Development of Molecularly Imprinted Magnetic Amino Acid-Based Nanoparticles for Voltammetric Analysis of Lead Ions in Honey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. Treatment of Honey Samples
2.4. Synthesis of 2-Methacryloyl-Amido Cysteine (MAC) Functional Monomer
2.5. Pb (II) Ion-Imprinted Amino Acid-Based Magnetic Polymeric Nanoparticles
2.6. Preparation of CPE Sensors
2.7. Characterization Studies
3. Results and Discussion
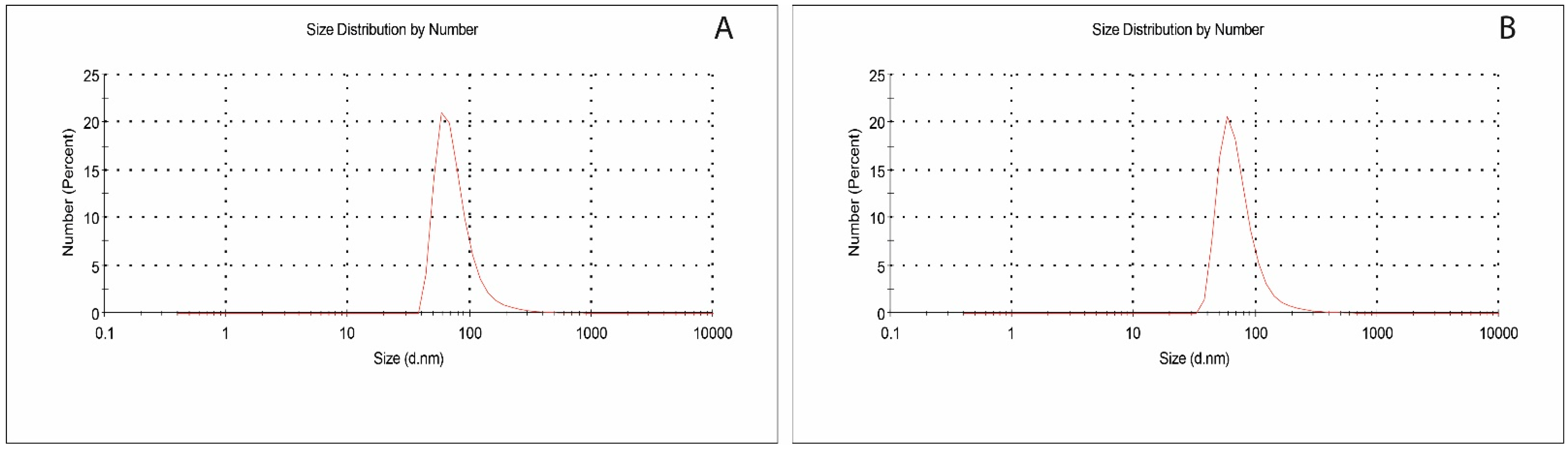
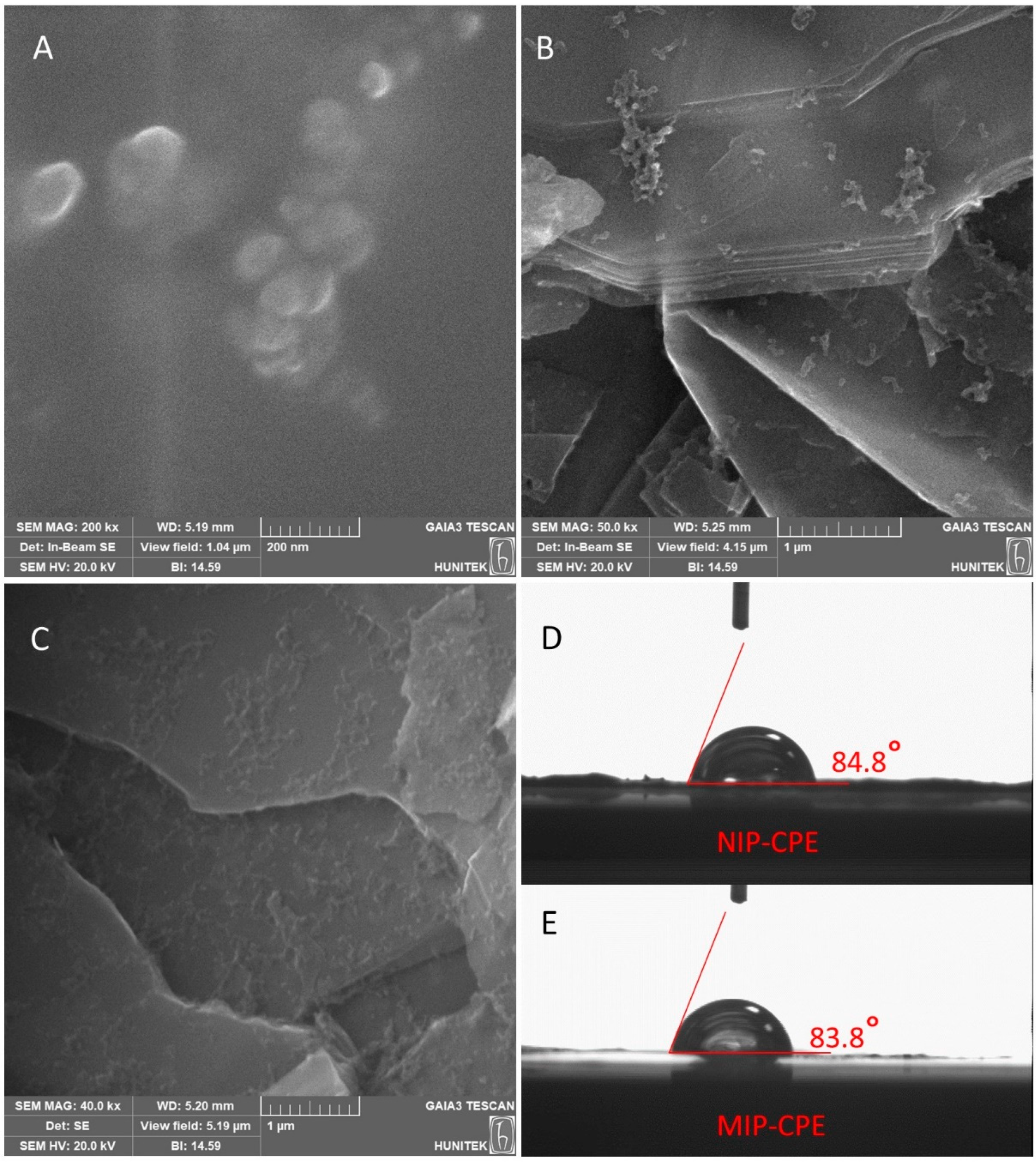
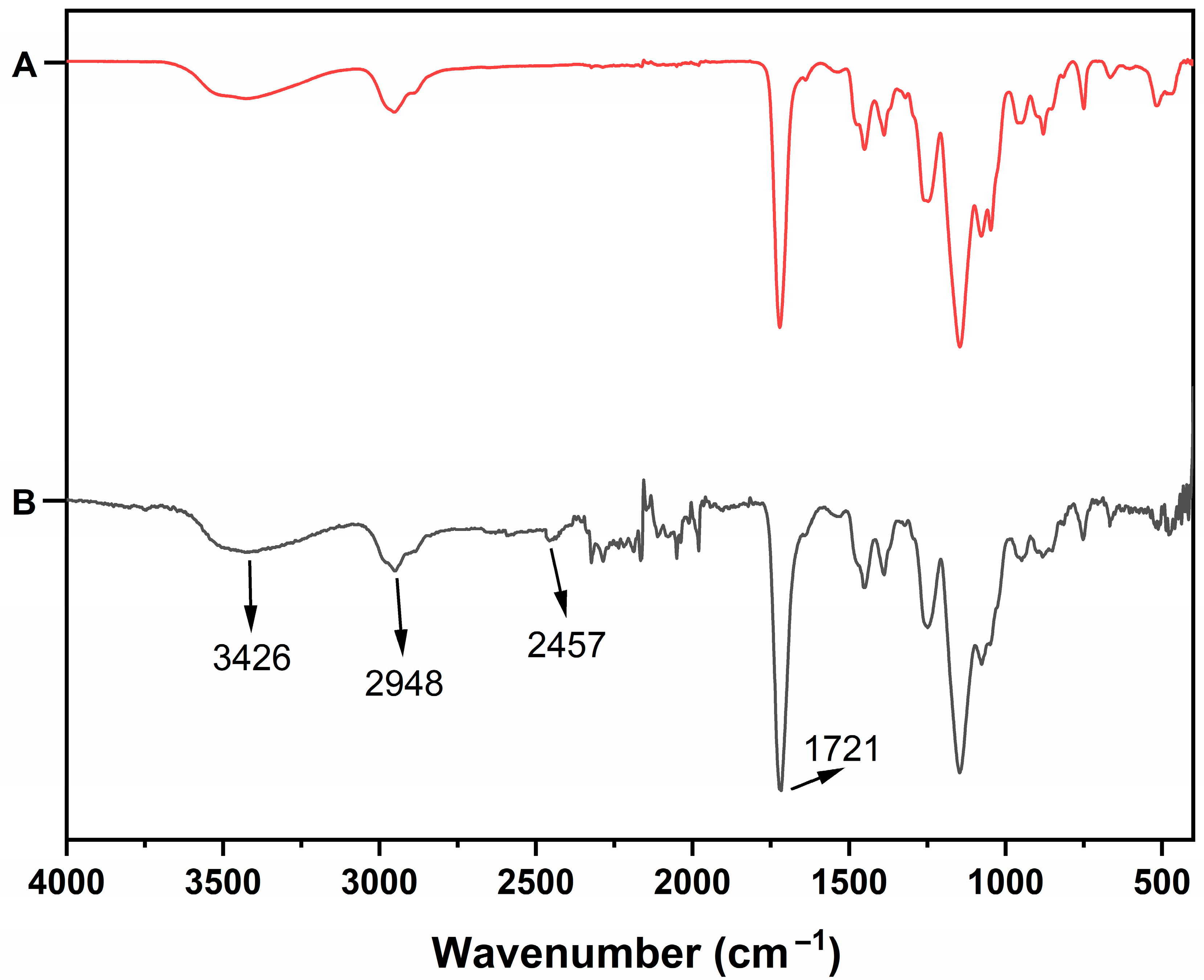
3.1. Characterization Studies
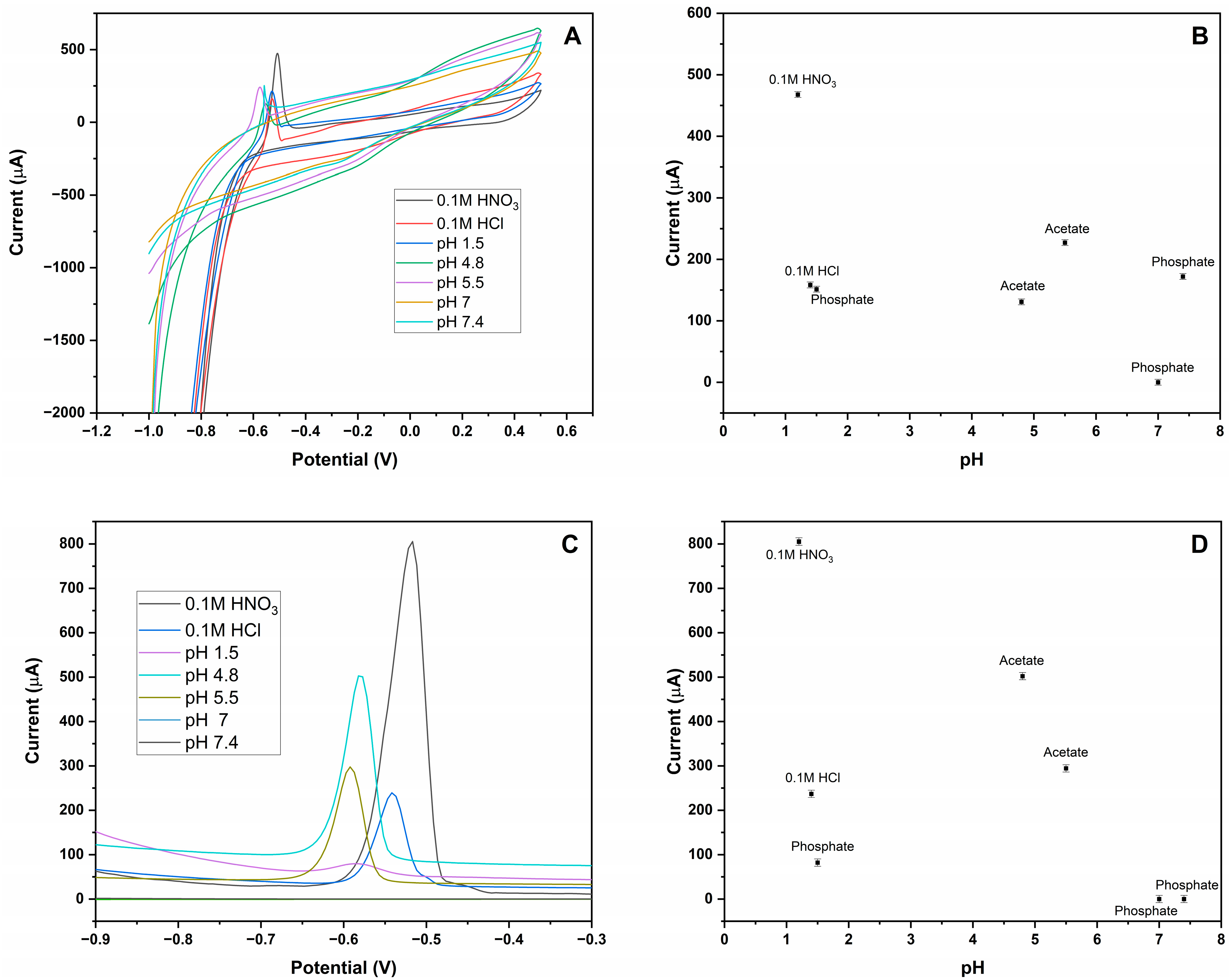
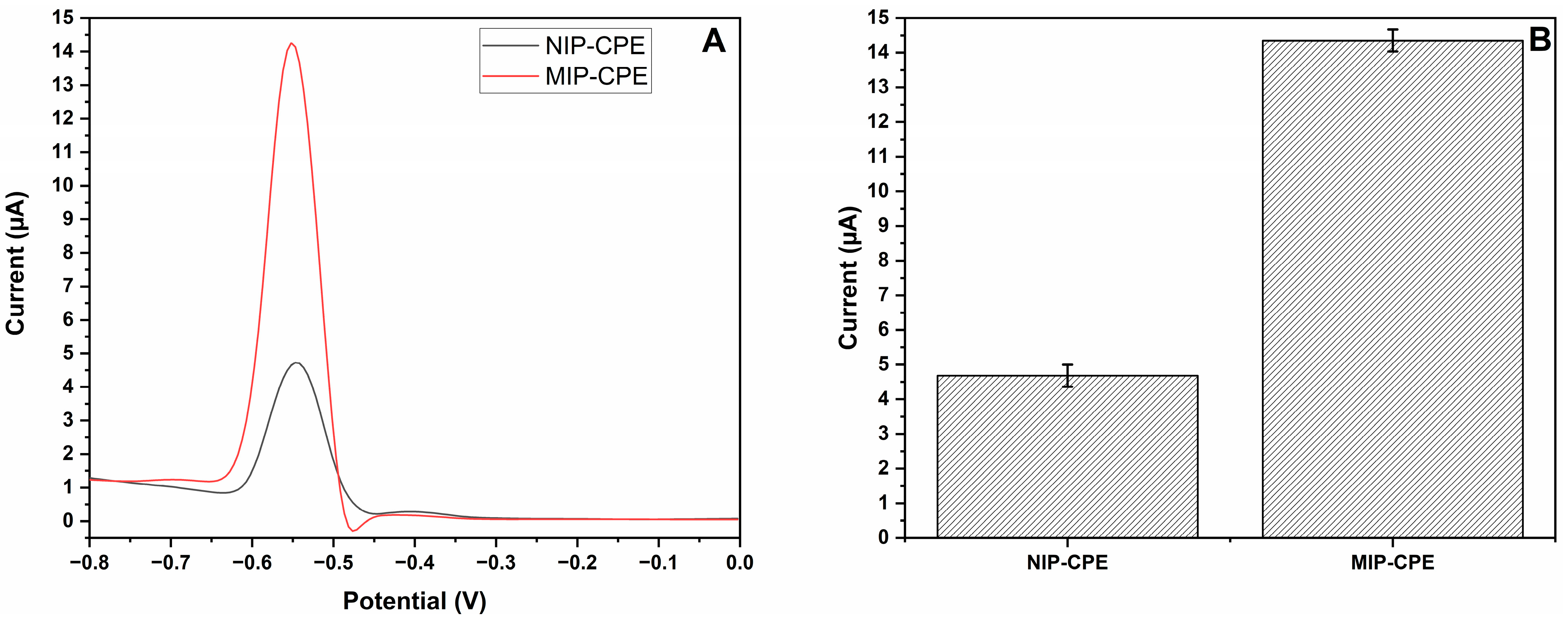
3.2. Optimization of MIP-CPE Sensor
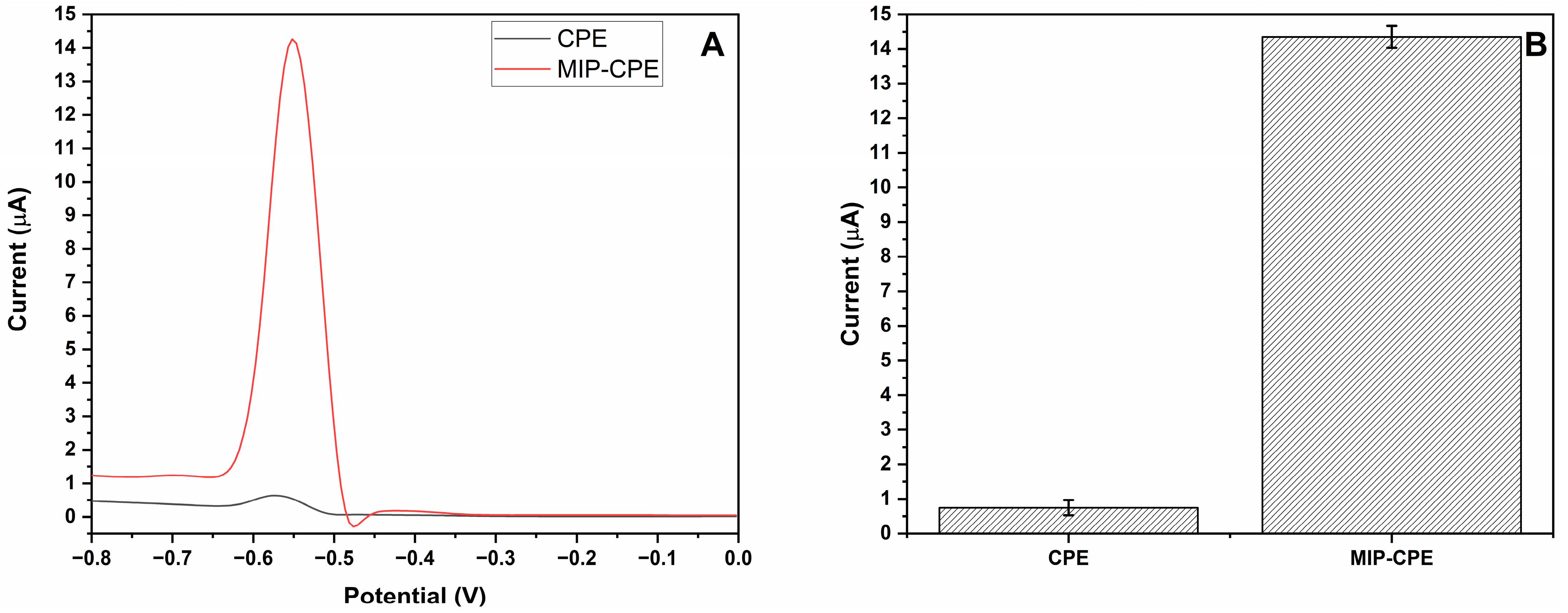
3.3. Analytical Performance of MIP-CPE Sensor
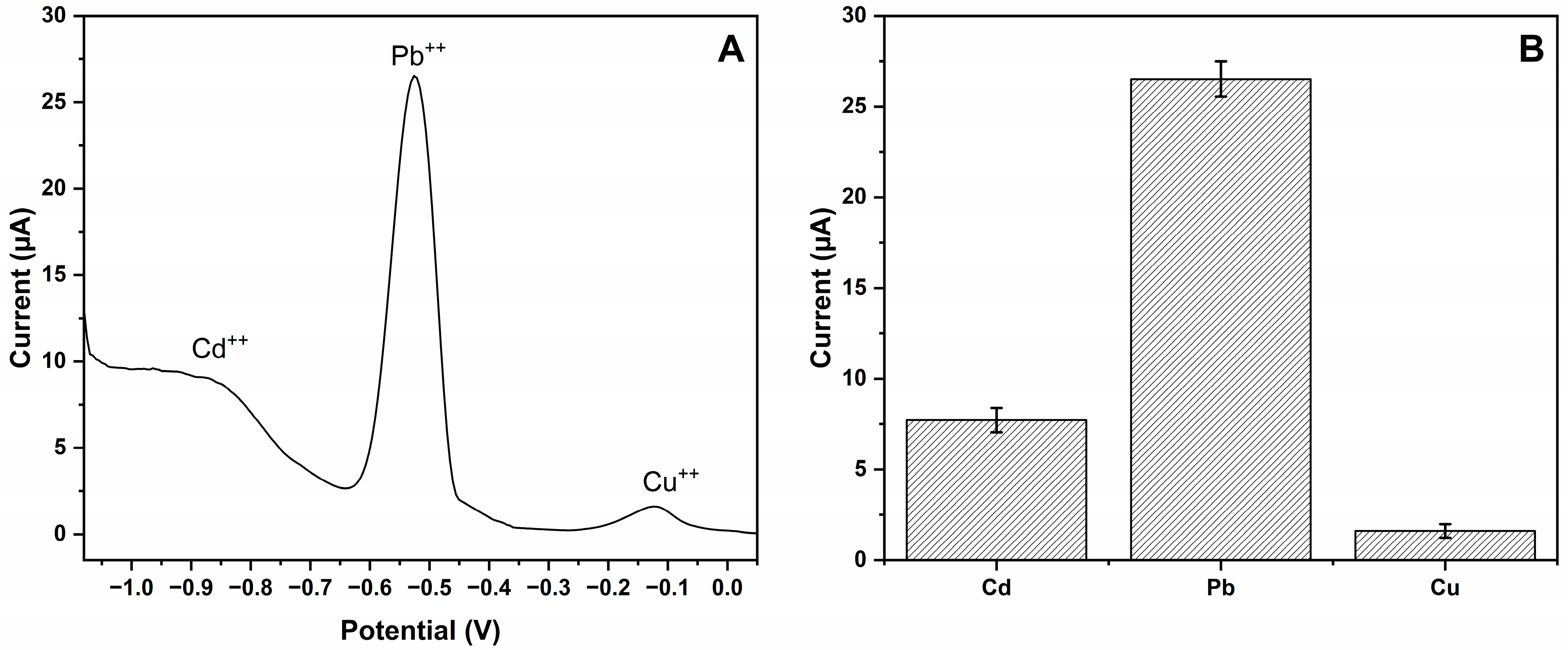
3.4. Estimation of Selectivity and Imprinting Efficiency
3.5. Comparison of MIP-CPE Sensor with Previous Studies
3.6. Determination of Pb (II) Ions in Real Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Morais, S.; e Costa, F.G.; de Lourdes Pereir, M. Heavy metals and human health. Environ. Health-Emerg. Issues Pract. 2012, 10, 227–245. [Google Scholar] [CrossRef]
- Pysz, K.; Leszczyńska, T.; Bieżanowska-Kopeć, R.; Kopeć, A. Chemical assessment of lead, cadmium, nitrate, and nitrite intakes with daily diets of children and adolescents from orphanages in Krakow, Poland. Environ. Sci. Pollut. Res. 2016, 23, 25200–25209. [Google Scholar] [CrossRef] [PubMed]
- Needleman, H.L.; Bellinger, D. The health effects of low level exposure to lead. Annu. Rev. Public Health 1991, 12, 111–140. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; von Schirnding, Y.E.; Prapamontol, T. Environmental lead exposure: A public health problem of global dimensions. Bull. World Health Organ. 2000, 78, 1068–1077. Available online: https://iris.who.int/handle/10665/268221 (accessed on 23 January 2024). [PubMed]
- Mielke, H.W.; Reagan, P.L. Soil is an important pathway of human lead exposure. Environ. Health Perspect. 1998, 106 (Suppl. S1), 217–229. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, E.; Tsuji, L.J.; Martin, I.D.; Liberda, E.N. Human biomonitoring issues related to lead exposure. Environ. Sci. Process. Impacts 2013, 15, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Elliott, M.L.; Abraham, W.C.; Broadbent, J.; Houts, R.M.; Ireland, D.; Knodt, A.R.; Poulton, R.; Ramrakha, S.; Hariri, A.R.; et al. Association of childhood lead exposure with MRI measurements of Structural Brain Integrity in Midlife. JAMA 2020, 324, 1970–1979. [Google Scholar] [CrossRef]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead exposure and cardiovascular disease—A systematic review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef]
- Šerevičienė, V.; Zigmontienė, A.; Paliulis, D. Heavy metals in honey collected from contaminated locations: A case of Lithuania. Sustainability 2022, 14, 9196. [Google Scholar] [CrossRef]
- Cunningham, M.M.; Tran, L.; McKee, C.G.; Ortega Polo, R.; Newman, T.; Lansing, L.; Griffiths, J.S.; Bilodeau, G.J.; Rott, M.; Marta Guarna, M. Honey bees as biomonitors of environmental contaminants, pathogens, and climate change. Ecol. Indic. 2022, 134, 108457. [Google Scholar] [CrossRef]
- Pohl, P. Determination of metal content in honey by atomic absorption and emission spectrometries. TrAC Trends Anal. Chem. 2009, 28, 117–128. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. General Standard for Contaminants and Toxins in Food and Feed, CXS 193-1995; Food and Agriculture Organization: Rome, Italy, 2019. [Google Scholar]
- Pehlivan, T.; Gul, A. Determination of Heavy Metals Contents of Some Monofloral Honey Produced in Turkey. J. Appl. Pharm. Sci. 2015, 5, 042–045. [Google Scholar] [CrossRef]
- Aghamirlou, H.; Khadem, M.; Rahmani, A.; Sadeghian, M.; Mahvi, A.; Akbarzadeh, A.; Nazmara, S. Heavy metals determination in honey samples using inductively coupled plasma-optical emission spectrometry. J. Environ. Health Sci. Eng. 2015, 13, 39. [Google Scholar] [CrossRef]
- Akbari, B.; Gharanfoli, F.; Khayyat, M.; Khashyarmanesh, Z.; Rezaee, R.; Karimi, G. Determination of heavy metals in different honey brands from Iranian markets. Food Addit. Contam. Part B 2012, 5, 105–111. [Google Scholar] [CrossRef]
- Kılıç Altun, S.; Dinç, H.; Paksoy, N.; Temamoğulları, F.K.; Savrunlu, M. Analyses of mineral content and heavy metal of honey samples from South and East Region of Turkey by using ICP-MS. Int. J. Anal. Chem. 2017, 2017, 6391454. [Google Scholar] [CrossRef] [PubMed]
- Sawan, S.; Maalouf, R.; Errachid, A.; Jaffrezic-Renault, N. Metal and metal oxide nanoparticles in the voltammetric detection of heavy metals: A review. Trac Trends Anal. Chem. 2020, 131, 116014. [Google Scholar] [CrossRef]
- Shama, N.; Aşır, S.; Ozsoz, M.; Göktürk, I.; Türkmen, D.; Yılmaz, F.; Denizli, A. Gold-modified molecularly imprinted N-methacryloyl-(l)-phenylalanine-containing electrodes for electrochemical detection of dopamine. Bioengineering 2022, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Shama, N.A.; Aşır, S.; Göktürk, I.; Yılmaz, F.; Türkmen, D.; Denizli, A. Electrochemical detection of cortisol by silver nanoparticle-modified molecularly imprinted polymer-coated pencil graphite electrodes. ACS Omega 2023, 8, 29202–29212. [Google Scholar] [CrossRef]
- Martín-Esteban, A. Recent molecularly imprinted polymer-based sample preparation techniques in environmental analysis. Trends Environ. Anal. Chem. 2016, 9, 8–14. [Google Scholar] [CrossRef]
- Mostafiz, B.; Bigdeli, S.A.; Banan, K.; Afsharara, H.; Hatamabadi, D.; Mousavi, P.; Hussain, C.M.; Keçili, R.; Ghorbani-Bidkorbeh, F. Molecularly imprinted polymer-carbon paste electrode (MIP-CPE)-based sensors for the sensitive detection of organic and inorganic environmental pollutants: A Review. Trends Environ. Anal. Chem. 2021, 32, e00144. [Google Scholar] [CrossRef]
- Bhateria, R.; Singh, R. A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J. Water Process Eng. 2019, 31, 100845. [Google Scholar] [CrossRef]
- Kong, D.; Qiao, N.; Wang, N.; Wang, Z.; Wang, Q.; Zhou, Z.; Ren, Z. Facile preparation of a nano-imprinted polymer on magnetite nanoparticles for the rapid separation of lead ions from aqueous solution. Phys. Chem. Chem. Phys. 2018, 20, 12870–12878. [Google Scholar] [CrossRef]
- Garipcan, B.; Andaç, M.; Uzun, L.; Denizli, A. Methacryloylamidocysteine functionalized poly(2-hydroxyethyl methacrylate) beads and its design as a metal-chelate affinity support for human serum albumin adsorption. React. Funct. Polym. 2004, 59, 119–128. [Google Scholar] [CrossRef]
- Bereli, N.; Denizli, A. Superior magnetic monodisperse particles for direct purification of immunoglobulin G under magnetic field. J. Macromol. Sci. Part A 2016, 53, 160–168. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, H.; Ping, J. Simultaneous determination of Cd(II) and Pb(II) ions in honey and milk samples using a single-walled carbon nanohorns modified screen-printed electrochemical sensor. Food Chem. 2019, 274, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Pudza, M.Y.; Abidin, Z.Z.; Abdul-Rashid, S.; Yasin, F.M.; Noor, A.S.; Abdullah, J. Selective and simultaneous detection of cadmium, lead and copper by tapioca-derived carbon dot–modified electrode. Environ. Sci. Pollut. Res. 2020, 27, 13315–13324. [Google Scholar] [CrossRef]
- Hu, S.; Xiong, X.; Huang, S.; Lai, X. Preparation of Pb(II) ion imprinted polymer and its application as the interface of an electrochemical sensor for trace lead determination. Anal. Sci. 2016, 32, 975–980. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Beltagi, A.M.; Hathoot, A.A.; Azzem, M.A. Simultaneous voltammetric sensing of Zn2+, Cd2+, and Pb2+ using an electrodeposited bi–SB nanocomposite modified carbon paste electrode. RSC Adv. 2023, 13, 7118–7128. [Google Scholar] [CrossRef]
- Malakootian, M.; Abolghasemi, H.; Mahmoudi-Moghaddam, H. A novel electrochemical sensor based on the modified carbon paste using Eu3+ − doped Nio for simultaneous determination of Pb (II) and Cd (II) in food samples. J. Electroanal. Chem. 2020, 876, 114474. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Celiesiute, R.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA_PANI/swcnts nanocomposite modified electrode for electrochemical determination of copper (II), lead (II) and Mercury (ii) ions. Electrochim. Acta 2018, 259, 930–938. [Google Scholar] [CrossRef]
- Lahari, S.A.; Amreen, K.; Dubey, S.K.; Ponnalagu, R.N.; Goel, S. Optimized porous carbon-fibre microelectrode for multiplexed, highly reproducible and repeatable detection of heavy metals in real water samples. Environ. Res. 2023, 220, 115192. [Google Scholar] [CrossRef] [PubMed]
- Raril, C.; Manjunatha, J.G. Fabrication of novel polymer-modified graphene-based electrochemical sensor for the determination of Mercury and lead ions in water and biological samples. J. Anal. Sci. Technol. 2020, 11, 3. [Google Scholar] [CrossRef]



| Electrode | Method | Electrolyte | a R2 | b LR (µM) | c LOD (µM) | Reference |
|---|---|---|---|---|---|---|
| MIP-CPE | DPV | 0.1 M HNO3 | 0.9954 | 5.0–100 | 0.0912 µM | This study |
| SPE/SWCNHs/BiF 1 | SWASV | 0.1 M ABS (pH 4.5) | 0.9866 | 4.83 × 10−3–0.290 µM | 1.93 × 10−3 µM | [27] |
| Eu3+-doped NiO/CPE 2 | SWASV | 0.1 M ABS (pH 4.5) | 0.997 | 3.86 × 10−3–0.796 µM | 4.83 × 10−4 µM | [31] |
| SPCE/CDs/AuNP 3 | DPV | 0.1 M PBS (pH 2.0) | 0.964 | 0.0483–1.30 µM | 0.02 µM | [28] |
| Pb(II)-IIP-GCE 4 | DPV | 0.2 M HAc-NaAc buffer (pH 4.5) | 0.9997 | 0.05–60 µM | 0.01 µM | [29] |
| Bi–Sb/CPE 5 | SWASV | ABS (pH 5.6) | 0.994 | 4.83 × 10−3–0.724 µM | 1.40 × 10−3 µM | [30] |
| EDTA_PANI/SWCNTs/SS 6 | DPV | 0.5 M H2SO4 | 0.9897 | 2–37 µM | 1.65 µM | [32] |
| AuNP-deposited porous carbon thread | DPV | HCl–KCl (pH 2) | 0.979 | 10–110 µM | 1.419 μM | [33] |
| PGMGPE 7 | CV | 0.1 M PBS (pH 4.5) | 0.996 | 200–450 μM and 500–1200 μM | 0.8 μM | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karagözlü, M.; Aşır, S.; Abu Shama, N.; Göktürk, I.; Yılmaz, F.; Türkmen, D.; Denizli, A.; Özgören, M. Development of Molecularly Imprinted Magnetic Amino Acid-Based Nanoparticles for Voltammetric Analysis of Lead Ions in Honey. Polymers 2024, 16, 1782. https://doi.org/10.3390/polym16131782
Karagözlü M, Aşır S, Abu Shama N, Göktürk I, Yılmaz F, Türkmen D, Denizli A, Özgören M. Development of Molecularly Imprinted Magnetic Amino Acid-Based Nanoparticles for Voltammetric Analysis of Lead Ions in Honey. Polymers. 2024; 16(13):1782. https://doi.org/10.3390/polym16131782
Chicago/Turabian StyleKaragözlü, Mehmet, Süleyman Aşır, Nemah Abu Shama, Ilgım Göktürk, Fatma Yılmaz, Deniz Türkmen, Adil Denizli, and Murat Özgören. 2024. "Development of Molecularly Imprinted Magnetic Amino Acid-Based Nanoparticles for Voltammetric Analysis of Lead Ions in Honey" Polymers 16, no. 13: 1782. https://doi.org/10.3390/polym16131782











