Enhanced Hydrogen Peroxide Decomposition in a Continuous-Flow Reactor over Immobilized Catalase with PAES-C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Process
2.2.1. Synthesis of Polyaryl Ether Sulfone Containing Carboxyl Side Chain
2.2.2. Preparation of Carrier
2.2.3. Preparation of Immobilized Catalase
2.2.4. Enzyme Activity of Free and Immobilized Enzyme
2.2.5. Selection of Cu2+ Concentration
2.2.6. Immobilized Enzyme Single-Factor Assay
2.2.7. Model of the Continuous Experimental Device
2.2.8. Continuous Catalytic H2O2 Decomposition Tests
2.3. Characterization
2.3.1. Fourier-Transform Infrared Spectra (FT-IR)
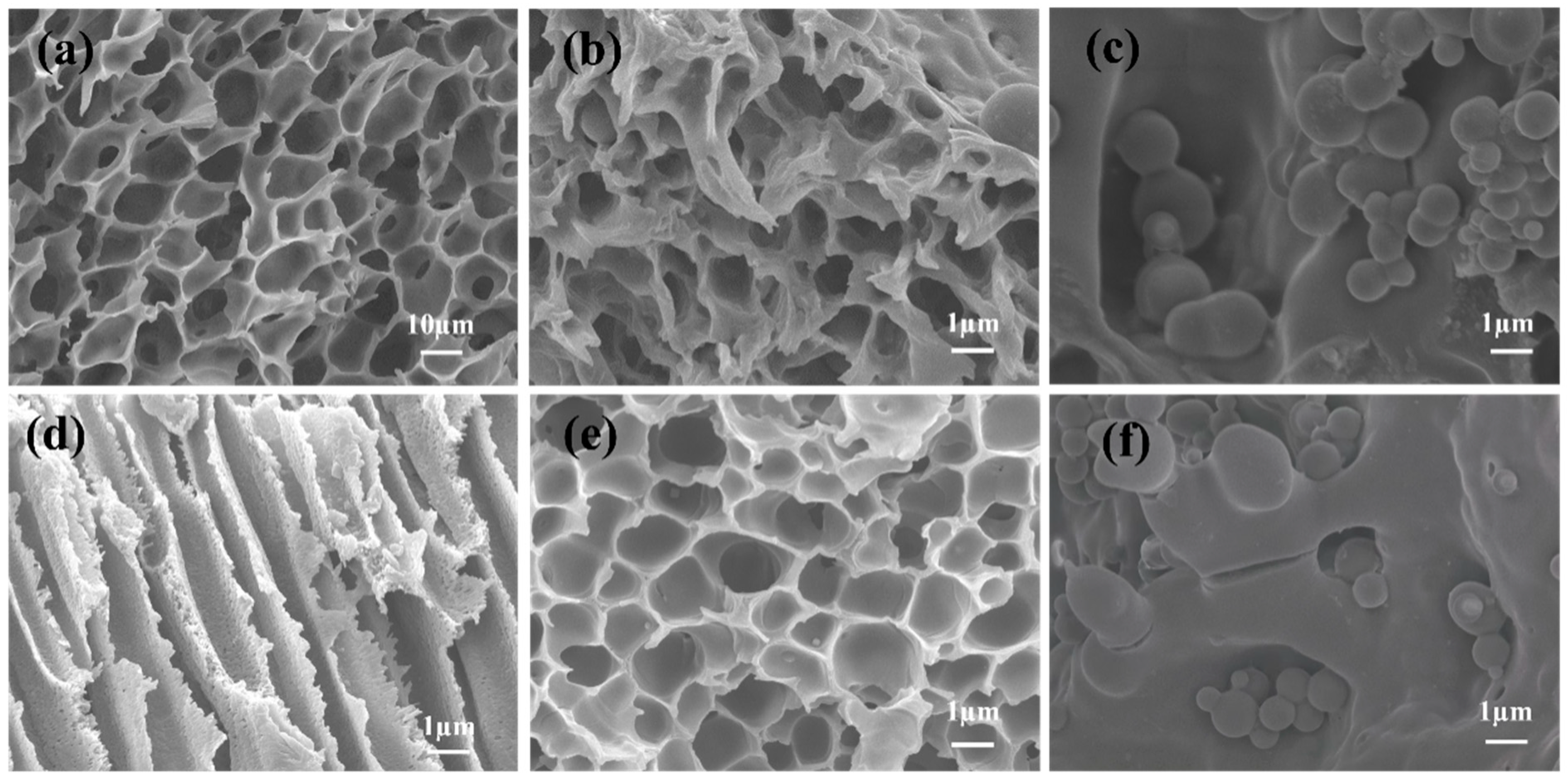
2.3.2. Scanning Electron Microscope (SEM)
2.3.3. 1H-NMR
2.3.4. Gel Permeation Chromatograpgy (GPC)
2.3.5. UV-Vis Spectrophotometry
2.4. Stability of Catalase Immobilized on PAES-C
2.4.1. pH Stability
2.4.2. Thermal Stability
2.4.3. Storage Stability
2.4.4. Operational Stability
3. Results
3.1. Characterization of PAES-C
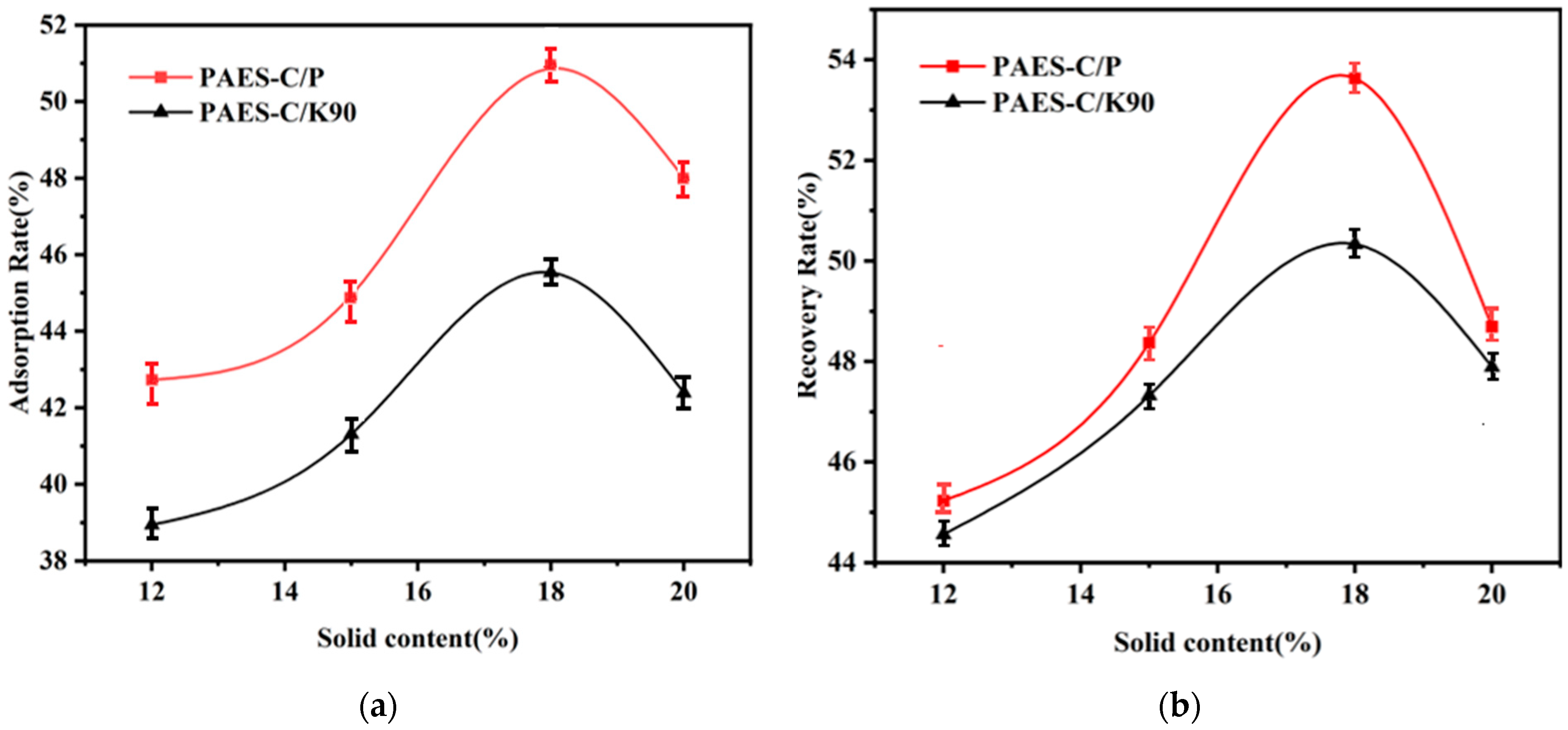
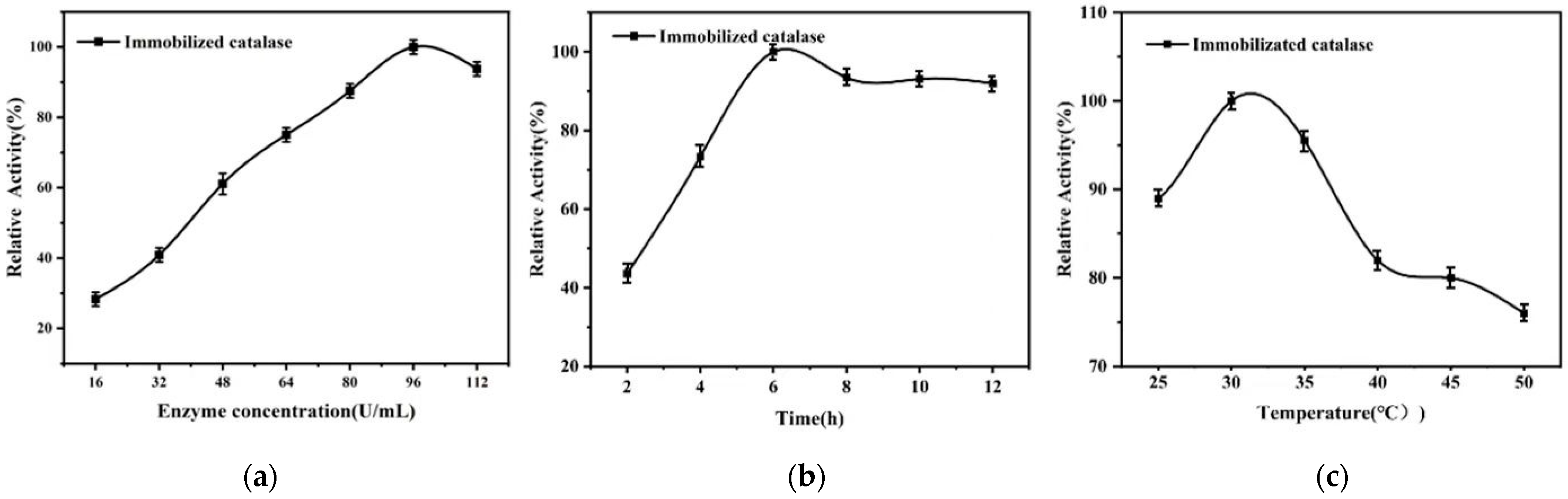
3.2. Immobilized Catalase Condition Optimization
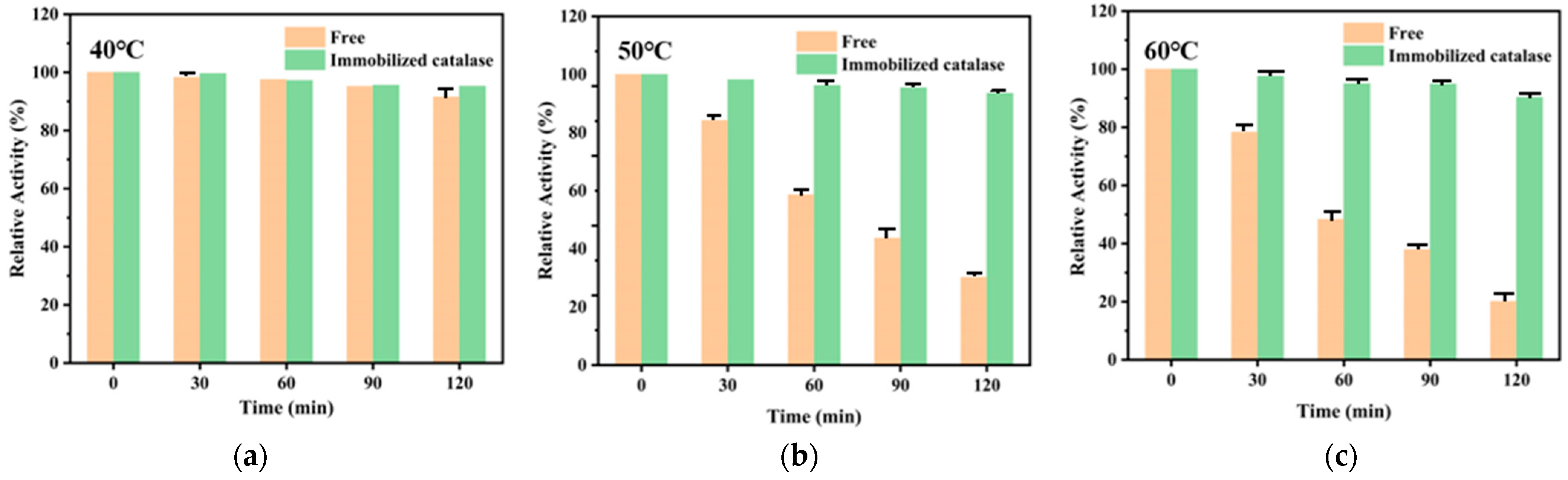
3.3. Stability of Catalase Immobilized on PAES-C
3.4. Catalytic Decomposition of H2O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miłek, J. Estimation of the kinetic parameters for H2O2 enzymatic decomposition and for catalase deactivation. Braz. J. Chem. Eng. 2018, 35, 995–1004. [Google Scholar] [CrossRef]
- Li, Y.; Buckin, V. Ultrasonic real-time monitoring of the process of decomposition of hydrogen peroxide in aqueous solutions. Anal. Methods 2016, 8, 4828–4834. [Google Scholar] [CrossRef]
- Sepasi-Tehrani, H.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Dumorné, K.; Córdova, D.K.; Astorga-Eló, M. A Potential Source for Industrial Applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, K.; Gursharan, S.S.; Arun, R. Catalase Enzyme: Application in Bioremediation and Food Industry. Biocatal. Agric. Biotechnol. 2018, 16, 192–199. [Google Scholar] [CrossRef]
- Takio, N.; Yadav, M.; Yadav, H.S. Catalase-mediated remediation of environmental pollutants and potential application—A review. Biocatal. Biotransfor. 2021, 39, 389–407. [Google Scholar] [CrossRef]
- Pang, J.; Jiang, M.; Liu, Y.X. Preparation and catalytic properties of catalase-inorganic hybrid nanoflowers. Chin. J. Biotechnol. 2022, 38, 4705–4718. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; El-Said, A.H.M. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Li, L.Y.; He, Y.H. Hydrolase-catalyzed asymmetric carbon–carbon bond formation in organic synthesis. RSC Adv. 2015, 5, 16801–16814. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Pelt, S.V. Enzyme immobilization in biocatalysis: What and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Oke, M.A.; Ojo, S.A.; Fasiku, S.A. Nanotechnology and enzyme immobilization: A review. Nanotechnology 2023, 34, 385101. [Google Scholar] [CrossRef]
- Arabaci, G.; Usluoglu, A. Immobilization of dill (Anethum Graveolens L.) catalase and its properties. Asia-Pac. J. Chem. Eng. 2012, 7, S296–S300. [Google Scholar] [CrossRef]
- Woo, W.X.; Tan, J.W.; Tan, J.P. An Insight into Enzymatic Immobilization Techniques on the Saccharification of Lignocellulosic Biomass. Ind. Eng. Chem. Res. 2022, 61, 10603–10615. [Google Scholar] [CrossRef]
- Abdulmalek, S.A.; Yan, Y. Recent developments of lipase immobilization technology and application of immobilized lipase mixtures for biodiesel production. Biofuels Bioprod. Biorefin. 2022, 16, 1062–1094. [Google Scholar] [CrossRef]
- De-Souza, W.F.C.; Almeida, F.L.C.; De-Melo, A.M. Immobilization Techniques on Bioprocesses: Current Applications Regarding Enzymes, Microorganisms, and Essential Oils. Food Bioprocess Technol. 2022, 15, 1449–1476. [Google Scholar] [CrossRef]
- Wu, C.; Ge, J.; Lucas, J.F. Enzyme or Whole Cell Immobilization for Efficient Biocatalysis: Focusing on Novel Supporting Platforms and Immobilization Techniques. Front. Bioeng. Biotechnol. 2021, 9, 620292. [Google Scholar] [CrossRef]
- Deska, M.; Konczak, B. Immobilized fungal laccase as “green catalyst” for the decolourization process—State of the art. Process Biochem. 2019, 84, 112–123. [Google Scholar] [CrossRef]
- Grigoras, A.G. Catalase immobilization—A review. Biochem. Eng. J. 2017, 117, 1–20. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Neto, F.S.; de Aguiar Falcão, I.R.; da Silva Souza, J.E.; de Moura, L.S., Jr.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M.; et al. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2020, 288, 119577. [Google Scholar] [CrossRef]
- Cao, S.; Xu, P.; Ma, Y.Z.; Yao, X.X.; Yao, Y.; Zong, M.H.; Li, X.H.; Lou, W.Y. Recent advances in immobilized enzymes on nanocarriers. Chin. J. Catal. 2016, 37, 1814–1823. [Google Scholar] [CrossRef]
- Zhu, Q.Q.; Zheng, Y.L.; Zhang, Z.J.; Chen, Y. Enzyme immobilization on covalent organic framework supports. Nat. Protoc. 2023, 18, 3080–3125. [Google Scholar] [CrossRef] [PubMed]
- Matura, A.; Köpke, D.; Marschelke, C. Funktionalisierte Kern-Schale-Partikel als Träger zur Enzymimmobilisierung und deren Anwendung. Chem. Ing. Tech. 2020, 92, 169–307. [Google Scholar] [CrossRef]
- Bebić, J.; Banjanac, K.; Rusmirović, J. Amino-modified kraft lignin microspheres as a support for enzyme immobilization. RSC Adv. 2020, 10, 21495–21508. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Bhardwaj, K.K.; Gupta, R. Immobilization and applications of esterases. Biocatal. Biotransfor. 2021, 40, 153–168. [Google Scholar] [CrossRef]
- Li, J.; Li, L.S.; Xu, L. Hierarchically macro/mesoporous silica sphere: A high efficient carrier for enzyme immobilization. Microporous Mesoporous Mater. 2016, 231, 147–153. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Simpson, B. Food enzymes immobilization: Novel carriers, techniques and applications. Curr. Opin. Food Sci. 2022, 43, 27–35. [Google Scholar] [CrossRef]
- Carvalho, T.; Pereira, A.D.S.; Bonomo, R.C.F.; Franco, M.; Finotelli, P.V.; Amaral, P.F.F. Simple physical adsorption technique to immobilize Yarrowia lipolytica lipase purified by different methods on magnetic nanoparticles: Adsorption isotherms and thermodynamic approach. Int. J. Biol. Macromol. 2020, 160, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.Z.A.S.; Borzouee, F.; Mofid, M.R.; Varshosaz, J. Immobilization of lactoperoxidase on graphene oxide nanosheets with improved activity and stability. Biotechnol. Lett. 2018, 40, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Montes, R.; Céspedes, F.; Gabriel, D.; Baeza, M. Electrochemical Biosensor Based on Optimized Biocomposite for Organophosphorus and Carbamates Pesticides Detection. J. Nanomater. 2018, 2018, 7093606. [Google Scholar]
- Herzog, V.; Fahimi, H.D. The effect of glutaraldehyde on catalase: Biochemical and cytochemical studies with beef liver catalase and rat liver peroxisomes. J. Cell Biol. 1974, 60, 303–311. [Google Scholar] [CrossRef]
- Kurzbaum, T.; Raizner, Y.; Kuc, M.E.; Kulikov, A.; Hakimi, B.; Kruh, L.; Menashe, O. Phenol biodegradation by bacterial cultures encapsulated in 3D microfiltration-membrane capsules. Environ. Technol. 2019, 41, 2875–2883. [Google Scholar] [CrossRef]
- Wang, Y.L.; Guan, Y.P.; Yang, Y.; Yu, P.; Huang, Y.Q. Enhancing the stability of immobilized catalase on activated carbon with gelatin encapsulation. J. Appl. Polym. Sci. 2013, 130, 1498–1502. [Google Scholar] [CrossRef]
- Matsumoto, M.; Sumi, N.; Ohmori, K.; Kondo, K. Immobilization of lipase in microcapsules prepared by organic and inorganic materials. Process Biochem. 1998, 33, 535–540. [Google Scholar] [CrossRef]
- Sel, E.; Ulu, A.; Ates, B.; Koytepe, S. Comparative study of catalase immobilization via adsorption on P(MMA-co-PEG500MA) structures as an effective polymer support. Polym. Bull. 2020, 78, 2663–2684. [Google Scholar] [CrossRef]
- Alptekin, Ö.; Tükel, S.S.; Yıldırım, D.; Alagöz, D. Immobilization of catalase onto Eupergit C and its characterization. J. Mol. Catal. B Enzym. 2010, 64, 177–183. [Google Scholar] [CrossRef]
- Yesmurzayeva, N.N.; Nurakhmetova, Z.A.; Tatykhanova, G.S.; Selenova, B.S.; Kudaibergenov, S.E. Catalytic Activity of Gold and Silver Nanoparticles Supported on Zinc Oxide. Supramol. Catal. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Kaushal, J.; Singh, G.; Arya, S.K. Immobilization of Catalase onto Chitosan and Chitosan–Bentonite Complex: A Comparative Study. Biotechnol. Rep. 2018, 18, e00258. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, X.J.; Chen, P.C.; Xu, Z.K. Polymer materials for enzyme immobilization and their application in bioreactors. BMB Rep. 2011, 44, 87–95. [Google Scholar] [PubMed]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of Polymers: A Review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef]
- Balakrishnan, P. Polymeric biomaterials: State-of-the-art and new challenges. In Fundamental Biomaterials: Polymers; Woodhead Publishing: Sawston, UK, 2018; pp. 1–20. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, S.S.; Li, Z.M. Poly(arylene ether sulfone) containing thioether units: Synthesis, oxidation and properties. RSC Adv. 2014, 4, 23191–23201. [Google Scholar] [CrossRef]
- Bi, H.Y.; Tian, J.Y.; Fu, Y. Immobi1ization of Catalase Using PAES-C polymer for Wastewater Biological Treatment Research. IOP Conf. Ser. Earth Environ. Sci. 2021, 661, 012004. [Google Scholar] [CrossRef]
- Yan, G.M.; Lu, H.R.; Yuan, S.S.; Deng, X.; Zhu, J.Y.; Zhang, G. Side grafting tertiary amine in polyaryl ether sulfone membranes for reversible-multiple separations. J. Membr. Sci. 2023, 667, 121641. [Google Scholar] [CrossRef]
- Ma, D.W.; Wang, D.Z.; Zhang, S.Y.; Cai, W.J. Brief Communication. Cell. Polym. 2016, 35, 209–216. [Google Scholar] [CrossRef]
- Michler, A. PVPylation of catalase. Stellenbosch Stellenbosch Univ. 2017, 18, 445–650. [Google Scholar]
- Sigel, H. Catalase and Peroxidase Activity of Cu2+ Complexes. Angew. Chem. Int. Ed. 1969, 8, 167–177. [Google Scholar] [CrossRef]
- Lukatkin, A.S.; Michailova, I.D.; Silva, J.A.T.D. Prooxidative and antioxidative properties of cucumber (Cucumis sativus L.) callus in vitro and young in vivo plantlets in response to copper ions. Folia Hortic. 2013, 25, 141–151. [Google Scholar] [CrossRef]
- Sigel, H.; Wyss, K.; Fischer, B.E.; Prijs, B. Metal ions and hydrogen peroxide.1 Catalase-like activity of Cu2+ in aqueous solution and its promotion by the coordination of 2, 2′-bipyridyl. Inorg. Chem. 1979, 18, 1354–1358. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Gascon, V.; Maquez-Alvarez, C.; Blanco, R.M.; Nidetzky, B. Oriented coimmobilization of oxidase and catalase on tailor-made ordered mesoporous silica. Langmuir 2017, 33, 5065–5076. [Google Scholar] [CrossRef]






| Type | K90 | PVP-P | ||||||
|---|---|---|---|---|---|---|---|---|
| Solid content (%) | 12 | 15 | 18 | 20 | 12 | 15 | 18 | 20 |
| DMSO (%) | 88 | 75 | 82 | 80 | 88 | 75 | 82 | 80 |
| Experimental Conditions | Concentration of Enzyme (U/mL) | Temperature (°C) | Time (h) |
|---|---|---|---|
| Concentration of enzyme (U/mL) | 16, 32, 48, 64, 80, 96, 112 | --- | --- |
| Temperature (°C) | --- | 25, 30, 35, 40, 45, 50 | --- |
| Time (h) | --- | --- | 2, 4, 6, 8, 10, 12 |
| Experimental Conditions | H2O2 Initial Concentration (mol/L) | Velocity of Flow (mL/min) | Mass of Immobilized Enzyme (g) |
|---|---|---|---|
| H2O2 initial concentration (mol/L) | 0.035, 0.045 | --- | --- |
| Velocity of flow (mL/min) | --- | 4, 8, 12 | --- |
| Mass of immobilized enzyme (g) | --- | --- | 0.1, 0.15, 0.2 |
| Activation | Suppression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mmol/L) | 1 | 2 | 3 | 4 | 5 | 10 | 20 | 30 | 40 | 50 |
| Relative Activity (%) | 125.34 | 143.58 | 181.53 | 152.48 | 102.60 | 28.06 | 23.84 | 14.71 | 8.78 | 5.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, Y.; Zhang, W.; Wu, H.; Zhang, S. Enhanced Hydrogen Peroxide Decomposition in a Continuous-Flow Reactor over Immobilized Catalase with PAES-C. Polymers 2024, 16, 1762. https://doi.org/10.3390/polym16131762
Li Y, Zhang Y, Zhang W, Wu H, Zhang S. Enhanced Hydrogen Peroxide Decomposition in a Continuous-Flow Reactor over Immobilized Catalase with PAES-C. Polymers. 2024; 16(13):1762. https://doi.org/10.3390/polym16131762
Chicago/Turabian StyleLi, Yunrui, Yu Zhang, Wenyu Zhang, Hao Wu, and Shaoyin Zhang. 2024. "Enhanced Hydrogen Peroxide Decomposition in a Continuous-Flow Reactor over Immobilized Catalase with PAES-C" Polymers 16, no. 13: 1762. https://doi.org/10.3390/polym16131762






