Physicochemical Characterization and Biological Properties of Polysaccharides from Alpiniae oxyphyllae Fructus
Abstract
1. Introduction
2. Result and Discussion
2.1. Extraction Yields and Physicochemical Properties of AOPs
2.1.1. Extraction Yields
2.1.2. Chemical Composition
2.1.3. Mw of AOPs
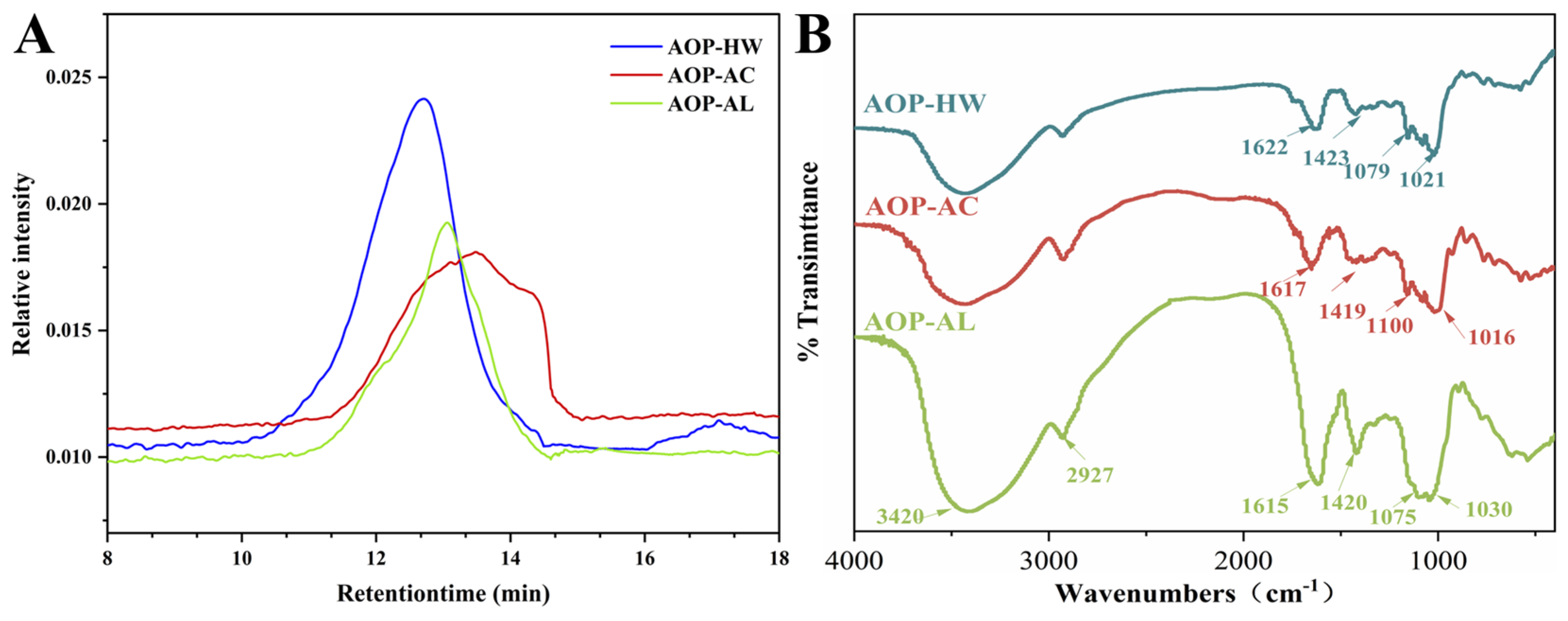
2.1.4. FT-IR Spectroscopy Analysis
2.2. Morphological Properties
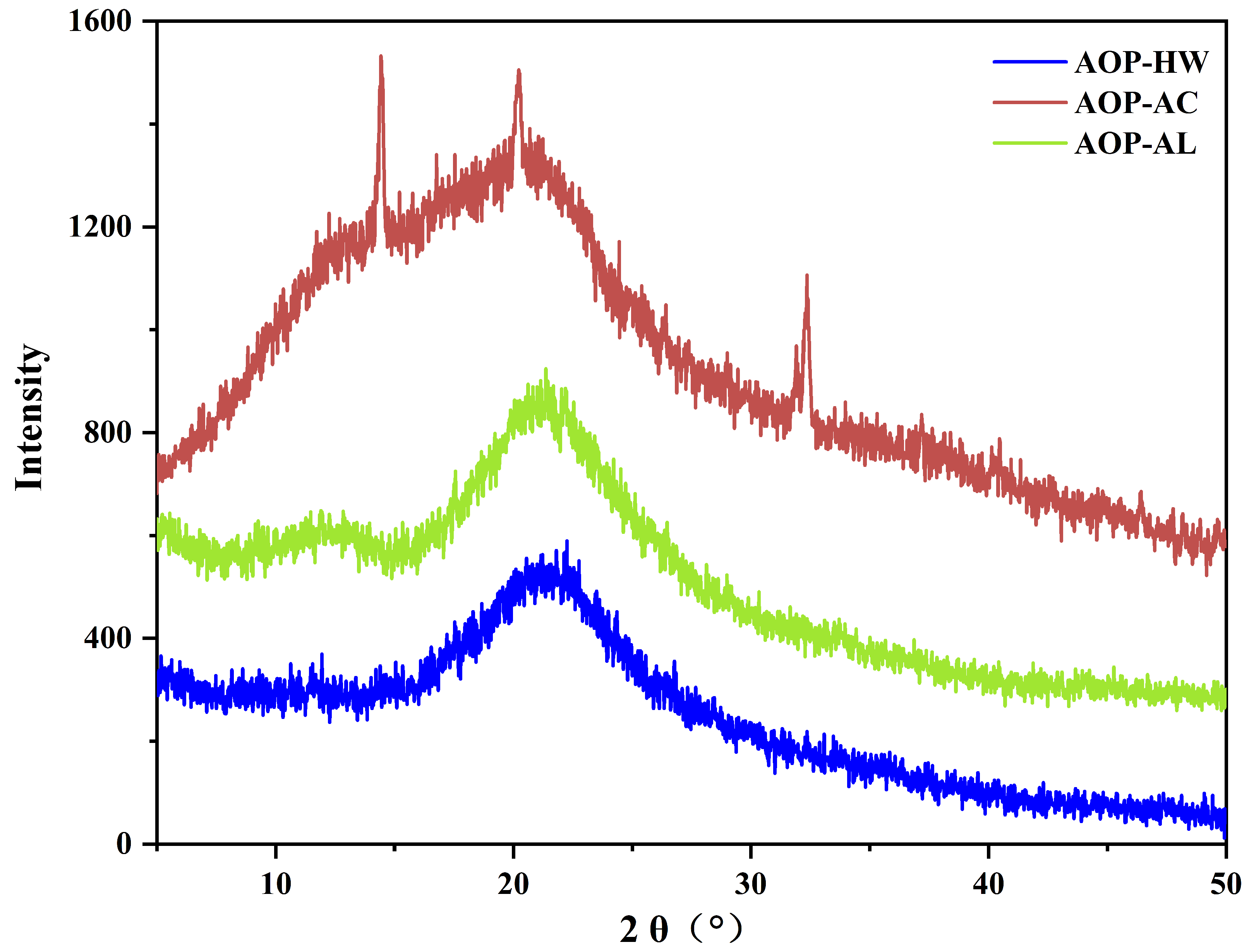
2.3. XRD Analysis
2.4. In vitro Antioxidant Activity
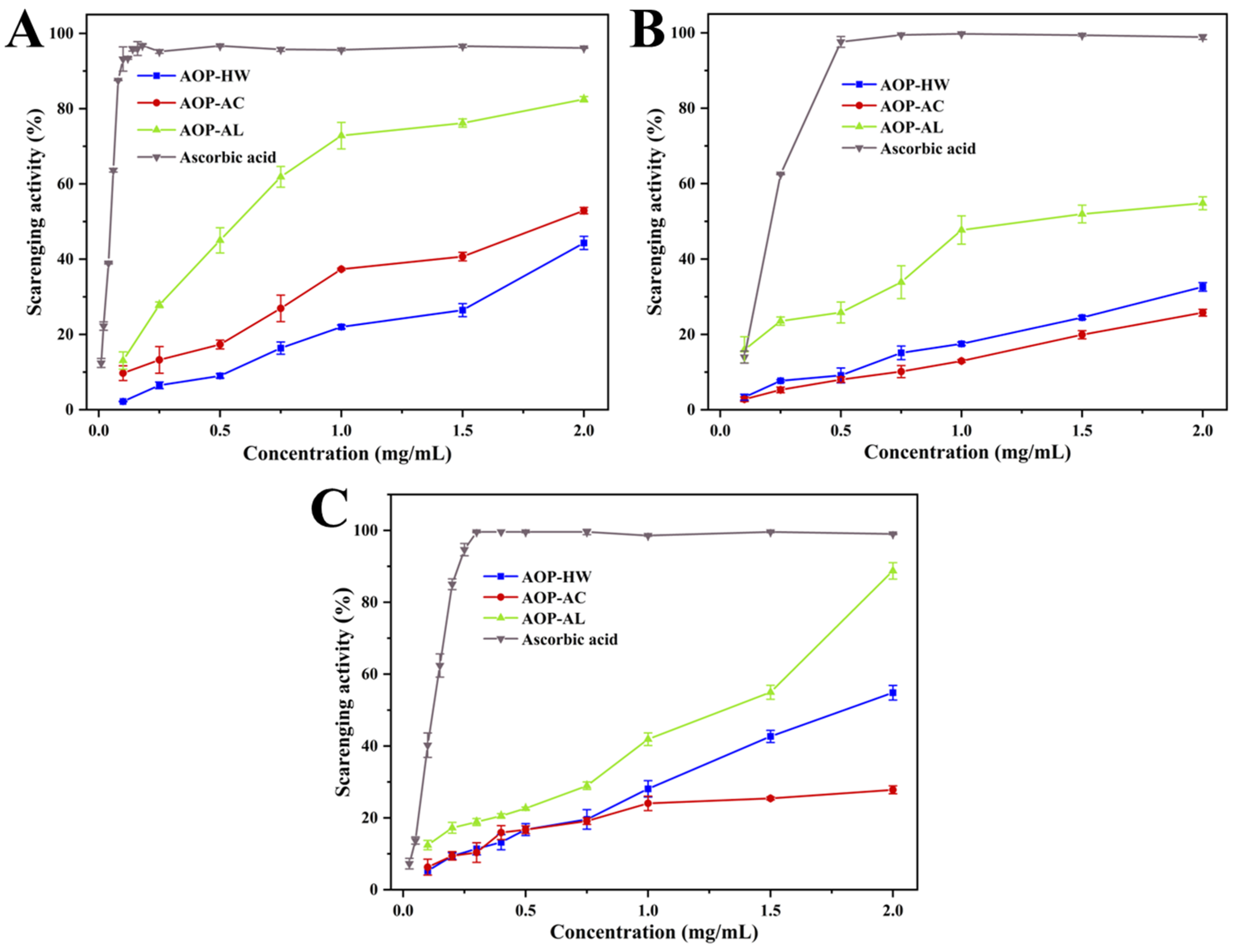
2.4.1. DPPH Radical Scavenging Activity
2.4.2. Hydroxyl Radical Scavenging Activity
2.4.3. ABTS Radical Scavenging Activity
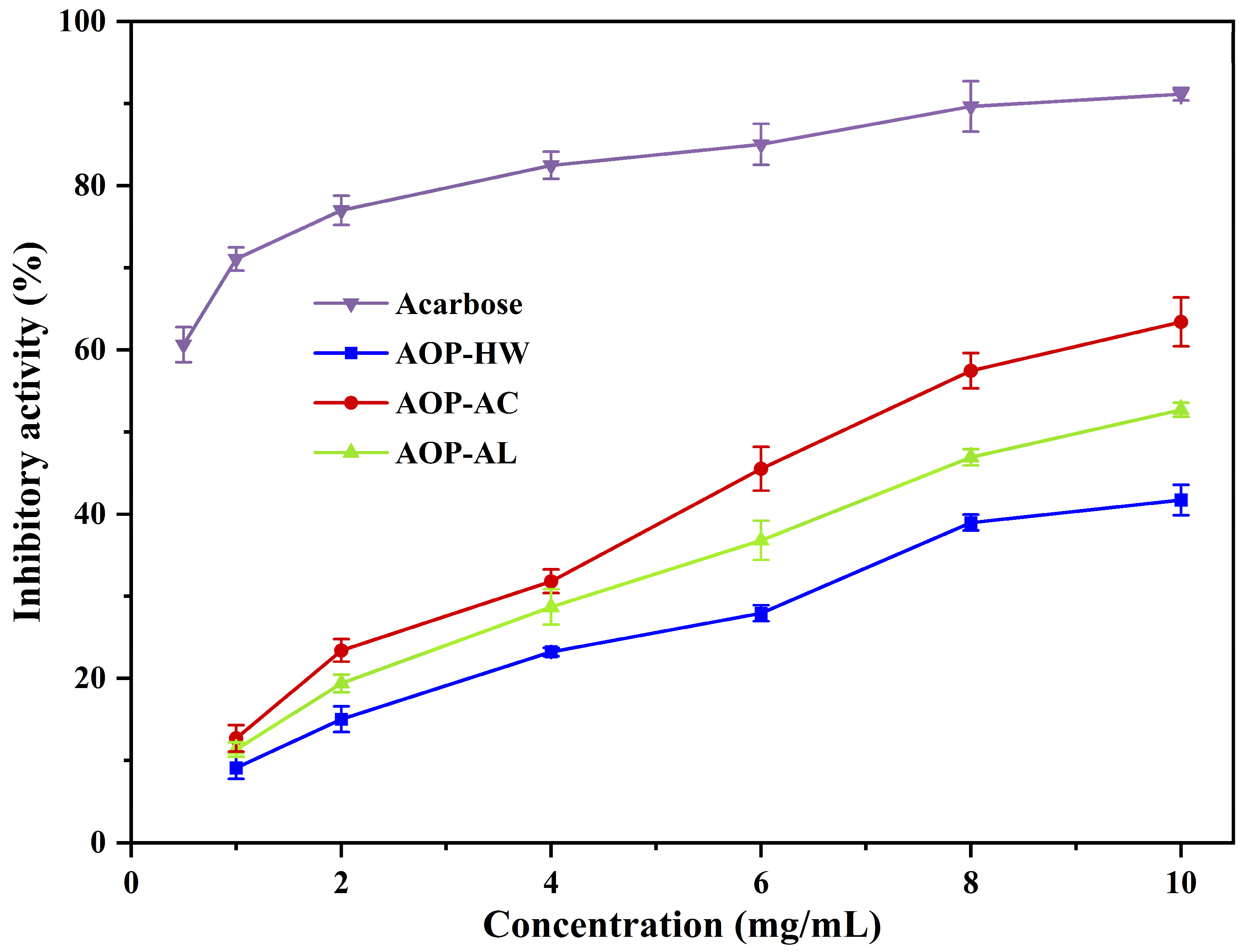
2.5. Inhibitory Effects on α-Amylase Activity
3. Materials and Methods
3.1. Materials and Reagents
3.2. Polysaccharide Extraction and Isolation
3.3. Determination of Chemical Composition
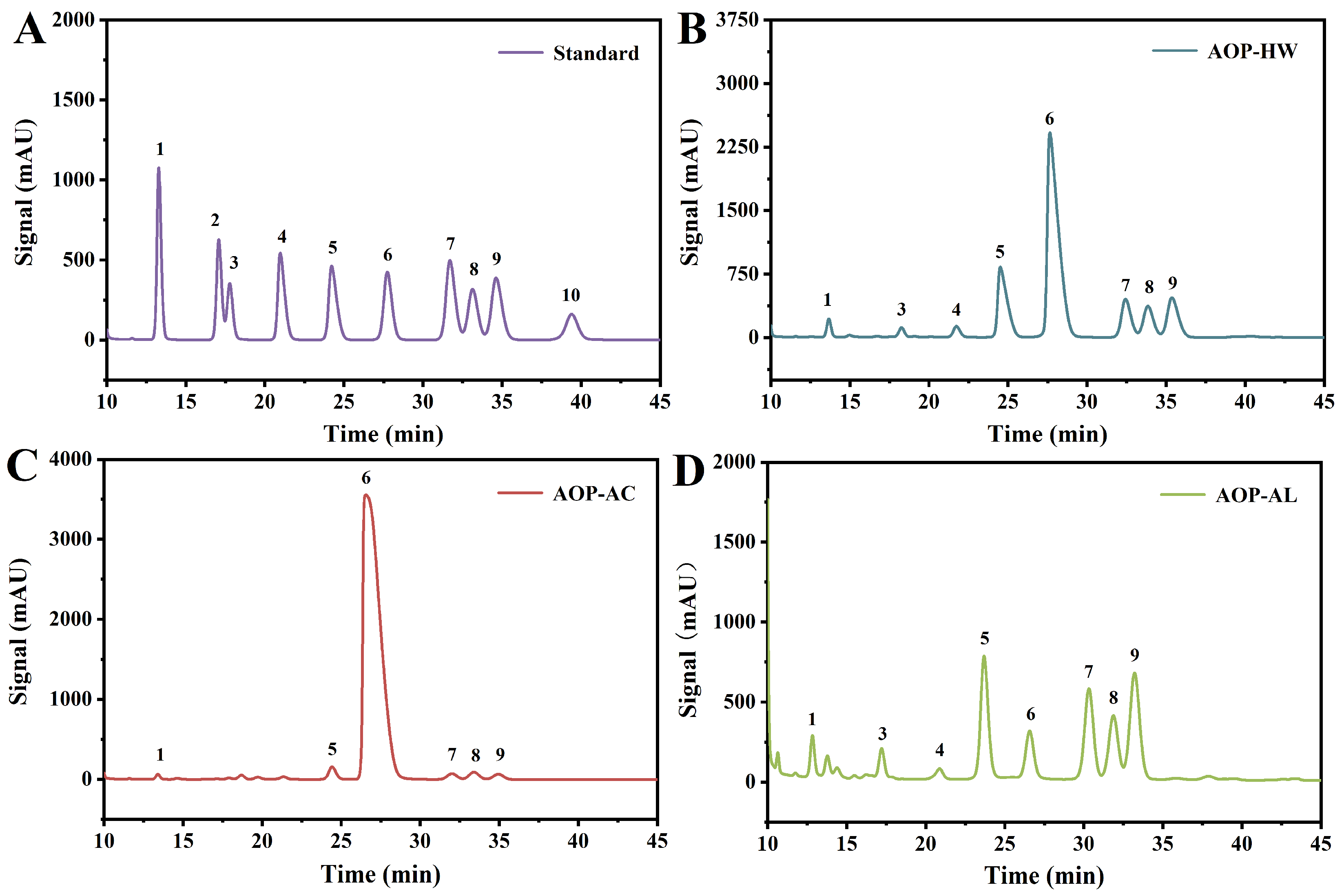
3.4. Monosaccharide Composition Analysis
3.5. Determination of Mw
3.6. FT-IR Spectroscopy
3.7. Scanning Electron Microscopy Analysis (SEM)
3.8. X-ray Diffraction Analysis (XRD)
3.9. Antioxidant Qctivities
3.9.1. DPPH Radical Scavenging Activity
3.9.2. Hydroxyl Radical Scavenging Activity
3.9.3. ABTS Radical Scavenging Activity
3.10. α-Amylase Inhibitory Activity
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, Y.; Wu, J.; Liu, Y.; Qi, J.; Wang, C.; Yu, T.; Xia, Y.; Li, H. Therapeutic effect and mechanism of polysaccharide from Alpiniae oxyphyllae fructus on urinary incontinence. Int. J. Biol. Macromol. 2019, 128, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, L.; Zhang, Y.; Zhang, Z.; Li, H.; Fan, F.; He, J.; Kang, J.; Zuo, L. Integrated untargeted and targeted metabolomics to reveal therapeutic effect and mechanism of Alpiniae oxyphyllae fructus on Alzheimer’s disease in APP/PS1 mice. Front. Pharmacol. 2023, 13, 1104954. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Lin, C.Y.; Chung, Y.P.; Liu, C.H.; Huang, K.T.; Guan, S.S.; Wu, C.T.; Liu, S.H. Protective effects of nootkatone on renal inflammation, apoptosis, and fibrosis in a unilateral ureteral obstructive mouse model. Nutrients 2021, 13, 3921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, Y.; Hu, X.; Hu, X.; Lv, W.; Lv, D.; Chen, J.; Wu, M.; Song, Q.; Shentu, J. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Alpinia oxyphylla Miguel: A review. J. Ethnopharmacol. 2018, 224, 149–168. [Google Scholar] [CrossRef]
- Shi, W.; Zhong, J.; Zhang, Q.; Yan, C. Structural characterization and antineuroinflammatory activity of a novel heteropolysaccharide obtained from the fruits of Alpinia oxyphylla. Carbohyd Polym. 2020, 229, 115405. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.; Chen, H.; Xu, T.; Li, C.; Zhou, R.; Gao, L.; Han, M.; He, X.; Chen, Y. Extraction, isolation, immunoregulatory activity, and characterization of Alpiniae oxyphyllae fructus polysaccharides. Int. J. Biol. Macromol. 2020, 155, 927–937. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, C.; Chen, Y.; Chen, H.; Yang, Y. Alpiniae oxyphyllae fructus polysaccharide 3 inhibits porcine epidemic diarrhea virus entry into IPEC-J2 cells. Res. Vet. Sci. 2022, 152, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Luo, Q.; Zhu, Y.; Du, H.; Liao, S.; Yang, Y.; Chen, H. Inhibition of porcine epidemic diarrhea virus by Alpiniae oxyphyllae fructus polysaccharide 3. Res. Vet. Sci. 2021, 141, 146–155. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, S.; Li, H.; An, J.; Li, C.; Zhou, R.; Teng, L.; Zhu, Y.; Liao, S.; Yang, Y.; et al. Structural characterization of Alpiniae oxyphyllae fructus polysaccharide 2 and its activation effects on RAW264.7 macrophages. Int. Immunopharmacol. 2021, 97, 107708. [Google Scholar] [CrossRef]
- Bian, Y.; Lei, C.; Li, N.; Xu, H.; Gong, W.; Gao, M.; Hu, Q.; Jia, L. Chemical characterization and bioactivity analysis of polysaccharides extracted from Ginkgo Folium residue with different solvents. Chromatographia 2023, 86, 375–385. [Google Scholar] [CrossRef]
- Dou, Z.M.; Chen, C.; Huang, Q.; Fu, X. Comparative study on the effect of extraction solvent on the physicochemical properties and bioactivity of blackberry fruit polysaccharides. Int. J. Biol. Macromol. 2021, 183, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, L.; Deng, D.; Tan, Y.; Wu, Y.; Ming, G.; Zhang, Q.; He, X.; Li, Y.; Wang, K. Protective effect of Alpinia oxyphylla fructus polysaccharides extracted with different solvents on dextran sulfate sodium-induced ulcerative colitis in mice. J. Food Biochem. 2023, 217, 1–19. [Google Scholar] [CrossRef]
- Fang, C.; Chen, G.; Kan, J. Comparison on characterization and biological activities of Mentha haplocalyx polysaccharides at different solvent extractions. Int. J. Biol. Macromol. 2020, 154, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Pei, J.J.; Ma, H.L.; Cai, P.F.; Yan, J.K. Effect of extraction media on preliminary characterizations and antioxidant activities of Phellinus linteus polysaccharides. Carbohyd Polym. 2014, 109, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Sun, Y.; Qi, Y.; Xu, J.; Sun, J.; Bai, Y.; Han, L.; Han, R.; Hou, L.; Sun, H. Structural characterization and induction of tumor cell apoptosis of polysaccharide from purple sweet potato (Ipomoea batatas (L.) Lam). Int. J. Biol. Macromol. 2023, 235, 123799. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cai, Y.; Zhao, Z.; Yang, D.; Xu, X. Optimization of extraction process, preliminary characterization and safety study of crude polysaccharides from Morindae Officinalis Radix. Foods 2023, 12, 1590. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lin, L.; Sun, B.; Zhao, M. A comparison study on polysaccharides extracted from Laminaria japonica using different methods: Structural characterization and bile acid-binding capacity. Food Funct. 2017, 8, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Campestrini, L.H.; Rasera, G.B.; de Camargo, A.C.; Franchin, M.; Nani, B.D.; Rosalen, P.L.; Canniatti-Brazaca, S.G.; Telles Biasoto, A.C.; Shahidi, F.; Alencar, S.M. Alkaline conditions better extract anti-inflammatory polysaccharides from winemaking by-products. Food Res. Int. 2020, 131, 108532. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Dai, B.; An, Y.; Yu, L. Structural, thermal, and anti-inflammatory properties of a novel pectic polysaccharide from Alfalfa (Medicago sativa L.) Stem. J. Agric. Food Chem. 2015, 63, 3219–3228. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Zhang, F.; Yang, X.; Ni, L.; Zhang, W.; Liu, Z.; Zhang, Y. Improving viscosity and gelling properties of leaf pectin by comparing five pectin extraction methods using green tea leaf as a model material. Food Hydrocoll. 2020, 98, 105246. [Google Scholar] [CrossRef]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Wanlapa, S. Characteristics and antioxidant of Ulva intestinalis sulphated polysaccharides extracted with different solvents. Int. J. Biol. Macromol. 2015, 81, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hua, X.; Wang, M.; Zhang, W.; Yang, R. Effect of extraction pH on the yield and physicochemical properties of polysaccharides extracts from peanut sediment of aqueous extraction process. LWT-Food Sci. Technol. 2019, 106, 137–144. [Google Scholar] [CrossRef]
- Liao, D.W.; Cheng, C.; Liu, J.P.; Zhao, L.Y.; Huang, D.C.; Chen, G.T. Characterization and antitumor activities of polysaccharides obtained from ginger (Zingiber officinale) by different extraction methods. Int. J. Biol. Macromol. 2020, 152, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ji, H.; Feng, Y.; Yu, J.; Liu, A. Structural characterization and antitumor activity of polysaccharides from Kaempferia galanga L. Oxid. Med. Cell Longev. 2018, 2018, 9579262. [Google Scholar] [CrossRef]
- Qiu, S.; Yadav, P.M.; Chau, K.H.; Yin, L.J. Physicochemical characterization and rheological behavior of hemicelluloses isolated from sorghum bran, sorghum bagasse and sorghum biomass. Food Hydrocoll. 2020, 100, 105382. [Google Scholar] [CrossRef]
- Yin, J.; Chen, H.; Lin, H.; Xie, M.; Nie, S. Structural features of alkaline extracted polysaccharide from the seeds of Plantago asiatica L. and its rheological properties. Molecules 2016, 21, 1181. [Google Scholar] [CrossRef]
- Bai, L.; Zhu, P.; Wang, W.; Wang, M. The influence of extraction pH on the chemical compositions, macromolecular characteristics, and rheological properties of polysaccharide: The case of okra polysaccharide. Food Hydrocoll. 2020, 102, 105586. [Google Scholar] [CrossRef]
- Peng, Z.; Tian, S.; Li, H.; Zhu, L.; Zhao, Z.; Zheng, G.; Wen, Q.; Tian, H.; Yang, D. Extraction, characterization, and antioxidant properties of cell wall polysaccharides from the pericarp of Citrus Reticulata cv. Chachiensis. Food Hydrocoll. 2023, 136, 108237. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, W.; Tang, T.; Chen, H.; Zhou, X. Structural characteristics, antioxidant and hypoglycemic activities of polysaccharides from Mori Fructus based on different extraction methods. Front. Nutr. 2023, 10, 1125831. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, R.; Cao, S.; Ismael, M.; Wang, X.; Lue, X. A galacturonic acid-rich polysaccharide from Diospyros kaki peel: Isolation, characterization, rheological properties and antioxidant activities in vitro. Food Chem. 2023, 416, 135781. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Ding, Z.C.; Gao, X.; Wang, Y.Y.; Yang, Y.; Wu, D.; Zhang, H.N. Comparative study of physicochemical properties and bioactivity of Hericium erinaceus polysaccharides at different solvent extractions. Carbohyd Polym. 2018, 193, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, W.; Wen, P.; Shen, M.; Li, H.; Ren, Y.; Xiao, Y.; Song, Q.; Chen, Y.; Yu, Q.; et al. Two water-soluble polysaccharides from mung bean skin: Physicochemical characterization, antioxidant and antibacterial activities. Food Hydrocoll. 2020, 100, 105412. [Google Scholar] [CrossRef]
- Li, X.; Wang, L. Effect of extraction method on structure and antioxidant activity of Hohenbuehelia serotina polysaccharides. Int. J. Biol. Macromol. 2016, 83, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Z.; Ma, M.G.; Ji, X.X.; Choi, S.E.; Si, C. Recent developments and applications of hemicellulose from wheat straw: A review. Front. Bioeng. Biotech. 2021, 9, 690773. [Google Scholar] [CrossRef] [PubMed]
- Abuduaini, M.; Li, J.; Ruan, J.H.; Zhao, Y.X.; Maitinuer, M.; Aisa, H.A. Bioassay-guided preparation of antioxidant, anti-inflammatory active fraction from crabapples (Malus prunifolia (Willd.) Borkh.). Food Chem. 2023, 406, 135091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, J.; Shang, H.; Guo, Y.; Chen, S. Extraction, purification, hypoglycemic and antioxidant activities of red clover (Trifolium pratense L.) polysaccharides. Int. J. Biol. Macromol. 2020, 148, 750–760. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y.H. Ultrasound-assisted polysaccharide extraction from Cercis chinensis and properites, antioxidant activity of polysaccharide. Ultrason. Sonochem. 2023, 96, 106422. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Wang, Z.; Kan, J. A comparison of a polysaccharide extracted from ginger (Zingiber officinale) stems and leaves using different methods: Preparation, structure characteristics, and biological activities. Int. J. Biol. Macromol. 2020, 151, 635–649. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Zhang, C.; Kang, W. Physicochemical properties and biological activities of Tremella hydrocolloids. Food Chem. 2023, 407, 135164. [Google Scholar] [CrossRef]
- Amamou, S.; Lazreg, H.; Hafsa, J.; Majdoub, H.; Rihouey, C.; Le Cerf, D.; Achour, L. Effect of extraction condition on the antioxidant, antiglycation and α-amylase inhibitory activities of Opuntia macrorhiza fruit peels polysaccharides. LWT-Food Sci. Technol. 2020, 127, 109411. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, D.D.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. 2019, 59, S96–S115. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zou, S.; Liang, D.; Luan, L. Structural characterization, antioxidant and hepatoprotective activities of polysaccharides from Sophorae tonkinensis Radix. Carbohyd Polym. 2018, 184, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wu, Z.; Zhao, T.; Sun, Y.; Ye, H.; Xu, R.; Zeng, X. Characterization, antioxidant and hepatoprotective activities of polysaccharides from Ilex latifolia Thunb. Carbohyd Polym. 2014, 101, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Shang, H.; Chen, S.; Li, R.; Wu, H. Physicochemical properties and activities of comfrey polysaccharides extracted by different techniques. Int. J. Biol. Macromol. 2018, 115, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shang, H.; Yang, J.; Li, R.; Wu, H. Effects of different extraction techniques on physicochemical properties and activities of polysaccharides from comfrey (Symphytum officinale L.) root. Ind. Crop Prod. 2018, 121, 18–25. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Rahman, A.; Li, J.H.; Wei, C.Y.; Chen, J.L.; Linhardt, R.J.; Ye, X.Q.; Chen, S.G. Extraction methods affect the structure of Goji (Lycium barbarum) polysaccharides. Molecules 2020, 25, 936. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.K.; Smith, F. Reduction of the products of periodate oxidation of carbohydrates. III. The constitution of amylopectin1. J. Am. Chem. Soc. 1956, 78, 5910–5912. [Google Scholar] [CrossRef]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef]
- Compton, S.J.; Jones, C.G. Mechanism of dye response and interference in the bradford protein assay. Anal. Biochem. 1985, 151, 369–374. [Google Scholar] [CrossRef] [PubMed]
- von Hunolstein, C.; Parisi, L.; Bottaro, D. Simple and rapid technique for monitoring the quality of meningococcal polysaccharides by high performance size-exclusion chromatography. J. Biochem. Biophys. Methods. 2003, 56, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Niu, X.; Dai, T.; Hua, H.; Feng, S.; Liu, C.; McClements, D.J.; Liang, R. Amino acid-amidated pectin: Preparation and characterization. Food Chem. 2020, 309, 125768. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.Y.; Chen, W.; Zhang, W.M.; Zhang, H. Adulteration identification of some fungal polysaccharides with SEM, XRD, IR and optical rotation: A primary approach. Carbohyd Polym. 2009, 78, 620–625. [Google Scholar] [CrossRef]

| Sample | Yield (%) | Neutral Sugar (%) | Uronic Acid (%) | Protein (%) | Mw (kDa) |
|---|---|---|---|---|---|
| AOP-HW | 4.54 ± 0.46 c | 69.62 ± 2.11 b | 19.51 ± 1.21 b | 1.12 ± 0.95 b | 385.42 ± 5.64 a |
| AOP-AC | 13.76 ± 0.29 a | 80.57 ± 0.72 a | 13.15 ± 1.10 c | 1.24 ± 0.76 b | 121.28 ± 5.18 c |
| AOP-AL | 8.88 ± 0.21 b | 28.28 ± 1.14 c | 63.13 ± 2.58 a | 3.37 ± 1.30 a | 232.40 ± 3.93 b |
| Samples | Man | Rha | GlcA | GalA | Glc | Gal | Xyl | Ara |
|---|---|---|---|---|---|---|---|---|
| AOP-HW | 1.52 ± 0.05 b | 2.59 ± 0.03 b | 1.65 ± 0.04 b | 13.54 ± 0.13 b | 52.31 ± 0.44 b | 6.86 ± 0.10 b | 10.08 ± 0.06 a | 10.81 ± 0.16 b |
| AOP-AC | 0.30 ± 0.01 c | N.D. | N.D. | 1.42 ± 0.04 c | 94.77 ± 0.36 a | 0.78 ± 0.06 c | 1.74 ± 0.02 b | 1.00 ± 0.04 c |
| AOP-AL | 3.45 ± 0.11 a | 9.84 ± 0.23 a | 3.24 ± 0.02 a | 17.09 ± 0.21 a | 10.33 ± 0.19 c | 17.64 ± 0.14 a | 10.00 ± 0.07 a | 28.42 ± 0.21 a |
| Samples | IC50 (mg/mL) | ||
|---|---|---|---|
| DPPH Radical | Hydroxyl Radical | ABTS Radical | |
| AOP-HW | 2.16 ± 0.06 a | 2.29 ± 0.14 b | 2.10 ± 0.07 b |
| AOP-AC | 1.74 ± 0.08 b | 2.79 ± 0.12 a | 3.63 ± 0.10 a |
| AOP-AL | 0.56 ± 0.04 c | 1.07 ± 0.06 c | 1.40 ± 0.08 c |
| Ascorbic acid | 0.05 ± 0.00 d | 0.32 ± 0.02 d | 0.12 ± 0.03 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Ruan, X.; Chen, J.; Deng, L.; Zhou, W.; Shuai, X.; Liang, R.; Dai, T. Physicochemical Characterization and Biological Properties of Polysaccharides from Alpiniae oxyphyllae Fructus. Polymers 2024, 16, 1705. https://doi.org/10.3390/polym16121705
Wang R, Ruan X, Chen J, Deng L, Zhou W, Shuai X, Liang R, Dai T. Physicochemical Characterization and Biological Properties of Polysaccharides from Alpiniae oxyphyllae Fructus. Polymers. 2024; 16(12):1705. https://doi.org/10.3390/polym16121705
Chicago/Turabian StyleWang, Risi, Xinmei Ruan, Jun Chen, Lizhen Deng, Wei Zhou, Xixiang Shuai, Ruihong Liang, and Taotao Dai. 2024. "Physicochemical Characterization and Biological Properties of Polysaccharides from Alpiniae oxyphyllae Fructus" Polymers 16, no. 12: 1705. https://doi.org/10.3390/polym16121705
APA StyleWang, R., Ruan, X., Chen, J., Deng, L., Zhou, W., Shuai, X., Liang, R., & Dai, T. (2024). Physicochemical Characterization and Biological Properties of Polysaccharides from Alpiniae oxyphyllae Fructus. Polymers, 16(12), 1705. https://doi.org/10.3390/polym16121705








