Characterization of Microalgae Biomass-Based Composites Obtained through Rotational Molding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Strain Isolation and Outdoor Cultivation
2.1.2. Biomass Harvesting and Processing
2.2. Composite Processing
2.3. Characterization
2.3.1. Mechanical Characterization
2.3.2. Thermal Behavior
2.3.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.4. Microscopic Observations
2.3.5. Water Absorption
2.4. Determination of the Total Phenolic Compounds
2.5. Statistical Analysis
3. Results
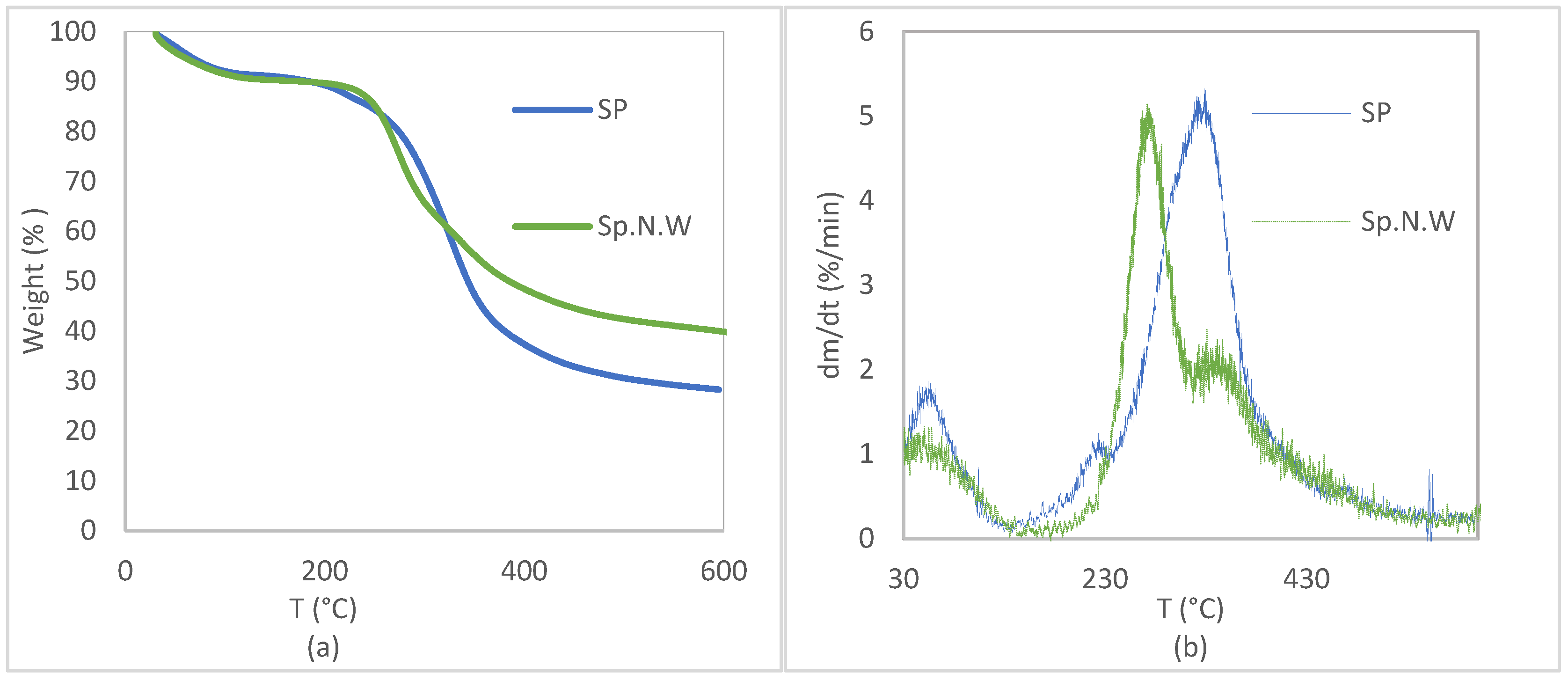
3.1. Biomass Characterization
3.2. Rotomolded Composites
3.2.1. Visual Evaluation
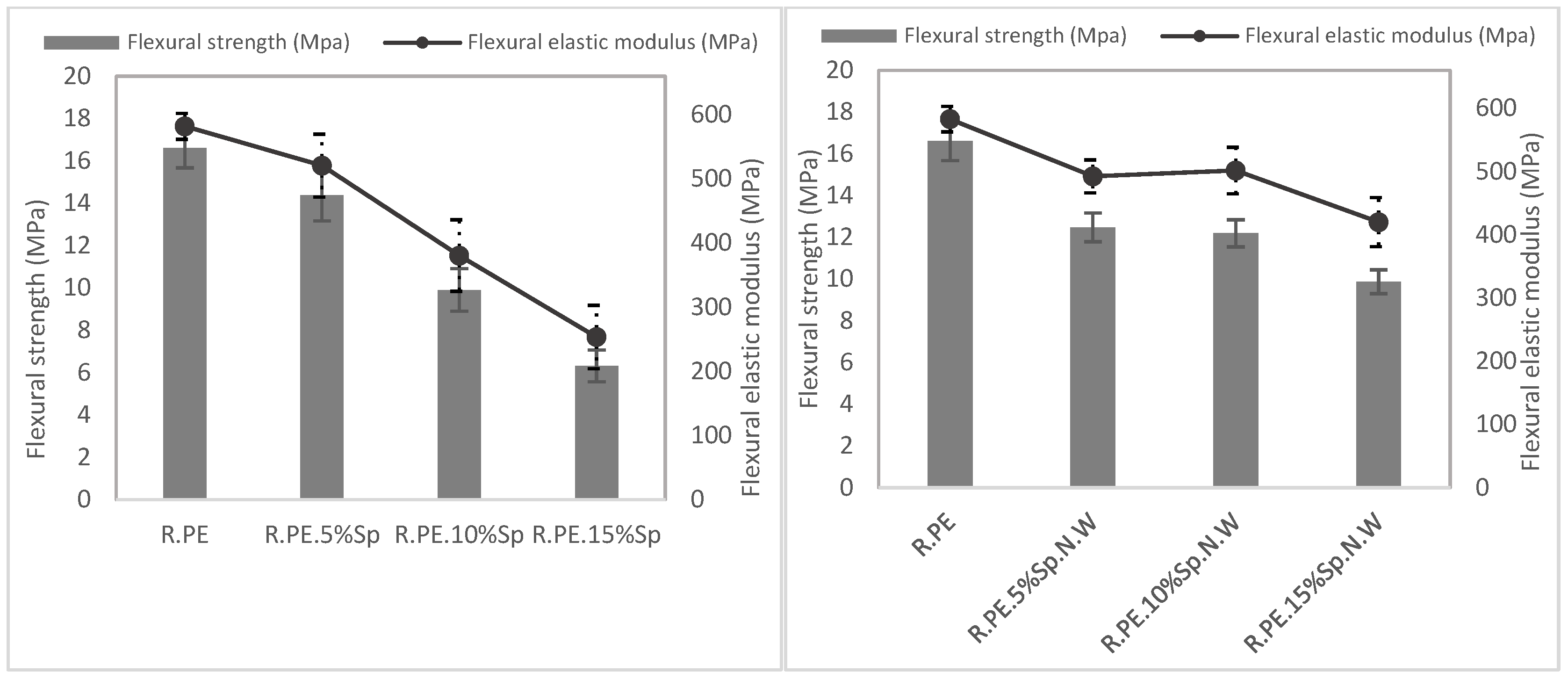
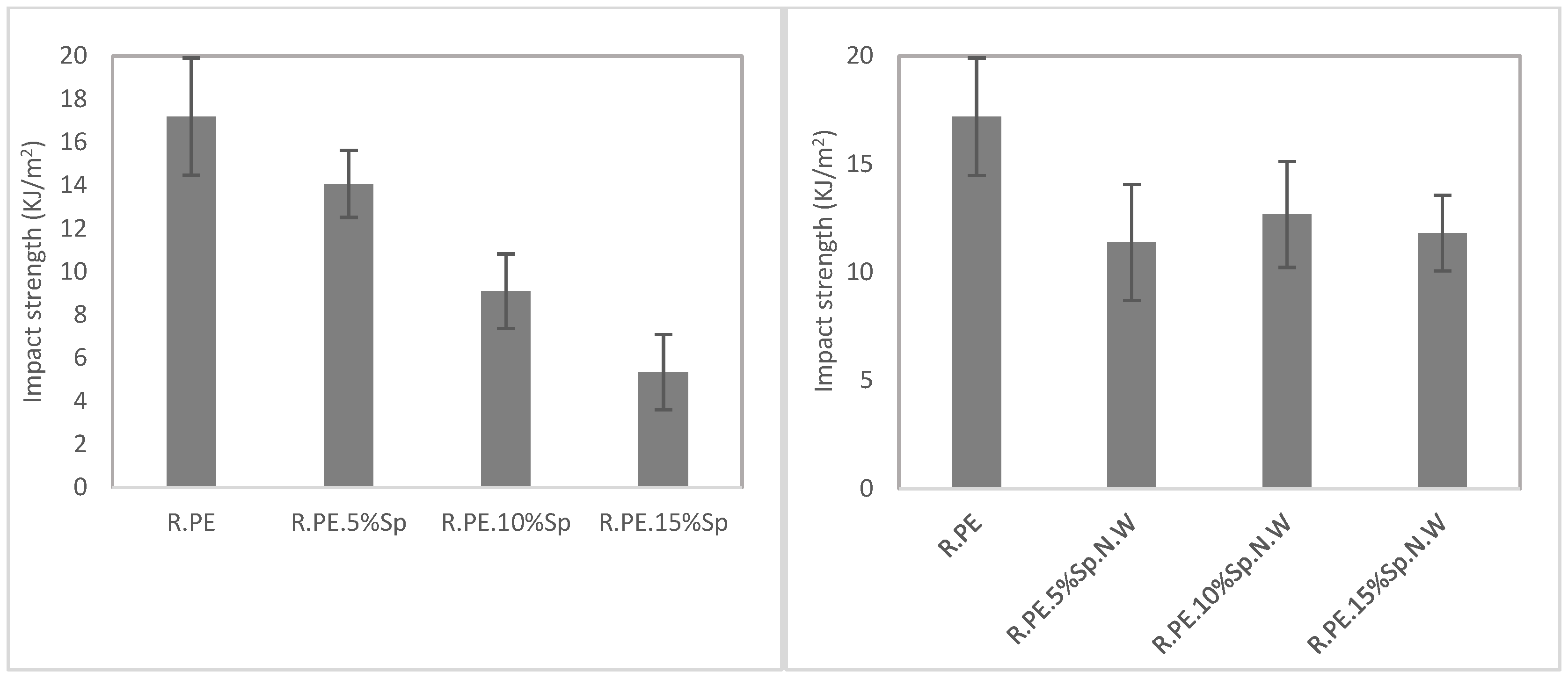
3.2.2. Mechanical Characterization
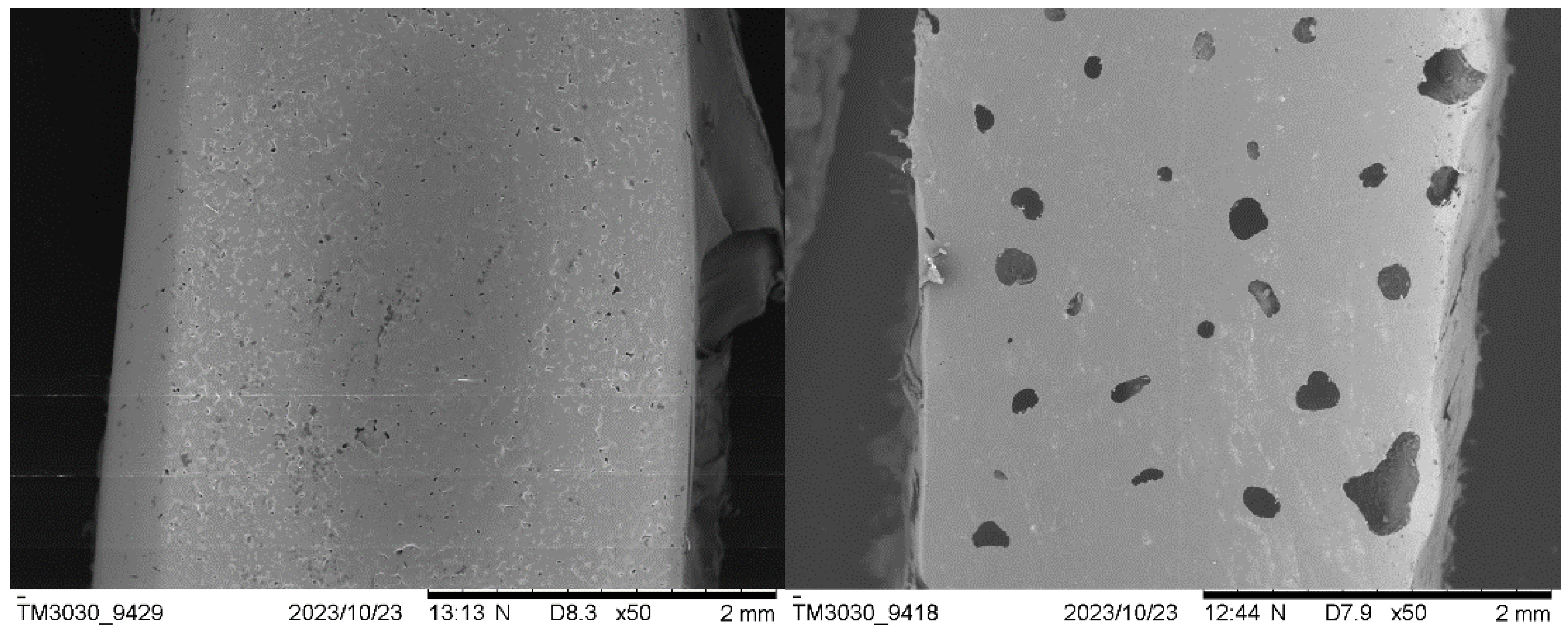
3.2.3. Scanning Electron Microscopy (SEM)
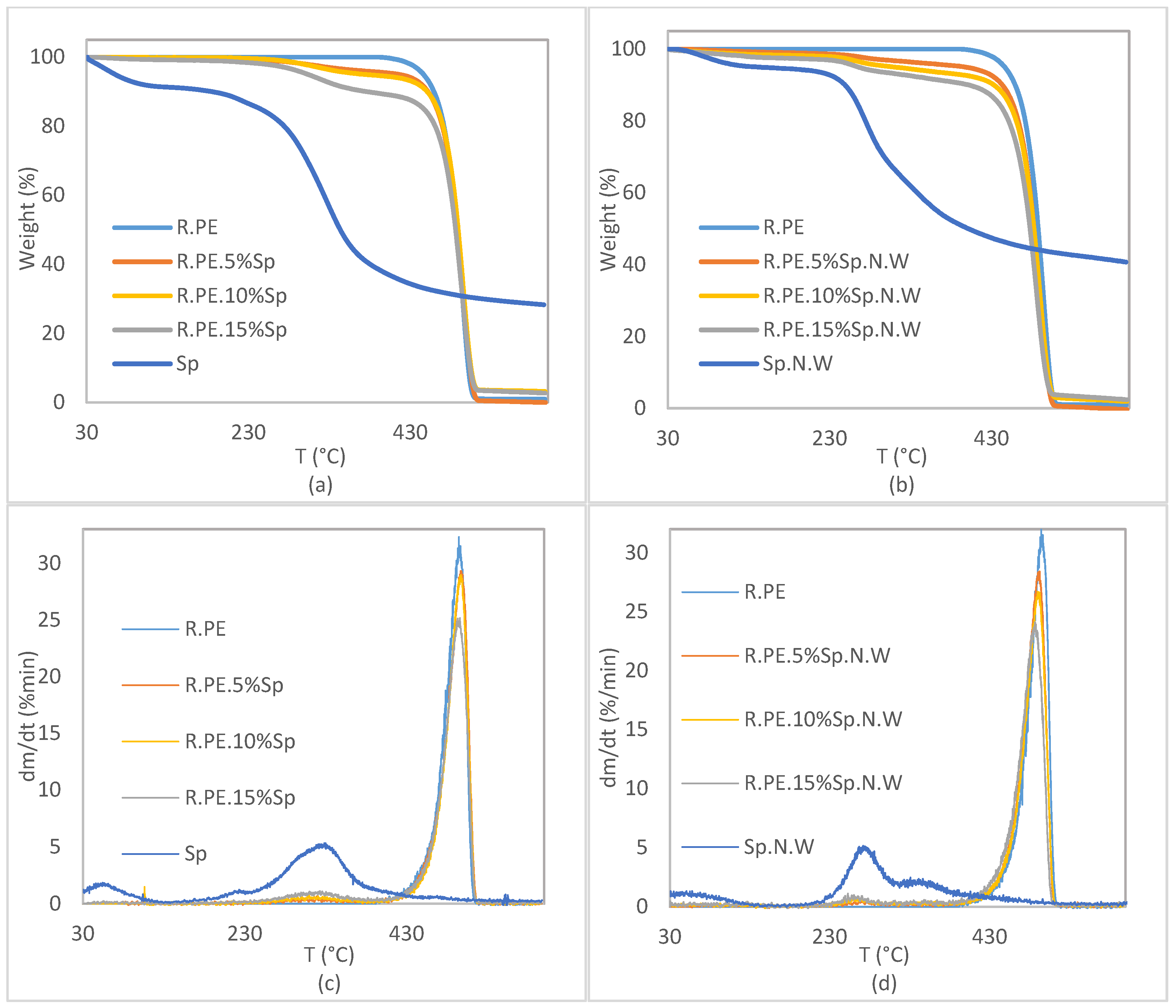
3.2.4. Thermal Characterization of Composites
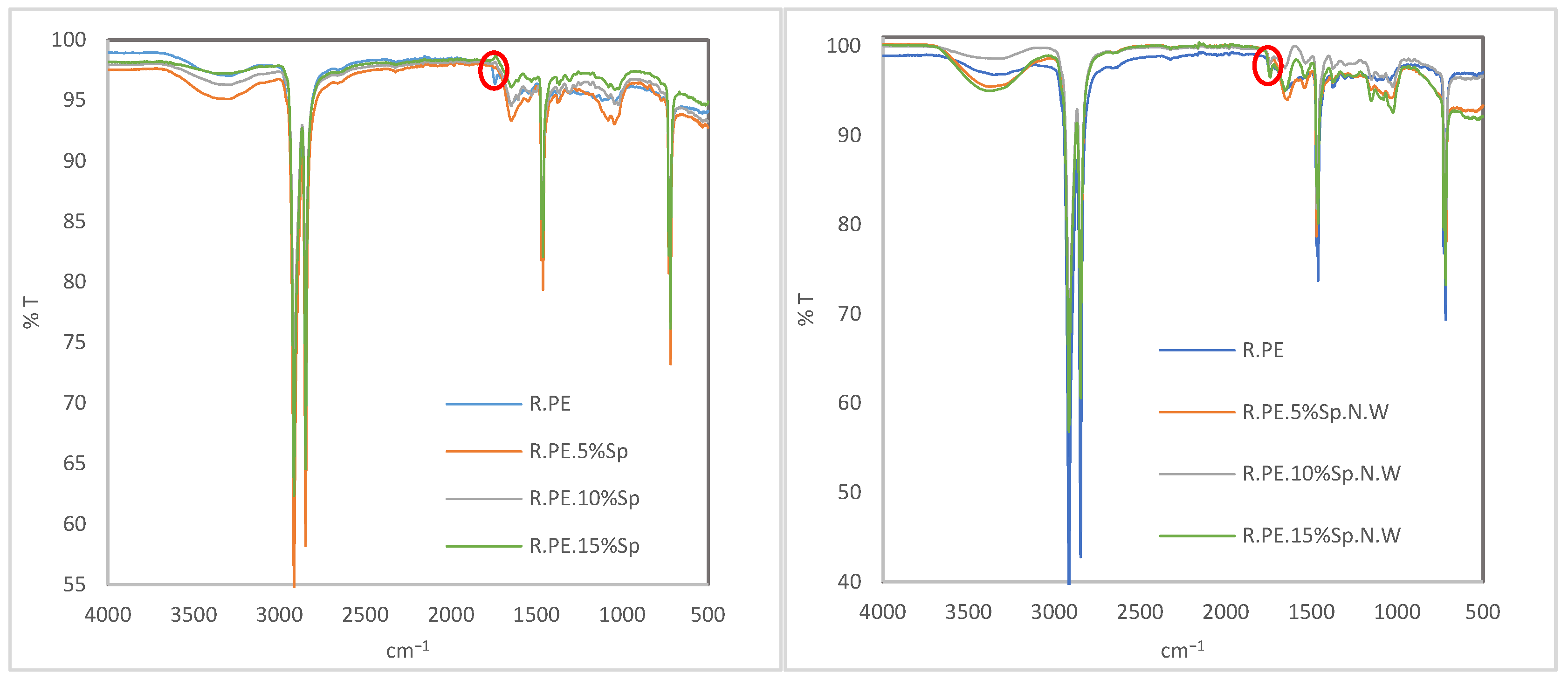
3.2.5. FTIR
3.2.6. Water Absorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaaba, N.; Ismail, H. Thermoplastic/Natural Filler Composites: A Short Review. J. Phys. Sci. 2019, 30, 81–99. [Google Scholar] [CrossRef]
- MacArthur, D.E. Beyond Plastic Waste. Science 2017, 358, 843. [Google Scholar] [CrossRef]
- Shanmugam, V.; Mensah, R.A.; Försth, M.; Sas, G.; Restás, Á.; Addy, C.; Xu, Q.; Jiang, L.; Neisiany, R.E.; Singha, S.; et al. Circular Economy in Biocomposite Development: State-of-the-Art, Challenges and Emerging Trends. Compos. Part C Open Access 2021, 5, 100138. [Google Scholar] [CrossRef]
- Tran, D.T.; Lee, H.R.; Jung, S.; Park, M.S.; Yang, J.W. Lipid-Extracted Algal Biomass Based Biocomposites Fabrication with Poly(Vinyl Alcohol). Algal Res. 2018, 31, 525–533. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kao, N. PLA Based Biopolymer Reinforced with Natural Fibre: A Review. J. Polym. Environ. 2011, 19, 714–725. [Google Scholar] [CrossRef]
- Ortega, Z.; Romero, F.; Paz, R.; Suárez, L.; Benítez, A.N.; Marrero, M.D. Valorization of Invasive Plants from Macaronesia as Filler Materials in the Production of Natural Fiber Composites by Rotational Molding. Polymers 2021, 13, 2220. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Andrzejewski, J.; Kosmela, P.; Piasecki, A.; Szostak, M.; Kuang, T. Rotational Molding of Linear Low-Density Polyethylene Composites Filled with Wheat Bran. Polymers 2020, 12, 1004. [Google Scholar] [CrossRef]
- Hanana, F.E.; Rodrigue, D. Rotational Molding of Polymer Composites Reinforced with Natural Fibers. Plast. Eng. 2015, 71, 28–31. [Google Scholar] [CrossRef]
- Barczewski, M.; Ortega, Z.; Piaskowski, P.; Aniśko, J.; Kosmela, P.; Szulc, J. A Case Study on the Rotomolding Behavior of Black Tea Waste and Bio-Based High-Density Polyethylene Composites: Do Active Compounds in the Filler Degrade during Processing? Compos. Part C Open Access 2024, 13, 100437. [Google Scholar] [CrossRef]
- Kelly-Walley, J.; Martin, P.; Ortega, Z.; Pick, L.; McCourt, M. Recent Advancements towards Sustainability in Rotomoulding. Materials 2024, 17, 2607. [Google Scholar] [CrossRef]
- Ortega, Z.; Monzón, M.D.; Benítez, A.N.; Kearns, M.; McCourt, M.; Hornsby, P.R. Banana and Abaca Fiber-Reinforced Plastic Composites Obtained by Rotational Molding Process. Mater. Manuf. Process. 2013, 28, 879–883. [Google Scholar] [CrossRef]
- Cisneros-López, E.O.; Pérez-Fonseca, A.A.; González-García, Y.; Ramírez-Arreola, D.E.; González-Núñez, R.; Rodrigue, D.; Robledo-Ortíz, J.R. Polylactic Acid–Agave Fiber Biocomposites Produced by Rotational Molding: A Comparative Study with Compression Molding. Adv. Polym. Technol. 2018, 37, 2528–2540. [Google Scholar] [CrossRef]
- Aniśko, J.; Barczewski, M. Uniaxial Rotational Molding of Bio-Based Low-Density Polyethylene Filled with Black Tea Waste. Materials 2023, 16, 3641. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Z.; McCourt, M.; Romero, F.; Suárez, L.; Cunningham, E. Recent Developments in Inorganic Composites in Rotational Molding. Polymers 2022, 14, 5260. [Google Scholar] [CrossRef] [PubMed]
- Cinar, S.O.; Chong, Z.K.; Kucuker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic Production from Microalgae: A Review. Int. J. Environ. Res. Public Health 2020, 17, 3842. [Google Scholar] [CrossRef] [PubMed]
- Dianursanti; Gozan, M.; Noviasari, C. The Effect of Glycerol Addition as Plasticizer in Spirulina Platensis Based Bioplastic. E3S Web Conf. 2018, 67, 03048. [Google Scholar] [CrossRef]
- Shi, B.; Wideman, G.; Wang, J.H. A New Approach of BioCO2 Fixation by Thermoplastic Processing of Microalgae. J. Polym. Environ. 2012, 20, 124–131. [Google Scholar] [CrossRef]
- Toro, C.; Reddy, M.M.; Navia, R.; Rivas, M.; Misra, M.; Mohanty, A.K. Characterization and Application in Biocomposites of Residual Microalgal Biomass Generated in Third Generation Biodiesel. J. Polym. Environ. 2013, 21, 944–951. [Google Scholar] [CrossRef]
- Torres, S.; Navia, R.; Campbell Murdy, R.; Cooke, P.; Misra, M.; Mohanty, A.K. Green Composites from Residual Microalgae Biomass and Poly(Butylene Adipate-Co-Terephthalate): Processing and Plasticization. ACS Sustain. Chem. Eng. 2015, 3, 614–624. [Google Scholar] [CrossRef]
- Díaz Guzmán, S.; Rios Santana, R.J.; Ortega, Z. Characterization of Microalgae Biomass/PE Biocomposites Obtained by Compression and Rotational Molding. In Advances in Manufacturing IV. Volume 1—Mechanical Engineering: Digitalization, Sustainability and Industry Applications; Gapiński, B., Ciszak, O., Ivanov, V., Machado, J.M., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024. [Google Scholar]
- Guidi, F.; Gojkovic, Z.; Venuleo, M.; Assunçao, P.A.C.J.; Portillo, E. Long-Term Cultivation of a Native Arthrospira Platensis (Spirulina) Strain in Pozo Izquierdo (Gran Canaria, Spain): Technical Evidence for a Viable Production of Food-Grade Biomass. Processes 2021, 9, 1333. [Google Scholar] [CrossRef]
- Zhu, N.; Ye, M.; Shi, D.; Chen, M. Reactive Compatibilization of Biodegradable Poly(Butylene Succinate)/Spirulina Microalgae Composites. Macromol. Res. 2017, 25, 165–171. [Google Scholar] [CrossRef]
- Khoobkar, Z.; Delavari Amrei, H.; Heydarinasab, A.; Mirzaie, M.A.M. Biofixation of CO2 and Biomass Production from Model Natural Gas Using Microalgae: An Attractive Concept for Natural Gas Sweetening. J. CO2 Util. 2022, 64, 102153. [Google Scholar] [CrossRef]
- European Commission. The Blue Economy Report 2021; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Zhang, F.; Endo, T.; Kitagawa, R.; Kabeya, H.; Hirotsu, T. Synthesis and Characterization of a Novel Blendof Polypropylene with Chlorella. J. Mater. Chem. 2000, 10, 2666–2672. [Google Scholar] [CrossRef]
- Zhang, F.; Kabeya, H.; Kitagawa, R.; Hirotsu, T.; Yamashita, M.; Otsuki, T. Exploratory Research of PVC-Chlorella Composite Material (PCCM) as Effective Utilization of Chlorella Biologically Fixing CO2. J. Mater. Sci. 2000, 35, 2603–2609. [Google Scholar] [CrossRef]
- Otsuki, J.; Zhang, F.; Kabeya, H.; Hirotsu, T. Synthesis and Tensile Properties of a Novel Composite of Chlorella and Polyethylene. J. Appl. Polym. Sci. 2004, 92, 812–816. [Google Scholar] [CrossRef]
- Zeller, M.A.; Hunt, R.; Jones, A.; Sharma, S. Bioplastics and Their Thermoplastic Blends from Spirulina and Chlorella Microalgae. J. Appl. Polym. Sci. 2013, 130, 3263–3275. [Google Scholar] [CrossRef]
- Wang, K. Bio-Plastic Potential of Spirulina Microalgae. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2014. [Google Scholar]
- Monshupanee, T.; Nimdach, P.; Incharoensakdi, A. Two-Stage (Photoautotrophy and Heterotrophy) Cultivation Enables Efficient Production of Bioplastic Poly-3-Hydroxybutyrate in Auto-Sedimenting Cyanobacterium. Sci. Rep. 2016, 6, 37121. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.K.; Costa, J.A.V.; de Morais, M.G. Polyhydroxybutyrate (PHB) Synthesis by Spirulina Sp. LEB 18 Using Biopolymer Extraction Waste. Appl. Biochem. Biotechnol. 2018, 185, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.M.; Ali, G.H. Analysis of Polyhydroxybutrate and Bioplastic Production from Microalgae. Bull. Natl. Res. Cent. 2019, 43, 97. [Google Scholar] [CrossRef]
- Rajpoot, A.S.; Choudhary, T.; Chelladurai, H.; Nath Verma, T.; Shende, V. A Comprehensive Review on Bioplastic Production from Microalgae. Mater. Today Proc. 2022, 56, 171–178. [Google Scholar] [CrossRef]
- Khalis, S.A. The Effect of Compatibilizer Addition on Chlorella Vulgaris Microalgae Utilization as a Mixture for Bioplastic. E3S Web Conf. 2018, 67, 03047. [Google Scholar] [CrossRef]
- Kim, G.M.; Chang, W.S.; Kim, Y.K. Biocomposites Using Whole or Valuable Component-Extracted Microalgae Blended with Polymers: A Review. Catalysts 2021, 12, 25. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae—A Green Multi-Product Biorefinery for Future Industrial Prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Aflori, M.; Serbezeanu, D.; Ipate, A.M.; Dobos, A.M.; Rusu, D. Development of New Polyimide/Spirulina Hybrid Materials: Preparation and Characterization. J. Compos. Sci. 2024, 8, 178. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Cao, G.; Wang, D.; Ho, S.H. A Sustainable Solution to Plastics Pollution: An Eco-Friendly Bioplastic Film Production from High-Salt Contained Spirulina Sp. Residues. J. Hazard. Mater. 2020, 388, 121773. [Google Scholar] [CrossRef]
- Saha, P.; Aloui, H.; Yun, J.H.; Kim, H.S.; Kim, B.S. Development of a Novel Composite Film Based on Polyurethane and Defatted Chlorella Biomass: Physical and Functional Characterization. J. Appl. Polym. Sci. 2021, 138, 50152. [Google Scholar] [CrossRef]
- Sabathini, H.A.; Windiani, L.; Dianursanti; Gozan, M. Mechanical Physicial Properties of Chlorella-PVA Based Bioplastic with Ultrasonic Homogenizer. E3S Web Conf. 2018, 67, 03046. [Google Scholar] [CrossRef]
- González-Balderas, R.M.; Felix, M.; Bengoechea, C.; Orta Ledesma, M.T.; Guerrero, A.; Velasquez-Orta, S.B. Development of Composites Based on Residual Microalgae Biomass Cultivated in Wastewater. Eur. Polym. J. 2021, 160, 110766. [Google Scholar] [CrossRef]
- Ortega, Z.; Douglas, P.; Hanna, P.R.; Kelly-Walley, J.; McCourt, M. Influence of Mold Pressurization on Cycle Time in Rotational Molding Composites with Welded Ignimbrite as Loading. Compos. Commun. 2024, 45, 101797. [Google Scholar] [CrossRef]
- Vignaud, J.; Loiseau, C.; Hérault, J.; Mayer, C.; Côme, M.; Martin, I.; Ulmann, L. Microalgae Produce Antioxidant Molecules with Potential Preventive Effects on Mitochondrial Functions and Skeletal Muscular Oxidative Stress. Antioxidants 2023, 12, 1050. [Google Scholar] [CrossRef]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural Antioxidants as Stabilizers for Polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Mysiukiewicz, O.; Kuzmin, A. Coffee Silverskin as a Multifunctional Waste Filler for High-Density Polyethylene Green Composites. J. Compos. Sci. 2021, 5, 44. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Mysiukiewicz, O. Comparative Analysis of the Coffee and Cocoa Industry By-Products on the Performance of Polyethylene-Based Composites. Waste Biomass Valoriz. 2023, 14, 2691–2706. [Google Scholar] [CrossRef]
- Mateescu, C.; Zaharescu, T.; Mariş, M. Chemiluminescence Study on the Radiochemical Stability of Polypropylene Modified with Microalgal Extracts. Radiat. Phys. Chem. 2021, 183, 109401. [Google Scholar] [CrossRef]
- Hosseini Tafreshi, S.A.; Aghaie, P.; Toghyani, M.A.; Ramazani-Moghaddam-Arani, A. Improvement of Ionizing Gamma Irradiation Tolerance of Chlorella Vulgaris by Pretreatment with Polyethylene Glycol. Int. J. Radiat. Biol. 2020, 96, 919–928. [Google Scholar] [CrossRef]
- UNE-EN ISO 178:2011; Determinación de las Propiedades de Flexión. AENOR: Madrid, Spain, 2011.
- UNE-EN ISO 527-2:2012; Determinación de las Propiedades en Tracción. AENOR: Madrid, Spain, 2012.
- UNE-EN ISO 180:2001/A2:2013; Determinación de la Resistencia al Impacto Izod. AENOR: Madrid, Spain, 2013.
- Romero, F.; Ortega, Z.; Castellano, J.; Benítez, A.N.; Marrero, M.D.; Suárez, L. Use of Ricinus Communis Shredded Material as Filler in Rotational Molded Parts to Improve the Bio-Disintegration Behavior. Polym. Bull. 2023, 80, 11295–11316. [Google Scholar] [CrossRef]
- UNE-EN ISO 11357-6:2018; Plásticos—Calorimetría Diferencial de Barrido (DSC) Parte 6: Determinación del Tiempo de Inducción a la Oxidación (OIT Isotérmico) y de la Temperatura de Inducción a la Oxidación (OIT Dinámica). AENOR: Madrid, Spain, 2018.
- UNE-EN ISO 62:2008; Plásticos—Determinación de la Absorción de Agua. AENOR: Madrid, Spain, 2008.
- Pérez-Jiménez, J.; Saura-Calixto, F. Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Royal Society of Chemistry: London, UK, 2018; ISBN 1788011066. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Jamilatun, S.; Budhijanto; Rochmadi; Budiman, A. Thermal Decomposition and Kinetic Studies of Pyrolysis of Spirulina Platensis Residue. Int. J. Renew. Energy Dev. 2017, 6, 193–201. [Google Scholar] [CrossRef]
- Bataller, B.G.; Capareda, S.C. A Rapid and Non-Destructive Method for Quantifying Biomolecules in Spirulina Platensis via Fourier Transform Infrared—Attenuated Total Reflectance Spectroscopy. Algal Res. 2018, 32, 341–352. [Google Scholar] [CrossRef]
- Ferro, L.; Gojkovic, Z.; Gorzsás, A.; Funk, C. Statistical Methods for Rapid Quantification of Proteins, Lipids, and Carbohydrates in Nordic Microalgal Species Using ATR–FTIR Spectroscopy. Molecules 2019, 24, 3237. [Google Scholar] [CrossRef]
- Sudhakar, K.; Premalatha, M. Characterization of Micro Algal Biomass through FTIR/TGA /CHN Analysis: Application to Scenedesmus sp. Energy Sources Part A Recovery Util. Environ. Eff. 2015, 37, 2330–2337. [Google Scholar] [CrossRef]
- Arif, M.; Li, Y.; El-Dalatony, M.M.; Zhang, C.; Li, X.; Salama, E.S. A Complete Characterization of Microalgal Biomass through FTIR/TGA/CHNS Analysis: An Approach for Biofuel Generation and Nutrients Removal. Renew. Energy 2021, 163, 1973–1982. [Google Scholar] [CrossRef]
- Michalak, I.; Mironiuk, M.; Godlewska, K.; Trynda, J.; Marycz, K. Arthrospira (Spirulina) Platensis: An Effective Biosorbent for Nutrients. Process Biochem. 2020, 88, 129–137. [Google Scholar] [CrossRef]
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Using FTIR Spectroscopy for Rapid Determination of Lipid Accumulation in Response to Nitrogen Limitation in Freshwater Microalgae. Bioresour. Technol. 2010, 101, 4499–4507. [Google Scholar] [CrossRef]
- León, L.D.V.E.; Escocio, V.A.; Visconte, L.L.Y.; Junior, J.C.J.; Pacheco, E.B.A.V. Rotomolding and Polyethylene Composites with Rotomolded Lignocellulosic Materials: A Review. J. Reinf. Plast. Compos. 2020, 39, 459–472. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Mysiukiewicz, O. Insights into the Processing, Structure, and Mechanical Performance of Polyethylene/Gypsum Composites. Compos. Theory Pract. 2024, 24, 17–24. [Google Scholar] [CrossRef]
- Abhilash, S.S.; Singaravelu, D.L. Effect of Fiber Content on Mechanical and Morphological Properties of Bamboo Fiber-Reinforced Linear Low-Density Polyethylene Processed by Rotational Molding. Trans. Indian Inst. Met. 2020, 73, 1549–1554. [Google Scholar] [CrossRef]
- Torres, F.G.; Aragon, C.L. Final Product Testing of Rotational Moulded Natural Fibre-Reinforced Polyethylene. Polym. Test. 2006, 25, 568–577. [Google Scholar] [CrossRef]
- Ortega, Z.; Suárez, L.; Kelly-Walley, J.; McCourt, M. Mechanical Properties of Rotomolded Parts with Abaca Fiber: Effect of Manufacturing with 1, 2 or 3 Layers. Compos. Theory Pract. 2023, 3, 158–166. [Google Scholar]
- Cisneros-López, E.O.; González-López, M.E.; Pérez-Fonseca, A.A.; González-Núñez, R.; Rodrigue, D.; Robledo-Ortíz, J.R. Effect of Fiber Content and Surface Treatment on the Mechanical Properties of Natural Fiber Composites Produced by Rotomolding. Compos. Interfaces 2017, 24, 35–53. [Google Scholar] [CrossRef]
- López GonzalezNúñez, R.G.; Vázquez-Fletes, R.C.; Ortega-Gudiño, P.; Vázquez-Lepe, M.O.; Rodrigue, D.; González-Núñez, R. Rotational Molding of Compatibilized PA6/LLDPE Blends. Polym. Eng. Sci. 2021, 61, 1007–1017. [Google Scholar] [CrossRef]
- Greco, A.; Maffezzoli, A. Rotational Molding of Poly(Lactic Acid): Effect of Polymer Grade and Granulometry. Adv. Polym. Technol. 2017, 36, 477–482. [Google Scholar] [CrossRef]
- Barczewski, M.; Aniśko, J.; Hejna, A.; Mysiukiewicz, O.; Kosmela, P.; Sałasińska, K.; Boczkowska, A.; Przybylska-Balcerek, A.; Stuper-Szablewska, K. Ground Lemon and Stevia Leaves as Renewable Functional Fillers with Antioxidant Activity for High-Density Polyethylene Composites. Clean Technol. Environ. Policy 2023, 25, 3345–3361. [Google Scholar] [CrossRef]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Aniśko, J.; Szulc, J.; Skórczewska, K.; Piasecki, A.; Kuang, T. More than Just a Beer—Brewers’ Spent Grain, Spent Hops, and Spent Yeast as Potential Functional Fillers for Polymer Composites. Waste Manag. 2024, 180, 23–35. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Kosmela, P.; Barczewski, M.; Hejna, A. Mechanical, Thermal and Rheological Properties of Polyethylene-Based Composites Filled with Micrometric Aluminum Powder. Materials 2020, 13, 1242. [Google Scholar] [CrossRef]
- Barczewski, M.; Hejna, A.; Sałasińska, K.; Aniśko, J.; Piasecki, A.; Skórczewska, K.; Andrzejewski, J. Thermomechanical and Fire Properties of Polyethylene-Composite-Filled Ammonium Polyphosphate and Inorganic Fillers: An Evaluation of Their Modification Efficiency. Polymers 2022, 14, 2501. [Google Scholar] [CrossRef]
- Ramesh Kumar, S.C.; Kaviti, R.V.P.; Mahesh, L.; Mohan Babu, B.M. Water Absorption Behavior of Hybrid Natural Fiber Reinforced Composites. Mater. Today Proc. 2022, 54, 187–190. [Google Scholar] [CrossRef]
- Gowda, A.H.R.; Goud, G.; Sathynarayana, K.; Puttegowda, M. Influence of Water Absorption on Mechanical and Morphological Behaviour of Roystonea-Regia/Banana Hybrid Polyester Composites. Appl. Sci. Eng. Prog. 2024, 17, 7074. [Google Scholar] [CrossRef]
- Aranha, R.; Filho, M.A.A.; de Lima Santos, C.; Fonseca, V.M.; Rivera, J.L.V.; de Lima, A.G.B.; de Amorim, W.F.; Carvalho, L.H. Water Sorption in Hybrid Polyester/Glass/Jute Composites Processed via Compression Molding and Vacuum-Assisted Resin Transfer Molding. Polymers 2023, 15, 4438. [Google Scholar] [CrossRef] [PubMed]
- Marcovich, N.E.; Reboredo, M.M.; Aranguren, M.I. Moisture Diffusion in Polyester-Woodflour Composites. Polymer 1999, 40, 7313–7320. [Google Scholar] [CrossRef]
- Andreopoulos, A.G.; Tarantili, P.A. Water Sorption Characteristics of Epoxy Resin-UHMPE Fibers Composites. J. Appl. Polym. Sci. 1998, 70, 747–755. [Google Scholar] [CrossRef]
- Suárez, L.; Hanna, P.R.; Ortega, Z.; Barczewski, M.; Kosmela, P.; Millar, B.; Cunningham, E. Influence of Giant Reed (Arundo Donax L.) Culms Processing Procedure on Physicochemical, Rheological, and Thermomechanical Properties of Polyethylene Composites. J. Nat. Fibers 2024, 21, 2296909. [Google Scholar] [CrossRef]
- Chen, H.; Miao, M.; Ding, X. Influence of Moisture Absorption on the Interfacial Strength of Bamboo/Vinyl Ester Composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 2013–2019. [Google Scholar] [CrossRef]
- Airinei, A.; Asandulesa, M.; Stelescu, M.D.; Tudorachi, N.; Fifere, N.; Bele, A.; Musteata, V. Dielectric, Thermal and Water Absorption Properties of Some EPDM/Flax Fiber Composites. Polymers 2021, 13, 2555. [Google Scholar] [CrossRef]
- Dhakal, H.N.; Zhang, Z.Y.; Richardson, M.O.W. Effect of Water Absorption on the Mechanical Properties of Hemp Fibre Reinforced Unsaturated Polyester Composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Akil, H.M.; Cheng, L.W.; Mohd Ishak, Z.A.; Abu Bakar, A.; Abd Rahman, M.A. Water Absorption Study on Pultruded Jute Fibre Reinforced Unsaturated Polyester Composites. Compos. Sci. Technol. 2009, 69, 1942–1948. [Google Scholar] [CrossRef]
- Robledo-Ortíz, J.R.; González-López, M.E.; Rodrigue, D.; Gutiérrez-Ruiz, J.F.; Prezas-Lara, F.; Pérez-Fonseca, A.A. Improving the Compatibility and Mechanical Properties of Natural Fibers/Green Polyethylene Biocomposites Produced by Rotational Molding. J. Polym. Environ. 2020, 28, 1040–1049. [Google Scholar] [CrossRef]
- Ferrero, B.; Fombuena, V.; Fenollar, O.; Boronat, T.; Balart, R. Development of Natural Fiber-reinforced Plastics (NFRP) Based on Biobased Polyethylene and Waste Fibers from Posidonia Oceanica Seaweed. Polym. Compos. 2015, 36, 1378–1385. [Google Scholar] [CrossRef]

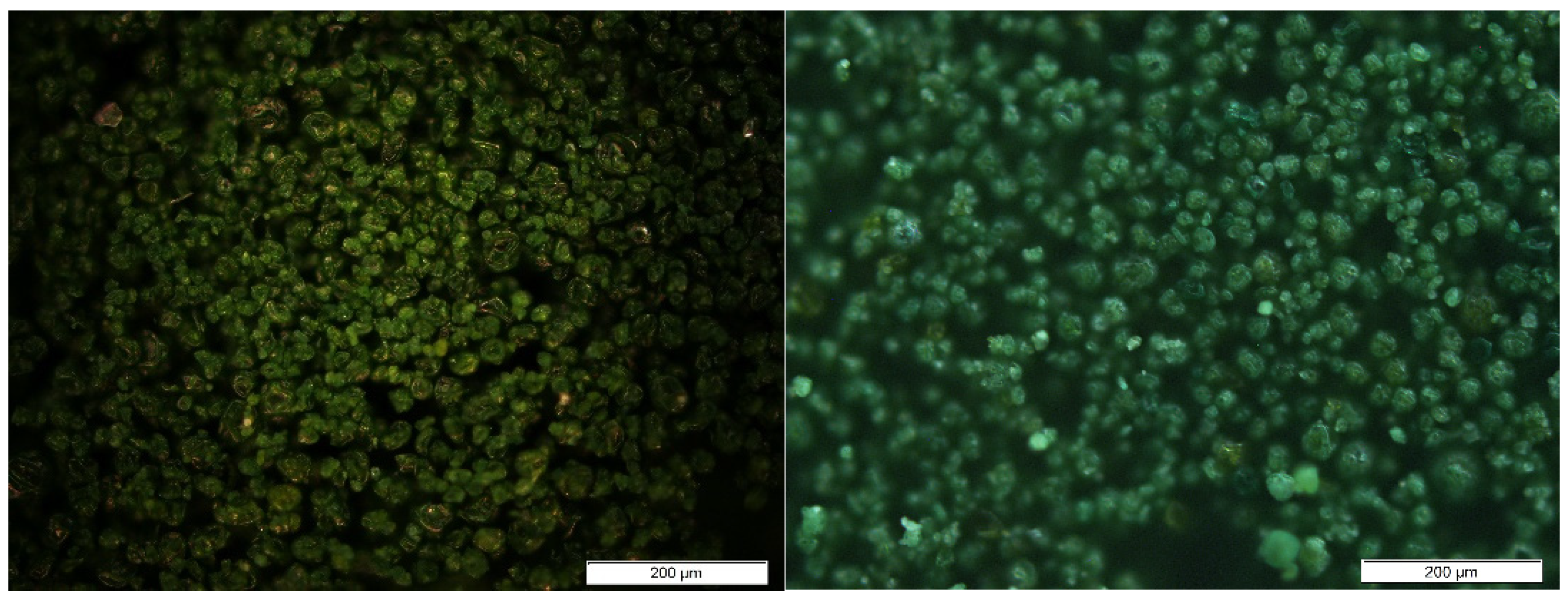



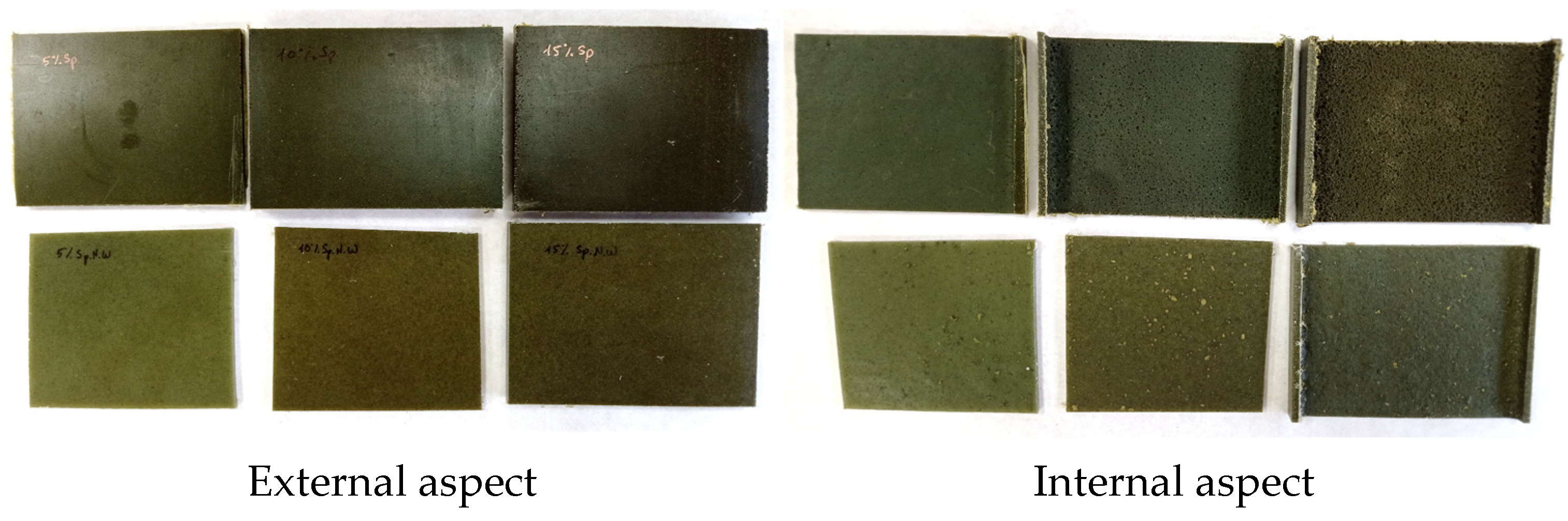


| Biomass | Specimen | % Biomass |
|---|---|---|
| R.PE | 0 | |
| Spirulina | R.PE.5%Sp | 5 |
| R.PE.10%Sp | 10 | |
| R.PE.15%Sp | 15 | |
| Spirulina non-washed | R.PE.5%Sp.N.W | 5 |
| R.PE.10%Sp.N.W | 10 | |
| R.PE.15%Sp.N.W | 15 |
| Microalgae | T Range (°C) | % Weight Loss | T Peak (°C) |
|---|---|---|---|
| Spirulina | 30–140 | 9.26 ± 1.12 | 60 |
| 140–240 | 5.73 ± 0.12 | 228 | |
| 240–600 | 56.87 ± 1.37 | 330 | |
| Non-washed Spirulina | 30–150 | 7.90 ± 2.46 | 60 |
| 150–320 | 29.40 ± 1.19 | 274 | |
| 320–600 | 22.25 ± 1.13 | 342 |
| Specimen | Flexural Test | Tensile Test | ||||
|---|---|---|---|---|---|---|
| Density (g/cm3) | Impact Strength (kJ/m2) | Flexural Strength (MPa) | Flexural Elastic Modulus (MPa) | Tensile Strength (MPa) | Tensile Elastic Modulus (MPa) | |
| R.PE | 0.892 ± 0.002 | 17.19 ± 2.72 a | 16.61 ± 0.94 a | 582.85 ± 20.15 a | 15.71 ± 0.54 a | 571.03 ± 40.65 a |
| R.PE.5%Sp | 0.852 ± 0.009 | 14.07 ± 1.56 a | 14.38 ± 1.21 a | 520.65 ± 49.10 a | 12.01 ± 0.30 ab | 452.83 ± 50.03 ab |
| R.PE.10%Sp | 0.807 ± 0.012 | 9.08 ± 1.73 ab | 9.91 ± 1.01 ab | 380.49 ± 55.78 ab | 8.40 ± 0.30 bc | 447.57 ± 37.66 ab |
| R.PE.15%Sp | 0.724 ± 0.018 | 5.34 ± 1.74 b | 6.32 ± 0.75 b | 253.44 ± 49.41 b | 4.61 ± 0.35 c | 315.01 ± 28.24 b |
| R.PE | 0.892 ± 0.002 | 17.19 ± 2.72 a | 16.61 ± 0.94 a | 582.85 ± 20.15 a | 15.71 ± 0.54 a | 571.03 ± 40.65 a |
| R.PE.5%Sp.N.W | 0.890 ± 0.002 | 11.38 ± 2.68 ab | 12.47 ± 0.69 ab | 492.41 ± 26.15 ab | 12.85 ± 0.45 ab | 598.58 ± 39.22 a |
| R.PE.10%Sp.N.W | 0.888 ± 0.003 | 12.67 ± 2.45 ab | 12.19 ± 0.65 ab | 501.59 ± 36.64 ab | 11.97 ± 0.51 bc | 587.71 ± 43.14 a |
| R.PE.15%Sp.N.W | 0.878 ± 0.011 | 11.81 ± 1.75 b | 9.87 ± 0.57 b | 419.92 ± 38.81 b | 10.45 ± 0.55 c | 542.04 ± 50.45 a |
| Property | a | b | R2 |
|---|---|---|---|
| Tensile strength | 15.72 | −0.739 | 0.999 |
| Tensile elastic modulus | 562.61 | −15.47 | 0.911 |
| Flexural strength | 17.109 | −0.707 | 0.985 |
| Flexural elastic modulus | 603.02 | −22.517 | 0.977 |
| Impact strength | 17.502 | −0.8104 | 0.993 |
| Specimen | T5% (°C) | T10% (°C) | T50% (°C) | DTG Peak [%/min; °C] | Residual Mass (%) |
|---|---|---|---|---|---|
| R.PE | 446.07 | 459.17 | 487.52 | 32.31; 494.64 | 0.92 |
| R.PE.5%Sp | 415.82 | 451.58 | 488.67 | 29.32; 497.18 | 0.00 |
| R.PE.10%Sp | 372.82 | 450.78 | 489.05 | 28.96; 497.03 | 3.17 |
| R.PE.15%Sp | 303.30 | 375.92 | 485.91 | 25.15; 495.93 | 2.70 |
| R.PE.5%Sp.N.W | 397.11 | 443.24 | 482.48 | 28.42; 492.63 | 0.00 |
| R.PE.10%Sp.N.W | 309.71 | 432.85 | 482.32 | 26.70; 490.96 | 1.94 |
| R.PE.15%Sp.N.W | 264.99 | 402.08 | 477.47 | 24.10; 486.78 | 2.36 |
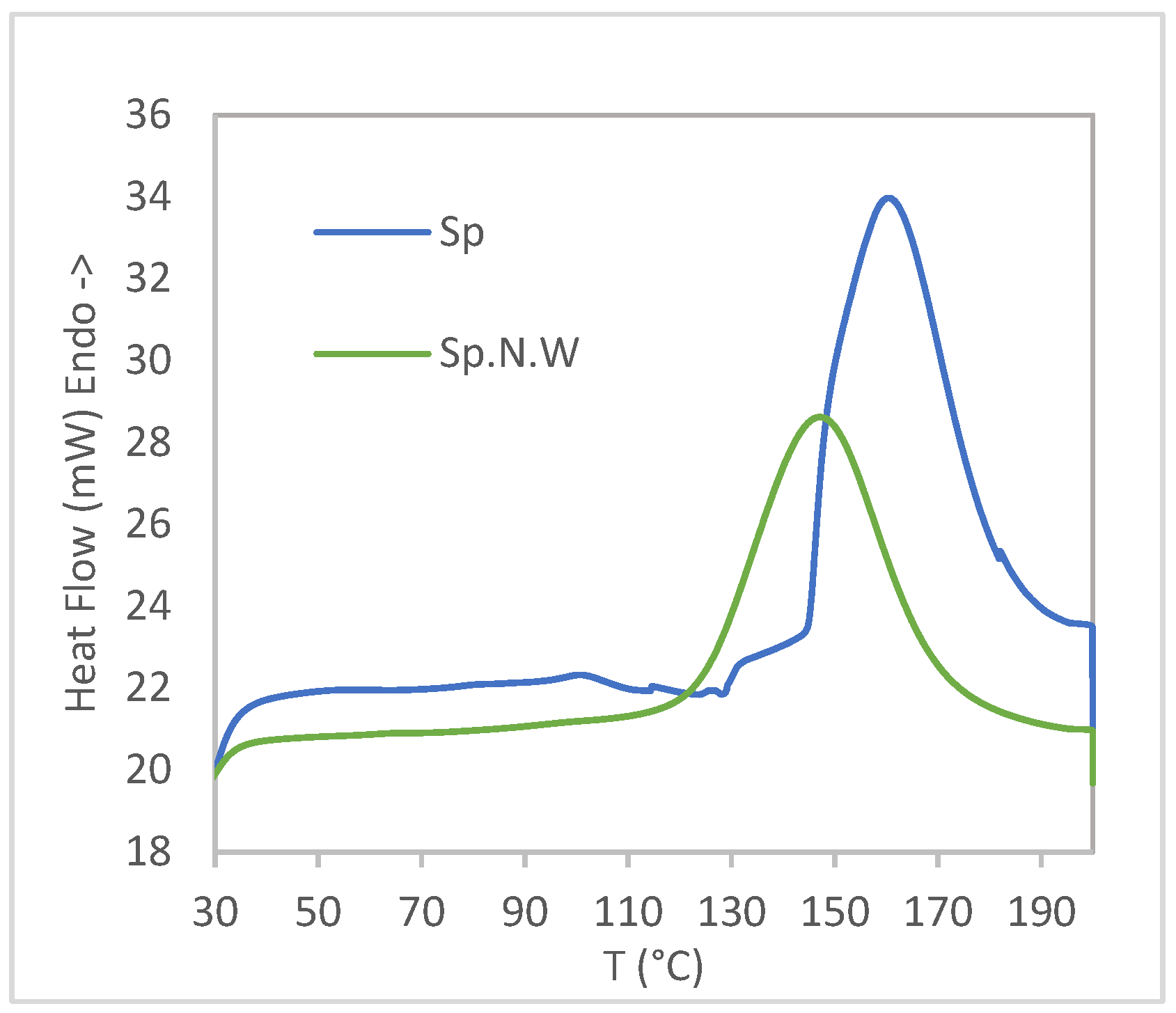
| Specimen | Tm1 | Tc | Tm2 | Xc | OIT (min) |
|---|---|---|---|---|---|
| R.PE | 128.56 | 108.92 | 127.55 | 35.99 | 0.0 |
| R.PE.5%Sp | 127.86 | 109.64 | 126.88 | 42.11 | 27.9 |
| R.PE.10%Sp | 130.64 | 109.38 | 127.26 | 41.03 | 58.0 |
| R.PE.15%Sp | 127.97 | 109.62 | 126.70 | 37.40 | 0.0 |
| R.PE.5%Sp.N.W | 128.58 | 109.26 | 127.41 | 41.72 | 14.6 |
| R.PE.10%Sp.N.W | 128.73 | 109.19 | 127.42 | 30.06 | 27.8 |
| R.PE.15%Sp.N.W | 128.80 | 109.68 | 126.96 | 40.07 | 41.5 |
| Specimen | % W | % Solubilization |
|---|---|---|
| R.PE | 0.34 ± 0.02 | - |
| R.PE.5%Sp | 3.63 ± 0.28 | 0.42 ± 0.04 |
| R.PE.10%Sp | 11.68 ± 0.20 | 1.01 ± 0.71 |
| R.PE.15%Sp | 18.77 ± 1.29 | 4.50 ± 0.99 |
| R.PE.5%Sp.N.W | 2.99 ± 0.43 | 0.72 ± 0.16 |
| R.PE.10%Sp.N.W | 4.49 ± 0.39 | 1.71 ± 0.18 |
| R.PE.15%Sp.N.W | 10.40 ± 1.38 | 4.63 ± 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, S.; Romero, F.; Suárez, L.; Ríos, R.; Alemán, M.; Venuleo, M.; Ortega, Z. Characterization of Microalgae Biomass-Based Composites Obtained through Rotational Molding. Polymers 2024, 16, 1807. https://doi.org/10.3390/polym16131807
Díaz S, Romero F, Suárez L, Ríos R, Alemán M, Venuleo M, Ortega Z. Characterization of Microalgae Biomass-Based Composites Obtained through Rotational Molding. Polymers. 2024; 16(13):1807. https://doi.org/10.3390/polym16131807
Chicago/Turabian StyleDíaz, Sara, Francisco Romero, Luis Suárez, Raúl Ríos, Monserrat Alemán, Marianna Venuleo, and Zaida Ortega. 2024. "Characterization of Microalgae Biomass-Based Composites Obtained through Rotational Molding" Polymers 16, no. 13: 1807. https://doi.org/10.3390/polym16131807
APA StyleDíaz, S., Romero, F., Suárez, L., Ríos, R., Alemán, M., Venuleo, M., & Ortega, Z. (2024). Characterization of Microalgae Biomass-Based Composites Obtained through Rotational Molding. Polymers, 16(13), 1807. https://doi.org/10.3390/polym16131807








