Composite Material Based on Polypropylene and Modified Natural Fillers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation or Modification of the Composition
2.3. Measurements
2.3.1. Determination of Physical and Mechanical Properties
2.3.2. Determination of Impact Strength
2.3.3. Determination of Melt Flow Index
2.3.4. Determination of Water Absorption
- m2 is the mass of the specimen after soaking in water, gram (g).
- m1 is the mass of the specimen after initial drying and before immersion into water, gram (g).
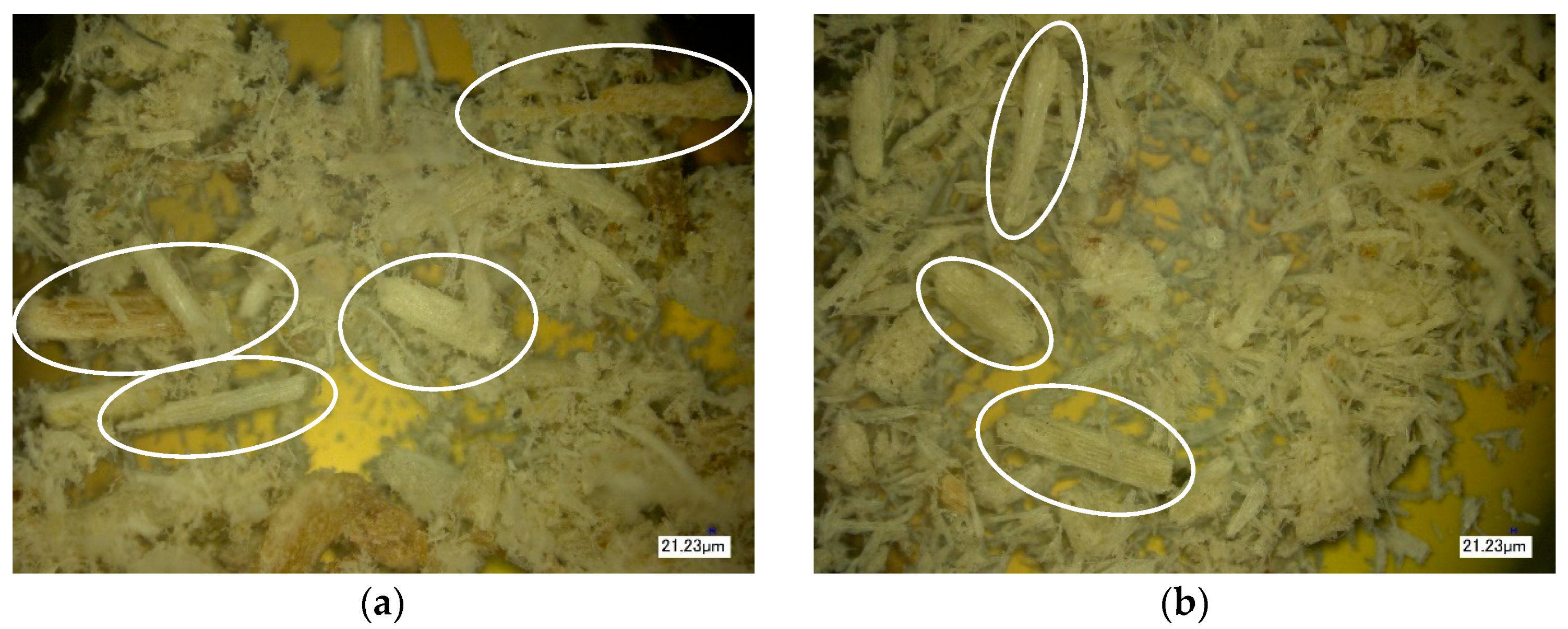
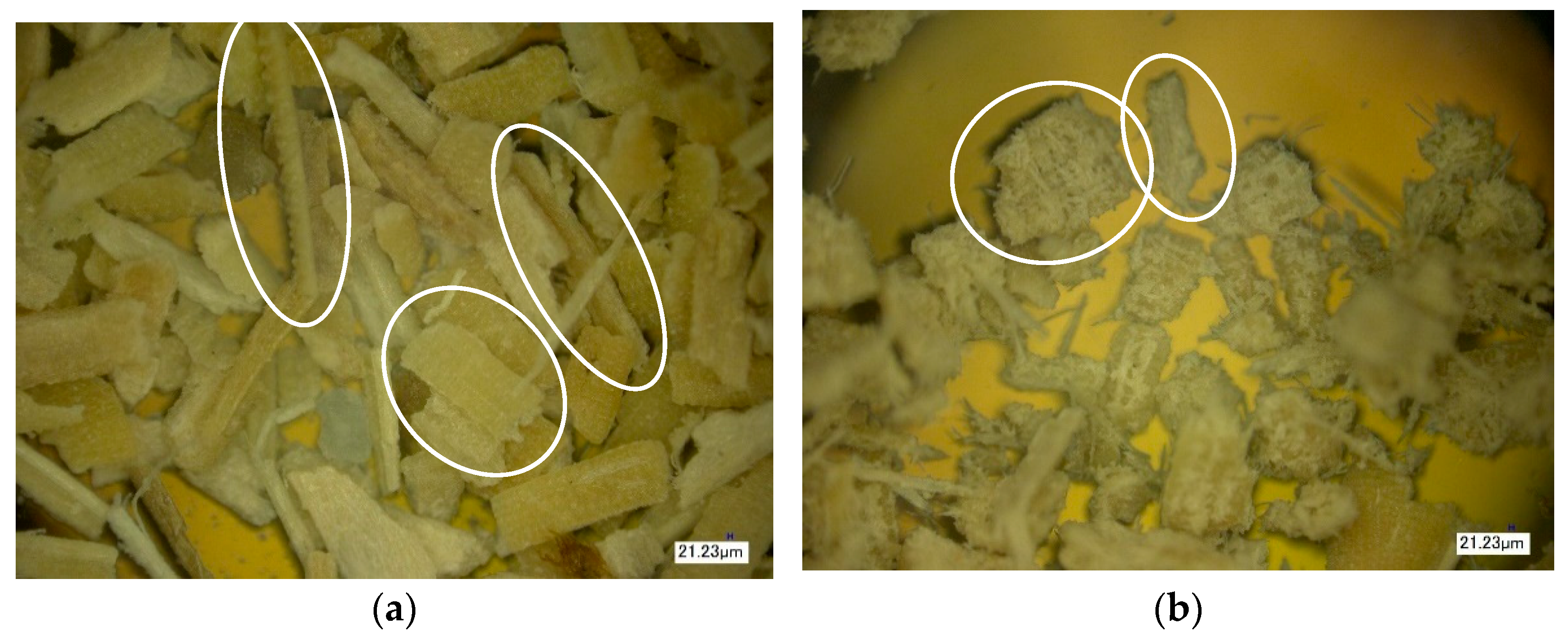
2.3.5. Optical (Light) Microscopy
2.3.6. Determination of Free Surface Energy
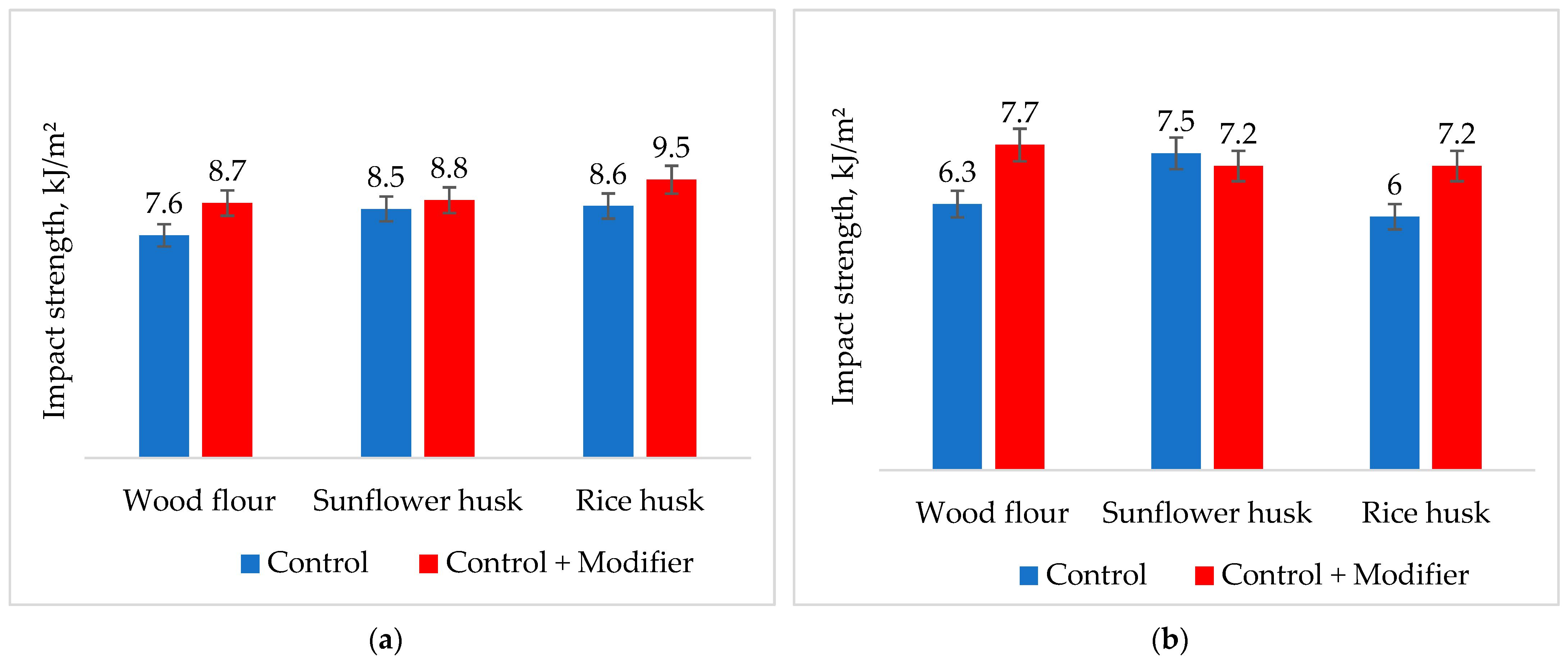
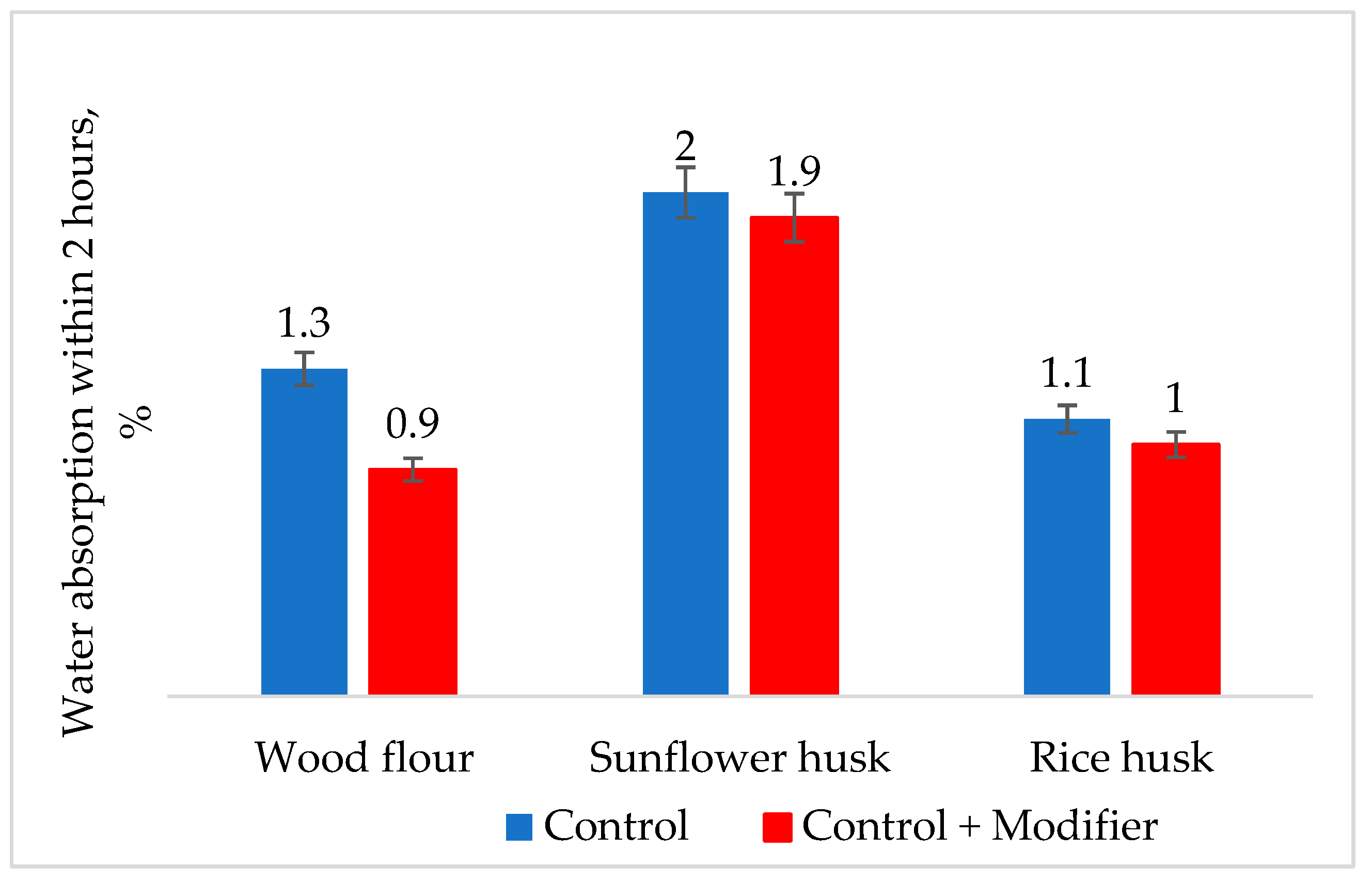
3. Results of the Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gorbachev, A.V.; Fayzullin, I.Z.; Wolfson, S.I.; Kanarsky, A.V.; Zakharov, I.V.; Kazakov, Y.M. Composite Material Based on Polyolefins and Modified Vegetable Fillers. Plast. Massy 2023, 1, 48–52. [Google Scholar] [CrossRef]
- Klyosov, A.A. Improving Wood–Polymer Composite Products: A Case Study. In Wood–Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2008; pp. 331–353. [Google Scholar]
- Shabarin, A.A.; Kuzmin, A.M.; Vodyakov, V.N.; Shabarin, I.A. Obtaining Biodegradable Composite Materials Based on Polyolefins and Husk of Sunflower Seeds. Izv. Vyss. Uchebnykh Zaved. Khimiya Khimicheskaya Tekhnologiya 2021, 64, 73–78. [Google Scholar] [CrossRef]
- Mekhonoshina, M.S. Research of the Dynamics of Ecological Indicators in Large Cities of the Russian Federation. Chem. Ecol. Urban Stud. 2021, 1, 53–57. [Google Scholar]
- Volfson, S.I.; Fayzullin, I.Z.; Musin, I.N.; Fayzullin, A.Z.; Grachev, A.N.; Pushkin, S.A. The Physicomechanical and Rheological Characteristics of Wood–Polymer Composites Based on Thermally and Mechanically Modified Filler. Int. Polym. Sci. Technol. 2017, 44, 49–54. [Google Scholar] [CrossRef]
- Žiganova, M.; Merijs-Meri, R.; Zicāns, J.; Ivanova, T.; Bochkov, I.; Kalniņš, M.; Błędzki, A.K.; Danilovas, P.P. Characterisation of Nanoclay and Spelt Husk Microfiller-Modified Polypropylene Composites. Polymers 2022, 14, 4332. [Google Scholar] [CrossRef]
- Fayzullin, I.Z.; Musin, I.N.; Volfson, S.I.; Nikiforov, A.A. Glass-Filled Wood-Polymer Composites Based on Polypropylene. Key Eng. Mater. 2019, 816, 197–201. [Google Scholar] [CrossRef]
- Fayzullin, I.Z.; Volfson, S.I.; Musin, I.N.; Fayzullin, A.Z.; Nikiforov, A.A. Influence of the Type of Wood Flour and Nanoadditives on the Structure and Mechanical Properties of Polypropylene-Based Wood-Polymer Composites; AIP Publishing LLC: Long Island, NY, USA, 2016; p. 040098. [Google Scholar]
- Wang, W.; Chen, H.; Li, J. Effects of Maleic Anhydride Grafted Polypropylene on the Physical, Mechanical and Flammability Properties of Wood-Flour/Polypropylene/Ammonium Polyphosphate Composites. Fibers Polym. 2021, 22, 1137–1144. [Google Scholar] [CrossRef]
- Hao, X.; Xu, J.; Zhou, H.; Tang, W.; Li, W.; Wang, Q.; Ou, R. Interfacial Adhesion Mechanisms of Ultra-Highly Filled Wood Fiber/Polyethylene Composites Using Maleic Anhydride Grafted Polyethylene as a Compatibilizer. Mater. Des. 2021, 212, 110182. [Google Scholar] [CrossRef]
- Rindayatno; Davidson, J.; Dayadi, I. Improved Quality of Wood Plastic Composite (WPC) Through the Addition of Maleic Anhydride (MAH). IOP Conf. Ser. Earth Environ. Sci. 2023, 1282, 012034. [Google Scholar] [CrossRef]
- Narimisa, M.; Ghobeira, R.; Onyshchenko, Y.; De Geyter, N.; Egghe, T.; Morent, R. Different Techniques Used for Plasma Modification of Polyolefin Surfaces. In Plasma Modification of Polyolefins: Synthesis, Characterization and Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 15–56. [Google Scholar]
- Johnsen, K.; Kirkhorn, S.; Olafsen, K.; Redford, K.; Stori, A. Modification of Polyolefin Surfaces by Plasma-Induced Grafting. J. Appl. Polym. Sci. 1996, 59, 1651–1657. [Google Scholar] [CrossRef]
- Farris, S.; Pozzoli, S.; Biagioni, P.; Duó, L.; Mancinelli, S.; Piergiovanni, L. The Fundamentals of Flame Treatment for the Surface Activation of Polyolefin Polymers—A Review. Polymer 2010, 51, 3591–3605. [Google Scholar] [CrossRef]
- Nosova, N.; Roiter, Y.; Samaryk, V.; Varvarenko, S.; Stetsyshyn, Y.; Minko, S.; Stamm, M.; Voronov, S. Polypropylene Surface Peroxidation with Heterofunctional Polyperoxides. Macromol. Symp. 2004, 210, 339–348. [Google Scholar] [CrossRef]
- Shkuro, A.; Chernysheva, A.; Krivonogov, P.; Artemov, A. Studying the Modificability of Wood-Polymer Composites by UV Radiation. Bull. Univ. Technol. 2019, 22, 84–87. [Google Scholar]
- Shpeizman, V.V.; Yakushev, P.N.; Smolyansky, A.S. Method for Radiation-Chemical Modification of Wood-Polymer Composites. RU2707936C1, 2 December 2019. Available online: https://patents.google.com/patent/RU2707936C1/en (accessed on 2 June 2024).
- Gorbachev, A.V.; Faizullin, I.Z.; Wolfson, S.I.; Kazakov, Y.M. Lignocellulose Fillers and Methods of Their Modification. Her. Technol. Univ. 2022, 25, 148–157. [Google Scholar] [CrossRef]
- Musa, O.M. (Ed.) Handbook of Maleic Anhydride Based Materials; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-29453-7. [Google Scholar]
- Zakharov, P.S.; Shkuro, A.E.; Glukhikh, V.V.; Kulazhenko, Y.M. Effect of Microcrystalline Cellulose Content in Mixture with Kraft Lignin on Properties of Wood-Polymer Composites; AIP Publishing LLC: Long Island, NY, USA, 2022; p. 020004. [Google Scholar]
- Fabiyi, J.S. Chemistry of Wood Plastic Composite Weathering; University of Idaho: Moscow, ID, USA, 2007. [Google Scholar]
- Shkuro, A.E.; Artyomov, A.V.; Savinovskikh, A.V. Physicochemical WPC Modification Techniques. Key Eng. Mater. 2021, 887, 144–150. [Google Scholar] [CrossRef]
- Kabir, M.M.; Wang, H.; Lau, K.T.; Cardona, F. Chemical Treatments on Plant-Based Natural Fibre Reinforced Polymer Composites: An Overview. Compos. Part B Eng. 2012, 43, 2883–2892. [Google Scholar] [CrossRef]
- Olkhov, A.A.; Vlasov, S.V.; Zaikov, G.E. Environmental Problems of Recycling Packaging Made of Polymeric Materials. All Materials. Encyclopedic Reference Book 4. 2012, pp. 34–43. Available online: https://elibrary.ru/item.asp?id=17839973 (accessed on 2 June 2024).
- Bugg, T.D.H.; Ahmad, M.; Hardiman, E.M.; Rahmanpour, R. Pathways for Degradation of Lignin in Bacteria and Fungi. Nat. Prod. Rep. 2011, 28, 1883. [Google Scholar] [CrossRef]
- Cragg, S.M.; Beckham, G.T.; Bruce, N.C.; Bugg, T.D.; Distel, D.L.; Dupree, P.; Etxabe, A.G.; Goodell, B.S.; Jellison, J.; McGeehan, J.E.; et al. Lignocellulose Degradation Mechanisms across the Tree of Life. Curr. Opin. Chem. Biol. 2015, 29, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.; Casal, M.; Cavaco-Paulo, A. Application of Enzymes for Textile Fibres Processing. Biocatal. Biotransform. 2008, 26, 332–349. [Google Scholar] [CrossRef]
- Khoshnava, S.M.; Rostami, R.; Ismail, M.; Valipour, A. The Using Fungi Treatment as Green and Environmentally Process for Surface Modification of Natural Fibres. Appl. Mech. Mater. 2014, 554, 116–122. [Google Scholar] [CrossRef]
- Kalia, S.; Thakur, K.; Celli, A.; Kiechel, M.A.; Schauer, C.L. Surface Modification of Plant Fibers Using Environment Friendly Methods for Their Application in Polymer Composites, Textile Industry and Antimicrobial Activities: A Review. J. Environ. Chem. Eng. 2013, 1, 97–112. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Bharadia, P.; Blaker, J.J.; Bismarck, A. Short Sisal Fibre Reinforced Bacterial Cellulose Polylactide Nanocomposites Using Hairy Sisal Fibres as Reinforcement. Compos. Part A Appl. Sci. Manuf. 2012, 43, 2065–2074. [Google Scholar] [CrossRef]
- Karaduman, Y.; Gokcan, D.; Onal, L. Effect of Enzymatic Pretreatment on the Mechanical Properties of Jute Fiber-Reinforced Polyester Composites. J. Compos. Mater. 2013, 47, 1293–1302. [Google Scholar] [CrossRef]
- Zanuso, E.; Gomes, D.G.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Enzyme Immobilization as a Strategy towards Efficient and Sustainable Lignocellulosic Biomass Conversion into Chemicals and Biofuels: Current Status and Perspectives. Sustain. Energy Fuels 2021, 5, 4233–4247. [Google Scholar] [CrossRef]
- López-Otín, C.; Bond, J.S. Proteases: Multifunctional Enzymes in Life and Disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, K.; Noman, A.; Xia, W.; Jiang, Q.; Xu, Y. Influence of the Enzymatic Hydrolysis Using Flavourzyme Enzyme on Functional, Secondary Structure, and Antioxidant Characteristics of Protein Hydrolysates Produced from Bighead Carp (Hypophthalmichthys Nobilis). Molecules 2023, 28, 519. [Google Scholar] [CrossRef] [PubMed]
- Luciński, R.; Adamiec, M. The Role of Plant Proteases in the Response of Plants to Abiotic Stress Factors. Front. Plant Physiol. 2023, 1, 1330216. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An Environmentally Friendly Method for Enzyme-Assisted Preparation of Microfibrillated Cellulose (MFC) Nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- George, M.; Mussone, P.G.; Bressler, D.C. Surface and Thermal Characterization of Natural Fibres Treated with Enzymes. Ind. Crop. Prod. 2014, 53, 365–373. [Google Scholar] [CrossRef]
- Nikitin, N.I. Chemistry of Wood and Cellulose; Publishing House of Academy of Sciences SSSR: Moscow, Russia, 1962. [Google Scholar]
- Rowell, R. The Chemistry of Solid Wood; Rowell, R., Ed.; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1984; Volume 207, ISBN 9780841207967. [Google Scholar]
- Chen, R.S.; Ahmad, S.; Gan, S. Characterization of Rice Husk-Incorporated Recycled Thermoplastic Blend Composites. BioResources 2016, 11, 8470–8482. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal Decomposition Kinetics of Natural Fibers: Activation Energy with Dynamic Thermogravimetric Analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Li, J.; Wang, F.; Chang, G.; Fu, T.; Zhou, W. Effects of Temperature on Cellulose Hydrogen Bonds during Dissolution in Ionic Liquid. Carbohydr. Polym. 2018, 201, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Ipatova, E.; Krutov, S.; Gribkov, I.; Sazanov, I. Solvolysis of Technical Lignin in Water and Alcohol Solutions of Sodium Hydroxide. Bull. High Educ. Inst. Lesn. Zhurnal. 2015, 345, 123–136. [Google Scholar] [CrossRef]
- Lin, B.-J.; Colin, B.; Chen, W.-H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Thermal Degradation and Compositional Changes of Wood Treated in a Semi-Industrial Scale Reactor in Vacuum. J. Anal. Appl. Pyrolysis 2018, 130, 8–18. [Google Scholar] [CrossRef]
- Popa, V.I.; Spiridon, J. Hemicelluloses: Structure and Properties. In Polysaccharides: Structural Diversity and Functional Versatility; Marcel Dekker: New York, NY, USA, 1998; pp. 297–311. [Google Scholar]
- Arjmandi, R.; Hassan, A.; Majeed, K.; Zakaria, Z. Rice Husk Filled Polymer Composites. Int. J. Polym. Sci. 2015, 2015, 501471. [Google Scholar] [CrossRef]
- Zellner, W.; Tubaña, B.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s Role in Plant Stress Reduction and Why This Element Is Not Used Routinely for Managing Plant Health. Plant Dis. 2021, 105, 2033–2049. [Google Scholar] [CrossRef]
- Keskisaari, A.; Butylina, S.; Kärki, T. Use of Construction and Demolition Wastes as Mineral Fillers in Hybrid Wood–Polymer Composites. J. Appl. Polym. Sci. 2016, 133, 43412. [Google Scholar] [CrossRef]
- Liikanen, M.; Grönman, K.; Deviatkin, I.; Havukainen, J.; Hyvärinen, M.; Kärki, T.; Varis, J.; Soukka, R.; Horttanainen, M. Construction and Demolition Waste as a Raw Material for Wood Polymer Composites—Assessment of Environmental Impacts. J. Clean. Prod. 2019, 225, 716–727. [Google Scholar] [CrossRef]
- Shukla, E.; Bendre, A.D.; Gaikwad, S.M. Hydrolases: The Most Diverse Class of Enzymes. In Hydrolases; IntechOpen: London, UK, 2022. [Google Scholar]
- Naguib, H.M.; Taha, E.O.; Ahmed, M.A.; Kandil, U.F. Enhanced Wooden Polymer Composites Based on Polyethylene and Nano-Modified Wooden Flour. Egypt J. Pet. 2022, 31, 39–45. [Google Scholar] [CrossRef]
- Politov, A.; Golyazimova, O. Increasing the Energy Yield of Mechanochemical Transformations: Selected Case Studies. Faraday Dis. 2014, 170, 345–356. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Mittal, K.L. Physicochemical Aspects of Polymer Surfaces; Plenum Pre.: New York, NY, USA, 1983; Volume 2. [Google Scholar]
- Ingle, E.; Lill, J. P138-T Microwave-Assisted Protein Hydrolysis. J. Biomol. Tech. JBT 2007, 18, 48. [Google Scholar]
- Shokri, Z.; Seidi, F.; Saeb, M.R.; Jin, Y.; Li, C.; Xiao, H. Elucidating the Impact of Enzymatic Modifications on the Structure, Properties, and Applications of Cellulose, Chitosan, Starch and Their Derivatives: A Review. Mater. Today Chem. 2022, 24, 100780. [Google Scholar] [CrossRef]
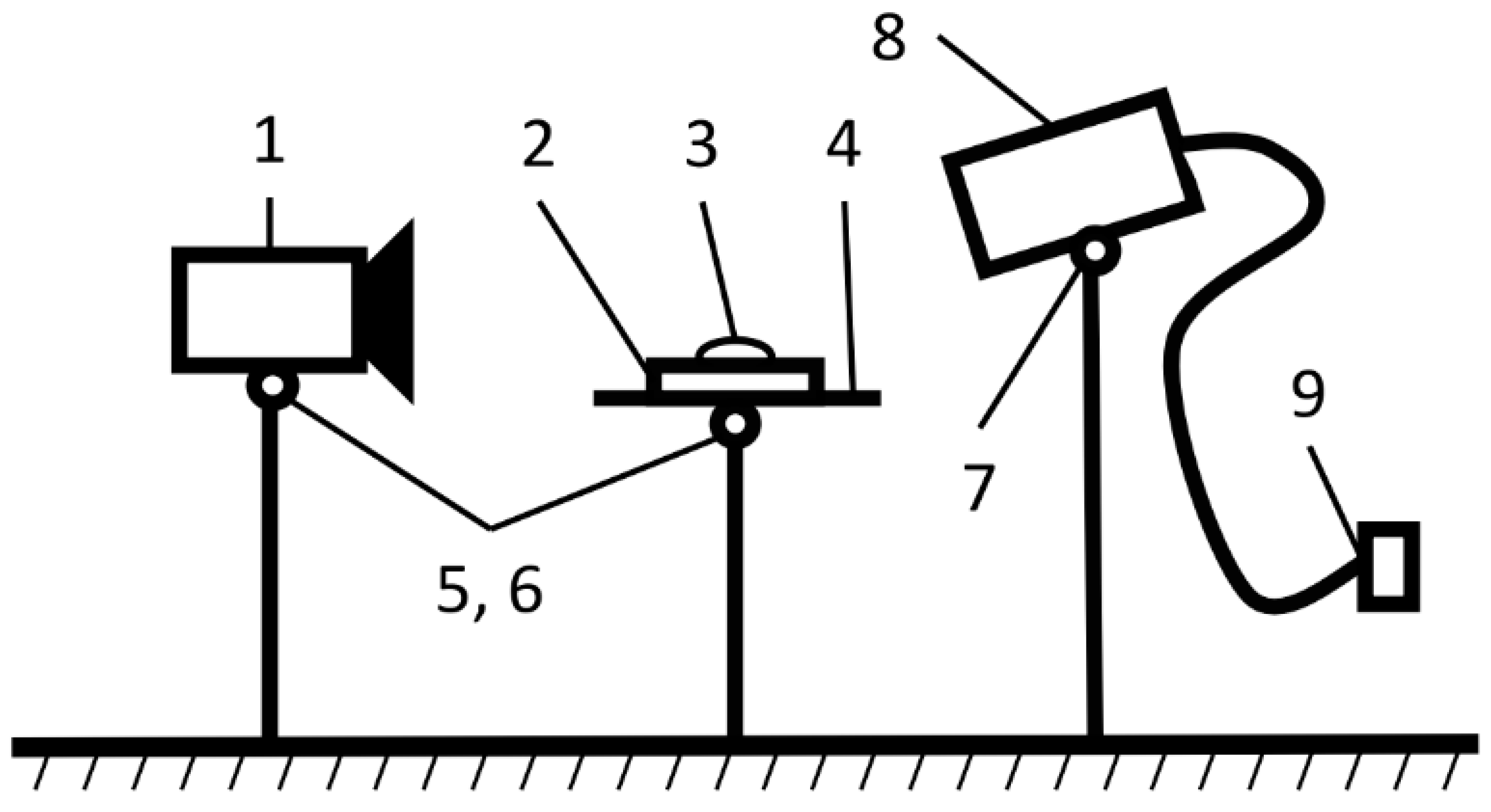

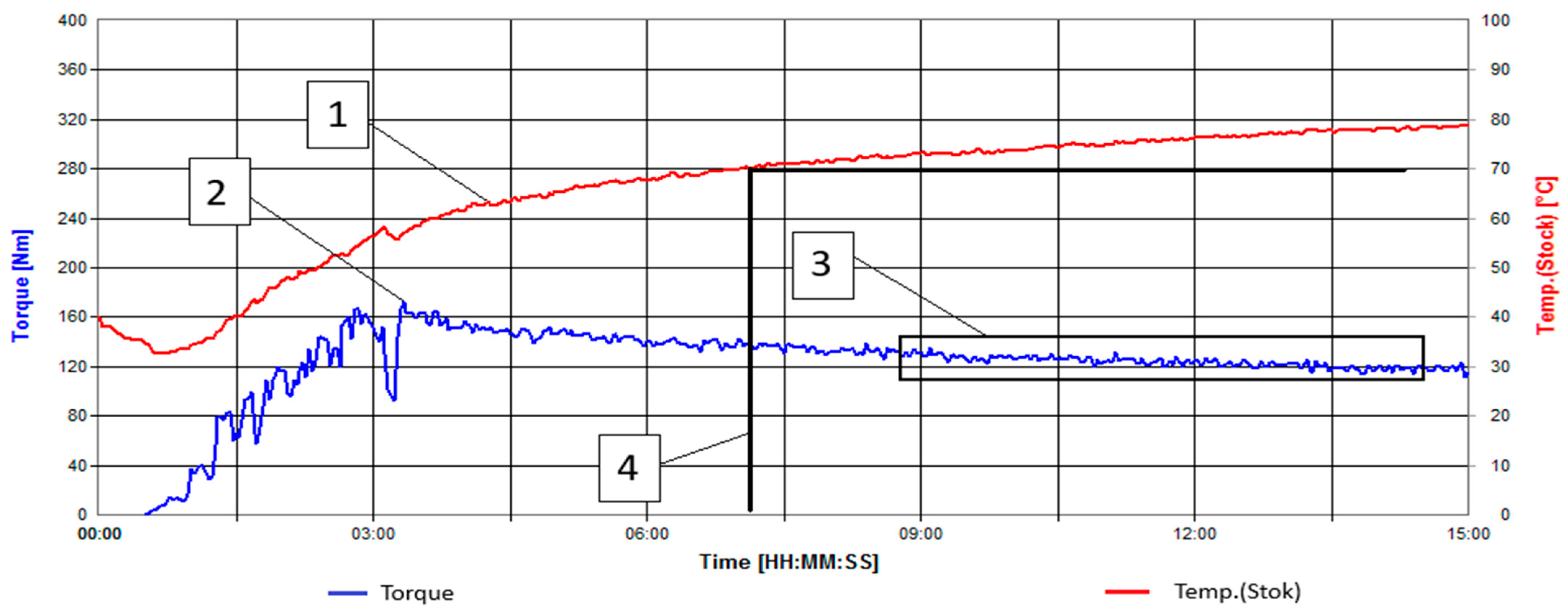
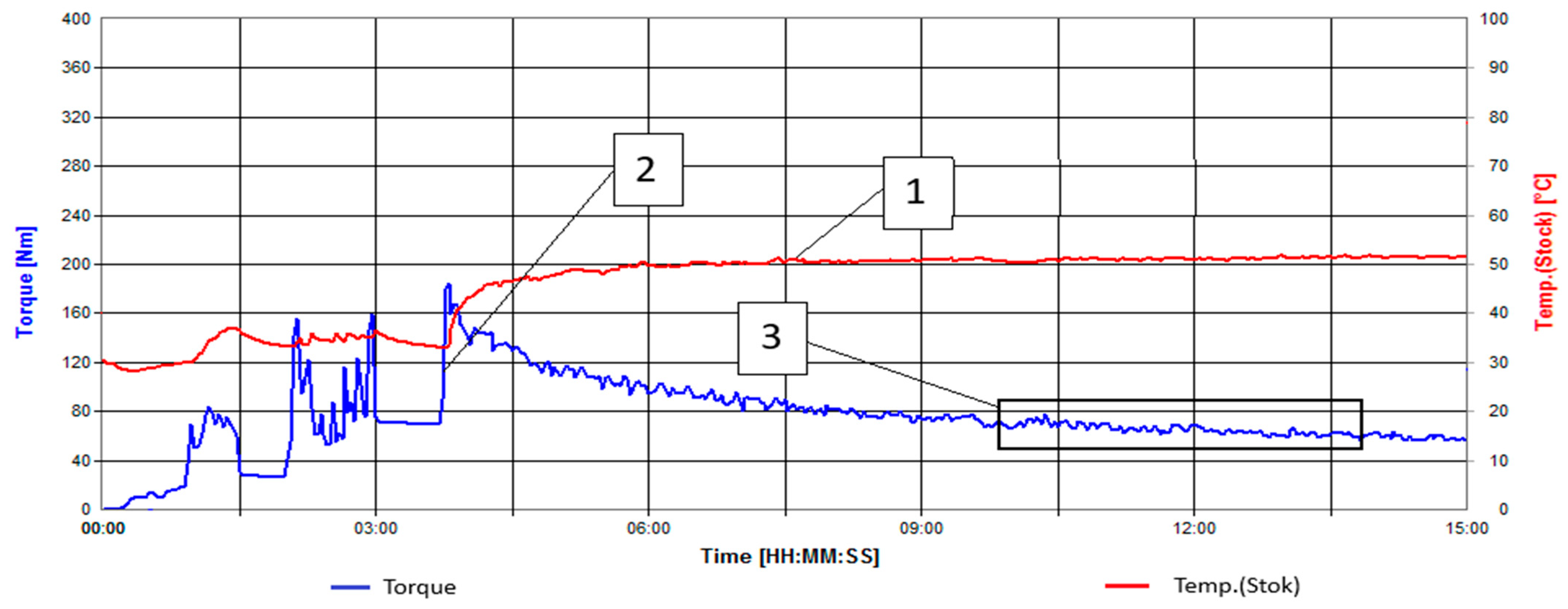
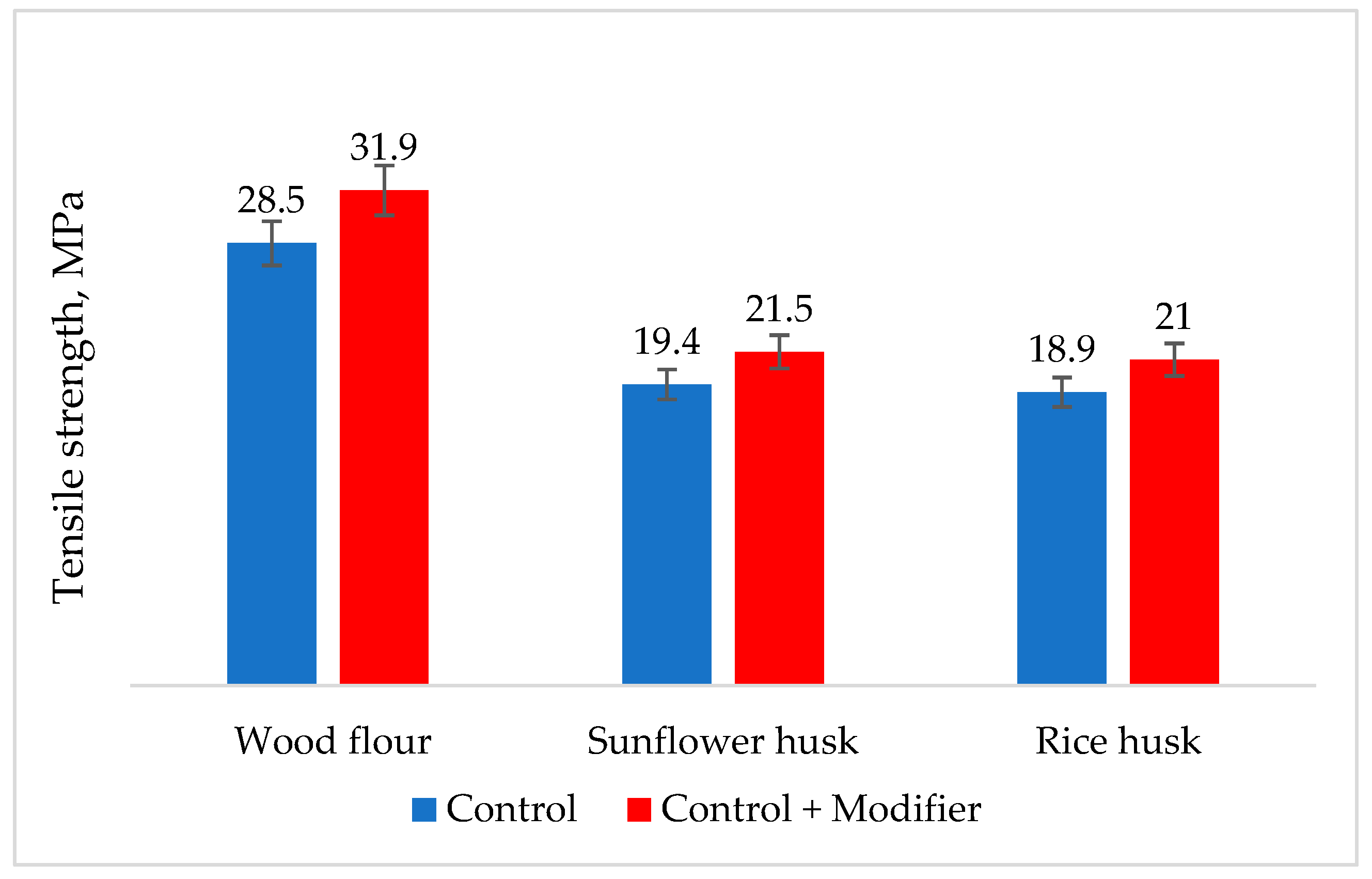







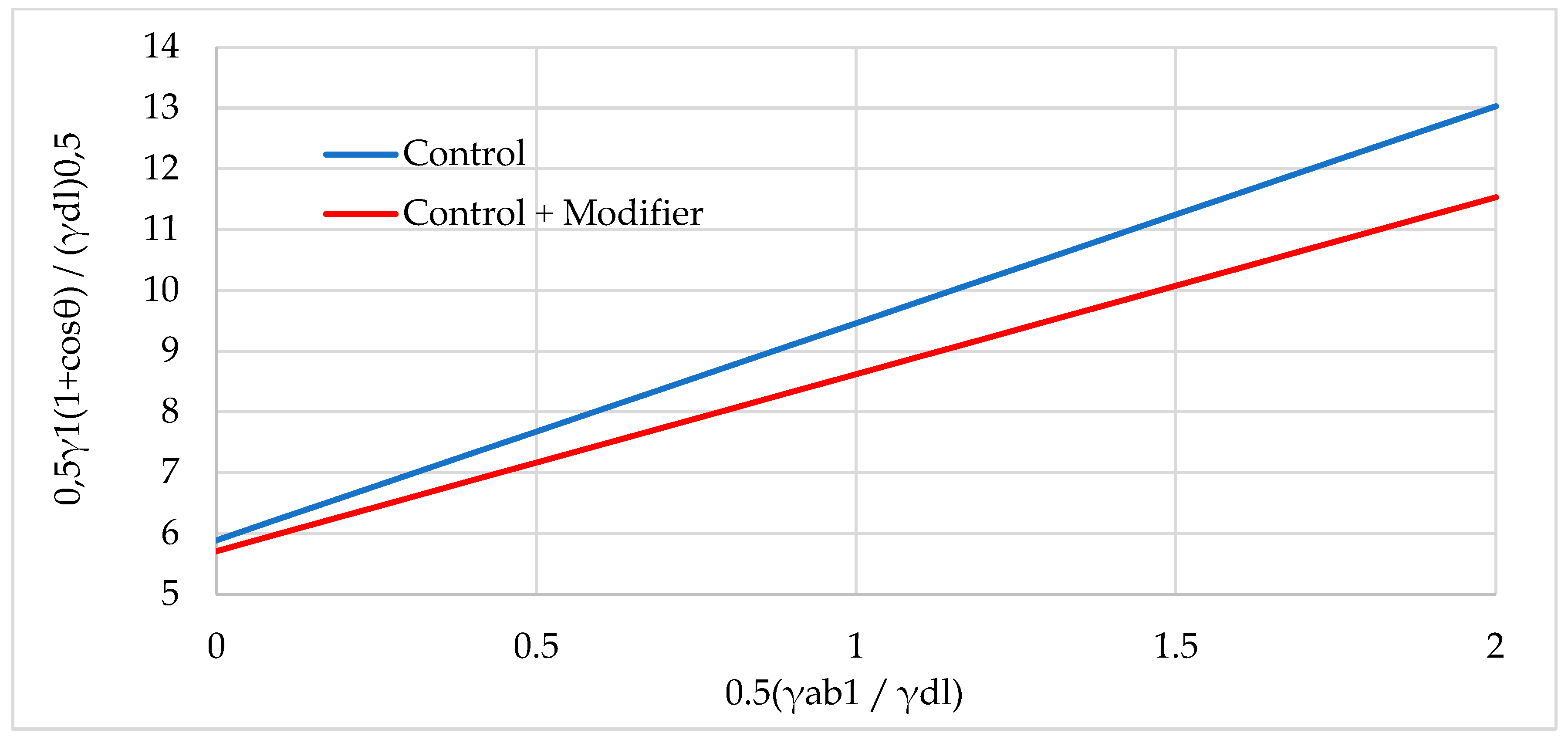
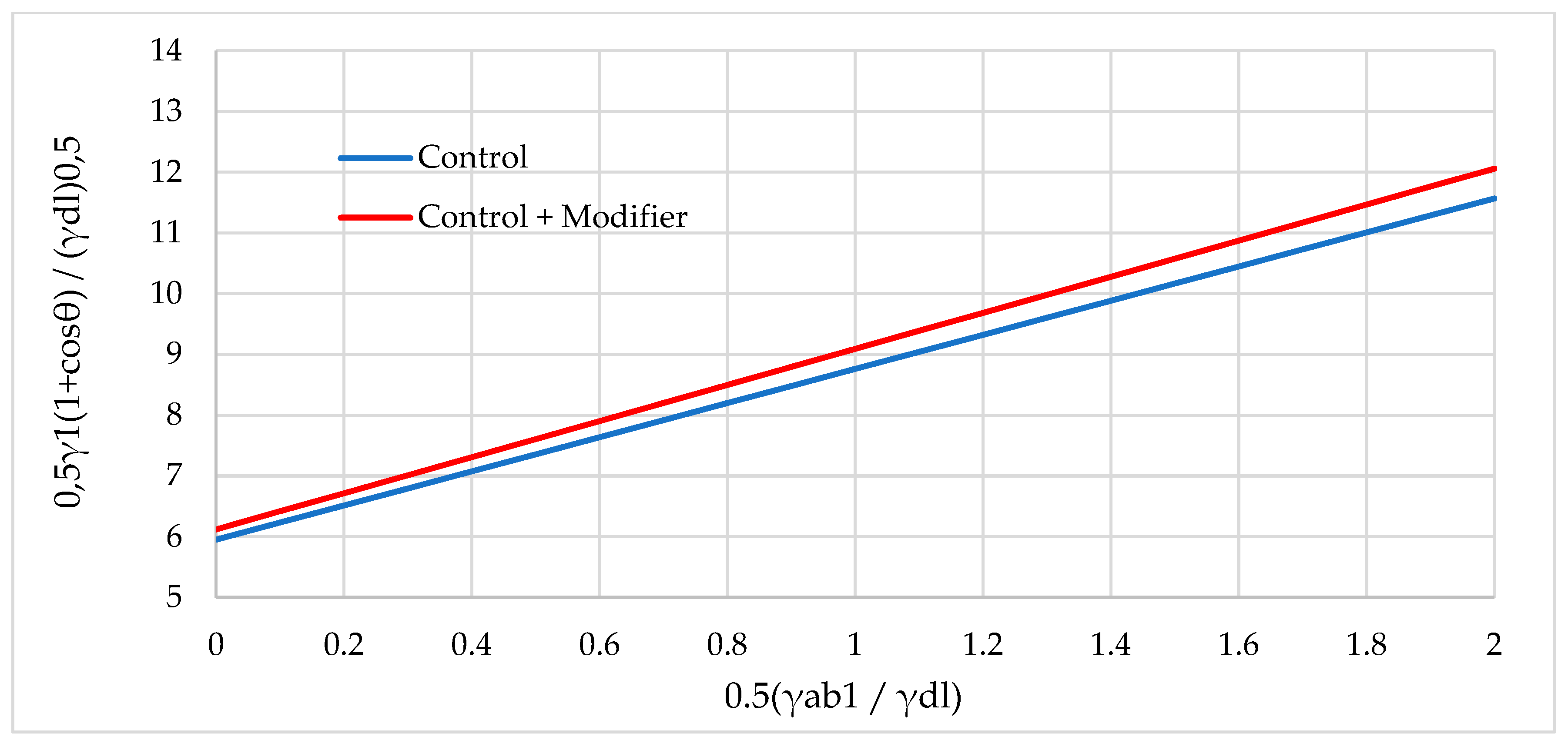

| Natural Fillers | Chemical Composition of Fillers | ||
|---|---|---|---|
| Cellulose, % by Mass | Lignin, % by Mass | Hemicellulose, % by Mass | |
| Rice husk | 28–45 | 12–16 | 23–28 |
| Sunflower husk | 36 | 26 | 25 |
| Wood flour | 43 | 20 | 26 |
| Filler | Content of Components, wt.-% | |||
|---|---|---|---|---|
| Polypropylene | Antioxidant | Natural Filler | Modifier | |
| Wood flour | 49.9 | 0.1 | 50.0 | - |
| 49.9 | 0.1 | 49.5 | 0.5 | |
| Rice husk | 49.9 | 0.1 | 50.0 | - |
| 49.9 | 0.1 | 49.5 | 0.5 | |
| Sunflower husk | 49.9 | 0.1 | 50.0 | - |
| 49.9 | 0.1 | 49.5 | 0.5 | |
| Sample | , mH/m | , mH/m | , mH/m |
|---|---|---|---|
| Polypropylene | 1.5 | 27.1 | 28.6 |
| Wood flour (Control) | 12.76 | 34.71 | 47.47 |
| Wood flour (Control + Modifier) | 8.46 | 32.67 | 41.13 |
| Rice husk (Control) | 8.82 | 37.46 | 46.29 |
| Rice husk (Control + Modifier) | 7.9 | 35.40 | 43.31 |
| Sunflower husk (Control) | 9.45 | 34.12 | 43.57 |
| Sunflower husk (Control + Modifier) | 8.52 | 29.23 | 37.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayzullin, I.; Gorbachev, A.; Volfson, S.; Serikbayev, Y.; Nakyp, A.; Akylbekov, N. Composite Material Based on Polypropylene and Modified Natural Fillers. Polymers 2024, 16, 1703. https://doi.org/10.3390/polym16121703
Fayzullin I, Gorbachev A, Volfson S, Serikbayev Y, Nakyp A, Akylbekov N. Composite Material Based on Polypropylene and Modified Natural Fillers. Polymers. 2024; 16(12):1703. https://doi.org/10.3390/polym16121703
Chicago/Turabian StyleFayzullin, Ilnur, Aleksandr Gorbachev, Svetoslav Volfson, Yerbol Serikbayev, Abdirakym Nakyp, and Nurgali Akylbekov. 2024. "Composite Material Based on Polypropylene and Modified Natural Fillers" Polymers 16, no. 12: 1703. https://doi.org/10.3390/polym16121703
APA StyleFayzullin, I., Gorbachev, A., Volfson, S., Serikbayev, Y., Nakyp, A., & Akylbekov, N. (2024). Composite Material Based on Polypropylene and Modified Natural Fillers. Polymers, 16(12), 1703. https://doi.org/10.3390/polym16121703









