Structural and Physiochemical Properties of Polyvinyl Alcohol–Succinoglycan Biodegradable Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Purification of SG
2.3. Preparation of PVA/SG Composite Films
2.4. Characterization
2.4.1. Light Transmission Measurements
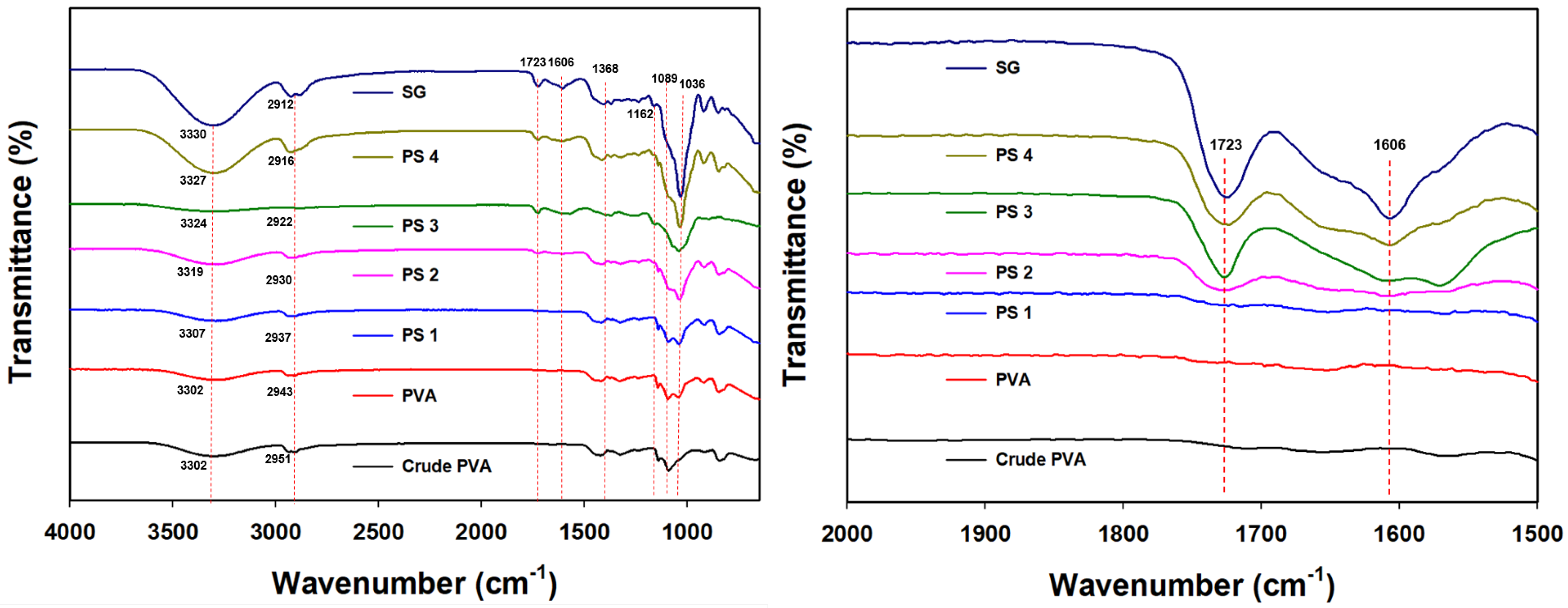
2.4.2. FTIR
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. X-ray Diffraction (XRD) Measurements
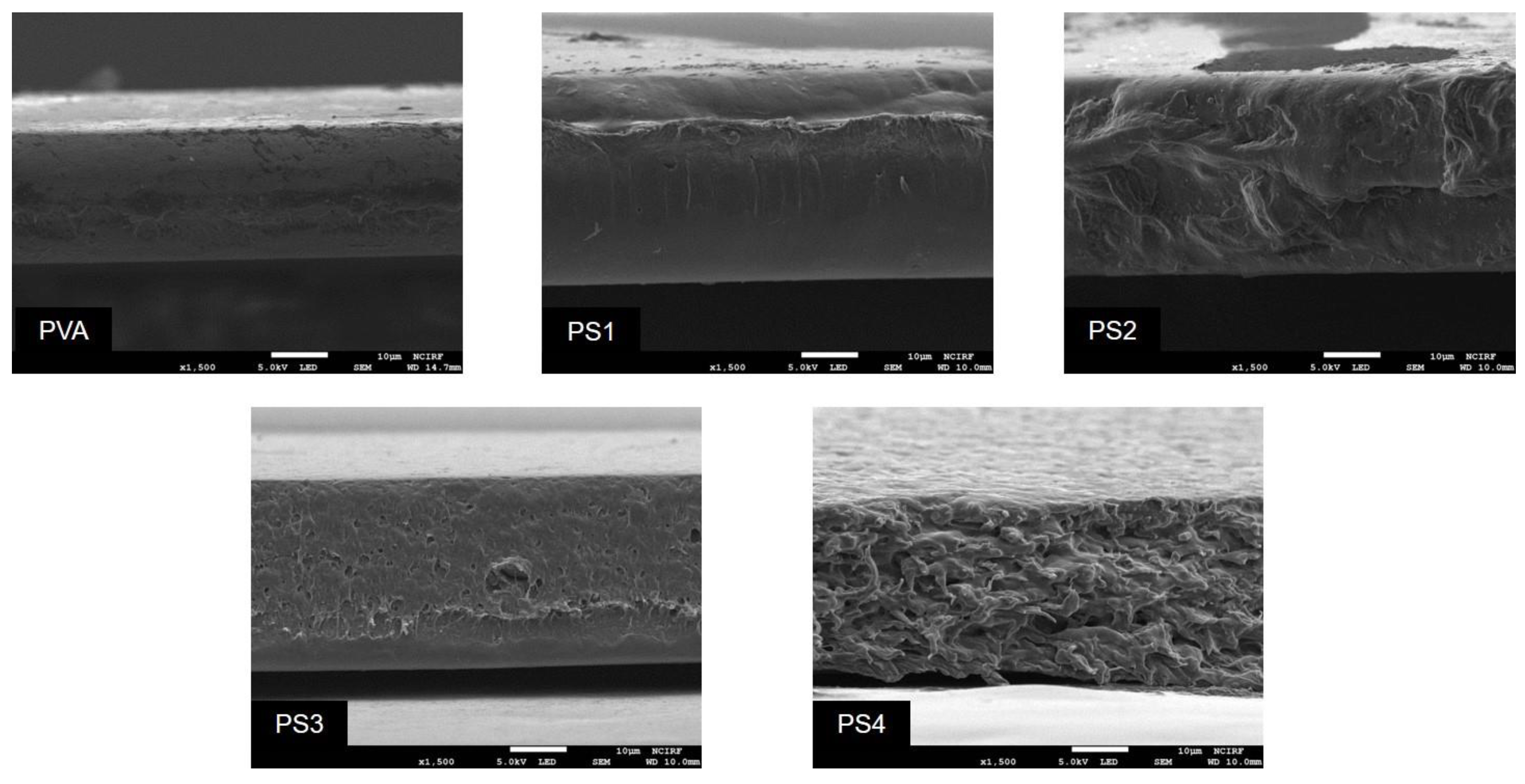
2.4.5. Field-Emission Scanning Electron Microscopy (FESEM)
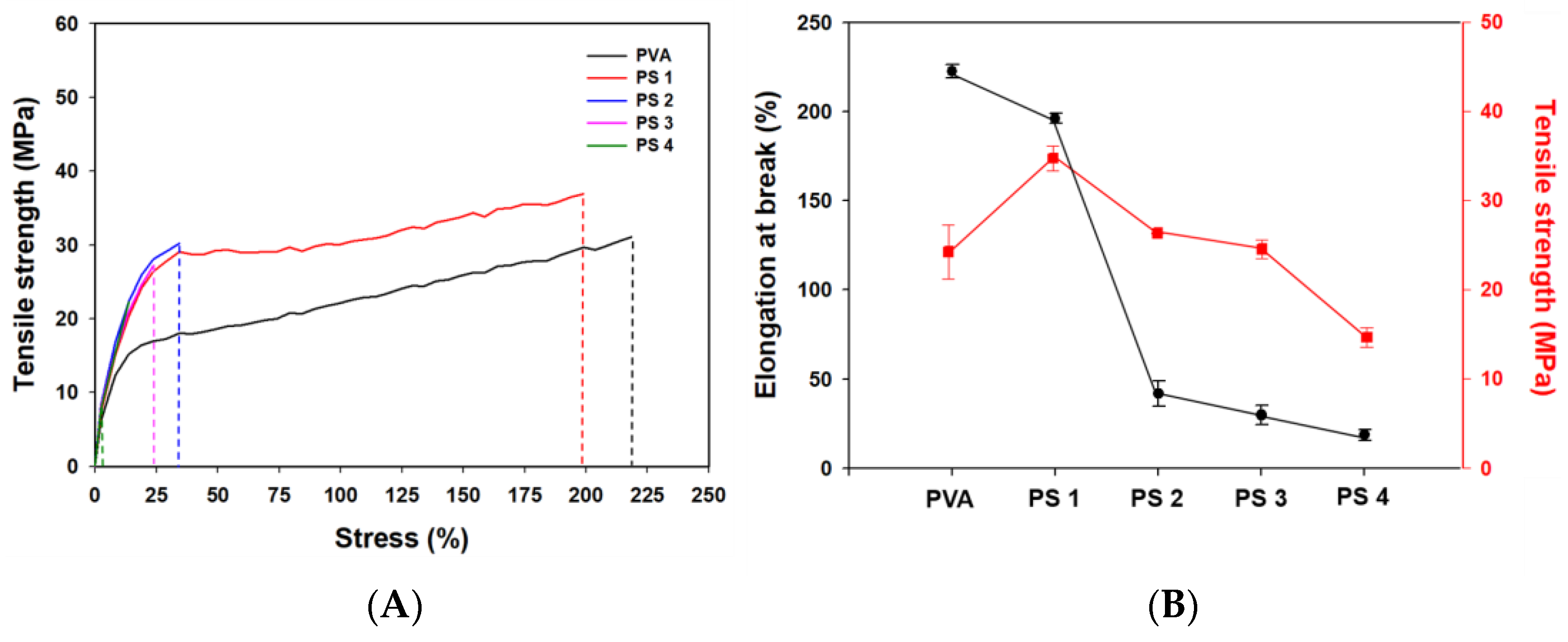
2.4.6. Mechanical Properties
2.5. Moisture Content (MC) and Water Solubility (WS) Measurement
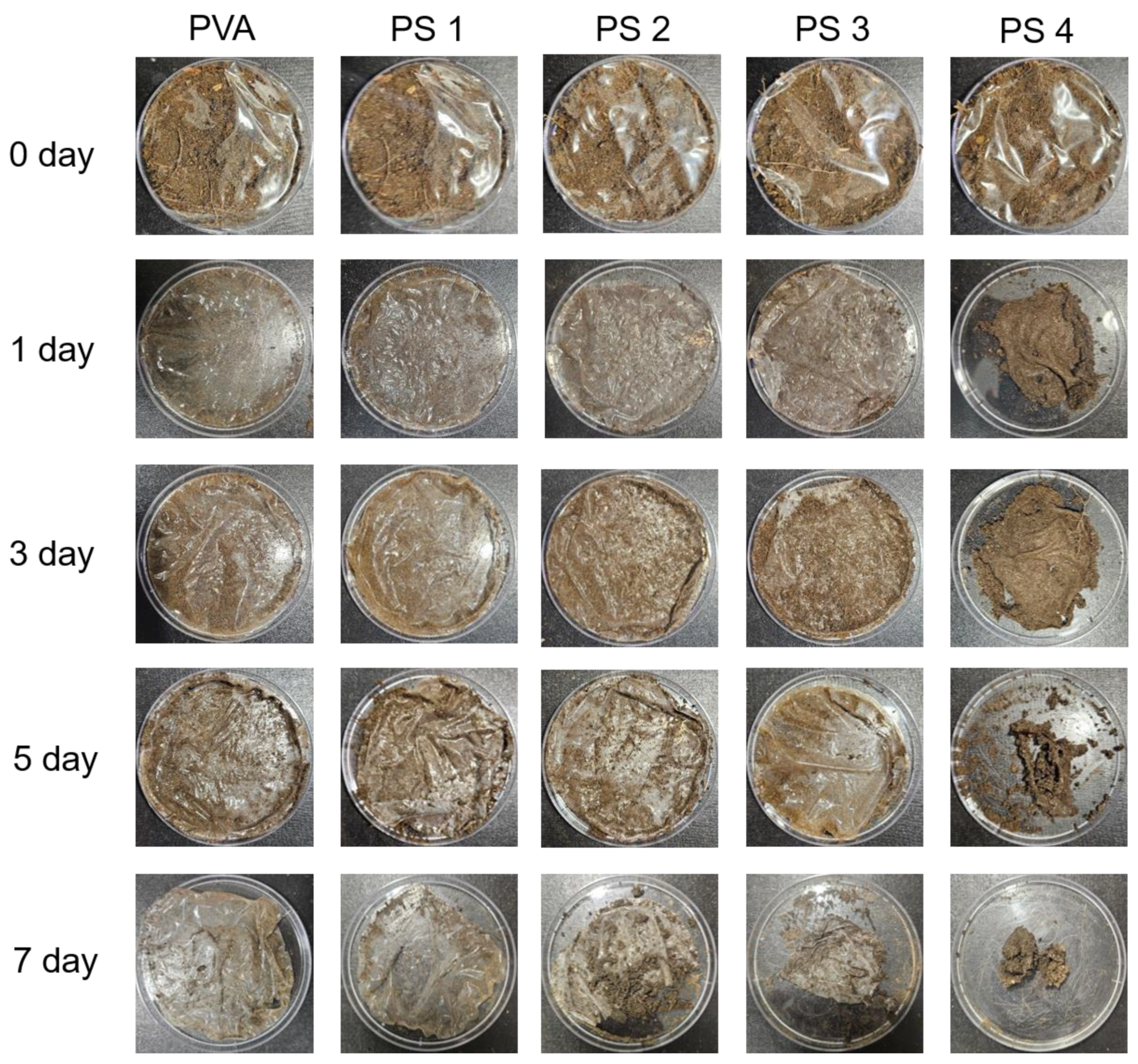
2.6. Biodegradability Test
3. Results
3.1. Optical Properties of PVA/SG Films
3.2. Characterization of the PVA/SG Films
3.2.1. FTIR Analysis
3.2.2. TGA and DTG Analysis
3.2.3. XRD Analysis
3.2.4. FE-SEM Analysis
3.2.5. Mechanical Properties
3.2.6. Moisture Content (MC) and Water Solubility (WS) of the Films
3.3. Biodegradation Test of the Films
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA blends, and their nanocomposites for biodegradable packaging application. Polym. -Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food packaging materials with special reference to biopolymers-properties and applications. Chem. Afr. 2023, 6, 117–144. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Madivoli, E. Polysaccharide based hydrogels in drug delivery systems, wound healing, and agriculture. Chem. Afr. 2023, 6, 2281–2295. [Google Scholar] [CrossRef]
- Yadav, H.; Karthikeyan, C. Natural Polysaccharides: Structural Features and Properties. In Polysaccharide Carriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–17. [Google Scholar]
- Arora, S.; Singh, D.; Rajput, A.; Bhatia, A.; Kumar, A.; Kaur, H.; Sharma, P.; Kaur, P.; Singh, S.; Attri, S. Plant-based polysaccharides and their health functions. Funct. Foods Health Dis. 2021, 11, 179–200. [Google Scholar] [CrossRef]
- Prasad, S.; Purohit, S.R. Microbial exopolysaccharide: Sources, stress conditions, properties and application in food and environment: A comprehensive review. Int. J. Biol. Macromol. 2023, 242, 124925. [Google Scholar] [CrossRef]
- Qamar, S.A.; Riasat, A.; Jahangeer, M.; Fatima, R.; Bilal, M.; Iqbal, H.M.; Mu, B.Z. Prospects of microbial polysaccharides-based hybrid constructs for biomimicking applications. J. Basic Microbiol. 2022, 62, 1319–1336. [Google Scholar] [CrossRef]
- Jeong, J.-P.; Kim, Y.; Hu, Y.; Jung, S. Bacterial succinoglycans: Structure, physical properties, and applications. Polymers 2022, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, S.; Kim, J.; Jeong, J.-P.; Jung, S. Rheological, antibacterial, antioxidant properties of D-mannitol-induced highly viscous succinoglycans produced by Sinorhizobium meliloti Rm 1021. Food Hydrocoll. 2024, 147, 109346. [Google Scholar] [CrossRef]
- Ahuja, V.; Bhatt, A.K.; Banu, J.R.; Kumar, V.; Kumar, G.; Yang, Y.-H.; Bhatia, S.K. Microbial exopolysaccharide composites in biomedicine and healthcare: Trends and advances. Polymers 2023, 15, 1801. [Google Scholar] [CrossRef]
- Panou, A.; Karabagias, I.K. Biodegradable packaging materials for foods preservation: Sources, advantages, limitations, and future perspectives. Coatings 2023, 13, 1176. [Google Scholar] [CrossRef]
- Bilanovic, D.; Starosvetsky, J.; Armon, R.H. Preparation of biodegradable xanthan–glycerol hydrogel, foam, film, aerogel and xerogel at room temperature. Carbohydr. Polym. 2016, 148, 243–250. [Google Scholar] [CrossRef]
- Bilanovic, D.; Starosvetsky, J.; Armon, R.H. Cross-linking xanthan and other compounds with glycerol. Food Hydrocoll. 2015, 44, 129–135. [Google Scholar] [CrossRef]
- De Morais Lima, M.; Bianchini, D.; Guerra Dias, A.; da Rosa Zavareze, E.; Prentice, C.; da Silveira Moreira, A. Biodegradable films based on chitosan, xanthan gum, and fish protein hydrolysate. J. Appl. Polym. Sci. 2017, 134, 44899. [Google Scholar] [CrossRef]
- Jaberifard, F.; Almajidi, Y.Q.; Arsalani, N.; Ghorbani, M. A self-healing crosslinked-xanthan gum/soy protein based film containing halloysite nanotube and propolis with antibacterial and antioxidant activity for wound healing. Int. J. Pharm. 2024, 656, 124073. [Google Scholar] [CrossRef]
- Huang, J.; Ren, J.; Chen, G.; Deng, Y.; Wang, G.; Wu, X. Evaluation of the xanthan-based film incorporated with silver nanoparticles for potential application in the nonhealing infectious wound. J. Nanomater. 2017, 2017, 6802397. [Google Scholar] [CrossRef]
- García-Betanzos, C.I.; Hernández-Sánchez, H.; Quintanar-Guerrero, D.; Del Real L, A.; de la Luz Zambrano-Zaragoza, M. The evaluation of mechanical, thermal, optical and microstructural properties of edible films with solid lipid nanoparticles-xanthan gum stored at different temperatures and relative humidities. Food Bioprocess Technol. 2016, 9, 1756–1768. [Google Scholar] [CrossRef]
- Bhunia, T.; Giri, A.; Nasim, T.; Chattopadhyay, D.; Bandyopadhyay, A. Uniquely different PVA-xanthan gum irradiated membranes as transdermal diltiazem delivery device. Carbohydr. Polym. 2013, 95, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, M.; Rengaramanujam, J.; Azarudeen, R.S.; Thirumarimurugan, M. Fabrication of polycaprolactone-xanthan gum-based membranes as potential drug carrier to control the growth of cancer cells and microbial strains. Polym. Bull. 2023, 81, 6823–6850. [Google Scholar] [CrossRef]
- Oun, A.A.; Shin, G.H.; Rhim, J.-W.; Kim, J.T. Recent advances in polyvinyl alcohol-based composite films and their applications in food packaging. Food Packag. Shelf Life 2022, 34, 100991. [Google Scholar] [CrossRef]
- Jana, S.; Sen, K.K. Gellan gum/PVA interpenetrating network micro-beads for sustained drug delivery. Mater. Today Proc. 2019, 11, 614–619. [Google Scholar] [CrossRef]
- Habibullah, S.; Swain, R.; Nandi, S.; Das, M.; Rout, T.; Mohanty, B.; Mallick, S. Nanocrystalline cellulose as a reinforcing agent for poly (vinyl alcohol)/gellan-gum-based composite film for moxifloxacin ocular delivery. Int. J. Biol. Macromol. 2024, 270, 132302. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, I.; Lüdtke, A.; Tavares, F.; Cholant, C.; Balboni, R.; Flores, W.H.; Galio, A.; Pawlicka, A.; Avellaneda, C.O. Ecologically friendly xanthan gum-PVA matrix for solid polymeric electrolytes. Ionics 2018, 24, 413–420. [Google Scholar] [CrossRef]
- Usawattanakul, N.; Torgbo, S.; Sukyai, P.; Khantayanuwong, S.; Puangsin, B.; Srichola, P. Development of nanocomposite film comprising of Polyvinyl Alcohol (PVA) incorporated with bacterial cellulose nanocrystals and magnetite nanoparticles. Polymers 2021, 13, 1778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, L.; Zhang, C.; Show, P.L.; Du, A.; Fu, J.; Ashokkumar, V. Preparation and characterization of curdlan/polyvinyl alcohol/thyme essential oil blending film and its application to chilled meat preservation. Carbohydr. Polym. 2020, 247, 116670. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.; Jeong, J.-P.; Kim, J.-M.; Kim, M.S.; Jung, S. A pH-sensitive drug delivery using biodegradable succinoglycan/chitosan hydrogels with synergistic antibacterial activity. Int. J. Biol. Macromol. 2023, 242, 124888. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Du, L.; Yang, Y.; Wang, L. Rheology of film-forming solutions and physical properties of tara gum film reinforced with polyvinyl alcohol (PVA). Food Hydrocoll. 2017, 63, 677–684. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of chitosan-based functional nanocomposite films: Effect of quercetin-loaded chitosan nanoparticles. Food Hydrocoll. 2021, 121, 107065. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Fernandes, S.C.; Sánchez, M.Á.A.; Labidi, J. Halochromic and antioxidant capacity of smart films of chitosan/chitin nanocrystals with curcuma oil and anthocyanins. Food Hydrocoll. 2022, 123, 107119. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Du, Y.; Wang, L.; Tong, C.; Hu, Y.; Pang, J.; Yan, Z. Preparation and characterization of konjac glucomannan-based bionanocomposite film for active food packaging. Food Hydrocoll. 2019, 89, 682–690. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT-Food Sci. Technol. 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Fernández-Marín, R.; Labidi, J.; Andrés, M.Á.; Fernandes, S.C. Using α-chitin nanocrystals to improve the final properties of poly (vinyl alcohol) films with Origanum vulgare essential oil. Polym. Degrad. Stab. 2020, 179, 109227. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, C.; Yao, Y.; Li, J.; He, S.; Zhou, Y.; Li, T.; Pan, X.; Yao, Y.; Hu, L. A strong, biodegradable and recyclable lignocellulosic bioplastic. Nat. Sustain. 2021, 4, 627–635. [Google Scholar] [CrossRef]
- Siemann, U. Solvent cast technology—A versatile tool for thin film production. In Scattering Methods and the Properties of Polymer Materials; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–14. [Google Scholar]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. In Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2005; pp. 403–433. [Google Scholar]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Xiao, X.; Duan, Q.; Bai, H.; Cao, Y.; Zhang, Y.; Alee, M.; Yu, L. Improved hydrophobicity, antibacterial and mechanical properties of polyvinyl alcohol/quaternary chitosan composite films for antibacterial packaging. Carbohydr. Polym. 2023, 312, 120755. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y.; Ma, Z.; Rao, Z.; Zheng, X.; Tang, K.; Liu, J. Effect of polyol plasticizers on properties and microstructure of soluble soybean polysaccharide edible films. Food Packag. Shelf Life 2023, 35, 101023. [Google Scholar] [CrossRef]
- Jipa, I.M.; Stoica, A.; Stroescu, M.; Dobre, L.-M.; Dobre, T.; Jinga, S.; Tardei, C. Potassium sorbate release from poly(vinyl alcohol)-bacterial cellulose films. Chem. Pap. 2012, 66, 138–143. [Google Scholar] [CrossRef]
- Shin, Y.; Hu, Y.; Park, S.; Jung, S. Novel succinoglycan dialdehyde/aminoethylcarbamoyl-β-cyclodextrin hydrogels for pH-responsive delivery of hydrophobic drugs. Carbohydr. Polym. 2023, 305, 120568. [Google Scholar] [CrossRef]
- Tretinnikov, O.; Zagorskaya, S. Determination of the degree of crystallinity of poly(vinyl alcohol) by FTIR spectroscopy. J. Appl. Spectrosc. 2012, 79, 521–526. [Google Scholar] [CrossRef]
- Abral, H.; Mahardika, M.; Handayani, D.; Sugiarti, E.; Muslimin, A.N. Characterization of disintegrated bacterial cellulose nanofibers/PVA bionanocomposites prepared via ultrasonication. Int. J. Biol. Macromol. 2019, 135, 591–599. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Qi, G.; Li, C.; Xu, P.; Zheng, T.; Zhu, X.; Kenny, J.M.; Puglia, D.; Ma, P. Highly transparent PVA/nanolignin composite films with excellent UV shielding, antibacterial and antioxidant performance. React. Funct. Polym. 2021, 162, 104873. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, D.; Lee, H.; Kim, D.; Jung, S. Succinoglycan dialdehyde-reinforced gelatin hydrogels with toughness and thermal stability. Int. J. Biol. Macromol. 2020, 149, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Kavitake, D.; Delattre, C.; Devi, P.B.; Pierre, G.; Michaud, P.; Shetty, P.H.; Andhare, P. Physical and functional characterization of succinoglycan exopolysaccharide produced by Rhizobium radiobacter CAS from curd sample. Int. J. Biol. Macromol. 2019, 134, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Suganthi, S.; Vignesh, S.; Kalyana Sundar, J.; Raj, V. Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications. Appl. Water Sci. 2020, 10, 100. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Jiao, C.; Zhao, Y.; Zhang, J.; Wang, H. Self-assembled polyvinyl alcohol–tannic acid hydrogels with diverse microstructures and good mechanical properties. ACS Omega 2018, 3, 11788–11795. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shin, Y.; Park, S.; Jeong, J.-P.; Kim, Y.; Jung, S. Multifunctional oxidized succinoglycan/poly (N-isopropylacrylamide-co-acrylamide) hydrogels for drug delivery. Polymers 2022, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zong, L.; Wang, J.; Xie, J. Microfibrillated cellulose reinforced starch/polyvinyl alcohol antimicrobial active films with controlled release behavior of cinnamaldehyde. Carbohydr. Polym. 2021, 272, 118448. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.; Zheng, X.; Chen, M.; Tang, K. Soluble soybean polysaccharide/nano zinc oxide antimicrobial nanocomposite films reinforced with microfibrillated cellulose. Int. J. Biol. Macromol. 2020, 159, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015, 44, 172–182. [Google Scholar] [CrossRef]
- Abdolshahi, A.; Monjazeb Marvdashti, L.; Salehi, B.; Maggi, F.; Sharifi-Rad, J. Active packaging film based on lysozyme/polyvinyl alcohol/alyssum homalocarpum seeds gum. J. Chem. Health Risks 2020, 10, 261–275. [Google Scholar]
- Kang, S.; Xiao, Y.; Guo, X.; Huang, A.; Xu, H. Development of gum arabic-based nanocomposite films reinforced with cellulose nanocrystals for strawberry preservation. Food Chem. 2021, 350, 129199. [Google Scholar] [CrossRef]
- Chaichi, M.; Hashemi, M.; Badii, F.; Mohammadi, A. Preparation and characterization of a novel bionanocomposite edible film based on pectin and crystalline nanocellulose. Carbohydr. Polym. 2017, 157, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Marvdashti, L.M.; Koocheki, A.; Yavarmanesh, M. Alyssum homolocarpum seed gum-polyvinyl alcohol biodegradable composite film: Physicochemical, mechanical, thermal and barrier properties. Carbohydr. Polym. 2017, 155, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Follain, N.; Joly, C.; Dole, P.; Bliard, C. Properties of starch based blends. Part 2. Influence of poly vinyl alcohol addition and photocrosslinking on starch based materials mechanical properties. Carbohydr. Polym. 2005, 60, 185–192. [Google Scholar] [CrossRef]
- Tilwani, Y.M.; Lakra, A.K.; Domdi, L.; Arul, V. Preparation and functional characterization of the bio-composite film based on chitosan/polyvinyl alcohol blended with bacterial exopolysaccharide EPS MC-5 having antioxidant activities. Int. J. Biol. Macromol. 2023, 245, 125496. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Development of ultrasound treated polyvinyl alcohol/tea polyphenol composite films and their physicochemical properties. Ultrason. Sonochem. 2019, 51, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Goudar, N.; Vanjeri, V.N.; Dixit, S.; Hiremani, V.; Sataraddi, S.; Gasti, T.; Vootla, S.K.; Masti, S.P.; Chougale, R.B. Evaluation of multifunctional properties of gallic acid crosslinked Poly(vinyl alcohol)/Tragacanth Gum blend films for food packaging applications. Int. J. Biol. Macromol. 2020, 158, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, M.; Mujumdar, A.S.; Adhikari, B.; Wang, H. Preparation of a novel carbon dot/polyvinyl alcohol composite film and its application in food preservation. ACS Appl. Mater. Interfaces 2022, 14, 37528–37539. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, J.; Zhou, H.; Zhou, S.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Wang, H. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocoll. 2022, 129, 107665. [Google Scholar] [CrossRef]






| Film Name | PVA | SG | Glycerol | Water |
|---|---|---|---|---|
| PVA | 2 g | - | 30 wt % | 100 mL |
| PS 1 | 1.6 g | 0.4 g | ||
| PS 2 | 1.2 g | 0.8 g | ||
| PS 3 | 0.8 g | 1.2 g | ||
| PS 4 | 0.4 g | 1.6 g |
| Film Name | Thickness (μm) | Opacity | T 280 | T 600 |
|---|---|---|---|---|
| PVA | 22.7 ± 0.18 | 1.53 ± 0.13 | 80.5 ± 0.32 | 89.67 ± 0.75 |
| PS 1 | 36.6 ± 0.57 | 1.18 ± 0.29 | 73.6 ± 0.52 | 89.2 ± 0.59 |
| PS 2 | 34.7 ± 0.36 | 1.38 ± 0.42 | 52.6 ± 0.65 | 86.6 ± 0.72 |
| PS 3 | 32.3 ± 0.48 | 2.03 ± 0.31 | 26.6 ± 0.57 | 73.3 ± 0.53 |
| PS 4 | 35.1 ± 1.86 | 2.53 ± 0.85 | 20.1 ± 0.45 | 68.1 ± 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-p.; Yoon, I.; Kim, K.; Jung, S. Structural and Physiochemical Properties of Polyvinyl Alcohol–Succinoglycan Biodegradable Films. Polymers 2024, 16, 1783. https://doi.org/10.3390/polym16131783
Jeong J-p, Yoon I, Kim K, Jung S. Structural and Physiochemical Properties of Polyvinyl Alcohol–Succinoglycan Biodegradable Films. Polymers. 2024; 16(13):1783. https://doi.org/10.3390/polym16131783
Chicago/Turabian StyleJeong, Jae-pil, Inwoo Yoon, Kyungho Kim, and Seunho Jung. 2024. "Structural and Physiochemical Properties of Polyvinyl Alcohol–Succinoglycan Biodegradable Films" Polymers 16, no. 13: 1783. https://doi.org/10.3390/polym16131783
APA StyleJeong, J.-p., Yoon, I., Kim, K., & Jung, S. (2024). Structural and Physiochemical Properties of Polyvinyl Alcohol–Succinoglycan Biodegradable Films. Polymers, 16(13), 1783. https://doi.org/10.3390/polym16131783








