; Li, W.K.; Zhu, Y.H.; Chen, G. Effect of Micro-Si3N4-nano-Al2O3 Co-filled Particles on Thermal Conductivity, Dielectric and Mechanical Properties of Silicone Rubber Composites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1989–1996. [Google Scholar] [CrossRef]
- Yan, H.; Li, J.; Fan, H.W.; Ji, S.L.; Zhang, G.J.; Zhang, Z.G. Sonication-enhanced in Situ Assembly of Organic/Inorganic Hybrid Membranes: Evolution of Nanoparticle Distribution and Pervaporation Performance. J. Membr. Sci. 2015, 481, 94–105. [Google Scholar] [CrossRef]
- Furtado, C.R.G.; Leblanc, J.L.; Nunes, R.C.R. Mica as Additional Filler in SBR–silica Compounds. Eur. Polym. J. 2000, 8, 1717–1723. [Google Scholar] [CrossRef]
- Mateusz, I.; Rafał, A.; Dariusz, M.B.; Magdalena, L.; Przemysław, R.; Bartłomiej, S. Synergistic Effect of Mica, Glass Frit, and Melamine Cyanurate for Improving Fire Resistance of Styrene-Butadiene Rubber Composites Destined for Ceramizable Coatings. Coatings 2019, 9, 170. [Google Scholar] [CrossRef]
- Skelhorn, D.; Rothon, R.N. Particulate-Filled Polymer Composites; Rapra Technology Limited: Shrewsbury, UK, 2003. [Google Scholar]
- Hanu, L.G.; Simon, G.P.; Cheng, Y.B. Preferential Orientation of Muscovite in Ceramifiable Silicone Composites. Mater. Sci. Eng. A 2005, 398, 180–187. [Google Scholar] [CrossRef]
- Uno, H.; Tamura, K.; Yamada, H.; Umeyama, K.; Hatta, T.; Moriyoshi, Y. Preparation and Mechanical Properties of Exfoliated Mica-polyamide 6 Nanocomposites Using Sericite Mica. Appl. Clay Sci. 2009, 46, 81–87. [Google Scholar] [CrossRef]
- Guo, J.; Gao, W.; Wang, Y.; Liang, D.; Li, H.J.; Zhang, X. Effect of Glass Frit with Low Softening Temperature on the Properties, Microstructure and Formation Mechanism of Polysiloxane Elastomer-based Ceramizable Composites. Polym. Degrad. Stabil. 2017, 136, 71–79. [Google Scholar] [CrossRef]
- Wang, J.H.; Ji, C.T.; Yan, Y.T.; Zhao, D.; Shi, L.Y. Mechanical and Ceramifiable Properties of Silicone Rubber Filled with Different Inorganic Fillers. Polym. Degrad. Stabil. 2015, 121, 149–156. [Google Scholar] [CrossRef]
- Zhang, M.; Li, R.G. Research Progress on Silicone Flame Retardants. Silicone Mater. 2009, 23, 51–54. [Google Scholar]
- He, C.J.; Dang, J.; Yang, W.J.; Pei, D.F.; Zheng, H. Study on Flame Retardant Silicone Rubber with Synergistic Effect form Carbon Black and Magnesium Hydroxide. China Rubber Ind. 2010, 57, 730–733. [Google Scholar]
- Li, H.J.; Guo, J.H.; Gao, W.; Zeng, X.R. Effect of Silica on Ceramifying Properties of Silicone Rubber-based Ceramizable Composites. Silicone Mater. 2015, 29, 360–365. [Google Scholar]
- Gupta, V.B.; Brahatheeswaran, C. Molecular Packing and Free Volume in Crosslinked Epoxy Networks. Polymer 1991, 32, 1875–1884. [Google Scholar] [CrossRef]
- Kirkpatrick, S. Percolation and Conduction. Rev. Mod. Phys. 1973, 45, 574. [Google Scholar] [CrossRef]
- Zallen, R. The Physics of Amorphous Solids; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Payandehpeyman, J.; Mazaheri, M.; Zeraati, A.S.; Jamasb, S.; Sundararaj, U. Physics-Based Modeling and Experimental Study of Conductivity and Percolation Threshold in Carbon Black Polymer Nanocomposites. Appl. Compos. Mater. 2024, 31, 127–147. [Google Scholar] [CrossRef]
- Stauffer, D. Scaling Theory of Percolation Clusters. Phys. Rep. 1979, 54, 1–74. [Google Scholar] [CrossRef]
- Park, S.H.; Hwang, J.; Park, G.S.; Ha, J.H.; Zhang, M.; Kim, D.; Yun, D.J.; Lee, S.; Lee, S.H. Modeling the Electrical Resistivity of Polymer Composites with Segregated Structures. Nat. Commun. 2019, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Liu, Z.Y. Dielectric Physics; Mechanical Industry Press: Beijing, China, 1982; p. 151. [Google Scholar]
- Zhao, X.T.; Liao, R.J.; Liang, N.C.; Yang, L.J.; Li, J.; Li, J.Y. Role of Defects in Determining the Electrical Properties of ZnO Ceramics. J. Appl. Phys. 2014, 116, 014103. [Google Scholar] [CrossRef]
- Zhao, X.T.; Liao, R.J.; Li, J.Y.; Wang, F.P. Effect of Direct Current Degradation on Dielectric Property of CaCu3Ti4O12 Ceramic. Acta Phys. Sin. 2015, 64, 127701. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.H.; Liang, D.; Yan, Y.Q.; Zhang, Y.; Li, H.J.; Zeng, R.X.; Luo, Q.K. Effect of Fluorophlogopite on Ceramic Properties of Ceramification Silicone Rubber. Spec. Purp. Rubber Prod. 2017, 38, 1–6. [Google Scholar]
- Zhang, Y.; Guo, J.H.; Liang, D.; Wang, Y.; Li, H.J. Effect of Heating Rate on Ceramifying Properties of Silicone Rubber-based Ceramizable Composites. China Rubber Ind. 2018, 65, 689–694. [Google Scholar]
- Imiela, M.; Anyszka, R.; Bieliński, M.D.; Pędzich, Z.; Zarzecka-Napierała, M.; Szumera, M. Effect of Carbon Fibers on Thermal Properties and Mechanical Strength of Ceramizable Composites Based on Silicone Rubber. J. Therm. Anal. Calorim. 2016, 124, 197–203. [Google Scholar] [CrossRef]
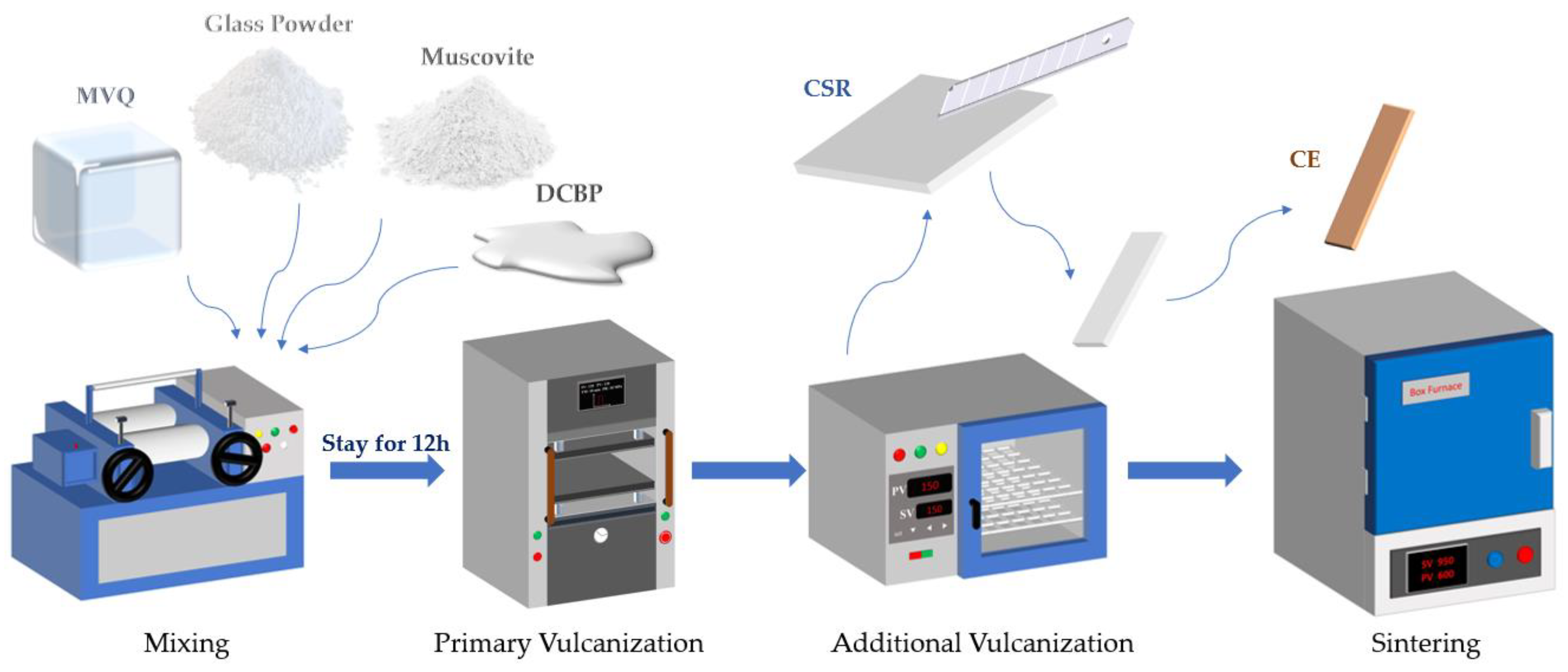





| Raw Materials | Suppliers |
|---|---|
| Methyl Vinyl Silicone Rubber | Wacker-Chemie GmbH (Munich, Germany) |
| Glass Powder | Lianyungang Oawa Materials Technology Co., Ltd. (Lianyungang, China) |
| Wollastonite | Jiangxi Kete Fine Powder Co., Ltd. (Yichun, China) |
| Muscovite | Jiangxi Aote Technology Co., Ltd. (Yichun, China) |
| 2,4-Dichloro-Benzoyl Peroxide (DCBP) | Dongguan Cai Meijia Electronic Technology Co., Ltd. (Dongguan, China) |
| Formulation | Content/g |
|---|---|
| Methyl Vinyl Silicone Rubber | 100 |
| Glass Powder | 30 |
| Wollastonite | 40/30/20/10/0 |
| Muscovite | 0/10/20/30/40 |
| 2,4-Dichloro-Benzoyl Peroxide (DCBP) | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Qiao, J.; Su, B.; Xiao, Y.; Kang, S.; Li, Y.; Cao, H.; Tang, H.; Zhao, X. Effect of Inorganic Fillers on Electrical and Mechanical Properties of Ceramizable Silicone Rubber. Polymers 2024, 16, 1695. https://doi.org/10.3390/polym16121695
Yang M, Qiao J, Su B, Xiao Y, Kang S, Li Y, Cao H, Tang H, Zhao X. Effect of Inorganic Fillers on Electrical and Mechanical Properties of Ceramizable Silicone Rubber. Polymers. 2024; 16(12):1695. https://doi.org/10.3390/polym16121695
Chicago/Turabian StyleYang, Mingyuan, Jingqi Qiao, Bolin Su, Yongjian Xiao, Shenglin Kang, Yuchen Li, Hanzhong Cao, Hongchuan Tang, and Xuetong Zhao. 2024. "Effect of Inorganic Fillers on Electrical and Mechanical Properties of Ceramizable Silicone Rubber" Polymers 16, no. 12: 1695. https://doi.org/10.3390/polym16121695





