Imparting Photocatalytic and Antioxidant Properties to Electrospun Poly(L-lactide-co-D,L-lactide) Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
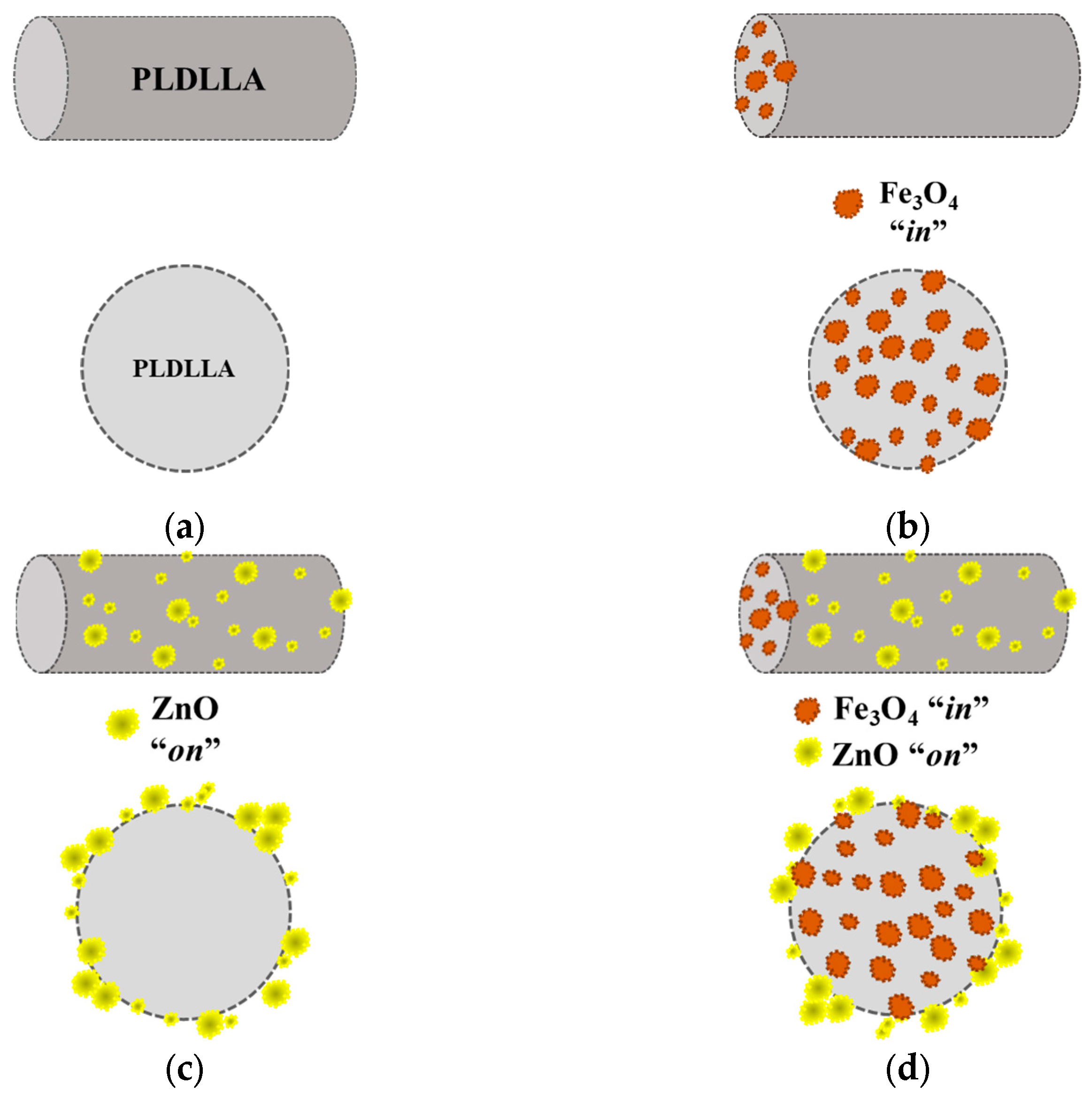
2.2. Preparation of Electrospun Materials
2.3. Characterization
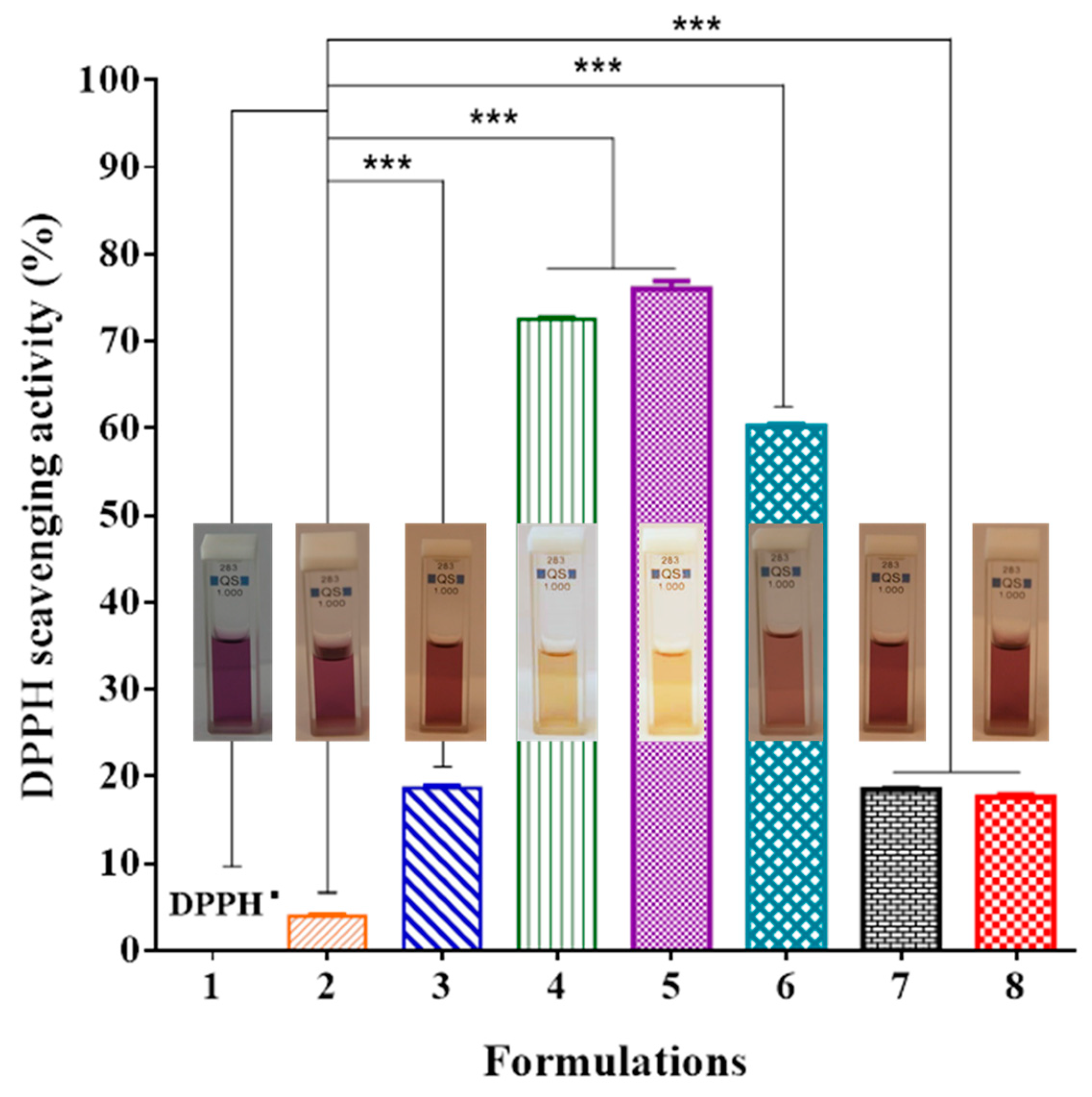
2.4. Antioxidant Activity
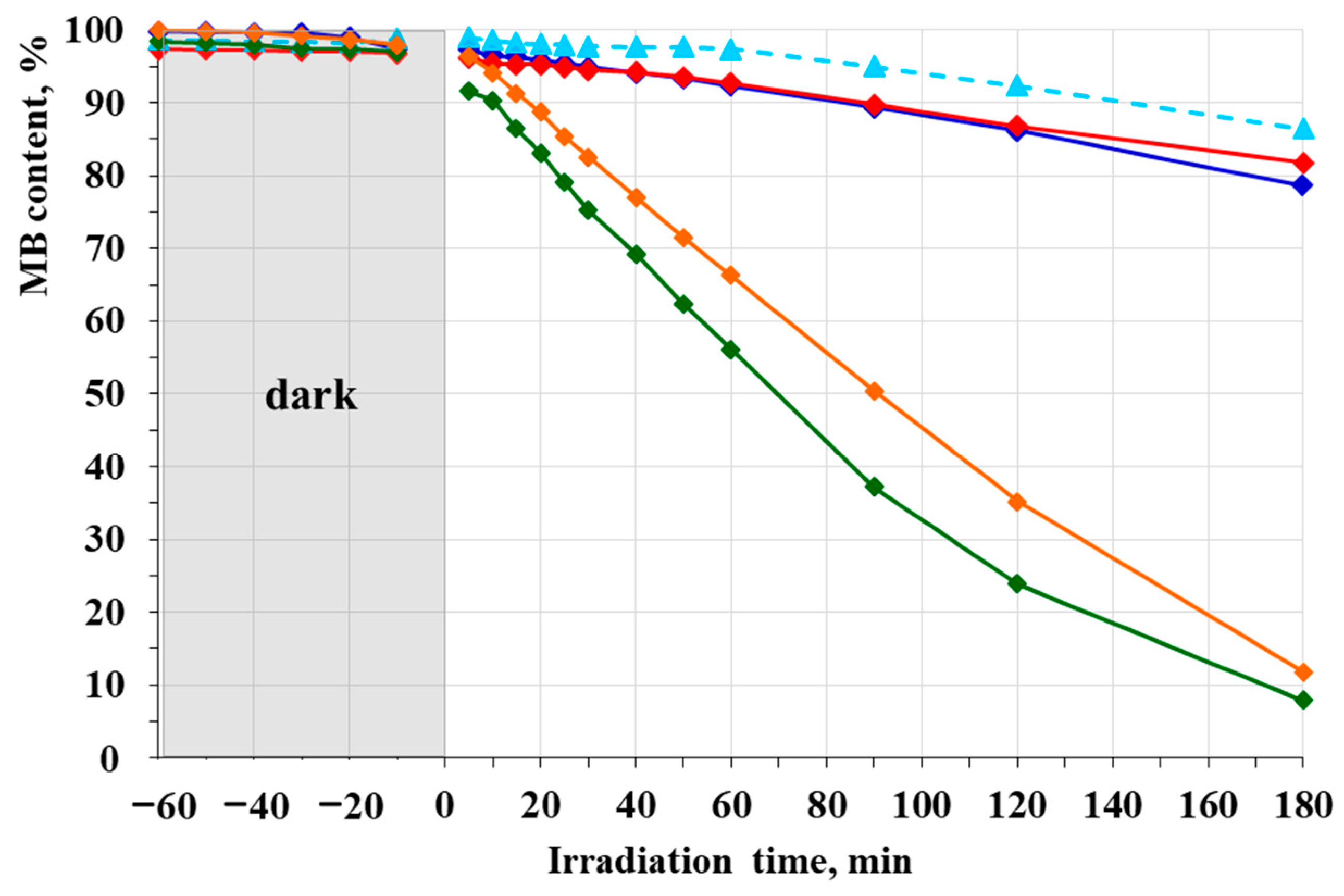
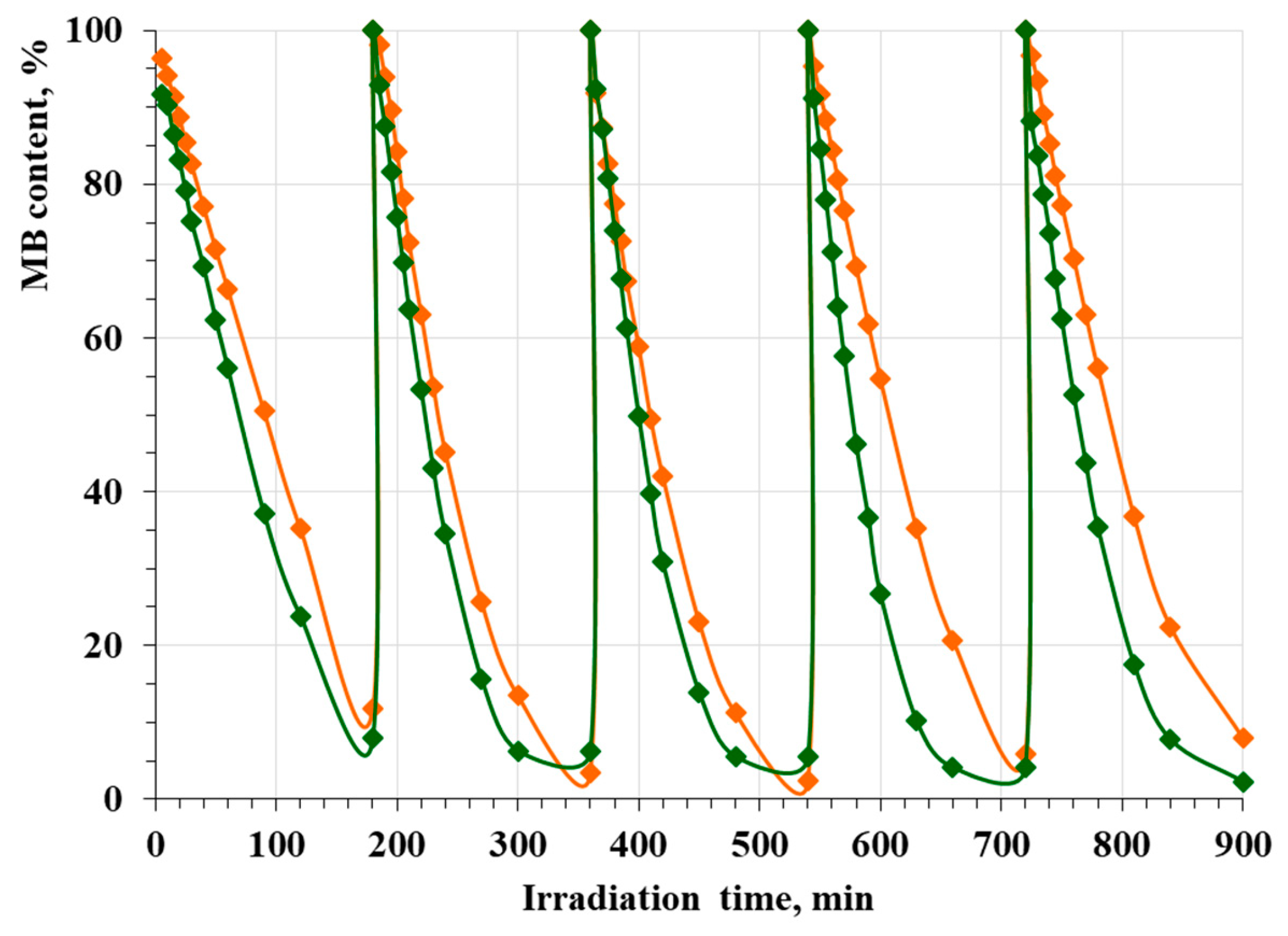
2.5. Photocatalytic Activity and Reusability Test
3. Results and Discussion
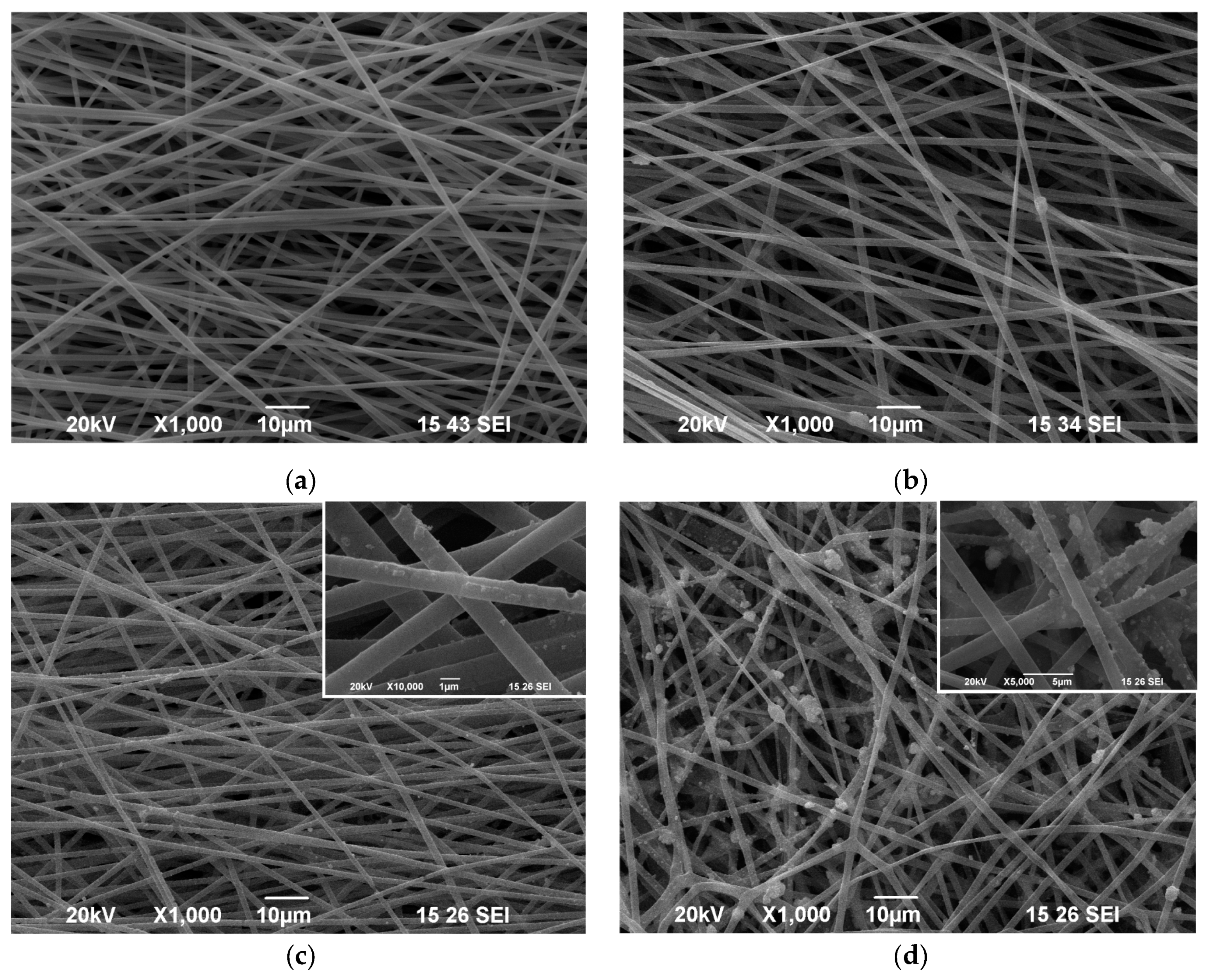
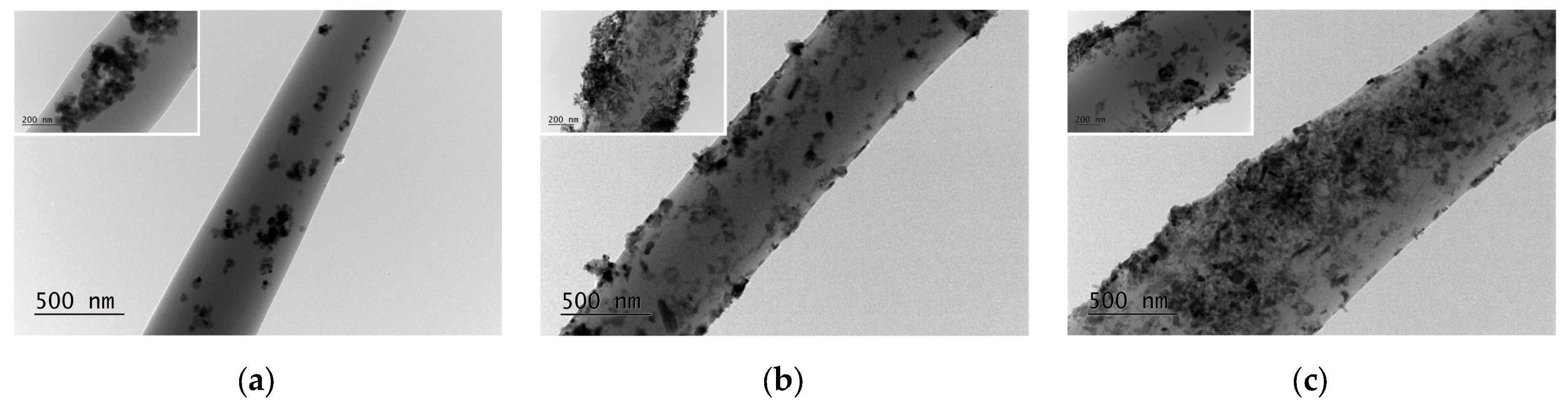
3.1. Morphological Characterization of the Electrospun Materials
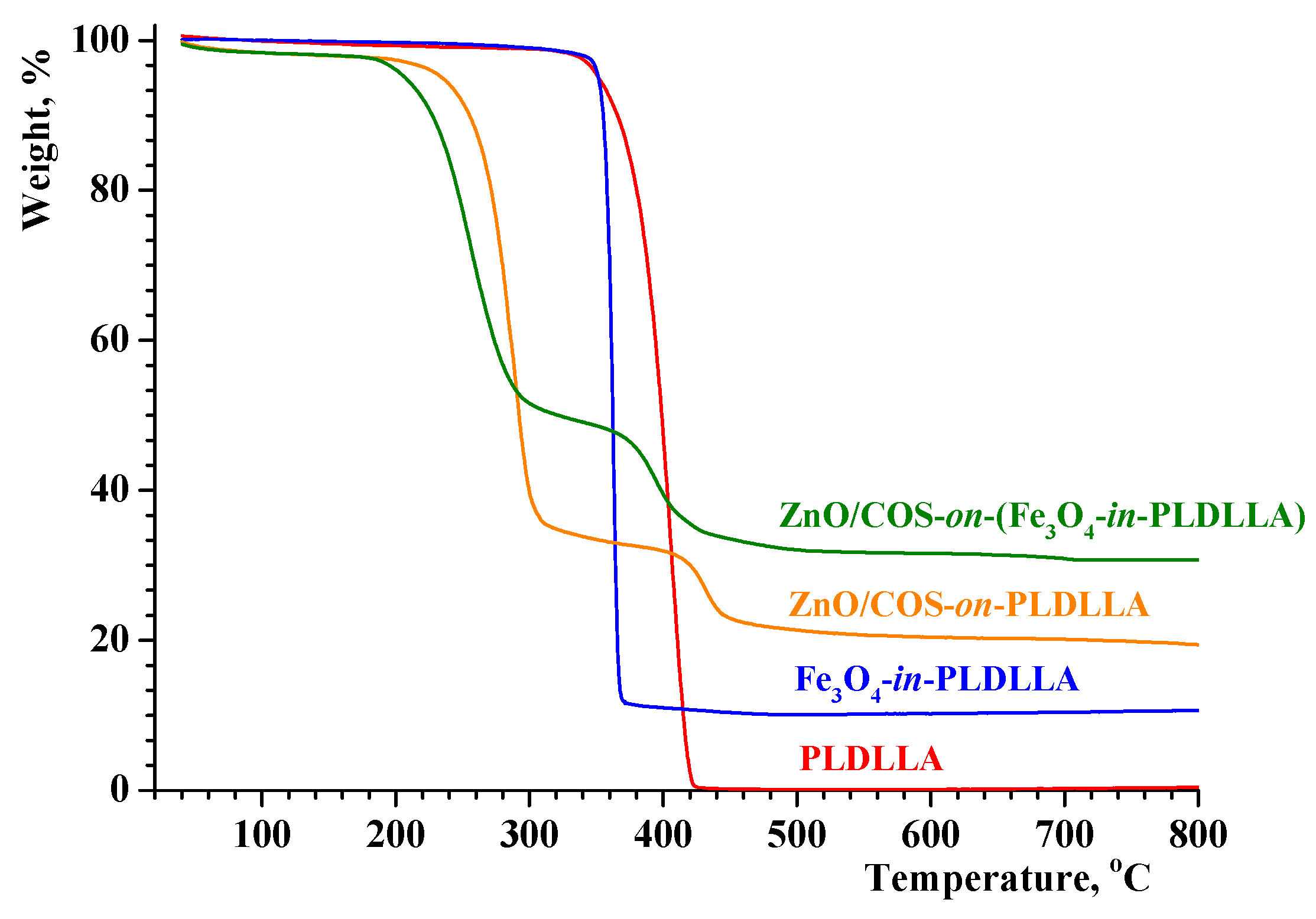
3.2. Thermal Behavior and Crystallinity of the Electrospun Materials
3.3. Antioxidant Activity
3.4. Photocatalytic Activity and Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Matamoros, V.; Rodríguez, Y.; Albaigés, J. A comparative assessment of intensive and extensive wastewater treatment technolo-gies for removing emerging contaminants in small communities. Water Res. 2016, 88, 777–785. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Q.; Cao, J.; Fang, F.; Feng, Q. Importance of monitor and control on new-emerging pollutants in conventional wastewater treatment plants. J. Geosci. Environ. Prot. 2018, 6, 55–58. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous photocatalysis: Recent advances and applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Massima Mouele, E.S.; Tijani, J.O.; Masikini, M.; Fatoba, O.O.; Eze, C.P.; Onwordi, C.T.; Zar Myint, M.T.; Kyaw, H.H.; Al-Sabahi, J.; Al-Abri, M.; et al. Spectroscopic measurements of dissolved O3, H2O2 and OH radicals in double cylindrical dielectric barrier discharge technology: Treatment of methylene blue dye simulated wastewater. Plasma 2020, 3, 59–91. [Google Scholar] [CrossRef]
- Vallejo, W.; Diaz-Uribe, C.; Cantillo, A. Methylene blue photocatalytic degradation under visible irradiation on TiO2 thin films sensitized with Cu and Zn tetracarboxy-phthalocyanines. J. Photochem. Photobiol. A Chem. 2015, 299, 80–86. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green approach for fabrication and applications of zinc oxide nanoparticles green approach for fabrication and applications of zinc oxide nanoparticles. Bioinorg. Chem. Appl. 2014, 2014, 523869. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Devi Rajeswari, V. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano 2018, 3, 48–55. [Google Scholar] [CrossRef]
- Supraja, N.; Prasad, T.N.V.K.V.; Krishna, T.G. Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated zinc oxide nanoparticles. Appl. Nanosci. 2016, 6, 581–590. [Google Scholar] [CrossRef]
- Sun, J.H.; Dong, S.Y.; Feng, J.L.; Yin, X.J.; Zhao, X.C. Enhanced sunlight photocatalytic performance of Sn-doped ZnO for Methylene Blue degradation. J. Mol. Catal. A Chem. 2011, 335, 145–150. [Google Scholar] [CrossRef]
- Muruganandham, M.; Wu, J.J. Synthesis, characterization and catalytic activity of easily recyclable zinc oxide nanobundles. Appl. Catal. B Environ. 2008, 80, 32–41. [Google Scholar] [CrossRef]
- Khassin, A.A.; Yurieva, T.M.; Kaichev, V.V.; Bukhtiyarov, V.I.; Budneva, A.A.; Paukshtis, E.A.; Parmon, V.N. Metal–support interactions in cobalt-aluminum co-precipitated catalysts: XPS and CO adsorption studies. J. Mol. Catal. A Chem. 2001, 175, 189–204. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Recent developments in electrospun ZnO nanofibers: A short review. J. Eng. Fibers Fabr. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Shah, A.P.; Jain, S.; Mokale, V.J.; Shimpi, N.G. High performance visible light photocatalysis of electrospun PAN/ZnO hybrid nanofibers. J. Ind. Eng. Chem. 2019, 77, 154–163. [Google Scholar] [CrossRef]
- Trabelsi, M.; Mamun, A.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Ehrmann, A. Increased mechanical properties of carbon nanofiber mats for possible medical applications. Fibers 2019, 7, 98. [Google Scholar] [CrossRef]
- Kancheva, M.; Toncheva, A.; Paneva, D.; Manolova, N.; Rashkov, I.; Markova, N. Materials from nanosized ZnO and polyacrylonitrile: Properties depending on the design of fibers (electrospinning or electrospinning/electrospraying). J. Inorg. Organomet. Polym. Mater. 2017, 27, 912–922. [Google Scholar] [CrossRef]
- Patil, P.T.; Anwane, R.S.; Kondawar, S.B. Development of electrospun polyaniline/ZnO composite nanofibers for LPG sensing. Proc. Mat. Sci. 2015, 10, 195–204. [Google Scholar] [CrossRef]
- Shingange, K.; Swart, H.C.; Mhlongo, G.H. H2S detection capabilities with fibrous-like La-doped ZnO nanostructures: A comparative study on the combined effects of La-doping and post-annealing. J. Alloy Compd. 2019, 797, 284–301. [Google Scholar] [CrossRef]
- Matysiak, W.; Tański, T. Novel bimodal ZnO (amorphous)/ZnO NPs (crystalline) electrospun 1D nanostructure and their optical characteristic. Appl. Surf. Sci. 2019, 474, 232–242. [Google Scholar] [CrossRef]
- Huang, X.; Or, S.W. Unique electromagnetic loss properties of Co-doped ZnO Nanofiber. Mater. Lett. 2019, 238, 271–274. [Google Scholar] [CrossRef]
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Multifunctional hybrid materials from poly(3-hydroxybutyrate), TiO2 nanoparticles, and chitosan oligomers by combining electrospinning/electrospraying and impregnation. Macromol. Biosci. 2013, 13, 707–716. [Google Scholar] [CrossRef]
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Poly(3-hydroxybutyrate)-based hybrid materials with photocatalytic and magnetic properties prepared by electrospinning and electrospraying. J. Mater. Sci. 2014, 49, 2144–2153. [Google Scholar] [CrossRef]
- Korina, E.; Stoilova, O.; Manolova, N.; Rashkov, I. Polymer fibers with magnetic core decorated with titanium dioxide prospective for photocatalytic water treatment. J. Environ. Chem. Eng. 2018, 6, 2075–2084. [Google Scholar] [CrossRef]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Electrospinning/electrospraying vs. electrospinning: A comparative study on the design of poly (L-lactide)/zinc oxide non-woven textile. Appl. Surf. Sci. 2014, 311, 842–850. [Google Scholar] [CrossRef]
- Virovska, D.; Paneva, D.; Manolova, N.; Rashkov, I.; Karashanova, D. Photocatalytic self-cleaning poly (L-lactide) materials based on a hybrid between nanosized zinc oxide and expanded graphite or fullerene. Mater. Sci. Eng. C 2016, 60, 184–194. [Google Scholar] [CrossRef]
- Wuisman, P.I.J.M.; Smit, T.H. Bioresorbable polymers: Heading for a new generation of spinal cages. Eur. Spine J. 2006, 15, 133–148. [Google Scholar] [CrossRef]
- Ignatova, M.; Petkova, Z.; Manolova, N.; Markova, N.; Rashkov, I. Non-woven fibrous materials with antibacterial properties prepared by tailored attachment of quaternized chitosan to electrospun mats from maleic anhydride copolymer. Macromol. Biosci. 2012, 12, 104–115. [Google Scholar] [CrossRef]
- Polnok, A.; Borchar, G.; Verhoe, J.C.; Sarisut, N.; Junginger, H.E. Influence of methylation process on the degree of quaternization of N-trimethyl chitosan chloride. Eur. J. Pharm. Biopharm. 2004, 57, 77–83. [Google Scholar] [CrossRef]
- Runarsson, Ö.V.; Holappa, J.; Nevalainen, T.; Hjálmarsdottir, M.; Järvinen, T.; Loftsson, T.H.; Einarsson, J.M.; Jónsdóttir, S.; Valdimarsdóttir, M.; Másson, M. Antibacterial activity of methylated chitosan and chitooligomer derivatives: Synthesis and structure activity relationships. Eur. Polym. J. 2007, 43, 2660–2671. [Google Scholar] [CrossRef]
- Kluger, P.J.; Wyrwa, R.; Weisser, J.; Maierle, J.; Votteler, M.; Rode, C.; Schnabelrauch, M.; Walles, H.; Schenke-Layland, K. Electrospun poly (D/L-lactide-co-L-lactide) hybrid matrix: A novel scaffold material for soft tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 2665–2671. [Google Scholar] [CrossRef]
- Du, L.; Xu, H.; Zhang, Y.; Zou, F. Electrospinning of polycaprolatone nanofibers with DMF additive: The effect of solution proprieties on jet perturbation and fiber morphologies. Fibers Polym. 2016, 17, 751–759. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; Chen, X.; Ji, X.; Li, P. Hydroxyl radicals scavenging activity of N-substituted chitosan and quaternized chitosan. Bioorg. Med. Chem. Lett. 2007, 16, 6348–6350. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.K.; Lee, T.S.; Park, W.H. Synthesis of chitooligosaccharide derivative with quaternary ammonium group and its antimicrobial activity against Streptococcus mutans. Int. J. Biol. Macromol. 2003, 32, 23–27. [Google Scholar] [CrossRef]
- Huang, R.; Mendis, E.; Rajapakse, N.; Kim, S.-K. Strong electronic charge as an important factor for anticancer activity of chitooligosaccharides (COS). Life Sci. 2006, 78, 2399–2408. [Google Scholar] [CrossRef]
- Ignatova, M.; Nachev, N.; Spasova, M.; Manolova, N.; Rashkov, I.; Naydenov, M. Electrospun 5-chloro-7-iodo-8-hydroxyquinoline (clioquinol)-containing poly(3-hydroxy-butyrate)/polyvinylpyrrolidone antifungal materials prospective as active dressings against Esca. Polymers 2022, 14, 367. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA-PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA-PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef]
- Abe, H.; Takahashi, N.; Kim, K.J.; Mochizuki, M.; Doi, Y. Thermal degradation processes of end-capped poly (L-lactide)s in the presence and absence of residual zinc catalyst. Biomacromolecules 2004, 5, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Wei, L.; Zhang, J.; Tan, W.; Chen, Y.; Dong, F.; Li, Q.; Guo, Z. Preparation and characterization of quaternized chitosan derivatives and assessment of their antioxidant activity. Molecules 2018, 23, 516. [Google Scholar] [CrossRef]
- Das, D.; Nath, B.C.; Phukon, P.; Kalita, A.; Dolui, S.K. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surf. B 2013, 111, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Wan, A.; Xu, Q.; Sun, Y.; Li, H. Antioxidant activity of high molecular weight chitosan and N,O-quaternized chitosans. J. Agric. Food. Chem. 2013, 61, 6921–6928. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasova, I.; Tsekova, P.; Ignatova, M.; Stoilova, O. Imparting Photocatalytic and Antioxidant Properties to Electrospun Poly(L-lactide-co-D,L-lactide) Materials. Polymers 2024, 16, 1814. https://doi.org/10.3390/polym16131814
Anastasova I, Tsekova P, Ignatova M, Stoilova O. Imparting Photocatalytic and Antioxidant Properties to Electrospun Poly(L-lactide-co-D,L-lactide) Materials. Polymers. 2024; 16(13):1814. https://doi.org/10.3390/polym16131814
Chicago/Turabian StyleAnastasova, Ina, Petya Tsekova, Milena Ignatova, and Olya Stoilova. 2024. "Imparting Photocatalytic and Antioxidant Properties to Electrospun Poly(L-lactide-co-D,L-lactide) Materials" Polymers 16, no. 13: 1814. https://doi.org/10.3390/polym16131814






