Advances in Polyvinyl Alcohol-Based Membranes for Fuel Cells: A Comprehensive Review on Types, Synthesis, Modifications, and Performance Optimization
Abstract
1. Introduction
| Device | Energy Density | Life Time | Benefits | Drawbacks | Refs. |
|---|---|---|---|---|---|
| Fuel Cell | Very high | 5000–10,000 (hours) | Modular and compact design Exceptional efficiency Swift hydrogen refueling Negligible emissions | Prolonged cold start Cost-intensive Risks associated with hydrogen Elevated fuel expenses | [7,9,10,11] |
| Battery | High | 4–6 (years) | Portable and rechargeable functionality Economical Well-established technology | Slow recharge rate Limited lifespan Battery preparation and recycling contribute to environmental pollution Flammable electrolyte | [12,13] |
| Supercapacitor | Very low | 10–20 (years) | Prompt recharging and response | Short time energy storage High cost | [14,15,16] |
| Photovoltaic panel | Medium | 25–30 (years) | Environmentally sustainable | Power output is intermittent Huge for light transport | [17,18] |
| Flywheels | High | 5–10 (years) | Elevated power Environmentally sustainable | Slow charging Heavy weight | [19,20] |
| Superconducting magnetic energy storage system | Low | 25–30 (years) | Elevated power output and performance Remarkable efficiency Environmentally conscious Rapid response time | Short-term energy storage High cost | [21,22,23] |

Fundamental Overview of PVA
2. Exploring the Role of PVA in Membrane Technology
2.1. Hydrogen Fuel Cells by PVA–Proton-Exchange Membranes
| Type of PEMFC | PEM | Water Uptake (%) | Thermal Stability Tm (°C) | Ion-Exchange Capacity (meq/g) | Proton Conductivity (S/cm) | Fuel Cell Performance (mV/cm2) |
|---|---|---|---|---|---|---|
| Low Temperature | PVA/SBA-15-propyl-SO3H | 80 (25 °C) | 150–185 | - | 0.006 (25 °C) | - |
| PVA/SGO | 58.3 (25 °C) | 214–223 | 0.92 (25 °C) | 0.050 (25 °C) | 16.15 (30 °C) | |
| SPEEK/PVA/TEOS | 65 (80 °C) | - | 2.02 (25 °C) | 0.084 (80 °C) | 336 (80 °C) | |
| PVA/SSA | - | - | - | 0.077 (70 °C) | 99 (70 °C) | |
| PVA/PAMPS/ZIF | 328 (25 °C) | <200 | 1.52 (25 °C) | 0.134 (80 °C) | - | |
| 25 kGy irradiation doses 10% PVA | - | >220 | - | 0.340 (25 °C) | - | |
| PVA/SSA/GO | - | 325 | - | 0.003 (30 °C) | 155.4 (23 °C) | |
| PVA/sulfonic acid | - | - | - | 0.156 × 10−3 (25 °C) | - | |
| High Temperature (>120 °C) | PVA/PAMPS/1.2.4-triazole | 248 (25 °C) | <250 | 1.62 (25 °C) | 0.002 (150 °C) | - |
| H3PO4-imbibed PAM/PVA | - | 100–200 | - | 0.053 (183 °C) | 225 (183 °C) |
2.2. PVA–Proton-Conducting and Methanol Barrier Membranes for Direct Methanol Cells
2.3. PVA–Anion-Exchange Membrane of Alkaline Fuel Cells
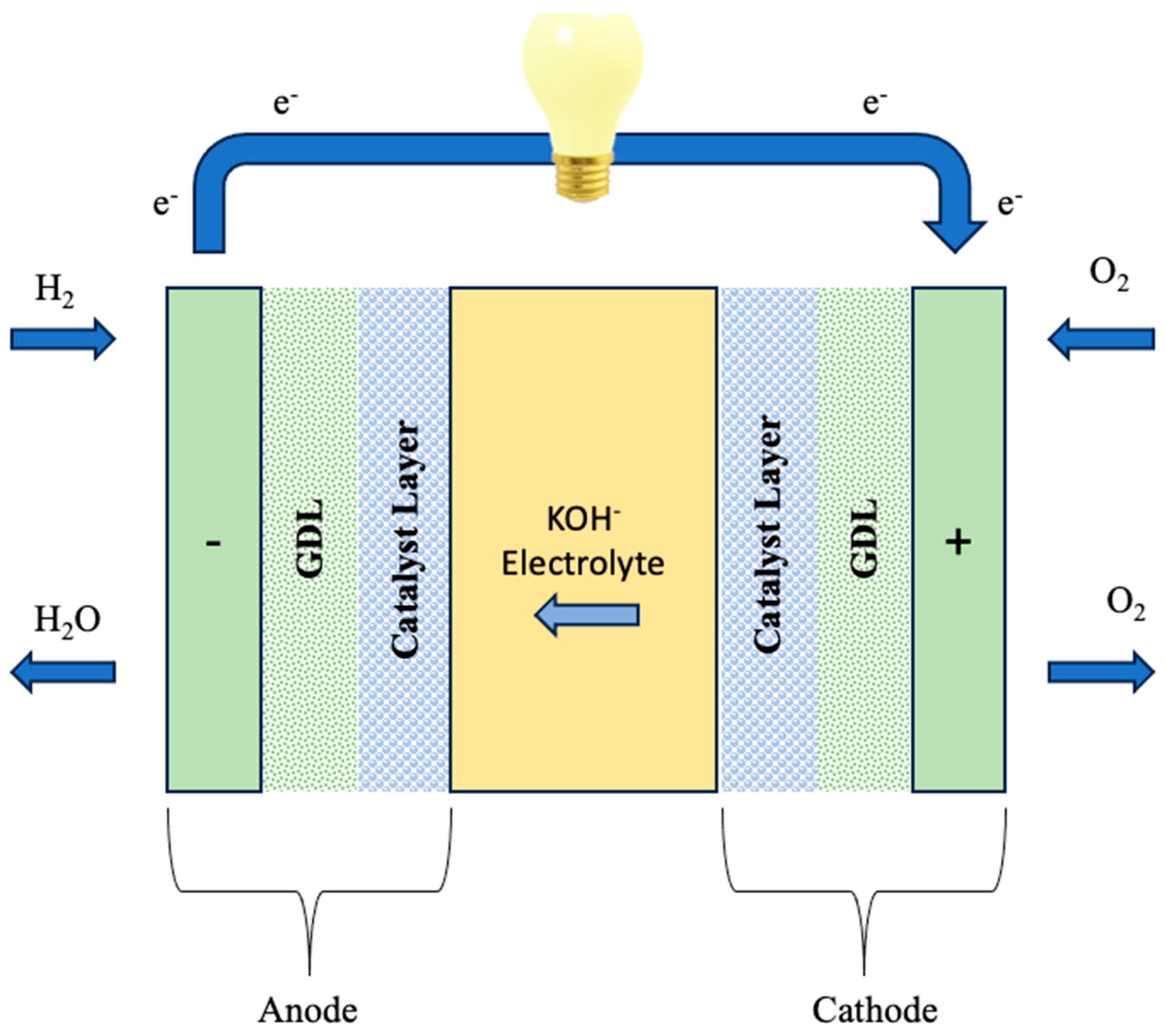
3. Crafting PVA-Based Membranes: A Synthesis Overview
3.1. Phase Inversion Technique
3.2. Electrospinning
4. Key Influencing Factors on the Performance of PVA-Based Membranes in Fuel Cells
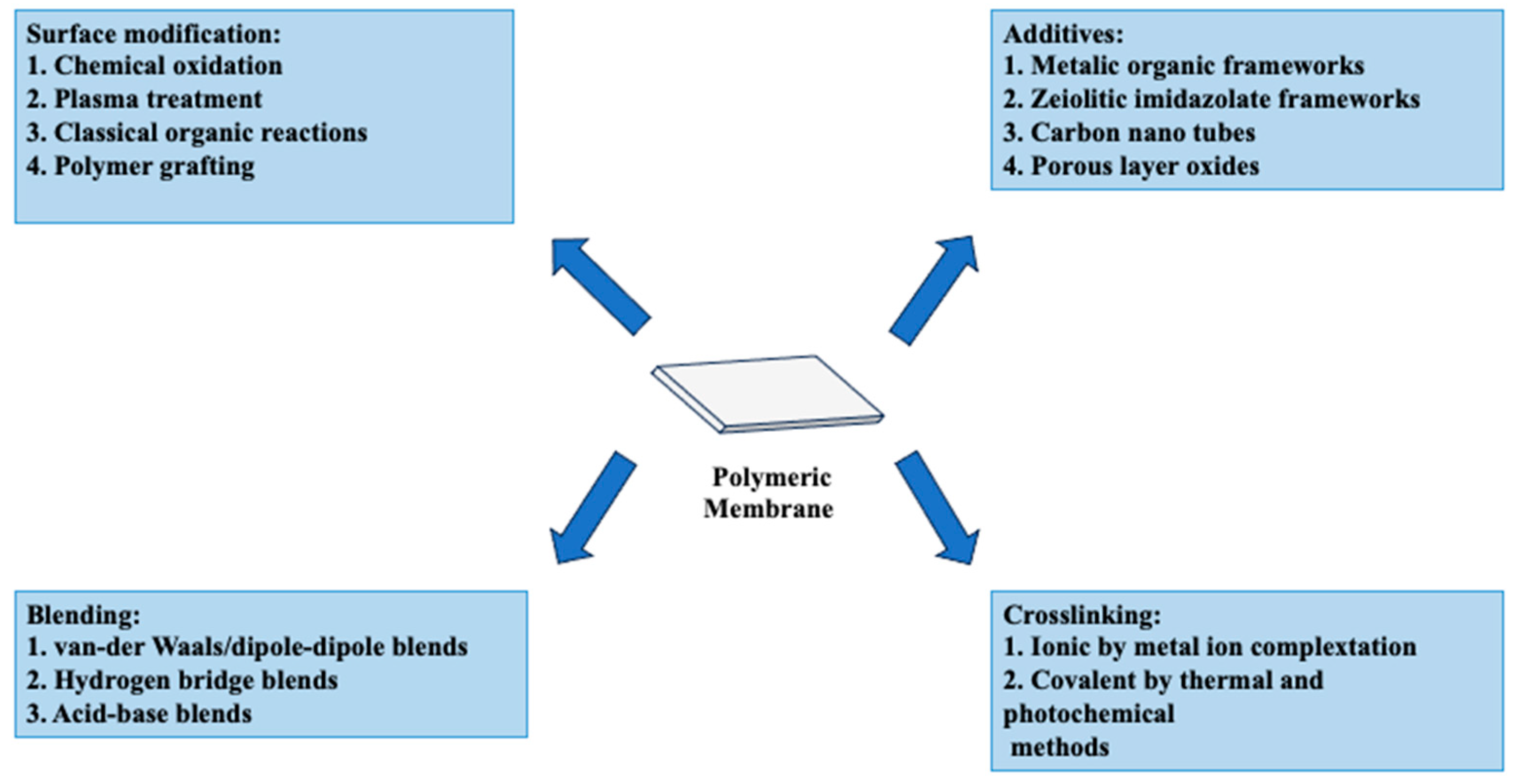
5. Enhancements and Modifications of PVA-Based Membranes for Fuel Cells
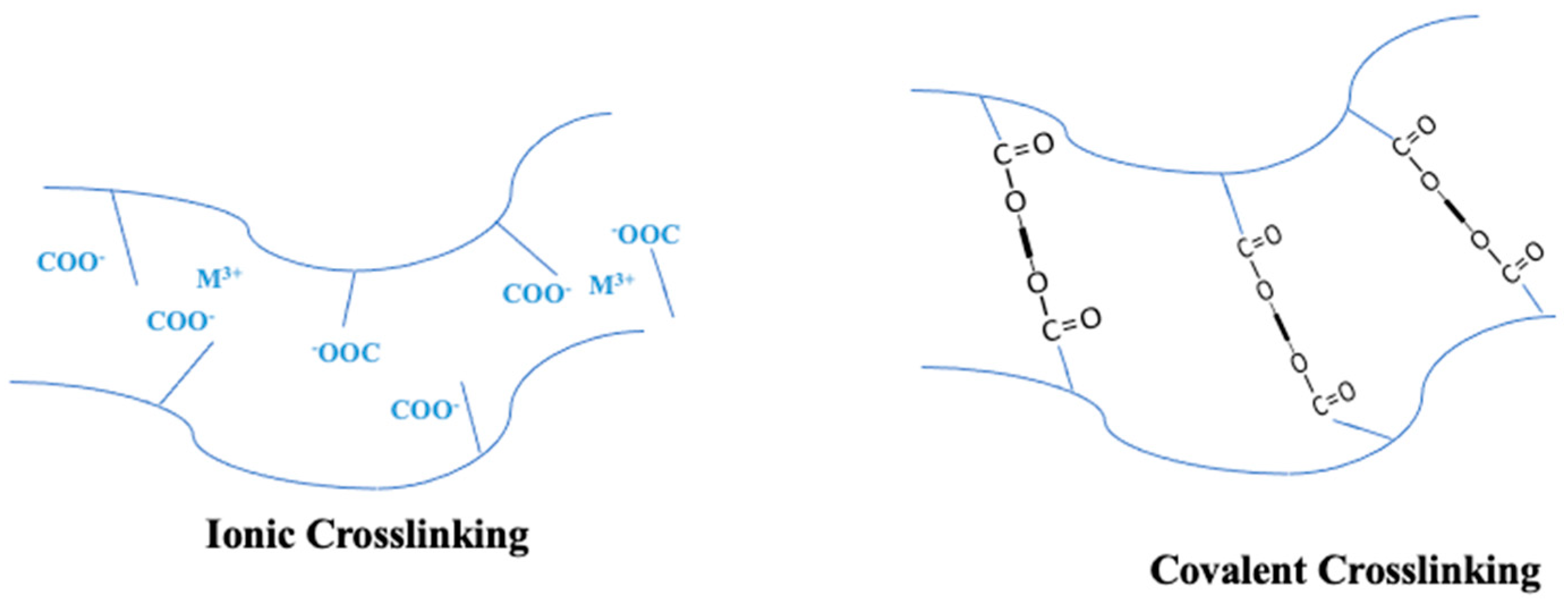
5.1. Enhancing PVA Membrane Performance through Cross-Linking/Copolymerization
5.2. Enhancing PVA Membrane Performance through Blending
6. Challenges Associated with PVA-Based Membranes for Fuel Cells and Future Aspects
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le, N.L.; Nunes, S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef]
- Xie, W.; Li, T.; Tiraferri, A.; Drioli, E.; Figoli, A.; Crittenden, J.C.; Liu, B. Toward the Next Generation of Sustainable Membranes from Green Chemistry Principles. ACS Sustain. Chem. Eng. 2021, 9, 50–75. [Google Scholar] [CrossRef]
- Roy, S.; Ragunath, S. Emerging Membrane Technologies for Water and Energy Sustainability: Future Prospects, Constraints and Challenges. Energies 2018, 11, 2997. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Kundu, P.P. Effect of the presence of partially sulfonated polyaniline on the proton and methanol transport behavior of partially sulfonated PVdF membrane. Polym. J. 2016, 48, 301–309. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Kundu, P.P. Polyaniline nanowhiskers induced low methanol permeability and high membrane selectivity in partially sulfonated PVdF-co-HFP membranes. RSC Adv. 2016, 6, 107960–107969. [Google Scholar] [CrossRef]
- Dutta, K.; Das, S.; Kundu, P.P. Highly methanol resistant and selective ternary blend membrane composed of sulfonated PVdF-co-HFP, sulfonated polyaniline and nafion. J. Appl. Polym. Sci. 2016, 133, 43294. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Pramuanjaroenkij, A.; Kakaç, S. The fuel cell electric vehicles: The highlight review. Int. J. Hydrogen Energy 2023, 48, 9401–9425. [Google Scholar] [CrossRef]
- Wang, C.-Y. Fundamental Models for Fuel Cell Engineering. Chem. Rev. 2004, 104, 4727–4766. [Google Scholar] [CrossRef]
- Corbo, P.; Migliardini, F.; Veneri, O. PEFC stacks as power sources for hybrid propulsion systems. Int. J. Hydrogen Energy 2009, 34, 4635–4644. [Google Scholar] [CrossRef]
- Halder, P.; Babaie, M.; Salek, F.; Shah, K.; Stevanovic, S.; Bodisco, T.A.; Zare, A. Performance, emissions and economic analyses of hydrogen fuel cell vehicles. Renew. Sustain. Energy Rev. 2024, 199, 114543. [Google Scholar] [CrossRef]
- Waseem, M.; Ahmad, M.; Parveen, A.; Suhaib, M. Battery technologies and functionality of battery management system for EVs: Current status, key challenges, and future prospectives. J. Power Sources 2023, 580, 233349. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A. Rechargeable batteries: Technological advancement, challenges, current and emerging applications. Energy 2023, 266, 126408. [Google Scholar] [CrossRef]
- Adedoja, O.S.; Sadiku, E.R.; Hamam, Y. An Overview of the Emerging Technologies and Composite Materials for Supercapacitors in Energy Storage Applications. Polymers 2023, 15, 2272. [Google Scholar] [CrossRef] [PubMed]
- Oyedotun, K.O.; Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J.; Adegoke, K.A. Advances in Supercapacitor Development: Materials, Processes, and Applications. J. Electron. Mater. 2023, 52, 96–129. [Google Scholar] [CrossRef]
- Sree Raj, K.A.; Rout, C.S. Recent developments, challenges and future prospects of magnetic field effects in supercapacitors. J. Mater. Chem. A 2023, 11, 5495–5519. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Z.; Zhu, C.; Yang, R.; Yan, B.; Jiang, G. Green or not? Environmental challenges from photovoltaic technology. Environ. Pollut. 2023, 320, 121066. [Google Scholar] [CrossRef] [PubMed]
- Artaş, S.B.; Kocaman, E.; Bilgiç, H.H.; Tutumlu, H.; Yağlı, H.; Yumrutaş, R. Why PV panels must be recycled at the end of their economic life span? A case study on recycling together with the global situation. Process. Saf. Environ. Prot. 2023, 174, 63–78. [Google Scholar] [CrossRef]
- Gharahbagh, A.A.; Hajihashemi, V.; da Silva Tavares, J.M.R.; Sadi, M.; Singh, A.K.; Arabkoohsar, A. 13—Flywheel energy storage. In Future Grid-Scale Energy Storage Solutions; Arabkoohsar, A., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 507–541. [Google Scholar] [CrossRef]
- Xu, K.; Guo, Y.; Lei, G.; Zhu, J. A Review of Flywheel Energy Storage System Technologies. Energies 2023, 16, 6462. [Google Scholar] [CrossRef]
- Papageorgiou, P.G.; Oureilidis, K.O.; Christoforidis, G.C. A systematic review of hybrid superconducting magnetic/battery energy storage systems: Applications, control strategies, benefits, limitations and future prospects. Renew. Sustain. Energy Rev. 2023, 183, 113436. [Google Scholar] [CrossRef]
- Khaleel, M.; Yusupov, Z.; Nassar, Y.; El-Khozondar, H.J.; Ahmed, A.; Alsharif, A. Technical challenges and optimization of superconducting magnetic energy storage in electrical power systems. E-Prime—Adv. Electr. Eng. Electron. Energy 2023, 5, 100223. [Google Scholar] [CrossRef]
- Ghiasi, N.S.; Ghiasi, S.M.S.; Ghiasi, N.S.; Ghiasi, S.M.S. A Review on Superconducting Magnetic Energy Storage System Applications. In Energy Storage Applications in Power Systems; IntechOpen: London, UK,, 2023. [Google Scholar] [CrossRef]
- Hanapi, I.; Kamarudin, S.; Zainoodin, A.; Hasran, U. Membrane-less micro fuel cell system design and performance: An overview. Int. J. Energy Res. 2019, 43, 8956–8972. [Google Scholar] [CrossRef]
- Nafion Membrane, Chemours Nafion, Proton Exchange Membrane. Available online: https://www.nafion.com/en/products/sulfonic-membranes (accessed on 15 January 2024).
- de Melo, S.V.S.; Yahyaoui, I.; Fardin, J.F.; Encarnação, L.F.; Tadeo, F. Power unit SOFC-MTG model in Electromagnetic Transient Software PSCAD. Int. J. Hydrogen Energy 2017, 43, 5386–5397. [Google Scholar] [CrossRef]
- Asensio, J.A.; Sánchez, E.M.; Gómez-Romero, P. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chem. Soc. Rev. 2010, 39, 3210–3239. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Lee, Y.M. Anion exchange polyelectrolytes for membranes and ionomers. Prog. Polym. Sci. 2021, 113, 101345. [Google Scholar] [CrossRef]
- Kang, N.R.; Pham, T.H.; Nederstedt, H.; Jannasch, P. Durable and highly proton conducting poly(arylene perfluorophenylphosphonic acid) membranes. J. Membr. Sci. 2021, 623, 119074. [Google Scholar] [CrossRef]
- Miyake, J.; Miyatake, K. Fluorine-free sulfonated aromatic polymers as proton exchange membranes. Polym. J. 2017, 49, 487–495. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Moliner-Estopiñán, C.; Imrie, C.T.; Ribes-Greus, A. Characterization of crosslinked poly(vinyl alcohol)-based membranes with different hydrolysis degrees for their use as electrolytes in direct methanol fuel cells. J. Appl. Polym. Sci. 2012, 124, 1000–1011. [Google Scholar] [CrossRef]
- Dadfar, S.M.M.; Kavoosi, G. Investigation of mechanical properties, antibacterial features, and water vapor permeability of polyvinyl alcohol thin films reinforced by glutaraldehyde and multiwalled carbon nanotube. Polym. Compos. 2014, 35, 1736–1743. [Google Scholar] [CrossRef]
- Wu, H.F.; Yue, L.Z.; Jiang, S.L.; Lu, Y.Q.; Wu, Y.X.; Wan, Z.Y. Biodegradation of polyvinyl alcohol by different dominant degrading bacterial strains in a baffled anaerobic bioreactor. Water Sci. Technol. 2019, 79, 2005–2012. [Google Scholar] [CrossRef]
- Ben Halima, N. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Kim, S.-T. Polyvinyl Acetate, Alcohol and Derivatives, Polystyrene, and Acrylics. In Patty’s Toxicology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 1–21. [Google Scholar] [CrossRef]
- Kaco, H.; Zakaria, S.; Chia, C.H.; Zhang, L. Transparent and Printable Regenerated Kenaf Cellulose/PVA Film. BioResources 2014, 9, 2167–2178. [Google Scholar] [CrossRef]
- Mokhtar, M.; Majlan, E.H.; Ahmad, A.; Tasirin, S.M.; Daud, W.R.W. Effect of ZnO Filler on PVA-Alkaline Solid Polymer Electrolyte for Aluminum-Air Battery Applications. J. Electrochem. Soc. 2018, 165, A2483–A2492. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Liu, Q.L.; Jiang, Z.Y.; Chen, Y. Anti-trade-off in dehydration of ethanol by novel PVA/APTEOS hybrid membranes. J. Membr. Sci. 2007, 287, 237–245. [Google Scholar] [CrossRef]
- Luo, J.; Wu, C.; Xu, T.; Wu, Y. Diffusion dialysis-concept, principle and applications. J. Membr. Sci. 2011, 366, 1–16. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Luo, J.; Xu, T.; Fu, Y. Anion exchange hybrid membranes from PVA and multi-alkoxy silicon copolymer tailored for diffusion dialysis process. J. Membr. Sci. 2010, 356, 96–104. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Li, Y.; Xu, T.; Fu, Y. PVA–silica anion-exchange hybrid membranes prepared through a copolymer crosslinking agent. J. Membr. Sci. 2010, 350, 322–332. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Biswal, A.K.; Chang, Y.-H.; Misra, P.K.; Yang, J.-M. Synthesis and Characterization of Modified Poly(vinyl alcohol) Membrane and Study of Its Enhanced Water-Induced Shape-Memory Behavior. J. Polym. Environ. 2022, 30, 3409–3419. [Google Scholar] [CrossRef]
- Cardona, E.S.M.; Camargo, J.R.; Monsalve, Y.A.C. A review of polyvinyl alcohol derivatives: Promising materials for pharmaceutical & biomedical applications. Afr. J. Pharm. Pharmacol. 2014, 8, 674–684. [Google Scholar] [CrossRef]
- Samsudin, A.M.; Bodner, M.; Hacker, V. A Brief Review of Poly(Vinyl Alcohol)-Based Anion Exchange Membranes for Alkaline Fuel Cells. Polymers 2022, 14, 3565. [Google Scholar] [CrossRef]
- Patel, P.; Rodriguez, F.; Moloney, G. N-methyl-2-pyrrolidone as a solvent for poly(vinyl alcohol). J. Appl. Polym. Sci. 1979, 23, 2335–2342. [Google Scholar] [CrossRef]
- Briscoe, B.; Luckham, P.; Zhu, S. The effects of hydrogen bonding upon the viscosity of aqueous poly(vinyl alcohol) solutions. Polymer 2000, 41, 3851–3860. [Google Scholar] [CrossRef]
- Chana, J.; Forbes, B.; Jones, S.A. The synthesis of high molecular weight partially hydrolysed poly(vinyl alcohol) grades suitable for nanoparticle fabrication. J. Nanosci. Nanotechnol. 2008, 8, 5739–5747. [Google Scholar] [CrossRef] [PubMed]
- Susanto, H.; Samsudin, A.M.; Faz, M.W.; Rani, M.P.H. Impact of post-treatment on the characteristics of electrospun poly (vinyl alcohol)/chitosan nanofibers. AIP Conf. Proc. 2016, 1725, 020087. [Google Scholar] [CrossRef]
- Sau, S.; Pandit, S.; Kundu, S. Crosslinked poly (vinyl alcohol): Structural, optical and mechanical properties. Surf. Interfaces 2021, 25, 101198. [Google Scholar] [CrossRef]
- Aruldass, S.; Mathivanan, V.; Mohamed, A.; Tye, C. Factors affecting hydrolysis of polyvinyl acetate to polyvinyl alcohol. J. Environ. Chem. Eng. 2019, 7, 103238. [Google Scholar] [CrossRef]
- Julinová, M.; Vaňharová, L.; Jurča, M. Water-soluble polymeric xenobiotics—Polyvinyl alcohol and polyvinylpyrrolidone—And potential solutions to environmental issues: A brief review. J. Environ. Manag. 2018, 228, 213–222. [Google Scholar] [CrossRef] [PubMed]
- DeMerlis, C.; Schoneker, D. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Kim, D.; Seo, J. Enhanced oxygen-barrier and water-resistance properties of poly(vinyl alcohol) blended with poly(acrylic acid) for packaging applications. Polym. Int. 2016, 65, 400–406. [Google Scholar] [CrossRef]
- Sapalidis, A.A.; Katsaros, F.K.; Steriotis, T.A.; Kanellopoulos, N.K. Properties of poly(vinyl alcohol)—Bentonite clay nanocomposite films in relation to polymer–clay interactions. J. Appl. Polym. Sci. 2012, 123, 1812–1821. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Ali Raza, Z. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Barton, A.F.M. Handbook of Poylmer-Liquid Interaction Parameters and Solubility Parameters; Routledge: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Assender, H.E.; Windle, A.H. Crystallinity in poly(vinyl alcohol) 2. Computer modelling of crystal structure over a range of tacticities. Polymer 1998, 39, 4303–4312. [Google Scholar] [CrossRef]
- Assender, H.E.; Windle, A.H. Crystallinity in poly(vinyl alcohol). 1. An X-ray diffraction study of atactic PVOH. Polymer 1998, 39, 4295–4302. [Google Scholar] [CrossRef]
- Bunn, C.W. Crystal Structure of Polyvinyl Alcohol. Nature 1948, 161, 929–930. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M.; Bagheri, A.; Salarizadeh, P.; Zahmatkesh, H.G.; Kashefi, S.; Kowsari, E. Novel nanocomposite membranes based on blended sulfonated poly(ether ether ketone)/poly(vinyl alcohol) containing sulfonated graphene oxide/Fe3O4 nanosheets for DMFC applications. RSC Adv. 2015, 5, 74054–74064. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Hussein, S.A.; Ahmed, H.M. Effect of PVA Blending on Structural and Ion Transport Properties of CS:AgNt-Based Polymer Electrolyte Membrane. Polymers 2017, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Gopi, K.H.; Dhavale, V.M.; Bhat, S.D. Development of polyvinyl alcohol/chitosan blend anion exchange membrane with mono and di quaternizing agents for application in alkaline polymer electrolyte fuel cells. Mater. Sci. Energy Technol. 2019, 2, 194–202. [Google Scholar] [CrossRef]
- Shabanpanah, S.; Omrani, A.; Lakouraj, M.M. Fabrication and characterization of PVA/NNSA/GLA/nano-silica proton conducting composite membranes for DMFC applications. Des. Monomers Polym. 2019, 22, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C. Fabrication and characterization of poly(vinyl alcohol)/montmorillonite/poly(styrene sulfonic acid) proton-conducting composite membranes for direct methanol fuel cells. Int. J. Hydrogen Energy 2011, 36, 4419–4431. [Google Scholar] [CrossRef]
- Chuang, W.-Y.; Young, T.-H.; Chiu, W.-Y.; Lin, C.-Y. The effect of polymeric additives on the structure and permeability of poly(vinyl alcohol) asymmetric membranes. Polymer 2000, 41, 5633–5641. [Google Scholar] [CrossRef]
- Yang, T. Preliminary study of SPEEK/PVA blend membranes for DMFC applications. Int. J. Hydrogen Energy 2008, 33, 6772–6779. [Google Scholar] [CrossRef]
- Gao, L.; Kong, T.; Guo, G.; Huo, Y. Proton conductive and low methanol permeable PVA-based zwitterionic membranes. Int. J. Hydrogen Energy 2016, 41, 20373–20384. [Google Scholar] [CrossRef]
- Hren, M.; Božič, M.; Fakin, D.; Kleinschek, K.S.; Gorgieva, S. Alkaline membrane fuel cells: Anion exchange membranes and fuels. Sustain. Energy Fuels 2021, 5, 604–637. [Google Scholar] [CrossRef]
- The Fuel Cell Industry Review 2019. France Hydrogène. Available online: https://www.france-hydrogene.org/publication/the-fuel-cell-industry-review-2019/ (accessed on 18 January 2024).
- Boddu, R.; Marupakula, U.K.; Summers, B.; Majumdar, P. Development of bipolar plates with different flow channel configurations for fuel cells. J. Power Sources 2009, 189, 1083–1092. [Google Scholar] [CrossRef]
- Mancino, A.N.; Menale, C.; Vellucci, F.; Pasquali, M.; Bubbico, R. PEM Fuel Cell Applications in Road Transport. Energies 2023, 16, 6129. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells. In Fuel Cell Technology: Reaching Towards Commercialization; in Engineering Materials and Processes; Sammes, N., Ed.; Springer: London, UK, 2006; pp. 27–51. [Google Scholar] [CrossRef]
- Proton Exchange Membrane Fuel Cell Bipolar Plate Analyses by GD-OES and Raman. Available online: https://www.horiba.com/aut/scientific/applications/energy/pages/proton-exchange-membrane-fuel-cell-bipolar-plate-analyses-by-gd-oes-and-raman/ (accessed on 18 January 2024).
- Pourrahmani, H.; Siavashi, M.; Yavarinasab, A.; Matian, M.; Chitgar, N.; Wang, L.; Van Herle, J. A Review on the Long-Term Performance of Proton Exchange Membrane Fuel Cells: From Degradation Modeling to the Effects of Bipolar Plates, Sealings, and Contaminants. Energies 2022, 15, 5081. [Google Scholar] [CrossRef]
- ERM. The Fuel Cell Industry Review. Available online: https://www.erm.com/service/capabilities/energy-climate-change/the-fuel-cell-industry-review/ (accessed on 25 May 2024).
- Zhang, Y.; Tu, Z. Flow-field design of the bipolar plates in polymer electrolyte membrane fuel cell: Problem, progress, and perspective. Appl. Energy Combust. Sci. 2024, 17, 100244. [Google Scholar] [CrossRef]
- de Bruijn, A.F.; Janssen, G.J.M. PEM Fuel Cell Materials: Costs, Performance and Durability. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2012; pp. 7694–7730. [Google Scholar] [CrossRef]
- Yang, D.; Tan, Y.; Li, B.; Ming, P.; Xiao, Q.; Zhang, C. A Review of the Transition Region of Membrane Electrode Assembly of Proton Exchange Membrane Fuel Cells: Design, Degradation, and Mitigation. Membranes 2022, 12, 306. [Google Scholar] [CrossRef]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef]
- Chae, J.E.; Yoo, S.J.; Kim, J.Y.; Jang, J.H.; Lee, S.Y.; Song, K.H.; Kim, H.-J. Hydrocarbon-based electrode ionomer for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2020, 45, 32856–32864. [Google Scholar] [CrossRef]
- Hydrocarbon-based PEM and polymer exceed industry durability targets. Membr. Technol. 2023, 2023, 1873–4049. [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Khalid, M.; Walvekar, R. Development of Poly(Vinyl Alcohol)-Based Polymers as Proton Exchange Membranes and Challenges in Fuel Cell Application: A Review. Polym. Rev. 2019, 60, 171–202. [Google Scholar] [CrossRef]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite Polymers Development and Application for Polymer Electrolyte Membrane Technologies—A Review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef] [PubMed]
- Mollá, S.; Compañ, V.; Gimenez, E.; Blazquez, A.; Urdanpilleta, I. Novel ultrathin composite membranes of Nafion/PVA for PEMFCs. Int. J. Hydrogen Energy 2011, 36, 9886–9895. [Google Scholar] [CrossRef]
- Yu, T.L.; Lin, H.-L.; Shen, K.-S.; Huang, L.-N.; Chang, Y.-C.; Jung, G.-B.; Huang, J.C. Nafion/PTFE Composite Membranes for Fuel Cell Applications. J. Polym. Res. 2004, 11, 217–224. [Google Scholar] [CrossRef]
- Yagizatli, Y.; Ulas, B.; Sahin, A.; Ar, I. Preparation and Characterization of SPEEK–PVA Blend Membrane Additives with Colloidal Silica for Proton Exchange Membrane Fuel Cell. J. Polym. Environ. 2024. [Google Scholar] [CrossRef]
- Muhmed, S.; Jaafar, J.; Ahmad, S.; Mohamed, M.; Ismail, A.; Ilbeygi, H.; Othman, M.; Rahman, M.A. Incorporating functionalized graphene oxide in green material-based membrane for proton exchange membrane fuel cell application. J. Environ. Chem. Eng. 2023, 11, 109547. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M.; Badiei, A. Cross-linked poly(vinyl alcohol)/sulfonated nanoporous silica hybrid membranes for proton exchange membrane fuel cell. J. Nanostruct. Chem. 2014, 4, 97. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M.; Kowsari, E. Synthesis and Characterization of Poly(vinyl alcohol)/Sulfonated Graphene Oxide Nanocomposite Membranes for Use in Proton Exchange Membrane Fuel Cells (PEMFCs). Ind. Eng. Chem. Res. 2014, 53, 16621–16632. [Google Scholar] [CrossRef]
- Sahin, A. The development of Speek/Pva/Teos blend membrane for proton exchange membrane fuel cells. Electrochim. Acta 2018, 271, 127–136. [Google Scholar] [CrossRef]
- Ebenezer, D.; Haridoss, P. Effect of crosslinked poly(vinyl alcohol)/sulfosuccinic acid ionomer loading on PEMFC electrode performance. Int. J. Hydrogen Energy 2017, 42, 4302–4310. [Google Scholar] [CrossRef]
- Ebenezer, D.; Deshpande, A.P.; Haridoss, P. Cross-linked poly (vinyl alcohol)/sulfosuccinic acid polymer as an electrolyte/electrode material for H2–O2 proton exchange membrane fuel cells. J. Power Sources 2016, 304, 282–292. [Google Scholar] [CrossRef]
- Erkartal, M.; Usta, H.; Citir, M.; Sen, U. Proton conducting poly(vinyl alcohol) (PVA)/poly(2-acrylamido-2-methylpropane sulfonic acid) (PAMPS)/zeolitic imidazolate framework (ZIF) ternary composite membrane. J. Membr. Sci. 2016, 499, 156–163. [Google Scholar] [CrossRef]
- Stoševski, I.; Krstić, J.; Vokić, N.; Radosavljević, M.; Popović, Z.K.; Miljanić, Š. Improved Poly(vinyl alcohol) (PVA) based matrix as a potential solid electrolyte for electrochemical energy conversion devices, obtained by gamma irradiation. Energy 2015, 90, 595–604. [Google Scholar] [CrossRef]
- González-Guisasola, C.; Ribes-Greus, A. Dielectric relaxations and conductivity of cross-linked PVA/SSA/GO composite membranes for fuel cells. Polym. Test. 2018, 67, 55–67. [Google Scholar] [CrossRef]
- Abd Ali, Z.D.; Aliami, S.A.; Jalal, N.M.; Ali, M.R. Solfonating PVA membrane for PEM hydrogen fuel cell. In Proceedings of the 2018 9th International Renewable Energy Congress (IREC), Hammamet, Tunisia, 20–22 March 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Erkartal, M.; Aslan, A.; Erkilic, U.; Dadi, S.; Yazaydin, O.; Usta, H.; Sen, U. Anhydrous proton conducting poly(vinyl alcohol) (PVA)/poly(2-acrylamido-2-methylpropane sulfonic acid) (PAMPS)/1,2,4-triazole composite membrane. Int. J. Hydrogen Energy 2016, 41, 11321–11330. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, K.; Qian, G.; Benicewicz, B.C. Phosphoric acid-imbibed three-dimensional polyacrylamide/poly(vinyl alcohol) hydrogel as a new class of high-temperature proton exchange membrane. J. Power Sources 2013, 229, 36–41. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, J.; Zhang, Y.; Liu, J.; Chen, J.; Li, M.; Wang, W.; Liu, X. Overcoming undesired fuel crossover: Goals of methanol-resistant modification of polymer electrolyte membranes. Renew. Sustain. Energy Rev. 2021, 138, 110660. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.; Jung, B. Proton conductivities and methanol permeabilities of membranes made from partially sulfonated polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene copolymers. J. Membr. Sci. 2002, 207, 129–137. [Google Scholar] [CrossRef]
- Mohamed, R. Synthesis and Characterisation of Proton Conducting Membranes for Direct Methanol Fuel Cell (DMFC) Applications. Master’s Thesis, University of the Western Cape, Cape Town, South Africa, 2005. Available online: https://etd.uwc.ac.za:443/xmlui/handle/11394/2089 (accessed on 19 January 2024).
- Bockris, J.O.; Srinivasan, S. Fuel Cells: Their Electrochemistry; McGraw-Hill: New York, NY, USA, 1969; Available online: http://books.google.com/books?id=DIU-AAAAIAAJ (accessed on 19 January 2024).
- Rhim, J.-W.; Yeom, C.-K.; Kim, S.-W. Modification of poly(vinyl alcohol) membranes using sulfur-succinic acid and its application to pervaporation separation of water-alcohol mixtures. J. Appl. Polym. Sci. 1998, 68, 1717–1723. [Google Scholar] [CrossRef]
- Chiang, W.; Hu, C. Separation of liquid mixtures by using polymer membranes. I. Water–alcohol separation by pervaporation through PVA-g-MMA/MA membrane. J. Appl. Polym. Sci. 1991, 43, 2005–2012. [Google Scholar] [CrossRef]
- Hamaya, T.; Inoue, S.; Qiao, J.; Okada, T. Novel proton-conducting polymer electrolyte membranes based on PVA/PAMPS/PEG400 blend. J. Power Sources 2006, 156, 311–314. [Google Scholar] [CrossRef]
- Pivovar, B.S.; Wang, Y.; Cussler, E. Pervaporation membranes in direct methanol fuel cells. J. Membr. Sci. 1999, 154, 155–162. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, H.B.; Rhim, J.W.; Lee, Y.M. Proton conductivity and methanol transport behavior of cross-linked PVA/PAA/silica hybrid membranes. Solid State Ion. 2005, 176, 117–126. [Google Scholar] [CrossRef]
- Guiver, M.D.; Seo, M.Y.; Cho, H.I.; Kim, D.H.; Rhim, J.W.; Moon, G.Y.; Nam, S.Y. Influence of silica content in crosslinked PVA/PSSA_MA/Silica hybrid membrane for direct methanol fuel cell (DMFC). Macromol. Res. 2007, 15, 412–417. [Google Scholar] [CrossRef]
- Kang, M.-S.; Kim, J.H.; Won, J.; Moon, S.-H.; Kang, Y.S. Highly charged proton exchange membranes prepared by using water soluble polymer blends for fuel cells. J. Membr. Sci. 2005, 247, 127–135. [Google Scholar] [CrossRef]
- Abu-Saied, M.A.; Soliman, E.A.; Abualnaj, K.M.; El Desouky, E. Highly Conductive Polyelectrolyte Membranes Poly(vinyl alcohol)/Poly(2-acrylamido-2-methyl propane sulfonic acid) (PVA/PAMPS) for Fuel Cell Application. Polymers 2021, 13, 2638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Y.; Chen, D.; Jin, Q.; Chen, J.; Cao, Y. Preparation of phosphotungstic acid hybrid proton exchange membranes by constructing proton transport channels for direct methanol fuel cells. Polymer 2023, 265, 125589. [Google Scholar] [CrossRef]
- Lue, S.J.; Wang, W.-T.; Mahesh, K.; Yang, C.-C. Enhanced performance of a direct methanol alkaline fuel cell (DMAFC) using a polyvinyl alcohol/fumed silica/KOH electrolyte. J. Power Sources 2010, 195, 7991–7999. [Google Scholar] [CrossRef]
- Seeponkai, N.; Wootthikanokkhan, J. Proton conductivity and methanol permeability of sulfonated poly(vinyl alcohol) membranes modified by using sulfoacetic acid and poly(acrylic acid). J. Appl. Polym. Sci. 2007, 105, 838–845. [Google Scholar] [CrossRef]
- Khalaf, M.; Saeed, A.M.; Ali, A.I.; Kamoun, E.A.; Fahmy, A. Polyelectrolyte membranes based on phosphorylated-PVA/cellulose acetate for direct methanol fuel cell applications: Synthesis, instrumental characterization, and performance testing. Sci. Rep. 2023, 13, 13011. [Google Scholar] [CrossRef]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Dicks, A.L.; Rand, D.A.J. Fuel Cell Systems Explained; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Brouzgou, A.; Podias, A.; Tsiakaras, P. PEMFCs and AEMFCs directly fed with ethanol: A current status comparative review. J. Appl. Electrochem. 2013, 43, 119–136. [Google Scholar] [CrossRef]
- Lin, B.Y.; Kirk, D.W.; Thorpe, S.J. Performance of alkaline fuel cells: A possible future energy system? J. Power Sources 2006, 161, 474–483. [Google Scholar] [CrossRef]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Kariduraganavar, M.; Nagarale, R.; Kittur, A.; Kulkarni, S. Ion-exchange membranes: Preparative methods for electrodialysis and fuel cell applications. Desalination 2006, 197, 225–246. [Google Scholar] [CrossRef]
- Fu, J.; Qiao, J.; Wang, X.; Ma, J.; Okada, T. Alkali doped poly(vinyl alcohol) for potential fuel cell applications. Synth. Met. 2010, 160, 193–199. [Google Scholar] [CrossRef]
- Wen, N.; Jiang, B.; Wang, X.; Shang, Z.; Jiang, D.; Zhang, L.; Sun, C.; Wu, Z.; Yan, H.; Liu, C.; et al. Overview of Polyvinyl Alcohol Nanocomposite Hydrogels for Electro-Skin, Actuator, Supercapacitor and Fuel Cell. Chem. Rec. 2020, 20, 773–792. [Google Scholar] [CrossRef]
- Samsudin, A.M.; Wolf, S.; Roschger, M.; Hacker, V. Poly(vinyl alcohol)-Based Anion Exchange Membranes for Alkaline Direct Ethanol Fuel Cells. Int. J. Renew. Energy Dev. 2021, 10, 435–443. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Qiao, J.; Baker, R.; Zhang, J. Alkaline polymer electrolyte membranes for fuel cell applications. Chem. Soc. Rev. 2013, 42, 5768–5787. [Google Scholar] [CrossRef]
- Cheng, J.; He, G.; Zhang, F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int. J. Hydrogen Energy 2015, 40, 7348–7360. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, R.; An, L.; Li, Y. Alkaline anion exchange membrane fuel cells for cogeneration of electricity and valuable chemicals. J. Power Sources 2017, 365, 430–445. [Google Scholar] [CrossRef]
- Higa, M.; Mehdizadeh, S.; Feng, S.; Endo, N.; Kakihana, Y. Cell performance of direct methanol alkaline fuel cell (DMAFC) using anion exchange membranes prepared from PVA-Based block copolymer. J. Membr. Sci. 2020, 597, 117618. [Google Scholar] [CrossRef]
- Truong, V.M.; Duong, N.B.; Wang, C.-L.; Yang, H. Effects of Cell Temperature and Reactant Humidification on Anion Exchange Membrane Fuel Cells. Materials 2019, 12, 2048. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. A review of quaternized polyvinyl alcohol as an alternative polymeric membrane in DMFCs and DEFCs. Int. J. Energy Res. 2020, 44, 6223–6239. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K. Performance of quaternized poly(vinyl alcohol)-based electrolyte membrane in passive alkaline DEFCs application: RSM optimization approach. J. Appl. Polym. Sci. 2019, 136, 47526. [Google Scholar] [CrossRef]
- Quaternary Ammonium Functionalized Poly(Arylene Ether Sulfone) Copolymer Ionomers: Synthesis, Processing, and Structure-property Relationships—ProQuest. Available online: https://www.proquest.com/openview/7c1569804803b661a0f05e9a82122a65/1?cbl=18750&diss=y&pq-origsite=gscholar (accessed on 22 January 2024).
- Jiang, X.; Sun, Y.; Zhang, H.; Hou, L. Preparation and characterization of quaternized poly(vinyl alcohol)/chitosan/MoS2 composite anion exchange membranes with high selectivity. Carbohydr. Polym. 2018, 180, 96–103. [Google Scholar] [CrossRef]
- Kumar, S.R.; Wang, J.-J.; Lue, S.J. An effective alkaline solid electrolyte with magnetic field-aligned Fe3O4–GO nanofillers in a polybenzimidazole membrane for methanol fuel cells. J. Power Sources 2023, 580, 233416. [Google Scholar] [CrossRef]
- Lin, J.-S.; Kumar, S.R.; Ma, W.-T.; Shih, C.-M.; Teng, L.-W.; Yang, C.-C.; Lue, S.J. Gradiently distributed iron oxide@graphene oxide nanofillers in quaternized polyvinyl alcohol composite to enhance alkaline fuel cell power density. J. Membr. Sci. 2017, 543, 28–39. [Google Scholar] [CrossRef]
- Yang, W.; Xu, P.; Li, X.; Xie, Y.; Liu, Y.; Zhang, B.; Zhang, Q.; Yan, Y. Mechanically robust semi-interpenetrating polymer network via thiol-ene chemistry with enhanced conductivity for anion exchange membranes. Int. J. Hydrogen Energy 2021, 46, 10377–10388. [Google Scholar] [CrossRef]
- Zhang, K.; McDonald, M.B.; Genina, I.E.A.; Hammond, P.T. A Highly Conductive and Mechanically Robust OH− Conducting Membrane for Alkaline Water Electrolysis. Chem. Mater. 2018, 30, 6420–6430. [Google Scholar] [CrossRef]
- Khan, M.I.; Mondal, A.N.; Tong, B.; Jiang, C.; Emmanuel, K.; Yang, Z.; Wu, L.; Xu, T. Development of BPPO-based anion exchange membranes for electrodialysis desalination applications. Desalination 2016, 391, 61–68. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Preparation of anion exchange membrane by efficient functionalization of polysulfone for electrodialysis. J. Membr. Sci. 2020, 596, 117591. [Google Scholar] [CrossRef]
- Vatanpour, V.; Teber, O.; Mehrabi, M.; Koyuncu, I. Polyvinyl alcohol-based separation membranes: A comprehensive review on fabrication techniques, applications and future prospective. Mater. Today Chem. 2023, 28, 101381. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Aiswarya, S.K.; Joseph, S. Synthesis of methanol blocking PVA-TiO2 cation exchange membrane for direct methanol alkaline fuel cell. Synth. Met. 2020, 266, 116442. [Google Scholar] [CrossRef]
- Karim, S.S.; Farrukh, S.; Hussain, A.; Younas, M.; Noor, T. The influence of polymer concentration on the morphology and mechanical properties of asymmetric polyvinyl alcohol (PVA) membrane for O2/N2 separation. Polym. Polym. Compos. 2022, 30, 09673911221090053. [Google Scholar] [CrossRef]
- Wang, X.; Fang, D.; Yoon, K.; Hsiao, B.S.; Chu, B. High performance ultrafiltration composite membranes based on poly(vinyl alcohol) hydrogel coating on crosslinked nanofibrous poly(vinyl alcohol) scaffold. J. Membr. Sci. 2006, 278, 261–268. [Google Scholar] [CrossRef]
- Sidharthan, K.A.; Joseph, S. Preparation and characterization of polyvinyl alcohol based nanocomposite membrane for direct methanol fuel cell. AIP Conf. Proc. 2019, 2162, 020143. [Google Scholar] [CrossRef]
- Khalid, F.; Roy, A.S.; Parveen, A.; Castro-Muñoz, R. Fabrication of the cross-linked PVA/TiO2/C nanocomposite membrane for alkaline direct methanol fuel cells. Mater. Sci. Eng. B 2024, 299, 116929. [Google Scholar] [CrossRef]
- Wibisono, Y.; Anggraeni, E.T.; Argo, B.D.; Nugroho, W.A.; Maharsih, I.K.; Bilad, M.R. Environmentally-safe anion exchange membranes of PVA/PDDA/SiO2composite for reverse electrodialysis. Int. J. Thermofluids 2023, 18, 100350. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Anton, F. Process and Apparatus for Preparing Artificial Threads. US1975504A, 2 October 1934. Available online: https://patents.google.com/patent/US1975504A/en (accessed on 16 January 2024).
- Mu’Min, M.S.; Komma, M.; Abbas, D.; Wagner, M.; Krieger, A.; Thiele, S.; Böhm, T.; Kerres, J. Electrospun phosphonated poly(pentafluorostyrene) nanofibers as a reinforcement of Nafion membranes for fuel cell application. J. Membr. Sci. 2023, 685, 121915. [Google Scholar] [CrossRef]
- Ge, J.C.; Wu, G.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Study on the Preparation and Lipophilic Properties of Polyvinyl Alcohol (PVA) Nanofiber Membranes via Green Electrospinning. Nanomaterials 2021, 11, 2514. [Google Scholar] [CrossRef]
- Zizhou, R.E.; Çay, A.; Kumbasar, E.P.A.; Çolpan, C. Production of poly(vinyl alcohol)/Nafion® nanofibers and their stability assessment for the use in direct methanol fuel cells. J. Ind. Text. 2021, 50, 773–793. [Google Scholar] [CrossRef]
- Liao, G.-M.; Li, P.-C.; Lin, J.-S.; Ma, W.-T.; Yu, B.-C.; Li, H.-Y.; Liu, Y.-L.; Yang, C.-C.; Shih, C.-M.; Lue, S.J. Highly conductive quasi-coaxial electrospun quaternized polyvinyl alcohol nanofibers and composite as high-performance solid electrolytes. J. Power Sources 2016, 304, 136–145. [Google Scholar] [CrossRef]
- Barlybayeva, A. Deep Eutectic Solvent Supported Polymer-Based High Performance Anion Exchange Membrane for Alkaline Fuel Cells. 2024. Available online: https://nur.nu.edu.kz/handle/123456789/7677 (accessed on 2 January 2024).
- Gómez, E.E.R.; Hernandez, J.H.M. Obtaining Electrospun Membranes of Chitosan/PVA and TiO2 as a Solid Polymer Electrolyte with Potential Application in Ion Exchange Membranes. Membranes 2023, 13, 862. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: An overview. Int. J. Energy Res. 2019, 43, 2756–2794. [Google Scholar] [CrossRef]
- Zhong, S.; Cui, X.; Cai, H.; Fu, T.; Zhao, C.; Na, H. Crosslinked sulfonated poly(ether ether ketone) proton exchange membranes for direct methanol fuel cell applications. J. Power Sources 2007, 164, 65–72. [Google Scholar] [CrossRef]
- Amnuaypanich, S.; Patthana, J.; Phinyocheep, P. Mixed matrix membranes prepared from natural rubber/poly(vinyl alcohol) semi-interpenetrating polymer network (NR/PVA semi-IPN) incorporating with zeolite 4A for the pervaporation dehydration of water–ethanol mixtures. Chem. Eng. Sci. 2009, 64, 4908–4918. [Google Scholar] [CrossRef]
- Xiao, S.; Huang, R.Y.; Feng, X. Preparation and properties of trimesoyl chloride crosslinked poly(vinyl alcohol) membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2006, 286, 245–254. [Google Scholar] [CrossRef]
- Rosiles-González, V.; Le Lagadec, R.; Varguez-Catzim, P.; Loria-Bastarrachea, M.I.; González-Díaz, A.; Hernández-Núñez, E.; Aguilar-Vega, M.; González-Díaz, M.O. Preparation and Characterization of Strongly Sulfonated Acid Block and Random Copolymer Membranes for Acetic Acid Esterification with 2-Propanol. Polymers 2022, 14, 2595. [Google Scholar] [CrossRef]
- Qiao, J.; Hamaya, T.; Okada, T. Chemically Modified Poly(vinyl alcohol)−Poly(2-acrylamido-2-methyl-1-propanesulfonic acid) as a Novel Proton-Conducting Fuel Cell Membrane. Chem. Mater. 2005, 17, 2413–2421. [Google Scholar] [CrossRef]
- Higa, M.; Feng, S.; Endo, N.; Kakihana, Y. Characteristics and direct methanol fuel cell performance of polymer electrolyte membranes prepared from poly(vinyl alcohol-b-styrene sulfonic acid). Electrochim. Acta 2015, 153, 83–89. [Google Scholar] [CrossRef]
- Sahu, A.; Selvarani, G.; Bhat, S.; Pitchumani, S.; Sridhar, P.; Shukla, A.; Narayanan, N.; Banerjee, A.; Chandrakumar, N. Effect of varying poly(styrene sulfonic acid) content in poly(vinyl alcohol)–poly(styrene sulfonic acid) blend membrane and its ramification in hydrogen–oxygen polymer electrolyte fuel cells. J. Membr. Sci. 2008, 319, 298–305. [Google Scholar] [CrossRef]
- Mishra, A.K.; Kuila, T.; Kim, N.H.; Lee, J.H. Polymer-Layered Silicate Nanocomposite Membranes for Fuel Cell Ap-plication. In Handbook of Polymernanocomposites; Processing, Performance and Application: Volume A: Layered Silicates; Pandey, J.K., Reddy, K.R., Mohanty, A.K., Misra, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 481–509. [Google Scholar] [CrossRef]
- Kim, D.S.; Guiver, M.D.; Nam, S.Y.; Yun, T.I.; Seo, M.Y.; Kim, S.J.; Hwang, H.S.; Rhim, J.W. Preparation of ion exchange membranes for fuel cell based on crosslinked poly(vinyl alcohol) with poly(styrene sulfonic acid-co-maleic acid). J. Membr. Sci. 2006, 281, 156–162. [Google Scholar] [CrossRef]
- Das, B.; Gaur, S.S.; Katha, A.R.; Wang, C.-T.; Katiyar, V. Crosslinked poly(vinyl alcohol) membrane as separator for domestic wastewater fed dual chambered microbial fuel cells. Int. J. Hydrogen Energy 2021, 46, 7073–7086. [Google Scholar] [CrossRef]
- Remiš, T.; Bělský, P.; Andersen, S.M.; Tomáš, M.; Kadlec, J.; Kovářík, T. Preparation and Characterization of Poly(Vinyl Alcohol) (PVA)/SiO2, PVA/Sulfosuccinic Acid (SSA) and PVA/SiO2/SSA Membranes: A Comparative Study. J. Macromol. Sci. Part B 2020, 59, 157–181. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, O.; Kim, H.; Kim, D.W.; Jeon, Y.; Ji, Y.; Jeon, O.S.; Lee, C.; Shul, Y.-G. Cross-Linked PVA/PAA Fibrous Web Composite Membrane for Enhanced Performance of PEM Fuel Cells under High-Temperature and Low-Humidity Conditions. J. Chem. Eng. Jpn. 2020, 53, 569–575. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Organic–inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed Matrix Membranes (MMMs) Comprising Organic Polymers with Dispersed Inorganic Fillers for Gas Separation. Scopus. Available online: https://scholarbank.nus.edu.sg/handle/10635/68310 (accessed on 23 January 2024).
- Pardhi, E.D.; Acharya, S.A.; Shirbhate, S. Development of PVA-ABO 3 organic-inorganic nanocomposite proton exchange membrane for HT-PEMFCs. Ferroelectrics 2023, 617, 134–145. [Google Scholar] [CrossRef]
- Martinelli, A. Structural Analysis of PVA-Based Proton Conducting Membranes. Solid State Ion. 2006. Available online: https://www.academia.edu/8565144/Structural_analysis_of_PVA_based_proton_conducting_membranes (accessed on 23 January 2024).
- Velayutham, P.; Sahu, A.K.; Parthasarathy, S. A Nafion-Ceria Composite Membrane Electrolyte for Reduced Methanol Crossover in Direct Methanol Fuel Cells. Energies 2017, 10, 259. [Google Scholar] [CrossRef]
- Balbaşı, M.; Gözütok, B. Poly(vinyl alcohol)-colloidal silica composite membranes for fuel cells. Synth. Met. 2010, 160, 150–155. [Google Scholar] [CrossRef]
- Vani, R.; Ramaprabhu, S.; Haridoss, P. Mechanically stable and economically viable polyvinyl alcohol-based membranes with sulfonated carbon nanotubes for proton exchange membrane fuel cells. Sustain. Energy Fuels 2020, 4, 1372–1382. [Google Scholar] [CrossRef]
- Merle, G.; Hosseiny, S.S.; Wessling, M.; Nijmeijer, K. New cross-linked PVA based polymer electrolyte membranes for alkaline fuel cells. J. Membr. Sci. 2012, 409–410, 191–199. [Google Scholar] [CrossRef]
- Li, Y.; Liang, L.; Liu, C.; Li, Y.; Xing, W.; Sun, J. Self-Healing Proton-Exchange Membranes Composed of Nafion–Poly(vinyl alcohol) Complexes for Durable Direct Methanol Fuel Cells. Adv. Mater. 2018, 30, e1707146. [Google Scholar] [CrossRef]
- Maiti, J.; Kakati, N.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Where do poly(vinyl alcohol) based membranes stand in relation to Nafion® for direct methanol fuel cell applications? J. Power Sources 2012, 216, 48–66. [Google Scholar] [CrossRef]
- Gouda, M.; Gouveia, W.; Afonso, M.; Šljukić, B.; El Essawy, N.; Nassr, A.; Santos, D. Poly(vinyl alcohol)-based crosslinked ternary polymer blend doped with sulfonated graphene oxide as a sustainable composite membrane for direct borohydride fuel cells. J. Power Sources 2019, 432, 92–101. [Google Scholar] [CrossRef]
- Gouda, M.H.; Elessawy, N.A.; Toghan, A. Development of effectively costed and performant novel cation exchange ceramic nanocomposite membrane based sulfonated PVA for direct borohydride fuel cells. J. Ind. Eng. Chem. 2021, 100, 212–219. [Google Scholar] [CrossRef]
- Kulasekaran, P.; Mahimai, B.M.; Deivanayagam, P. Novel cross-linked poly(vinyl alcohol)-based electrolyte membranes for fuel cell applications. RSC Adv. 2020, 10, 26521–26527. [Google Scholar] [CrossRef]
- Zhou, T. Application of a Novel PVA-based Proton Exchange Membrane Modified by Reactive Black KN-B for Low-temperature Fuel Cells. Int. J. Electrochem. Sci. 2019, 14, 8514–8531. [Google Scholar] [CrossRef]
- Zakil, F.A.; Kamarudin, S.K.; Basri, S. Modified Nafion membranes for direct alcohol fuel cells: An overview. Renew. Sustain. Energy Rev. 2016, 65, 841–852. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Wahid, K.A.A. Polymer electrolyte membrane modification in direct ethanol fuel cells: An update. J. Appl. Polym. Sci. 2023, 140, e53383. [Google Scholar] [CrossRef]
- Gouda, M.H.; Tamer, T.M.; Konsowa, A.H.; Farag, H.A.; Eldin, M.S.M. Organic-Inorganic Novel Green Cation Exchange Membranes for Direct Methanol Fuel Cells. Energies 2021, 14, 4686. [Google Scholar] [CrossRef]
- Tian, G. Organic-Inorganic Composite Membranes for Proton Exchange Membrane Fuel Cells. In Proton Exchange Membrane Fuel Cells; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 111–135. [Google Scholar] [CrossRef]
- Kerres, J. Blend Concepts for Fuel Cell Membranes. In Polymer Membranes for Fuel Cells; Javaid, Z.S.M., Matsuura, T., Eds.; Springer: Boston, MA, USA, 2009; pp. 1–37. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Feng, C.; Matsuura, T. The art of surface modification of synthetic polymeric membranes. J. Appl. Polym. Sci. 2010, 115, 855–895. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Gohil, J.M.; Dutta, K. Poly(vinyl alcohol)-based membranes for fuel cell and water treatment applications: A review on recent advancements. Polym. Adv. Technol. 2021, 32, 4175–4203. [Google Scholar] [CrossRef]
- Yadav, N.K.; Pramanik, H. Performance enhancement of NaOH doped physically crosslinked alkaline membrane electrolyte by addition of TEOS to PVA solution for direct sodium borohydride fuel cell application. Int. J. Hydrogen Energy 2024, 50, 1373–1394. [Google Scholar] [CrossRef]
- Hegde, S.; Munavalli, B.; Achari, D.; Gowda, R.; Kariduraganavar, M. Development of robust proton exchange membranes using a sPVA–silica composite with different crosslinkers and evaluation of their fuel cell performance. N. J. Chem. 2024, 48, 8799–8808. [Google Scholar] [CrossRef]
- Hunger, K.; Schmeling, N.; Jeazet, H.B.T.; Janiak, C.; Staudt, C.; Kleinermanns, K. Investigation of Cross-Linked and Additive Containing Polymer Materials for Membranes with Improved Performance in Pervaporation and Gas Separation. Membranes 2012, 2, 727–763. [Google Scholar] [CrossRef]
- Mukherjee, G.S. Modification of poly(vinyl alcohol) for improvement of mechanical strength and moisture resistance. J. Mater. Sci. 2005, 40, 3017–3019. [Google Scholar] [CrossRef]
- Krumova, M.; López, D.; Benavente, R.; Mijangos, C.; Pereña, J. Effect of crosslinking on the mechanical and thermal properties of poly(vinyl alcohol). Polymer 2000, 41, 9265–9272. [Google Scholar] [CrossRef]
- Li, G.; Zhang, W.; Yang, J.; Wang, X. Time-dependence of pervaporation performance for the separation of ethanol/water mixtures through poly(vinyl alcohol) membrane. J. Colloid Interface Sci. 2007, 306, 337–344. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Yagizatli, Y.; Sahin, A.; Ar, I. Effect of thermal crosslinking process on membrane structure and PEM fuel cell applications performed with SPEEK-PVA blend membranes. Int. J. Hydrogen Energy 2022, 47, 40445–40461. [Google Scholar] [CrossRef]
- Ng, W.W.; Thiam, H.S.; Pang, Y.L.; Lim, Y.S.; Wong, J.; Lai, S.O. Freeze-Thawed Nafion-Poly(vinyl alcohol) Self-healing Membranes for Direct Methanol Fuel Cells. Chem. Eng. Technol. 2023, 46, 2600–2607. [Google Scholar] [CrossRef]
- Kakati, N.; Maiti, J.; Das, G.; Lee, S.H.; Yoon, Y.S. An approach of balancing the ionic conductivity and mechanical properties of PVA based nanocomposite membrane for DMFC by various crosslinking agents with ionic liquid. Int. J. Hydrogen Energy 2015, 40, 7114–7123. [Google Scholar] [CrossRef]
- Rimpy; Ahuja, M. Chapter 6—Gum kondagogu as a potential material for micro- and nanoparticulate drug delivery. In Micro- and Nanoengineered Gum-Based Biomaterials for Drug Delivery and Biomedical Applications; in Micro and Nano Technologies; Jana, S., Jana, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 157–181. [Google Scholar] [CrossRef]
- Celli, A.; Sabaa, M.; Jyothi, A.; Kalia, S. Chitosan and Starch-Based Hydrogels Via Graft Copolymerization. In Polymeric Hydrogels as Smart Biomaterials; Springer Series on Polymer and Composite Materials; Kalia, S., Ed.; Springer: Cham, Switzerland, 2016; pp. 189–234. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Graft copolymers of natural fibers for green composites. Carbohydr. Polym. 2014, 104, 87–93. [Google Scholar] [CrossRef]
- Krishnamoorthi, S.; Singh, R.P. Synthesis, characterization, flocculation, and rheological characteristics of hydrolyzed and unhydrolyzed polyacrylamide-grafted poly(vinyl alcohol). J. Appl. Polym. Sci. 2006, 101, 2109–2122. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, Z.; Lu, D.; Wu, Q.; Lin, X. Graft copolymerization of water-soluble monomers containing quaternary ammonium group on poly(vinyl alcohol) using ceric ions. J. Appl. Polym. Sci. 2005, 97, 2186–2191. [Google Scholar] [CrossRef]
- Gupta, U.K.; Pramanik, H. A Study on Synthesis of Chemical Crosslinked Polyvinyl Alcohol-Based Alkaline Membrane for the Use in Low-Temperature Alkaline Direct Ethanol Fuel Cell. J. Electrochem. Energy Convers. Storage 2019, 16, 041005. [Google Scholar] [CrossRef]
- Awad, S.; Alomari, A.H.; Abdel-Hady, E.E.; Hamam, M.F.M. Characterization, nanostructure, and transport properties of styrene grafted PVA/SiO2 hybrid nanocomposite membranes: Positron lifetime study. Polym. Adv. Technol. 2021, 32, 1742–1751. [Google Scholar] [CrossRef]
- Danwanichakul, P.; Sirikhajornnam, P. An Investigation of Chitosan-Grafted-Poly(vinyl alcohol) as an Electrolyte Membrane. J. Chem. 2012, 2013, e642871. [Google Scholar] [CrossRef]
- Aoshima, S.; Ikeda, M.; Nakayama, K.; Kobayashi, E.; Ohgi, H.; Sato, T. Synthesis of Poly(vinyl alcohol) Graft Copolymers by Living Cationic Polymerization in the Presence of Added Bases I. Design and Synthesis of Poly(vinyl alcohol) Graft Copolymers with Well-Controlled Poly(vinyl ether) Grafts. Polym. J. 2001, 33, 610–616. [Google Scholar] [CrossRef]
- Qin, Y. 5—Applications of advanced technologies in the development of functional medical textile materials. In Medical Textile Materials; in Woodhead Publishing Series in Textiles; Qin, Y., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 55–70. [Google Scholar] [CrossRef]
- Girdhar, M.; Mohan, D.; Sharma, A. Blending Strategies of Natural Polymers: A Review. Trends Biomater. Artif. Organs 2016, 30, 61–76. [Google Scholar]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Arrighi, V.; Cowie, J.; Fuhrmann, S.; Youssef, A. Miscibility Criterion in Polymer Blends and its Determination. In Encyclopedia of Polymer Blends; Wiley: Hoboken, NJ, USA, 2016; pp. 153–198. [Google Scholar] [CrossRef]
- Srinivasa, P.; Ramesh, M.; Kumar, K. Tharanathan Properties and sorption studies of chitosan–polyvinyl alcohol blend films. Carbohydr. Polym. 2003, 53, 431–438. [Google Scholar] [CrossRef]
- Lewandowska, K. Miscibility and thermal stability of poly(vinyl alcohol)/chitosan mixtures. Thermochim. Acta 2009, 493, 42–48. [Google Scholar] [CrossRef]
- Park, S.; Jun, S.; Marsh, K. Physical properties of PVOH/chitosan-blended films cast from different solvents. Food Hydrocoll. 2001, 15, 499–502. [Google Scholar] [CrossRef]
- Qiao, J.; Hamaya, T.; Okada, T. New highly proton-conducting membrane poly(vinylpyrrolidone)(PVP) modified poly(vinyl alcohol)/2-acrylamido-2-methyl-1-propanesulfonic acid (PVA–PAMPS) for low temperature direct methanol fuel cells (DMFCs). Polymer 2005, 46, 10809–10816. [Google Scholar] [CrossRef]
- Gouda, M.H.; Elessawy, N.A.; Toghan, A. Novel Crosslinked Sulfonated PVA/PEO Doped with Phosphated Titanium Oxide Nanotubes as Effective Green Cation Exchange Membrane for Direct Borohydride Fuel Cells. Polymers 2021, 13, 2050. [Google Scholar] [CrossRef]
- Sidharthan, A.K.; Joseph, S. Green synthesis of polyvinyl-alcohol-montmorillonite clay cation exchange membrane for alkaline direct methanol fuel cell. Int. J. Hydrogen Energy 2023, 48, 39658–39672. [Google Scholar] [CrossRef]
- Samimi, F.; Rahimpour, M.R. Direct Methanol Fuel Cell. In Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 381–397. ISBN 9780444639035. [Google Scholar] [CrossRef]
- Corti, H. Membranes for Direct Alcohol Fuel Cells. In Direct Alcohol Fuel Cells: Materials, Performance, Durability and Applications; Springer: Dordrecht, The Netherlands, 2014; pp. 121–230. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, E.; Shin, G.; Han, J.; Mather, P.T. Poly(vinyl alcohol) (PVA)/sulfonated polyhedral oligosilsesquioxane (sPOSS) hybrid membranes for direct methanol fuel cell applications. Polym. Adv. Technol. 2007, 18, 535–543. [Google Scholar] [CrossRef]
- Gajbhiye, P.; Tiwari, A.; Mann, K.; Kahlon, J.; Upadhyay, H. Hybrid blend membrane using PVA–g–PMA for direct methanol fuel cell applications. Chem. Process. Eng. 2022, 43, 251–254. [Google Scholar] [CrossRef]
- Murmu, R.; Roy, D.; Patra, S.C.; Sutar, H.; Senapati, P. Preparation and characterization of the SPEEK/PVA/Silica hybrid membrane for direct methanol fuel cell (DMFC). Polym. Bull. 2022, 79, 2061–2087. [Google Scholar] [CrossRef]
- Pagidi, A.; Arthanareeswaran, G.; Seepana, M.M. Synthesis of highly stable PTFE-ZrP-PVA composite membrane for high-temperature direct methanol fuel cell. Int. J. Hydrogen Energy 2020, 45, 7829–7837. [Google Scholar] [CrossRef]
- Yagizatli, Y.; Ulas, B.; Cali, A.; Sahin, A.; Ar, I. Improved fuel cell properties of Nano-TiO2 doped Poly(Vinylidene fluoride) and phosphonated Poly(Vinyl alcohol) composite blend membranes for PEM fuel cells. Int. J. Hydrogen Energy 2020, 45, 35130–35138. [Google Scholar] [CrossRef]
- Punniakotti, G.; Sivasubramanian, G.; Thangavelu, S.A.G.; Deivanayagam, P. Sulfonated Poly(Vinyl Alcohol)/Fly Ash Composite Membranes for Polymer Electrolyte Membrane Fuel Cell Applications. Polym. Technol. Mater. 2021, 60, 571–578. [Google Scholar] [CrossRef]
- Gouda, M.; Gouveia, W.; Elessawy, N.; Šljukić, B.; Nassr, A.; Santos, D. Simple design of PVA-based blend doped with SO4(PO4)-functionalised TiO2 as an effective membrane for direct borohydride fuel cells. Int. J. Hydrogen Energy 2020, 45, 15226–15238. [Google Scholar] [CrossRef]
- Kim, J.-D.; Matsushita, S.; Tamura, K. Crosslinked Sulfonated Polyphenylsulfone-Vinylon (CSPPSU-vinylon) Membranes for PEM Fuel Cells from SPPSU and Polyvinyl Alcohol (PVA). Polymers 2020, 12, 1354. [Google Scholar] [CrossRef]
- Samsudin, A.M.; Hacker, V. Effect of Crosslinking on the Properties of QPVA/PDDA Anion Exchange Membranes for Fuel Cells Application. J. Electrochem. Soc. 2021, 168, 044526. [Google Scholar] [CrossRef]
- Yunphuttha, C.; Midpanon, S.; Marr, D.W.M.; Viravathana, P. Polyvinyl alcohol/nanocellulose nanocomposites from oil palm empty fruit bunch as anion exchange membranes for direct alcohol-hydrogen peroxide fuel cells. Cellulose 2024, 31, 1569–1601. [Google Scholar] [CrossRef]
- Hassan, M.F.; Al-Othman, A.; Tawalbeh, M.; Ka’ki, A.; Mohamad, S. Novel chitosan-ionic liquid immobilized membranes for PEM fuel cells operating above the boiling point of water. Int. J. Hydrogen Energy 2024, 53, 592–601. [Google Scholar] [CrossRef]
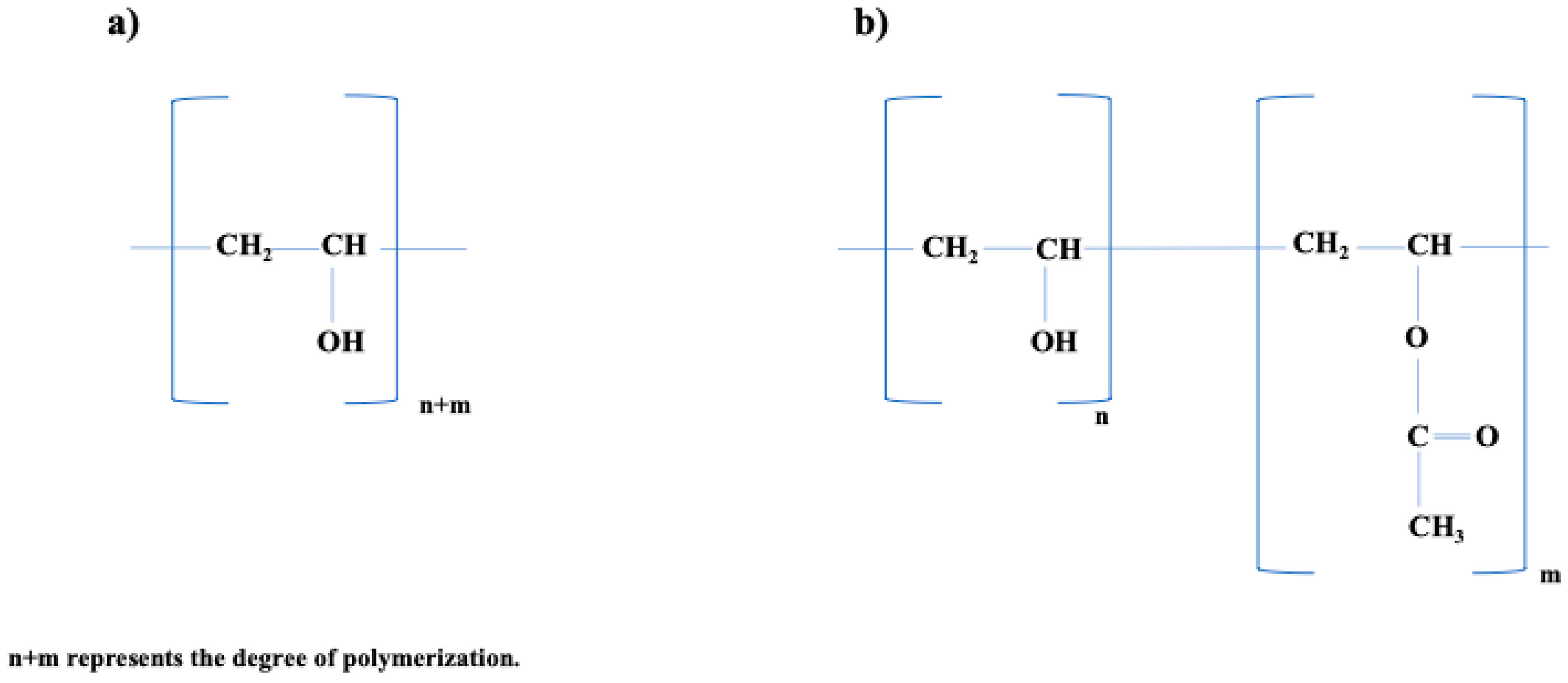

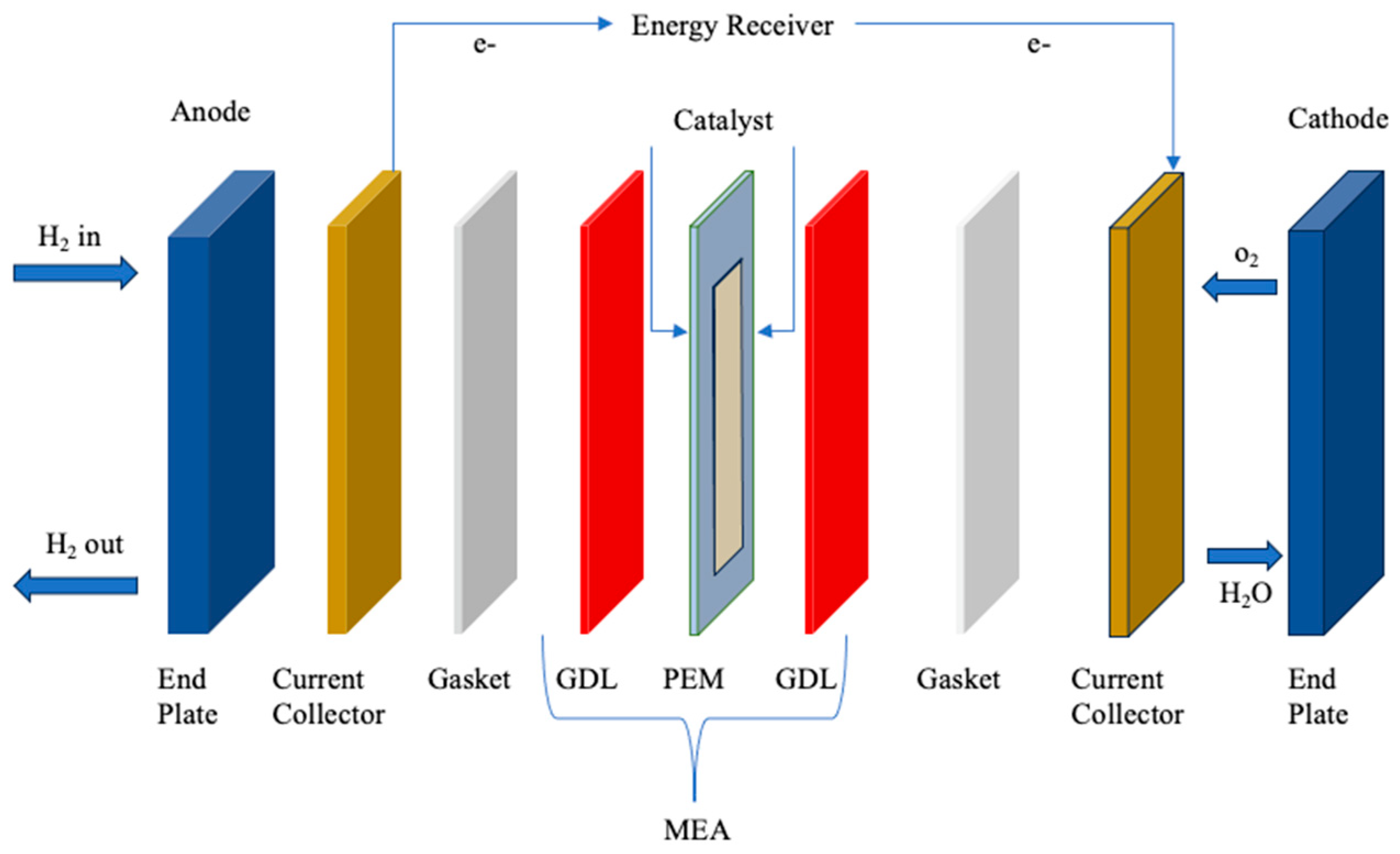
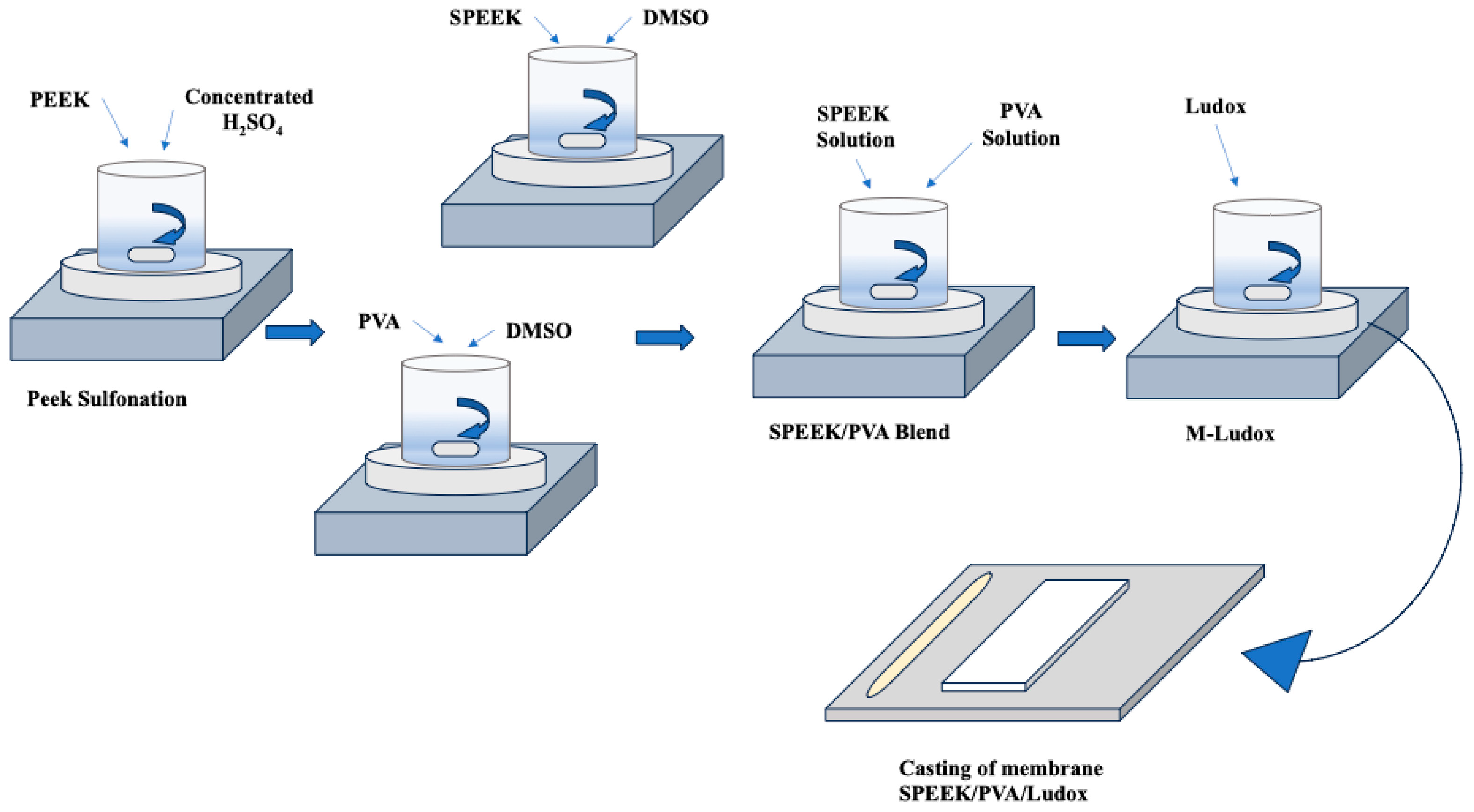

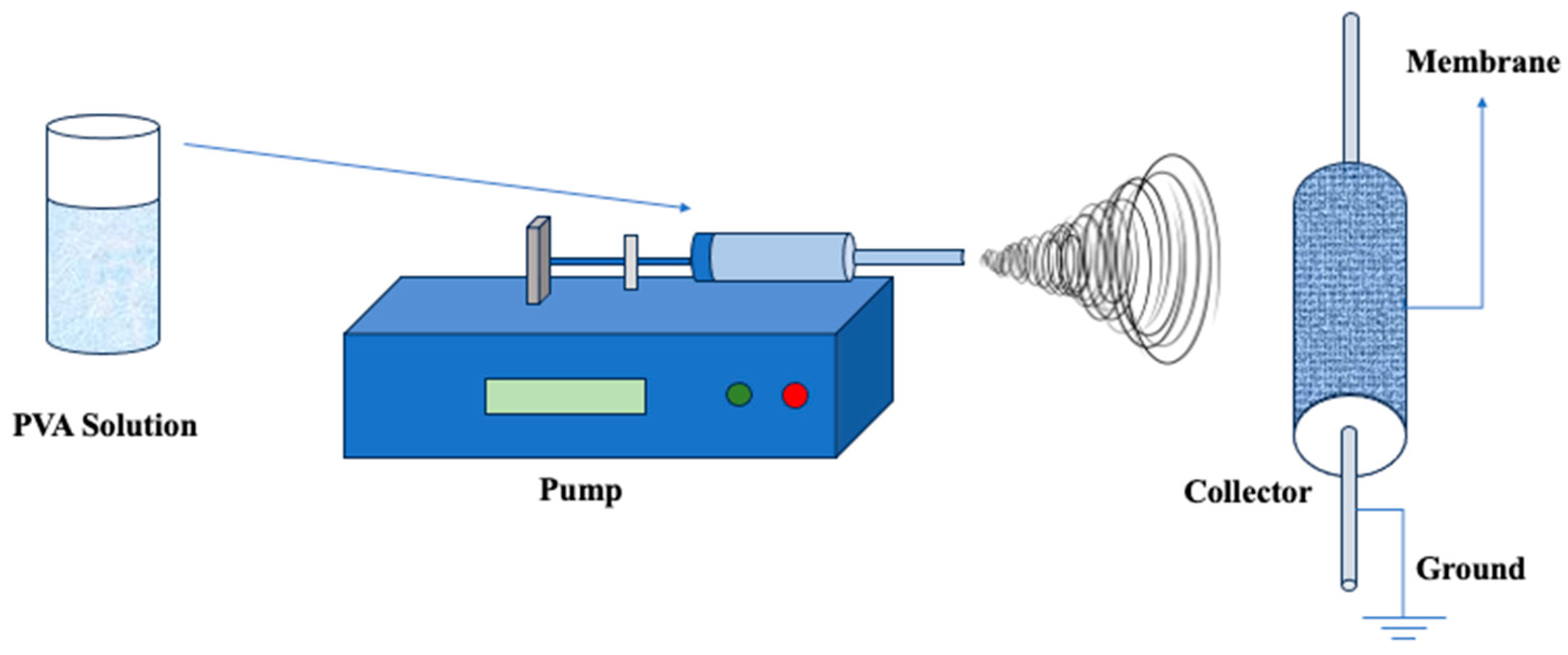
| Property | Description | References |
|---|---|---|
| Chemical Nature | Copolymer consisting of hydroxyl and acetyl units resulting from incomplete hydrolysis of poly(vinyl acetate). | [47] |
| Physical State | Odorless, tasteless, water-soluble polymer. | [34,48] |
| Crystallinity | Semi-crystalline. | [49] |
| Thermal Stability | Thermostable with melting points around 220–230 °C for fully hydrolyzed grades and 180–190 °C for partially hydrolyzed ones. | [34,50] |
| Biocompatibility | Non-toxic, biocompatible, and biodegradable in aerobic and anaerobic environments. | [51,52] |
| Optical Properties | Exhibits remarkable optical properties. | [53,54] |
| Charge Storage Ability and Dielectric Strength | Exceptional charge storage ability and high dielectric strength. | [55] |
| Solubility | Soluble in polar solvents like N-methyl-2-pyrrolidone, dimethyl sulfoxide, glycerol (hot), and others. Not soluble in lower alcohols, ketones, esters, and others. | [45,56] |
| Crystal Structure | Monoclinic crystal cell structure. | [57,58,59] |
| Membrane Composition | Methanol Permeability (cm 2/s) | Proton Conductivity (S/cm) | Ion Exchange Capacity (meq/g) | Remarks |
|---|---|---|---|---|
| Pure PVA | 3.57 × 10−7 | 3.06 × 10−3 | 0.462 | Pure PVA membrane with notable methanol barrier capability |
| PVA with sulfoacetic acid sodium salt (10 wt.%) | 5.21 × 10−9 | 0.57 × 10−3 | 0.092 | Improved methanol resistivity and moderate proton conductivity through chemical cross-linking |
| Phosphorylated PVA and grafted cellulose acetate (CA) | 1.08 × 10−10 | 0.035–0.05 | 2.1 | Low methanol permeability and enhanced proton conductivity and IEC with O-phosphoric acid (OPA) |
| PVA/PAA/SSA hybrid membrane | 10−8 to 10−7 | 10−3 to 10−2 | 0.46–1.58 | Cross-linked with sulfonic acid groups and silica particles to reduce methanol permeability while maintaining proton conductivity |
| PVA/PSSA_MA/Silica (20% TEOS) | 5.55 × 10−7 | 0.026 | 0.95 | High proton conductivity and low methanol permeability, with TEOS aiding in the formation of proton- conduction pathways |
| PVA/PSSA-MA (1:9 ratio) | 2.53 × 10−7 | 0.1 | 3.41 | Highly charged membranes with excellent proton addition |
| Membrane Composition | Ion-Exchange Capacity (mmol/g) | Tensile Strength (MPa) | Alkaline Stability | Notes | Ref. |
|---|---|---|---|---|---|
| PVA/Poly(2,6-dimethyl-1,4-phenylene oxide) (PPO) | 1.46 | 30.8 | Retains 66.5% conductivity after 1000 h in 1 M NaOH at 80 °C | High mechanical strength, relatively low swelling ratio, and peak power density of 78 mW/cm2 | [135] |
| PVA/qPPO (30% qPPO) | 1.28 | 126 (dry). 41 (wet) | Minimal permselectivity change (0.92 to 0.90) over 10 days in 6 M KOH at room temperature | High OH− conductivity, stable in alkaline water electrolysis, and retains permselectivity | [136] |
| Brominated PPO/N-methylmorpholine (NMM-18) | 1.74 | 22.2 | More stable than QPPPO membrane in 2 M NaOH at room temperature | High hydrophilicity, low membrane area resistance (1.5 Ω·cm2), and good electrodialytic performance | [137] |
| CMPSP with high DCM (1.95) | 1.65 | 0.07 | 9–13% loss in IEC after 48 h in 5.0 M KOH at 50 °C | High ion-exchange capacity, improved hydrophilicity, good desalination rate (94.5%), and low energy consumption (7.5 kWh/kg) | [138] |
| Impact Factors | Description | References |
|---|---|---|
| Mechanical Strength | Material qualities and reinforcing tactics determine mechanical strength | [176,177] |
| Reinforcement methods and fabrication procedures directly impact structural integrity | ||
| Durability | Environmental conditions during fuel cell operation affect membrane endurance | [178,179] |
| Resilience to chemical, thermal, and mechanical stresses is crucial for prolonged functionality | ||
| Processability | Influenced by material selection and fabrication techniques | [180,181] |
| Compatibility with industrial-scale production processes is a key consideration | ||
| Proton Conductivity and Swelling | PVA’s inherent lack of fixed charges and propensity to swell compromise proton conductivity and mechanical strength | [182,183] |
| Material Modifications | Enhancing proton conductivity involves combining PVA with materials possessing acid groups | [184,185] |
| Organic–Inorganic Composites | Examples include PAMPS, poly(styrene sulfonic acid), phosphor tungstic acid, or cross-linking with other substances | [186,187] |
| Addition of inorganic particles (e.g., AI22O3 and SiO2) prevents methanol crossover and improves conductivity and mechanical qualities |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhuranthakam, C.M.R.; Abudaqqa, W.S.K.; Fowler, M. Advances in Polyvinyl Alcohol-Based Membranes for Fuel Cells: A Comprehensive Review on Types, Synthesis, Modifications, and Performance Optimization. Polymers 2024, 16, 1775. https://doi.org/10.3390/polym16131775
Madhuranthakam CMR, Abudaqqa WSK, Fowler M. Advances in Polyvinyl Alcohol-Based Membranes for Fuel Cells: A Comprehensive Review on Types, Synthesis, Modifications, and Performance Optimization. Polymers. 2024; 16(13):1775. https://doi.org/10.3390/polym16131775
Chicago/Turabian StyleMadhuranthakam, Chandra Mouli R., Weam S. K. Abudaqqa, and Michael Fowler. 2024. "Advances in Polyvinyl Alcohol-Based Membranes for Fuel Cells: A Comprehensive Review on Types, Synthesis, Modifications, and Performance Optimization" Polymers 16, no. 13: 1775. https://doi.org/10.3390/polym16131775
APA StyleMadhuranthakam, C. M. R., Abudaqqa, W. S. K., & Fowler, M. (2024). Advances in Polyvinyl Alcohol-Based Membranes for Fuel Cells: A Comprehensive Review on Types, Synthesis, Modifications, and Performance Optimization. Polymers, 16(13), 1775. https://doi.org/10.3390/polym16131775








