Stretch-Induced Spin-Cast Membranes Based on Semi-Crystalline Polymers for Efficient Microfiltration
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Formulation and Preparation of Polyethylene and Filler Composite Membranes
2.3. Water Filtration Test
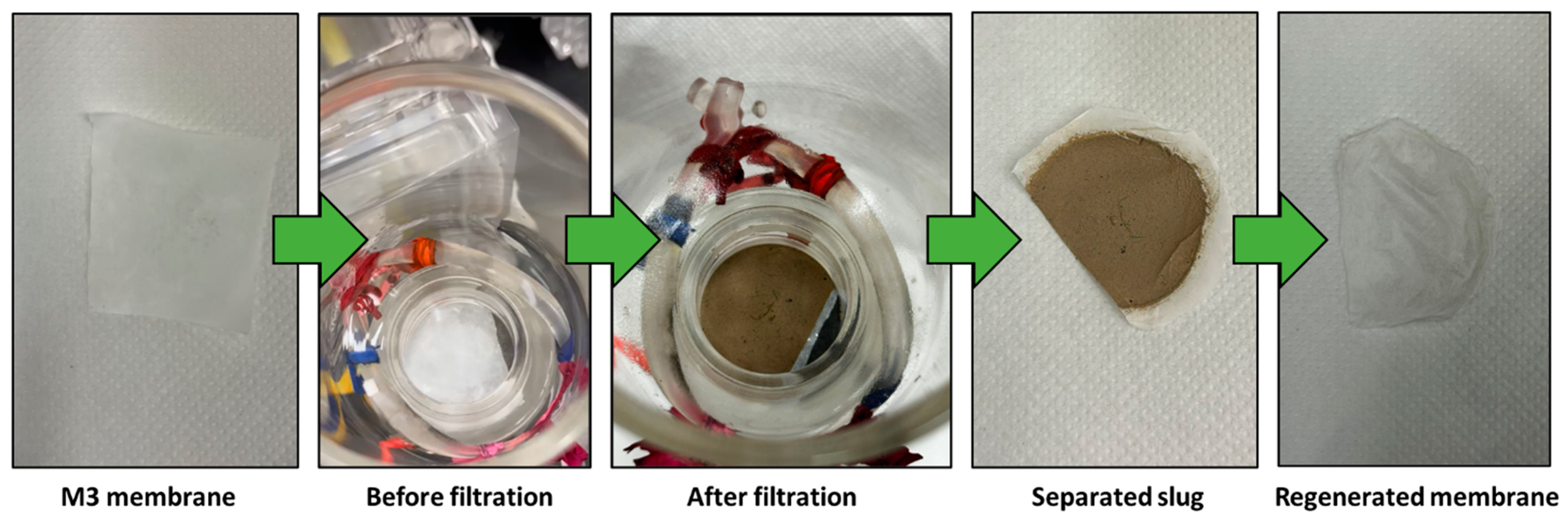
2.4. Membrane Regeneration
3. Results and Discussion
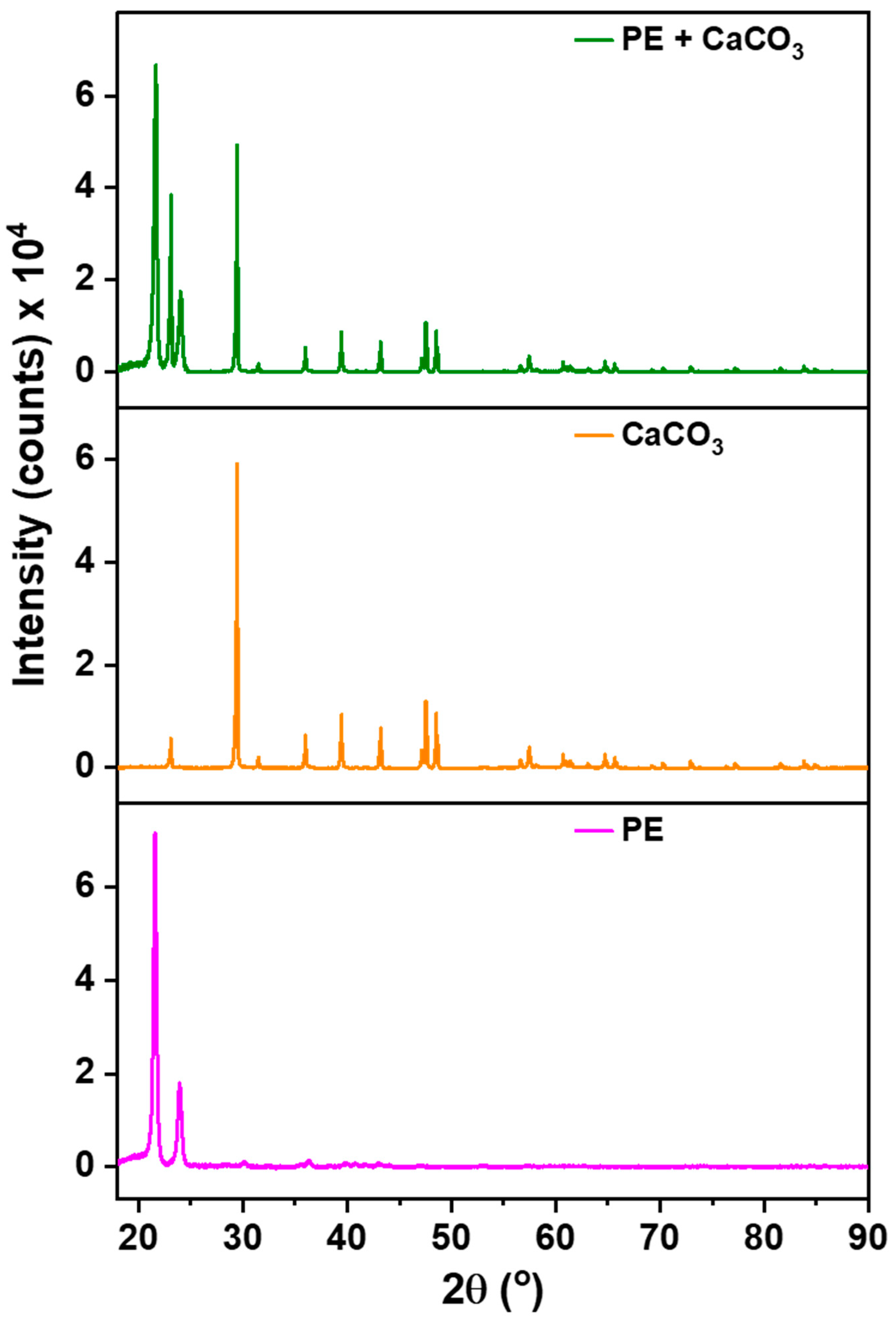
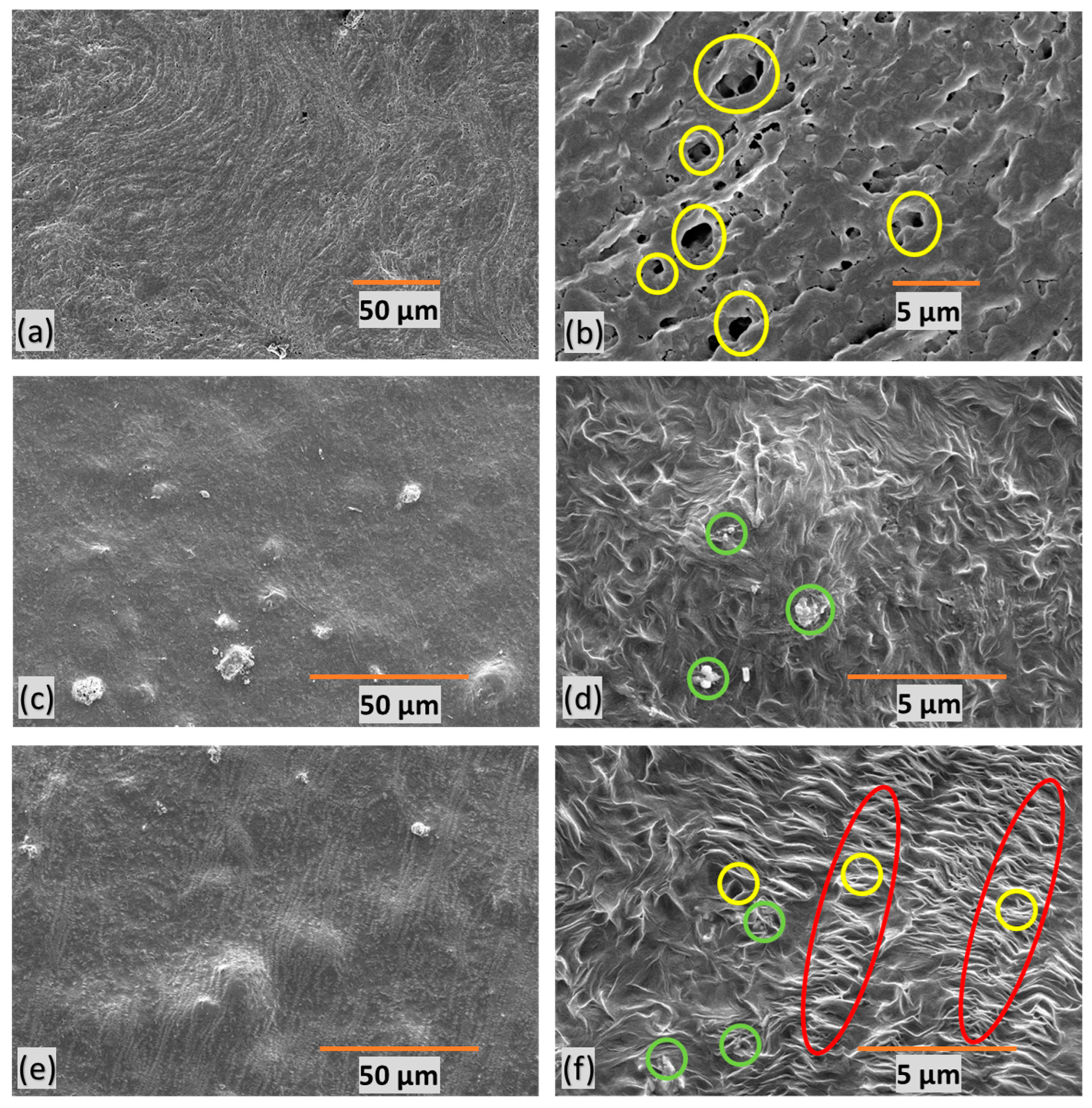
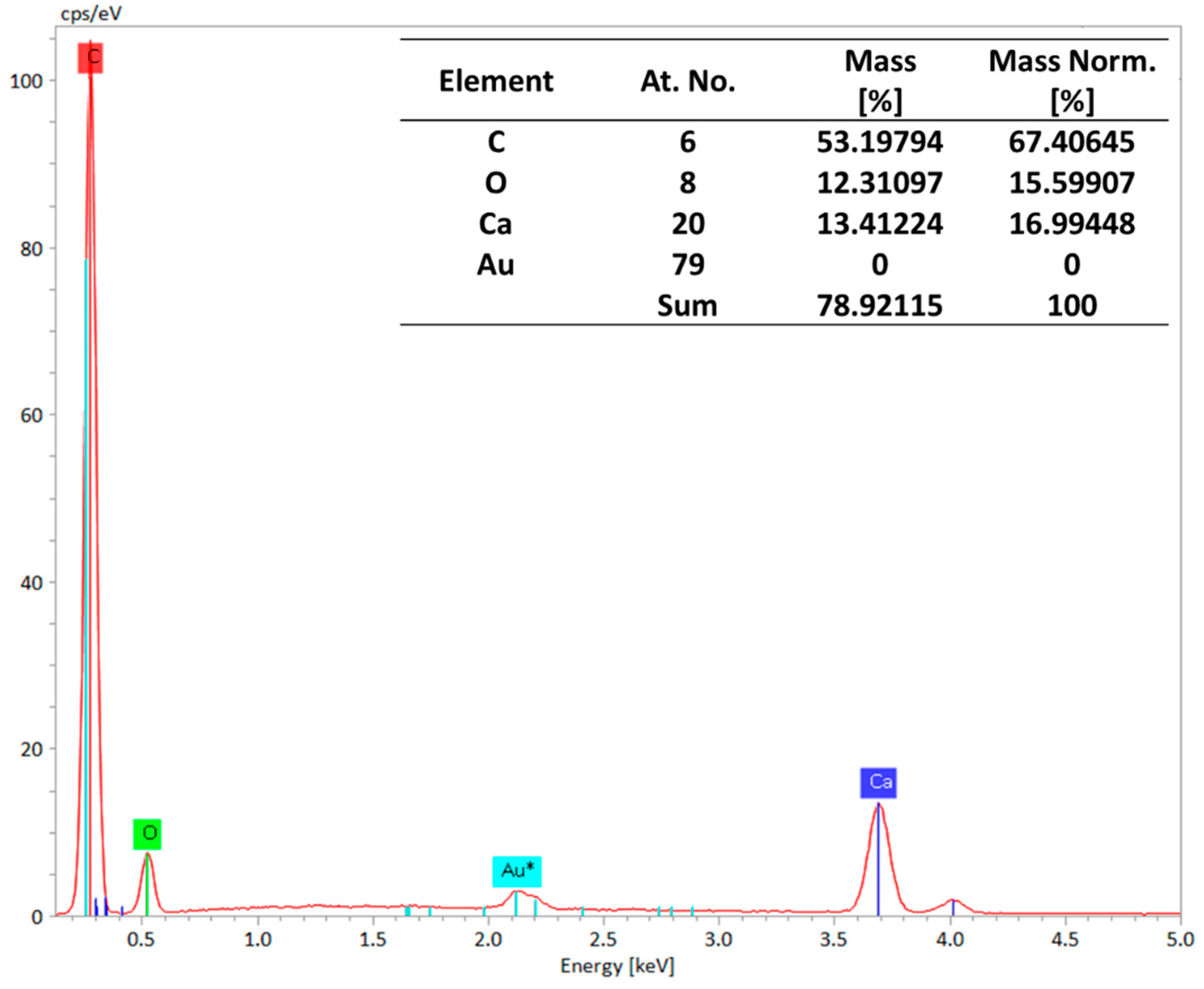
3.1. Material Characterization and Integration of Filler
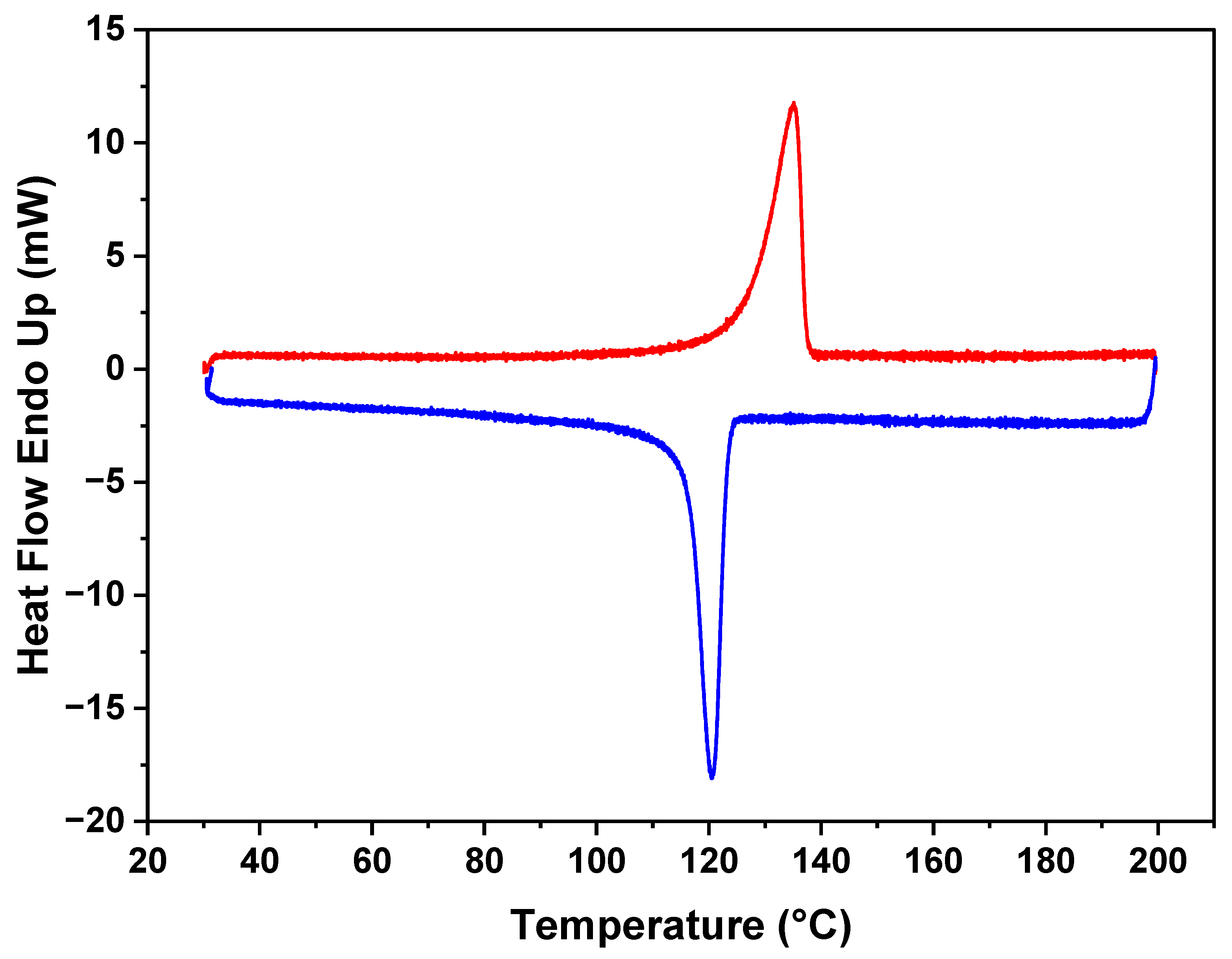
3.2. Thermal Behavior of the Composite
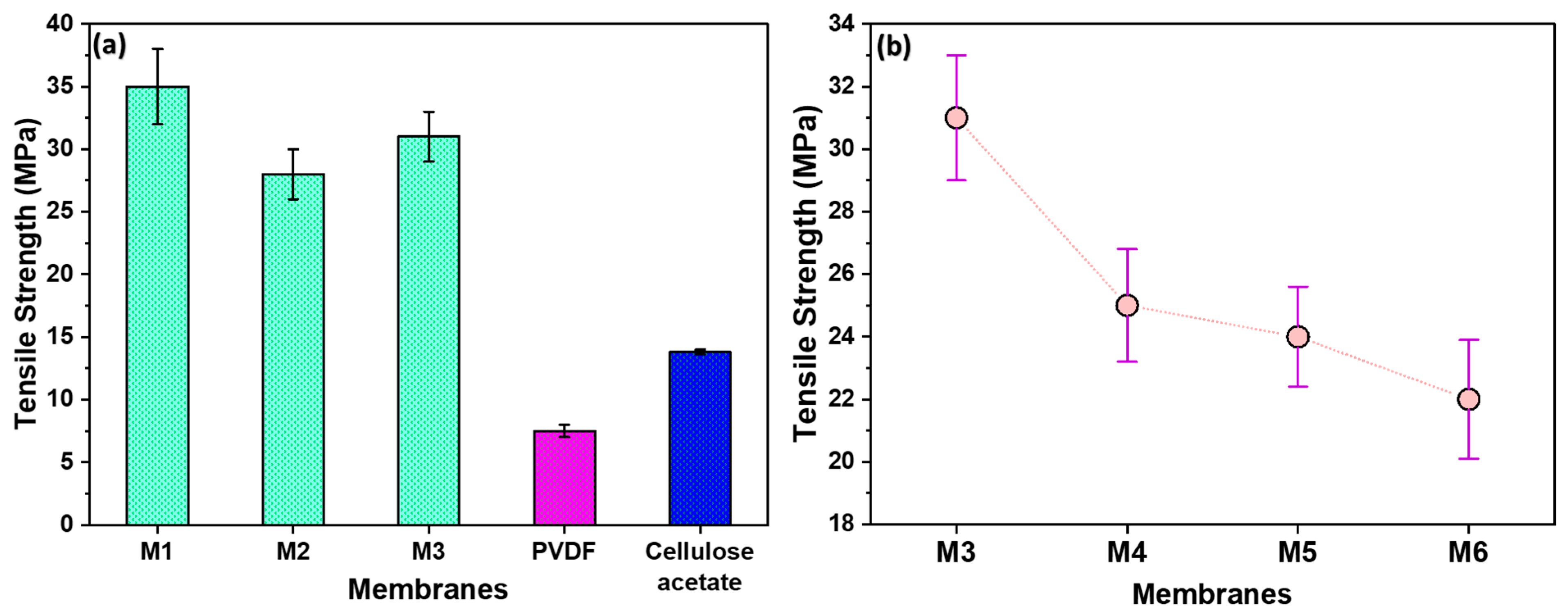
3.3. Mechanical Strength and Filler Content Influence
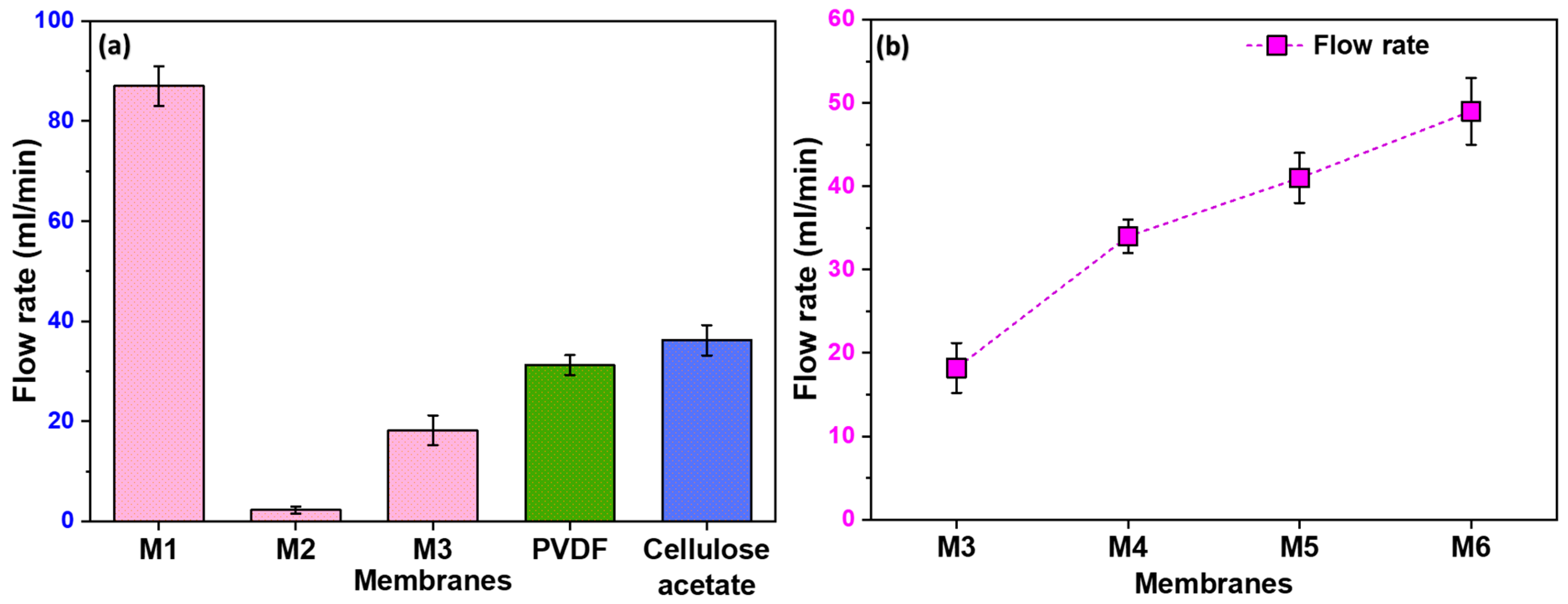
3.4. Pore Structure and Water Flow Rate
3.5. Influence of Filler Content on Flow Rate
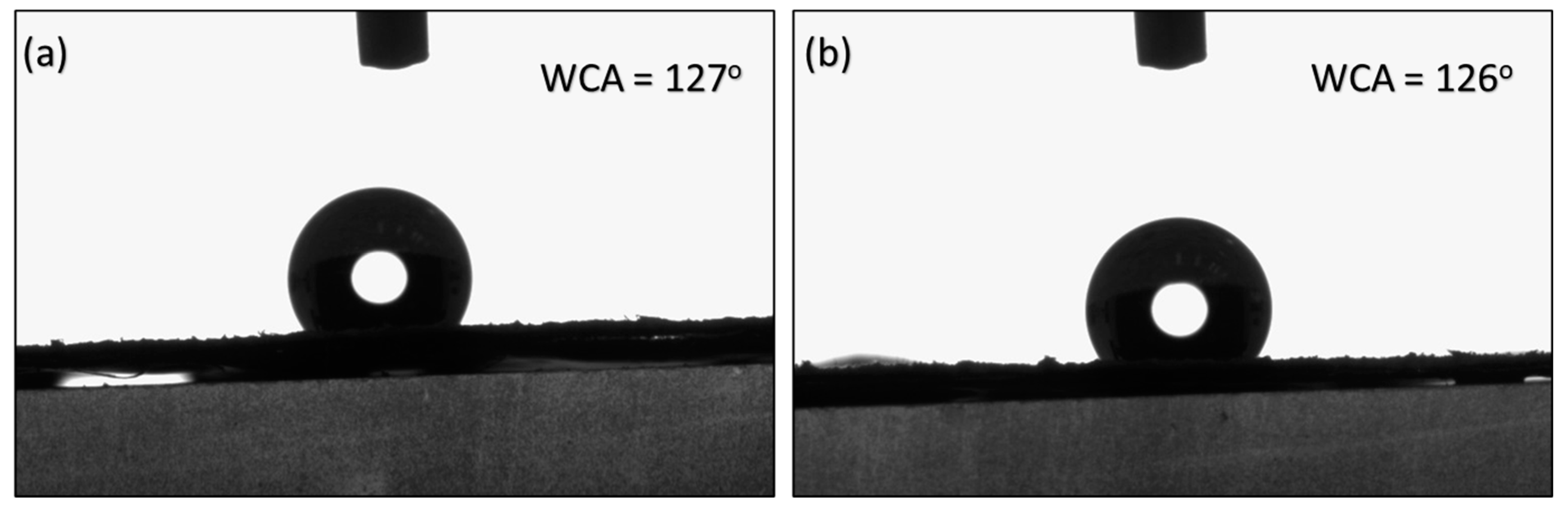
3.6. Surface Properties
3.7. Filtration and Membrane Regeneration
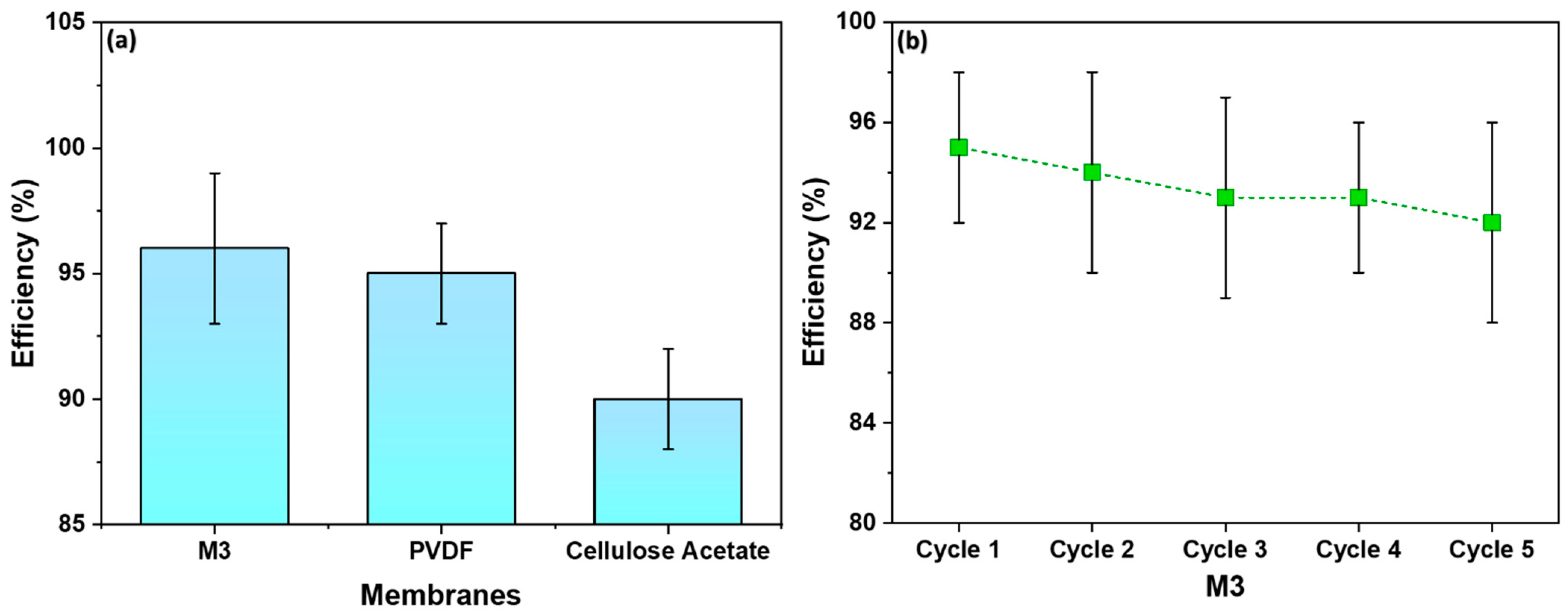
3.8. Filtration Efficiency Comparison and Recyclability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sholl, D.S.; Lively, R.P. Seven Chemical Separations to Change the World. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef]
- Zhuang, X.; Shi, L.; Jia, K.; Cheng, B.; Kang, W. Solution Blown Nanofibrous Membrane for Microfiltration. J. Membr. Sci. 2013, 429, 66–70. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Sajjad, S.; Saleem, J.; Alherbawi, M.; Mckay, G. A Review of the Removal of Dyestuffs from Effluents onto Biochar. Separations 2022, 9, 139. [Google Scholar] [CrossRef]
- Lin, H.; Ding, Y. Polymeric Membranes: Chemistry, Physics, and Applications. J. Polym. Sci. 2020, 58, 2433–2434. [Google Scholar] [CrossRef]
- Kuiper, S.; van Rijn, C.J.; Nijdam, W.; Elwenspoek, M. Development and Applications of Very High Flux Microfiltration Membranes. J. Membr. Sci. 1998, 150, 1–8. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; Luyt, A.S.; Shakoor, R.A.; McKay, G. Free-Standing Porous and Nonporous Polyethylene Thin Films Using Spin Coating: An Alternate to the Extrusion–Stretching Process. ACS Appl. Polym. Mater. 2023, 5, 2177–2184. [Google Scholar] [CrossRef]
- Saleem, J.; Tahir, F.; Baig, M.Z.K.; Al-Ansari, T.; McKay, G. Assessing the Environmental Footprint of Recycled Plastic Pellets: A Life-Cycle Assessment Perspective. Environ. Technol. Innov. 2023, 32, 103289. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; McKay, G. Up-Cycling Plastic Waste into Swellable Super-Sorbents. J. Hazard. Mater. 2023, 453, 131356. [Google Scholar] [CrossRef]
- Tahir, F.; Sbahieh, S.; Al-Ghamdi, S.G. Environmental Impacts of Using Recycled Plastics in Concrete. Mater. Today Proc. 2022, 62, 4013–4017. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental Impacts of Polypropylene (PP) Production and Prospects of Its Recycling in the GCC Region. Mater. Today Proc. 2022, 56, 2245–2251. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Life-Cycle Assessment of Polypropylene Production in the Gulf Cooperation Council (GCC) Region. Polymers 2021, 13, 3793. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future Scenarios of Global Plastic Waste Generation and Disposal. Palgrave Commun. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Mishra, S.; Charan Rath, C.; Das, A.P. Marine Microfiber Pollution: A Review on Present Status and Future Challenges. Mar. Pollut. Bull. 2019, 140, 188–197. [Google Scholar] [CrossRef]
- MacArthur, D.E. Rethinking the Future of Plastics Rethinking the Future of Plastics the New Plastics Economy; Ellen MacArthur Foundation: Cowes, UK, 2014; pp. 1–120. [Google Scholar]
- Pan, D.; Su, F.; Liu, C.; Guo, Z. Research Progress for Plastic Waste Management and Manufacture of Value-Added Products. Adv. Compos. Hybrid Mater. 2020, 3, 443–461. [Google Scholar] [CrossRef]
- Rahimi, A.R.; Garciá, J.M. Chemical Recycling of Waste Plastics for New Materials Production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Lase, I.S.; Tonini, D.; Caro, D.; Albizzati, P.F.; Cristóbal, J.; Roosen, M.; Kusenberg, M.; Ragaert, K.; Van Geem, K.M.; Dewulf, J.; et al. How Much Can Chemical Recycling Contribute to Plastic Waste Recycling in Europe? An Assessment Using Material Flow Analysis Modeling. Resour. Conserv. Recycl. 2023, 192, 106916. [Google Scholar] [CrossRef]
- Hale, W.R.; McGuire, J.; Sand, I.D.; Dohrer, K.K. Heat Setting of Stretched and Microvoided PE/CaCO3 Films. J. Appl. Polym. Sci. 2001, 82, 2454–2471. [Google Scholar] [CrossRef]
- Kono, K.; Mori, S.; Miyasaka, K.; Tabuchi, J. Polyethylene Microporous Membrane of Ultra High Molecular Weight. U.S. Patent 4,588,633, 13 May 1986. [Google Scholar]
- Zhang, X.; Rumierz, G. Biaxially Oriented Porous Membranes, Composites, and Methods of Manufacture and Use. U.S. Patent 2011/0223486 A1, 15 September 2011. [Google Scholar]
- Young-Keun, L.; Jang-Weon, R.; Jung-Moon, S.; Byoung-Cheon, J. Microporous High Density Polyethylene Film and Preparing Method Thereof. U.S. Patent US 2007/0218271 A1, 20 September 2007. [Google Scholar]
- Takeoka, S.; Saito, A.; Zhang, H.; Takamizawa, N. Ultra-Thin Polymer Film and Porous Ultra-Thin Polymer Film. U.S. Patent 10,858,490 B2, 8 December 2020. [Google Scholar]
- Ho-seok, C. Preparation Method for Free Standing Polymer Film with Through-Pore Structured Micropores. KR101714621B1, 9 March 2017. [Google Scholar]
- Herkenberg, W. Thin Flexible Sheet Sorption Material for the Removal of Oil Form Oil Spills. EP 0507784 B1, 9 March 2017. [Google Scholar]
- Raghavan, A.; Mohanty, S.; Biswal, M.; Vardikar, H.S.; GS, W.F.; Nayak, S.K. Development of Thermoplastic Recycled Blends from Biomedical Waste: Evaluation of Properties and Value Addition Thereof. Polym. Int. 2023, 72, 274–286. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.P.S.; Feo, L.; Fraternali, F. Recycling of Plastic Solid Waste: A State of Art Review and Future Applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and Recovery Routes of Plastic Solid Waste (PSW): A Review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Ujimatsu, H.F.; Im, Y.S.K.; Atsuzaki, H.M.; Akamura, A.N. Drawing Properties and Physical Properties of Ultrahigh-Molecular-Weight Polyethylene Swollen in Mixed Solvent. Polym. J. 2001, 33, 709–717. [Google Scholar] [CrossRef]
- Şirin, K.; Doǧan, F.; Balcan, M.; Kaya, I. Effect of CaCO3 Filler Component on Solid State Decomposition Kinetic of PP/LDPE/CaCO3 Composites. J. Macromol. Sci. Part A Pure Appl. Chem. 2009, 46, 949–958. [Google Scholar] [CrossRef]
- Hossain, S.S.; Saleem, J.; Al Ahmed, A.; Hossain, M.M.; Shaikh, M.N.; Rahman, S.U.; Mc Kay, G. Preparation and Evaluation of Nickel Oxide-Carbon Nanotube Supported Palladium as Anode Electrocatalyst for Formic Acid Fuel Cells. Int. J. Electrochem. Sci. 2016, 11, 2686–2708. [Google Scholar] [CrossRef]
- Saleem, J.; Safdar Hossain, S.; Al-Ahmed, A.; Rahman, A.; McKay, G.; Hossain, M.M. Evaluation of Pd Nanoparticle-Decorated CeO2-MWCNT Nanocomposite as an Electrocatalyst for Formic Acid Fuel Cells. J. Electron. Mater. 2018, 47, 2277–2289. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; McKay, G. Environmental Nanotechnology. In Handbook of Environmental Materials Management; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–32. [Google Scholar]


| Membrane | Polymer (mg) | CaCO3 (mg) | Stretching | |
|---|---|---|---|---|
| UHMWPE | HDPE | |||
| M1 | 700 | 300 | 0 | Yes |
| M2 | 700 | 300 | 100 | No |
| M3 | 700 | 300 | 100 | Yes |
| M4 | 700 | 300 | 400 | Yes |
| M5 | 700 | 300 | 700 | Yes |
| M6 | 700 | 300 | 1000 | Yes |
| Membrane | Polymer–CaCO3 | Uni-Axial Stretch |
|---|---|---|
| M1 | 10:0 | 1.8 |
| M2 | 10:1 | 0 |
| M3 | 10:1 | 1.8 |
| M4 | 10:4 | 1.8 |
| M5 | 10:7 | 1.8 |
| M6 | 10:10 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, J.; Moghal, Z.K.B.; Hafeez, A.; Sajjad, S.; Shoaib, M.; Alahmad, J.; McKay, G. Stretch-Induced Spin-Cast Membranes Based on Semi-Crystalline Polymers for Efficient Microfiltration. Polymers 2024, 16, 1799. https://doi.org/10.3390/polym16131799
Saleem J, Moghal ZKB, Hafeez A, Sajjad S, Shoaib M, Alahmad J, McKay G. Stretch-Induced Spin-Cast Membranes Based on Semi-Crystalline Polymers for Efficient Microfiltration. Polymers. 2024; 16(13):1799. https://doi.org/10.3390/polym16131799
Chicago/Turabian StyleSaleem, Junaid, Zubair Khalid Baig Moghal, Ahsan Hafeez, Samra Sajjad, Mohammad Shoaib, Johaina Alahmad, and Gordon McKay. 2024. "Stretch-Induced Spin-Cast Membranes Based on Semi-Crystalline Polymers for Efficient Microfiltration" Polymers 16, no. 13: 1799. https://doi.org/10.3390/polym16131799
APA StyleSaleem, J., Moghal, Z. K. B., Hafeez, A., Sajjad, S., Shoaib, M., Alahmad, J., & McKay, G. (2024). Stretch-Induced Spin-Cast Membranes Based on Semi-Crystalline Polymers for Efficient Microfiltration. Polymers, 16(13), 1799. https://doi.org/10.3390/polym16131799






