Synthesis and Characterization of Negative-Tone Photosensitive Polyimides with Low Coefficient of Thermal Expansion for Packaging Applications
Abstract
1. Introduction
2. Experimental
2.1. Materials
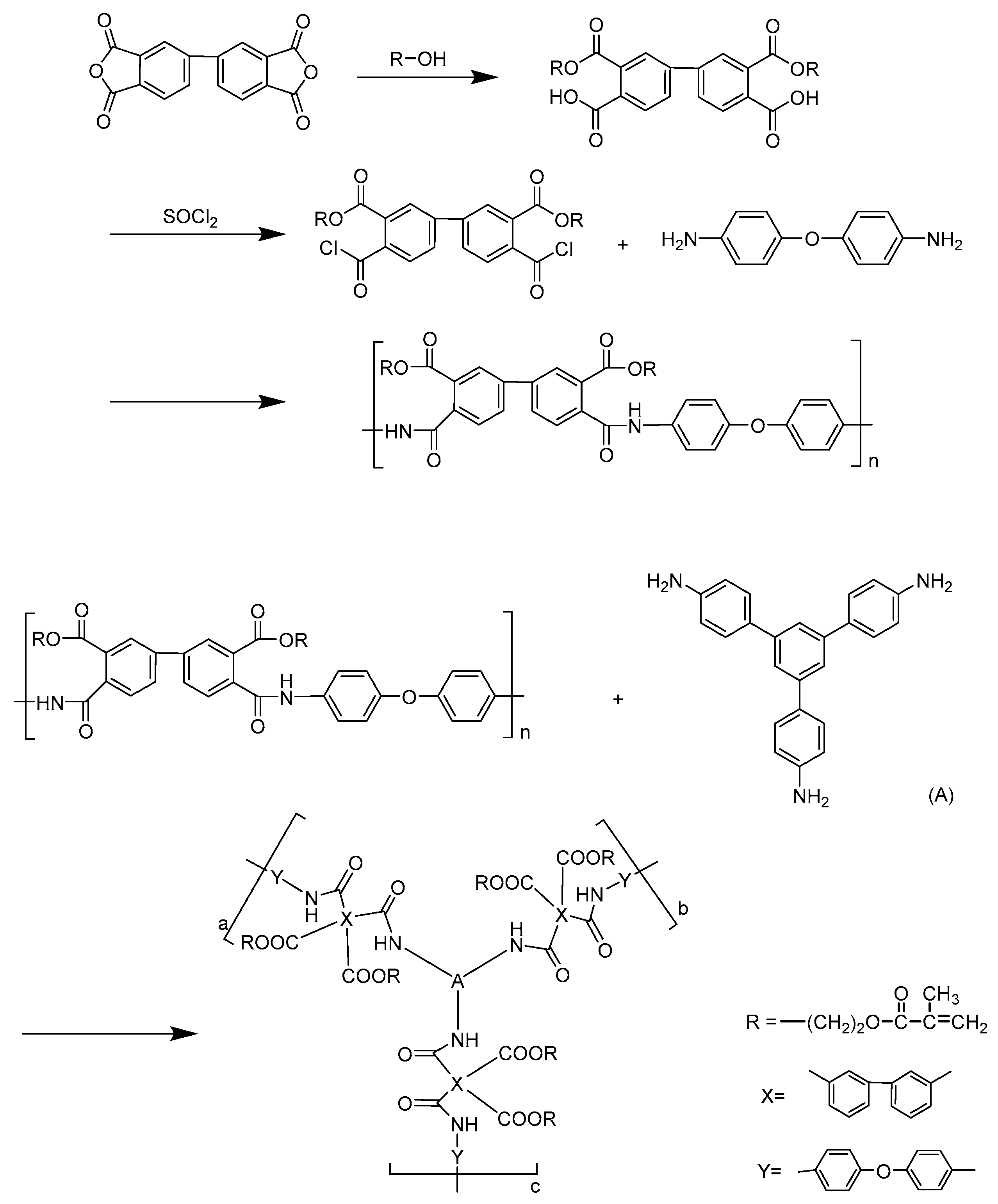
2.2. Synthesis of PAE Resin
2.3. Measurements
2.4. Photolithography Process
3. Results and Discussion
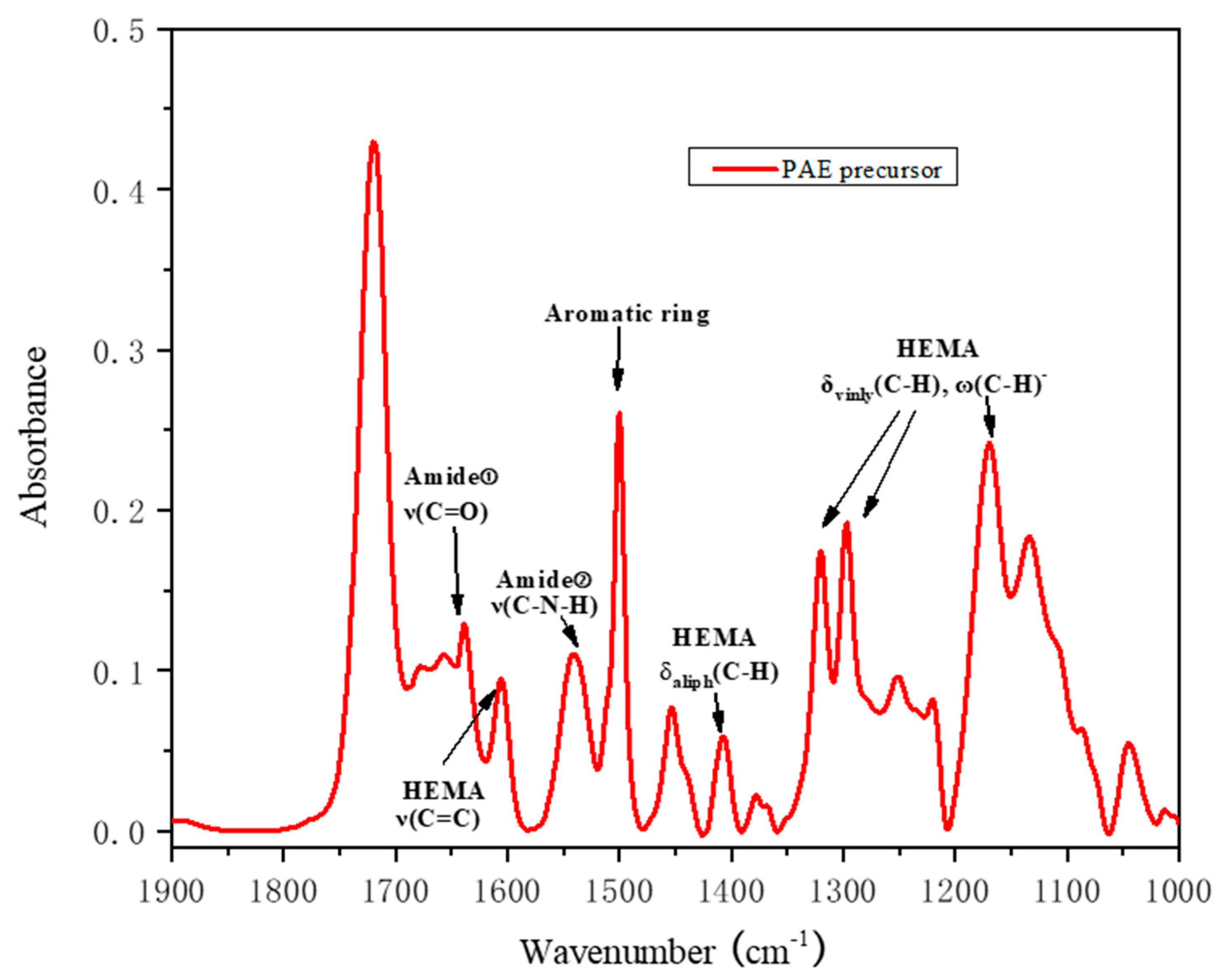
3.1. Synthesis and Characterization of PAE Resins
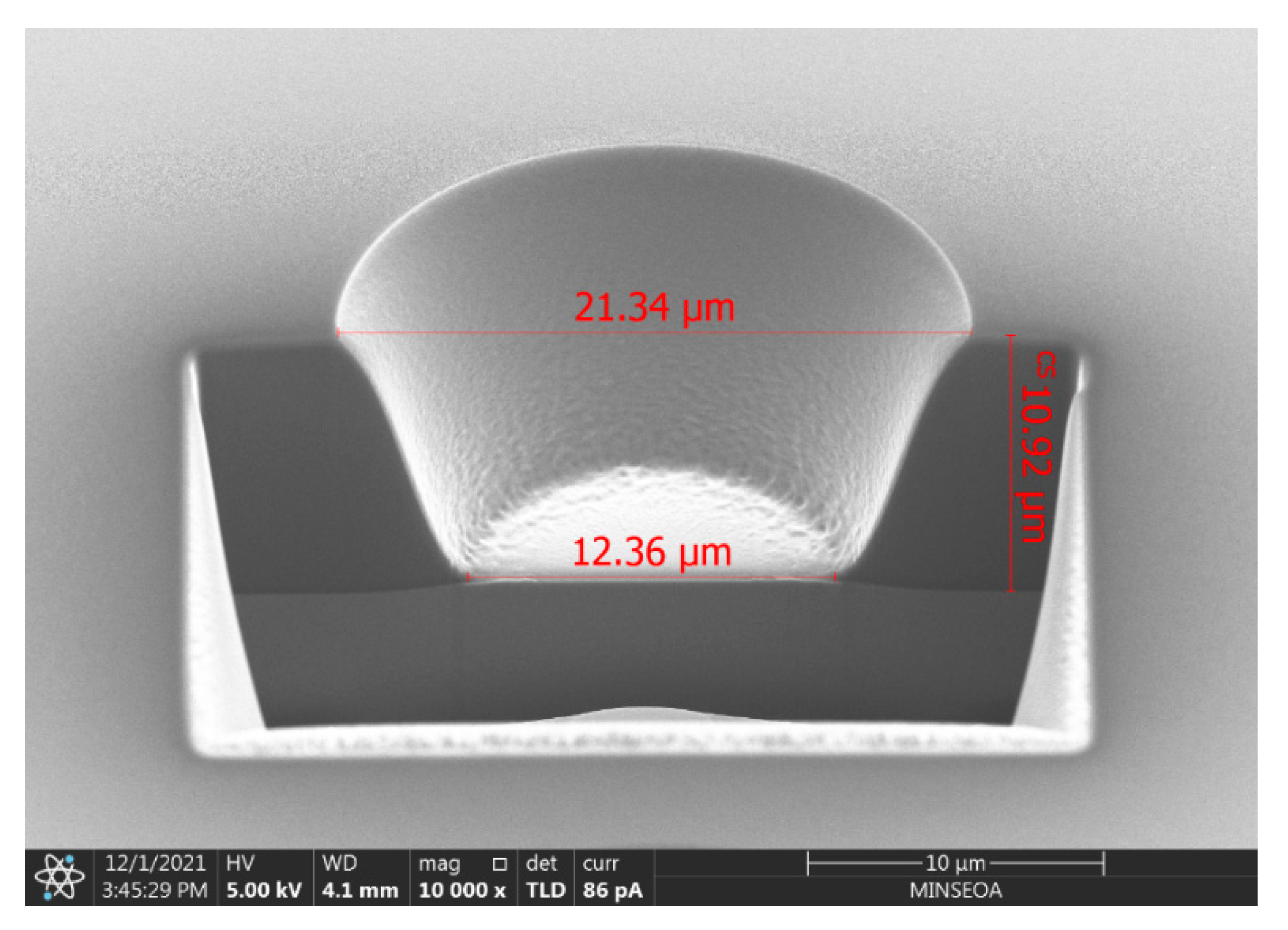
3.2. Lithographic Performance
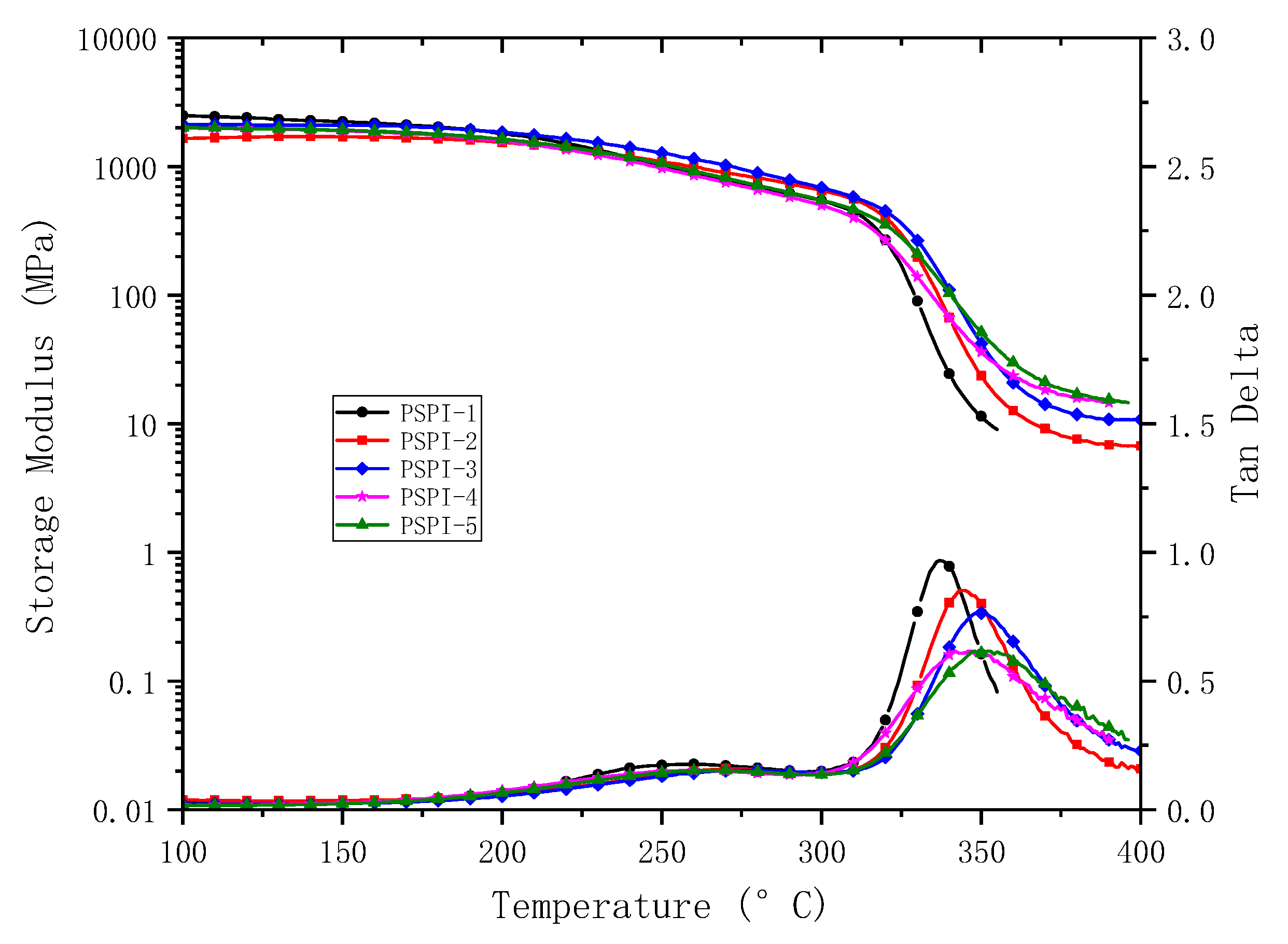
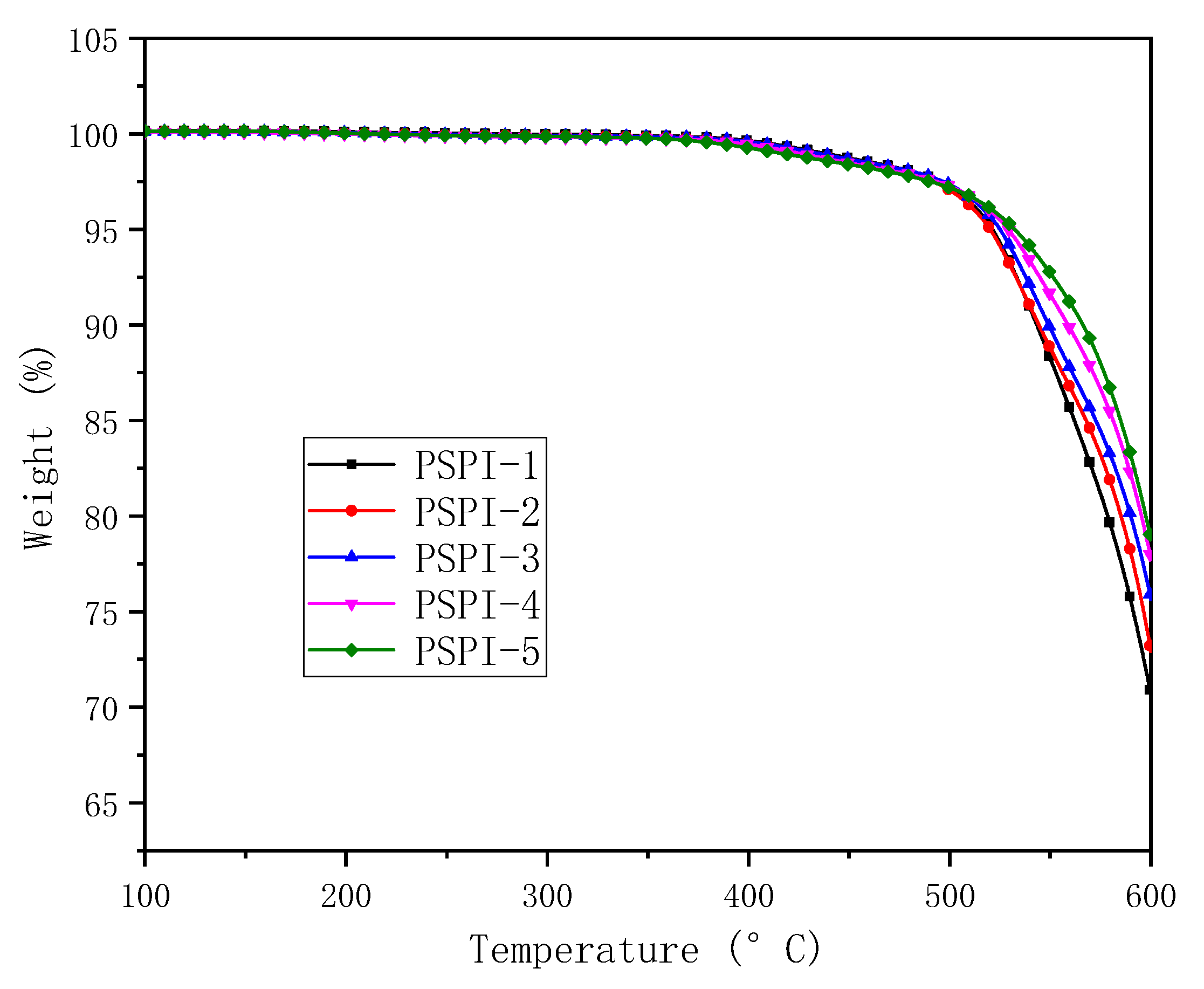
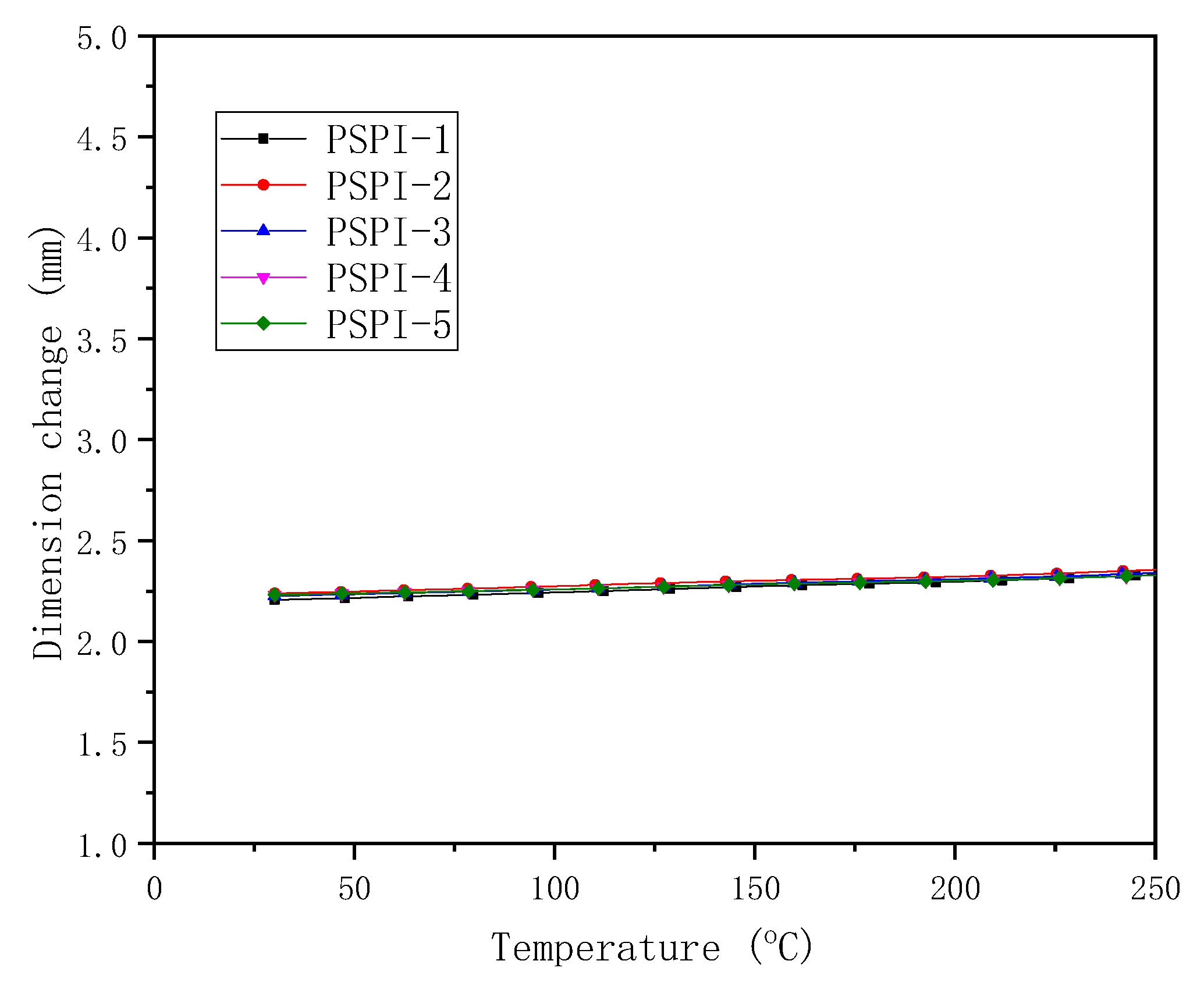
3.3. Thermal and Dielectric Properties
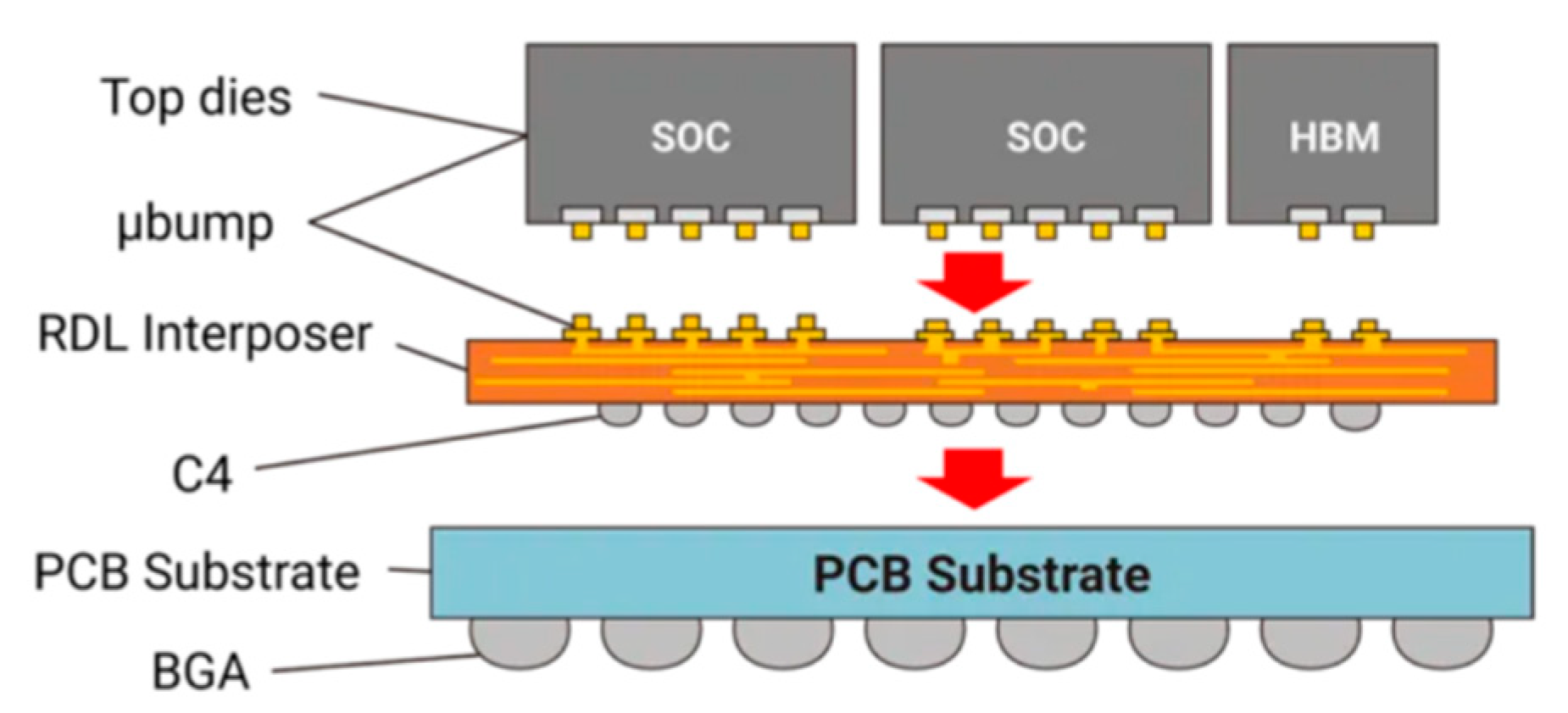
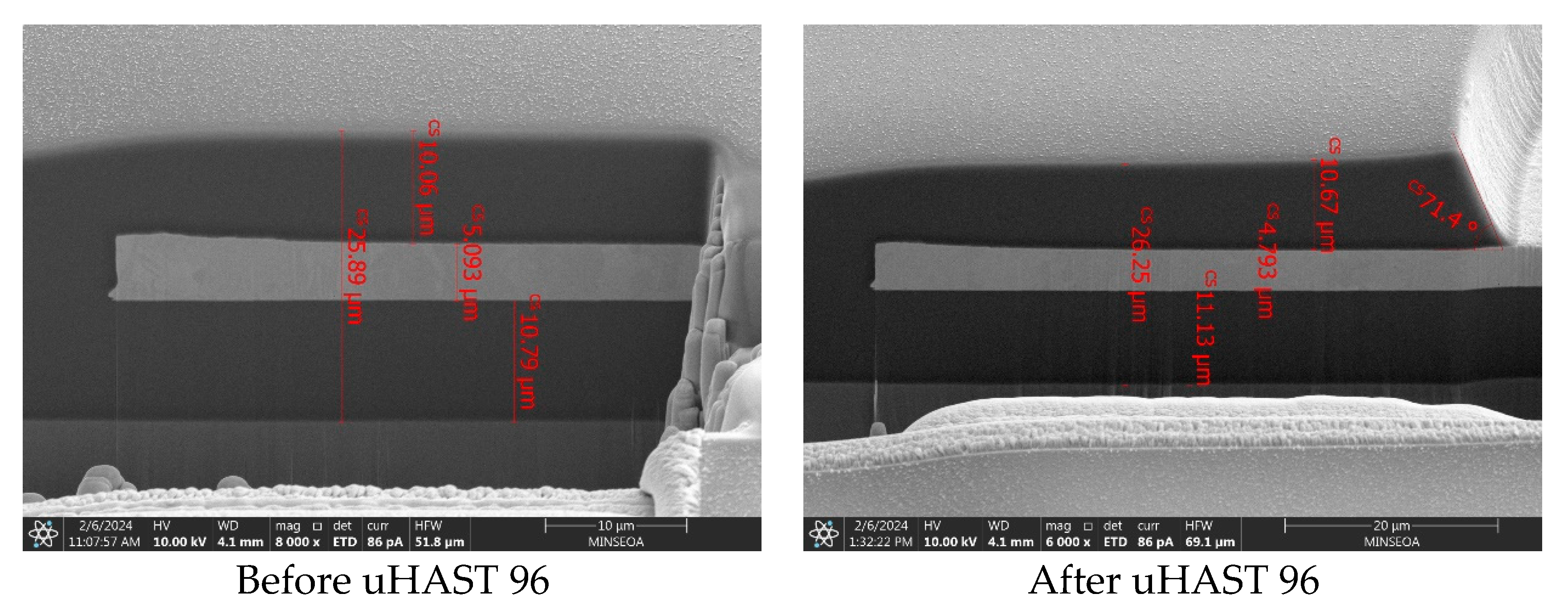
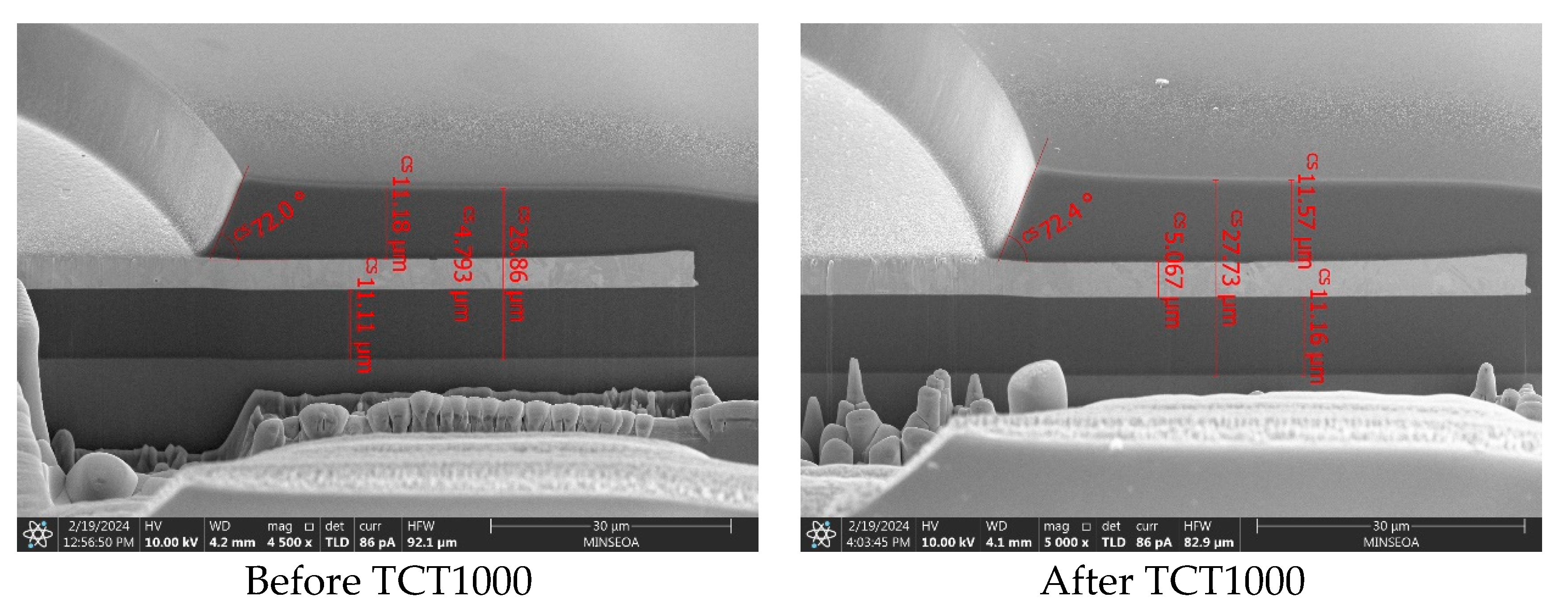
3.4. Practical Applications in RDL
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, H.; Jiang, J.; Ding, M. Ester-type precursor of polyimide and photosensitivity. Eur. Polym. J. 1999, 35, 1993–2000. [Google Scholar] [CrossRef]
- Fukukawa, K.I.; Ueda, M. Recent Development of Photosensitive Polybenzoxazoles. Polymer 2006, 38, 405–418. [Google Scholar]
- Le Thu, T.; Nguyen, H.N.N.; La, T.H.T. Synthesis and characterization of a photosensitive polyimide precursor and its photocuring behavior for lithography applications. Opt. Mater. 2007, 29, 610–618. [Google Scholar]
- Ahne, H.; Leuschner, R.; Rubner, R. Recent advances in photosensitive polyimides. Polym. Adv. Technol. 2010, 4, 217–233. [Google Scholar]
- Fukukawa, K.; Ueda, M. Recent Progress of Photosensitive Polyimides. Polym. J. 2008, 40, 281–296. [Google Scholar]
- Tomikawa, M.; Matsumura, K.; Shoji, Y.; Tatsuta, Y.; Okuda, R. Development of Photosensitve Polyimide B-stage Sheet Having High Cu Migration Resistance. J. Photopolym. Sci. Technol. 2017, 30, 181–185. [Google Scholar]
- Tumne, P.; Venkatadri, V.; Kudtarkar, S.; Delaus, M.; Santos, D.; Havens, R.; Srihari, K. Effect of Design Parameters on Drop Test Performance of Wafer Level Chip Scale Packages. J. Electron. Packag. 2012, 134, 020905. [Google Scholar]
- Lau, J.H.; Li, M.; Qingqian, M.L.; Chen, T.; Xu, I.; Yong, Q.X.; Cheng, Z.; Fan, N.; Kuah, E.; Li, Z.; et al. Fan-Out Wafer-Level Packaging for Heterogeneous Integration. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 8, 1544–1560. [Google Scholar]
- Cheng, H.C.; Liu, Y.C. Warpage Characterization of Molded Wafer for Fan-Out Wafer-Level Packaging. J. Electron. Packag. 2020, 142, 42–51. [Google Scholar] [CrossRef]
- Araki, H.; Kiuchi, Y.; Shimada, A.; Ogasawara, H.; Jukei, M.; Tomikawa, M. Low Df Polyimide with Photosensitivity for High Frequency Applications. J. Photopolym. Sci. Technol. 2020, 33, 165–170. [Google Scholar]
- Chen, M.-H.; Lai, C.-C.; Chen, H.-L.; Lin, Y.-H.; Huang, K.-Y.; Lin, C.-H.; Hsiao, H.-T.; Liu, L.-C.; Chen, C.-M. Preparation of photosensitive polyimides (PSPIs) and their feasible evaluation for lithographic insulation patterns (LIPs) of integrated circuits (ICs) without negative photoresists. Mater. Sci. Semicond. Process. 2018, 88, 132–138. [Google Scholar] [CrossRef]
- Ogura, T.; Higashihara, T.; Ueda, M. Low-CTE photosensitive polyimide based on semialicyclic poly(amic acid) and photobase generator. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1317–1323. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sakamoto, Y.; Tanaka, Y.; Kobayashi, Y. Poly(ester imide)s possessing low coefficients of thermal expansion (CTE) and low water absorption (III). Use of bis(4-aminophenyl)terephthalate and effect of substituents. Eur. Polym. J. 2010, 46, 1510–1524. [Google Scholar] [CrossRef]
- Hasegawa, M.; Tanaka, Y.; Koseki, K.; Tominaga, A. Positive-type Photo-patterning of Low-CTE, High-modulus Transparent Polyimide Systems. J. Photopolym. Sci. Technol. 2006, 19, 285–290. [Google Scholar] [CrossRef]
- Hao, J. Preparation of hyperbranched aromatic polyimide via A2 + B3 approach. Macromolecules 2002, 35, 5372–5381. [Google Scholar] [CrossRef]
- Yamanaka, K.; Jikei, M.; Kakimoto, M.-A.; Nihira, T.; Nakayama, T. Preparation, Dielectric and Optical Properties of Hyperbranched Aromatic Polyimide via Polyamic Acid Methyl Ester Precursor. J. Photopolym. Sci. Technol. 2000, 13, 321–322. [Google Scholar] [CrossRef]
- Kanehashi, S.; Sato, S.; Nagai, K. Membrane Gas Separation. In Synthesis and Gas Permeability of Hyperbranched and Cross-Linked Polyimide Membranes; Wiley Online Library: Hoboken, NJ, USA, 2010. [Google Scholar]
- Balasubramanian, R.; Kumutha, K.; Sarojadevi, M. Mechanical, thermal and electrical properties of polyimides containing 1, 2, 3-triazole ring prepared by click reaction. Polym. Bull. 2016, 73, 309–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J.; Li, Q.; Pang, L.; Zhang, Q.; Xu, Z.; Yeung, K.W.; Yi, C. Synthesis and characterization of novel hyperbranched polyimides/attapulgite nanocomposites. Compos. Part A Appl. Sci. Manuf. 2013, 55, 161–168. [Google Scholar] [CrossRef]
- Lanc, M.; Petr, M.; Soltys, M.; Stepanek, F.; Fonod, K.; Klepic, M.; Vopicka, O.; Lhotka, M.; Ulbrich, P.; Friess, K. Synthesis, preparation and characterization of novel hyperbranched 6FDA-TTM based polyimide membranes for effective CO2 separation: Effect of embedded mesoporous silica particles and siloxane linkages. Polymer 2018, 144, 33–42. [Google Scholar] [CrossRef]
- Lei, Y.; Shu, Y.; Peng, J.; Tang, Y.; Huo, J. Synthesis and properties of low coefficient of thermal expansion copolyimides derived from biphenyltetracarboxylic dianhydride with p-phenylenediamine and 4,4′-oxydialinine. e-Polymers 2016, 16, 295–302. [Google Scholar] [CrossRef]
- Miura, H.; Nishimura, A.; Kawai, S.; Murakami, G. Effect of package structure on the residual stress of silicon chips encapsulated in IC plastic packages. Trans. Jpn. Soc. Mech. Eng. Part A 1990, 56, 365–371. [Google Scholar] [CrossRef]
- Masaaki, K.; Toru, I.; Noriyuki, M. Evaluation of Residual Stress in Semiconductor Chips during Resin-Molding Process Using Piezoresistive Test Chips and a Finite Element Analysis Method. J. Jpn. Inst. Electron. Packag. 2010, 9, 186–194. [Google Scholar]


| PAEs | Molecular Weight | ||
|---|---|---|---|
| Mn (×104 g/mol) | Mw (×104 g/mol) | PDI | |
| PAE-1 | 1.2 | 2.5 | 2.1 |
| PAE-2 | 1.4 | 2.9 | 2.1 |
| PAE-3 | 1.6 | 3.2 | 2.1 |
| PAE-4 | 1.8 | 3.8 | 2.1 |
| PAE-5 | 2.0 | 4.2 | 2.1 |
| PSPIs | Resolution (µm) | Sensitivity (mJ/cm2) | Contrast |
|---|---|---|---|
| PSPI-1 | 8 | 87 | 2.5 |
| PSPI-2 | 8 | 90 | 2.3 |
| PSPI-3 | 10 | 93 | 2.0 |
| PSPI-4 | 12 | 93 | 1.9 |
| PSPI-5 | 15 | 94 | 1.7 |
| PSPIs | CTE (TMA, ppm/°C) | Tg (DMA, °C) | Decomposition Temperature (°C) | |
|---|---|---|---|---|
| Td5 | Td10 | |||
| PSPI-1 | 46 | 338 | 500 | 522 |
| PSPI-2 | 41 | 343 | 520 | 544 |
| PSPI-3 | 38 | 350 | 505 | 529 |
| PSPI-4 | 29 | 344 | 529 | 559 |
| PSPI-5 | 29 | 352 | 520 | 554 |
| PSPIs | Dk, ε′ (10 GHz) | Df, tanδ (10 GHz) |
|---|---|---|
| PSPI-1 | 2.89 | 0.0033 |
| PSPI-2 | 2.92 | 0.0031 |
| PSPI-3 | 2.90 | 0.0032 |
| PSPI-4 | 2.88 | 0.0028 |
| PSPI-5 | 2.90 | 0.0031 |
| PSPIs | Eb (%) | TM (GPa) | Ts (MPa) |
|---|---|---|---|
| PSPI-1 | 34.6 | 3.4 | 131.5 |
| PSPI-2 | 42.4 | 3.4 | 153.7 |
| PSPI-3 | 46.5 | 3.5 | 162.7 |
| PSPI-4 | 46.5 | 3.5 | 169.0 |
| PSPI-5 | 57.6 | 3.5 | 182.0 |
| Item. | Conditions | Standards |
|---|---|---|
| Precon condition | 125 °C, 24 h | JESD22-A113C |
| MSL3 | 30 °C, 60% RH, 192 h | JESD22-A113 |
| uHAST96 | RT~260 °C, 3 cycle | JESD22-A110B |
| TCT1000 | 130 °C, 85% RH, 2 atm, 96 h | JESD22-A104D |
| MR3X+UHAST96 | Condition B, −55~125 °C Stay 5 min, 20 min/cycle, 500 cycle | JESD22-A110B |
| MR5X+TCC200 | 150 °C, 500 h | JESD22-A104D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Wang, H.; Xia, P.; Chen, X.; Zhao, W.; Wang, C.; Meng, X.; Jia, B. Synthesis and Characterization of Negative-Tone Photosensitive Polyimides with Low Coefficient of Thermal Expansion for Packaging Applications. Polymers 2024, 16, 1805. https://doi.org/10.3390/polym16131805
Zhang P, Wang H, Xia P, Chen X, Zhao W, Wang C, Meng X, Jia B. Synthesis and Characterization of Negative-Tone Photosensitive Polyimides with Low Coefficient of Thermal Expansion for Packaging Applications. Polymers. 2024; 16(13):1805. https://doi.org/10.3390/polym16131805
Chicago/Turabian StyleZhang, Peng, Hehe Wang, Pengcheng Xia, Xiaolei Chen, Wei Zhao, Chengqian Wang, Xiao Meng, and Bin Jia. 2024. "Synthesis and Characterization of Negative-Tone Photosensitive Polyimides with Low Coefficient of Thermal Expansion for Packaging Applications" Polymers 16, no. 13: 1805. https://doi.org/10.3390/polym16131805
APA StyleZhang, P., Wang, H., Xia, P., Chen, X., Zhao, W., Wang, C., Meng, X., & Jia, B. (2024). Synthesis and Characterization of Negative-Tone Photosensitive Polyimides with Low Coefficient of Thermal Expansion for Packaging Applications. Polymers, 16(13), 1805. https://doi.org/10.3390/polym16131805






