Microspheres Based on Blends of Chitosan Derivatives with Carrageenan as Vitamin Carriers in Cosmeceuticals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Composition of the Cream Base
- Phase A:
- Deionized water—61.8%, improves the texture and moisturizes the skin;
- Sodium Phytate (Merck, Rahway, NJ, USA)—0.2%, chelating agent (sequestrant);
- Propanediol (Merck, Rahway, NJ, USA)—5%, solvent, improves hydration, and regulates viscosity;
- Panthenol—1% (Merck, Rahway, NJ, USA), provitamin B5, humectant, and a soothing and regenerating agent;
- Xanthan Gum—1% (Merck, Rahway, NJ, USA); emulsifying, emulsion stabilizing, and a skin conditioning agent.
- Phase B:
- Caprylic/Capric Acid Triglyceride (Cisme, Milan, Italy)—3%, emollient, and a viscosity regulator;
- Butyrospermum Parkii Butter (Clariant, Muttenz, Switzerland)—2%, emollient, moisturizing, and a conditioning agent;
- Prunus Amygdalus Dulcis Oil (Clariant, Muttenz, Switzerland)—5%, emollient, and a conditioning agent;
- Cetearyl Alcohol (Alfa Chemistry, Holbrook, NY, USA)—4%, emmolient and humectant;
- Polyglyceryl-6 Stearate Polyglyceryl-6 Behenate (Evonik, Piscataway, NJ, USA)—6%, emulsifier, and moisturizing;
- Pantolactone (Sigma-Aldrich, Saint Louis, MO, USA)—1%, humectant, and a moisturizing agent.
- Phase C:
- Microspheres filled with vitamins (A, E, or C) suspended in butylene glycol—10%, a carrier containing biologically active vitamins, and a bacteriostatic and antifungal agent.
2.3. Forming the Microparticles
2.4. Loading Efficiency of Vitamins
2.5. Morphology and Size Distribution of Microspheres
2.6. Study of Swelling and Solubility Degree of Microspheres
2.7. In Vitro Vitamin Release and Trans-Epidermal Absorption Studies
2.8. Assessment of Cytotoxicity
3. Results
3.1. Microsphere Properties
3.2. Studies on the Degree of Swelling and Dissolution of Microspheres
3.3. Kinetics of Vitamin Release and Trans-Epidermal Absorption
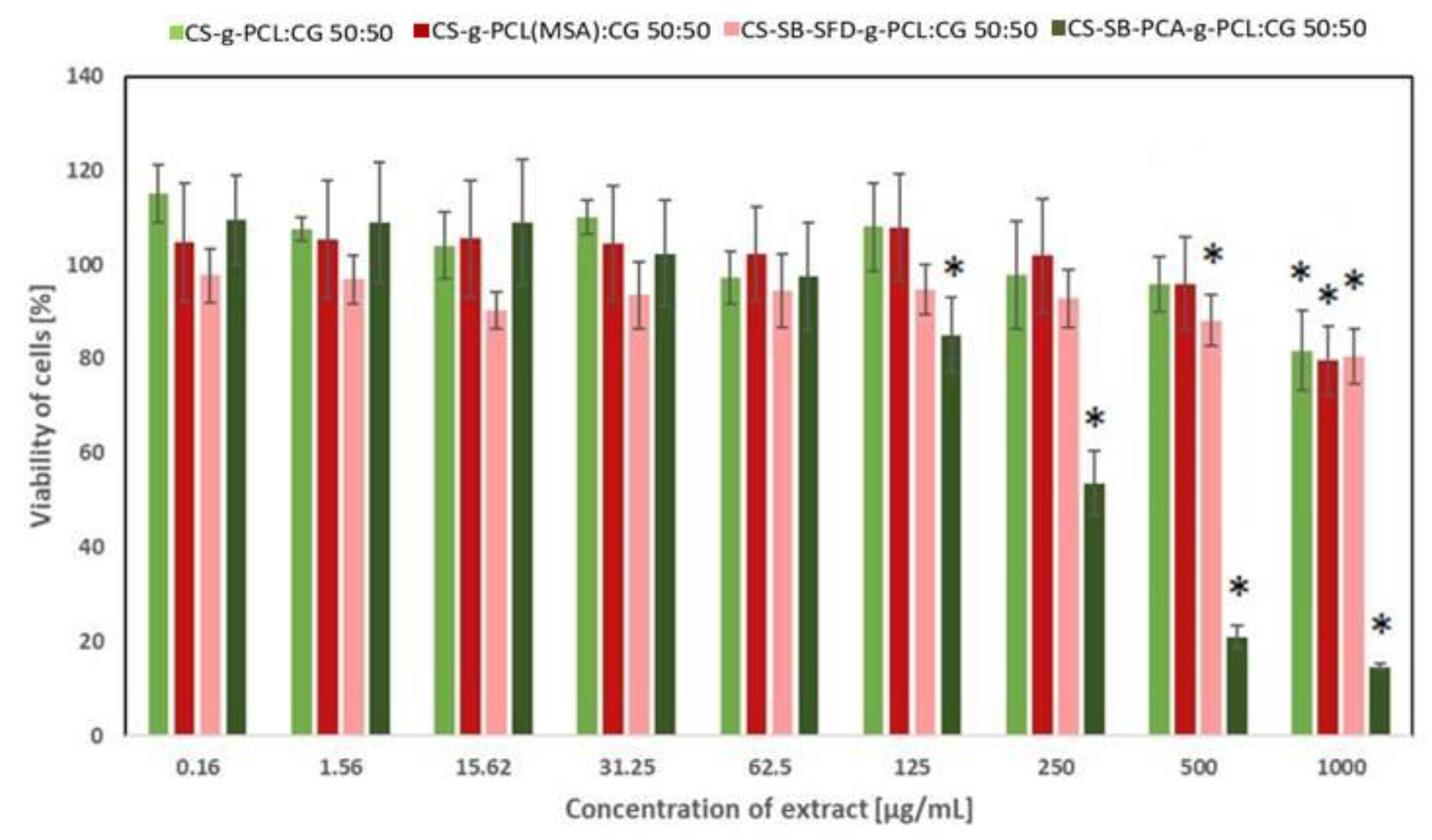
3.4. Assessment of Cytotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smola-Dmochowska, A.; Lewicka, K.; Macyk, A.; Rychter, P.; Pamuła, E.; Dobrzyński, P. Biodegradable Polymers and Polymer Composites with Antibacterial Properties. Int. J. Mol. Sci. 2023, 24, 7473. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Y.; Wu, Y.; Dai, F.; Yuan, M.; Wang, F.; Bai, Y.; Deng, H. Natural Polysaccharides Based Self-Assembled Nanoparticles for Biomedical Applications—A Review. Int. J. Biol. Macromol. 2021, 192, 1240–1255. [Google Scholar] [CrossRef]
- Ghalei, S.; Handa, H. A Review on Antibacterial Silk Fibroin-Based Biomaterials: Current State and Prospects. Mater. Today Chem. 2022, 23, 100673. [Google Scholar] [CrossRef]
- Rychter, P. Chitosan/Glyphosate Formulation as a Potential, Environmental Friendly Herbicide with Prolonged Activity. J. Environ. Sci. Health B 2019, 54, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, W.; Musioł, M.; Rydz, J.; Zięba, M.; Rychter, P.; Lewicka, K.; Šiškova, A.; Mosnáčková, K.; Kowalczuk, M.; Adamus, G. Prediction Studies of Environment-Friendly Biodegradable Polymeric Packaging Based on PLA. Influence of Specimens’ Thickness on the Hydrolytic Degradation Profile. Waste Manag. 2018, 78, 938–947. [Google Scholar] [CrossRef]
- Rychter, P.; Śmigiel-Gac, N.; Pamuła, E.; Smola-Dmochowska, A.; Janeczek, H.; Prochwicz, W.; Dobrzyński, P. Influence of Radiation Sterilization on Properties of Biodegradable Lactide/Glycolide/Trimethylene Carbonate and Lactide/Glycolide/ε-Caprolactone Porous Scaffolds with Shape Memory Behavior. Materials 2016, 9, 64. [Google Scholar] [CrossRef]
- Rychter, P.; Lewicka, K.; Pastusiak, M.; Domański, M.; Dobrzyński, P. PLGA–PEG Terpolymers as a Carriers of Bioactive Agents, Influence of PEG Blocks Content on Degradation and Release of Herbicides into Soil. Polym. Degrad. Stab. 2019, 161, 95–107. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; El-Hakim, Y.M.A.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.R.; Hirano, R.S.; Gaspar, A.L.; Chagas, E.G.L.; Carvalho, R.A.; Silva, F.V.; Leonardi, G.R.; Lopes, P.S.; Silva, C.F.; Yoshida, C.M.P. Biodegradable Antioxidant Chitosan Films Useful as an Anti-Aging Skin Mask. Int. J. Biol. Macromol. 2019, 132, 1262–1273. [Google Scholar] [CrossRef]
- Kulka, K.; Sionkowska, A. Chitosan Based Materials in Cosmetic Applications: A Review. Molecules 2023, 28, 1817. [Google Scholar] [CrossRef]
- Baek, J.; Ramasamy, M.; Willis, N.C.; Kim, D.S.; Anderson, W.A.; Tam, K.C. Encapsulation and Controlled Release of Vitamin C in Modified Cellulose Nanocrystal/Chitosan Nanocapsules. Curr. Res. Food Sci. 2021, 4, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.E.; Zevy, D.L.; Jafferany, M. Psychodermatology of Vitiligo: Psychological Impact and Consequences. Dermatol. Ther. 2020, 33, e13418. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.D.l.L.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Sadik, W.A.-A.; El-Demerdash, A.-G.M.; Hassan, H.S. Formulation of pH-Sensitive Aminated Chitosan–Gelatin Crosslinked Hydrogel for Oral Drug Delivery. J. Saudi Chem. Soc. 2021, 25, 101384. [Google Scholar] [CrossRef]
- Contri, R.V.; Kulkamp-Guerreiro, I.C.; da Silva, S.J.; Frank, L.A.; Pohlmann, A.R.; Guterres, S.S. Nanoencapsulation of Rose-Hip Oil Prevents Oil Oxidation and Allows Obtainment of Gel and Film Topical Formulations. AAPS PharmSciTech 2016, 17, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.-K. Biological Activities of Carrageenan. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 72, pp. 113–124. ISBN 978-0-12-800269-8. [Google Scholar]
- Maia Campos, P.M.B.G.; De Melo, M.O.; De Camargo Junior, F.B. Effects of Polysaccharide-Based Formulations on Human Skin. In Polysaccharides; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–18. ISBN 978-3-319-03751-6. [Google Scholar]
- Goyanes, S.N.; D’Accorso, N.B. (Eds.) Industrial Applications of Renewable Biomass Products; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-61287-4. [Google Scholar]
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of Algae in Cosmetics: An Overview. Int. J. Innov. Res. Sci. 2018, 7, 1269–1278. [Google Scholar] [CrossRef]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of New Chitosan/Carrageenan Nanoparticles for Drug Delivery Applications. J. Biomed. Mater. Res. 2010, 92A, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Naiel, B.H.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S.; Omer, A.M. Construction of Gastroretentive Aminated Chitosan Coated (Sunflower Oil/Alginate/i-Carrageenan) Floatable Polymeric Beads for Prolonged Release of Amoxicillin Trihydrate. J. Drug Deliv. Sci. Technol. 2023, 84, 104534. [Google Scholar] [CrossRef]
- Stavarache, C.E.; Ghebaur, A.; Dinescu, S.; Samoilă, I.; Vasile, E.; Vlasceanu, G.M.; Iovu, H.; Gârea, S.A. 5-Aminosalicylic Acid Loaded Chitosan-Carrageenan Hydrogel Beads with Potential Application for the Treatment of Inflammatory Bowel Disease. Polymers 2021, 13, 2463. [Google Scholar] [CrossRef]
- Athipornchai, A.; Pabunrueang, P.; Trakulsujaritchok, T. Mangiferin Loaded Carrageenan/Chitosan Core-Shell Hydrogel Beads: Preparation, Characterization and Proposed Application. Food Hydrocoll. 2024, 147, 109394. [Google Scholar] [CrossRef]
- Ammala, A. Biodegradable Polymers as Encapsulation Materials for Cosmetics and Personal Care Markets. Int. J. Cosmet. Sci. 2013, 35, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Liping, L.; Kexin, L.; Huipu, D.; Jia, L.; Jie, Z. Study on Preparation of a Chitosan/Vitamin C Complex and Its Properties in Cosmetics. Nat. Prod. Commun. 2020, 15, 1934578X2094687. [Google Scholar] [CrossRef]
- Guo, Y.; Qu, Y.; Yu, J.; Song, L.; Chen, S.; Qin, Z.; Gong, J.; Zhan, H.; Gao, Y.; Zhang, J. A Chitosan-Vitamin C Based Injectable Hydrogel Improves Cell Survival under Oxidative Stress. Int. J. Biol. Macromol. 2022, 202, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Wang, X.; Yin, Y.; Xia, J.; Mei, Y. Preparation and Evaluation of a Chitosan-Coated Antioxidant Liposome Containing Vitamin C and Folic Acid. J. Microencapsul. 2018, 35, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Mei Yee, C. Development of Carrageenan Hydrogel as a Sustained Release Matrix Containing Tocotrienol-Rich Palm-Based Vitamin E. J. Oil Palm Res. 2016, 28, 373–386. [Google Scholar] [CrossRef]
- Panyoyai, N.; Bannikova, A.; Small, D.M.; Kasapis, S. Controlled Release of Thiamin in a Glassy κ-Carrageenan/Glucose Syrup Matrix. Carbohydr. Polym. 2015, 115, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Buchholcz, G.; Kelemen, A.; Sovány, T.; Pintye-Hódi, K. Matrix Tablets Based on a Carrageenan with the Modified-Release of Sodium Riboflavin 5′-Phosphate. Pharm. Dev. Technol. 2015, 20, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and Brightening Effects of a Topical Treatment Containing Vitamin C, Vitamin E, and Raspberry Leaf Cell Culture Extract: A Split-face, Randomized Controlled Trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef]
- Liston, L.S.; Rivas, P.L.; Sakdiset, P.; See, G.L.; Arce, F. Chemical Permeation Enhancers for Topically-Applied Vitamin C and Its Derivatives: A Systematic Review. Cosmetics 2022, 9, 85. [Google Scholar] [CrossRef]
- Lupo, M.P. Antioxidants and Vitamins in Cosmetics. Clin. Dermatol. 2001, 19, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Colombo, F. Skin Anti-Aging Effect of Oral Vitamin A Supplementation in Combination with Topical Retinoic Acid Treatment in Comparison with Topical Treatment Alone: A Randomized, Prospective, Assessor-Blinded, Parallel Trial. Cosmetics 2023, 10, 144. [Google Scholar] [CrossRef]
- Goudon, F.; Clément, Y.; Ripoll, L. Controlled Release of Retinol in Cationic Co-Polymeric Nanoparticles for Topical Application. Cosmetics 2020, 7, 29. [Google Scholar] [CrossRef]
- Vaz, S.; Silva, R.; Amaral, M.H.; Martins, E.; Sousa Lobo, J.M.; Silva, A.C. Evaluation of the Biocompatibility and Skin Hydration Potential of Vitamin E-Loaded Lipid Nanosystems Formulations: In Vitro and Human in Vivo Studies. Colloids Surf. B Biointerfaces 2019, 179, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Role of Vitamin E as a Lipid-Soluble Peroxyl Radical Scavenger: In Vitro and in Vivo Evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1223 (accessed on 28 May 2024).
- Hill, G. Preservation of Cosmetics and Toiletries. In Handbook of Biocide and Preservative Use; Rossmoore, H.W., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 349–415. ISBN 978-94-011-1354-0. [Google Scholar]
- Amaral, L.F.B.; Camilo, N.S.; Pereda, M.D.C.V.; Levy, C.E.; Moriel, P.; Mazzola, P.G. Evaluation of Antimicrobial Effectiveness of C-8 Xylitol Monoester as an Alternative Preservative for Cosmetic Products. Int. J. Cosmet. Sci. 2011, 33, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-Preserving Cosmetics. Int. J. Cosmet. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Sekar, P.; Pasupathi, M.; Mukhopadhyay, T. Self-preserving personal care products. Int. J. Cosmet. Sci. 2017, 39, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Barbaud, A.; Lafforgue, C. Risks Associated with Cosmetic Ingredients. Ann. Dermatol. Vénéréol. 2021, 148, 77–93. [Google Scholar] [CrossRef]
- Hughes, O.B.; Maderal, A.D.; Tosti, A. Preservative Sensitization—Safety with and Safety without. Curr. Treat. Options Allergy 2016, 3, 345–358. [Google Scholar] [CrossRef]
- Chen, M.X.; Alexander, K.S.; Baki, G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. J. Pharm. 2016, 2016, 5754349. [Google Scholar] [CrossRef] [PubMed]
- Lewicka, K.; Smola-Dmochowska, A.; Śmigiel-Gac, N.; Kaczmarczyk, B.; Janeczek, H.; Barczyńska-Felusiak, R.; Szymanek, I.; Rychter, P.; Dobrzyński, P. Bactericidal Chitosan Derivatives and Their Superabsorbent Blends with ĸ-Carrageenan. Int. J. Mol. Sci. 2024, 25, 4534. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Yosipovitch, G. Skin pH: From Basic SciencE to Basic Skin Care. Acta Derm. Venerol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Choi, J.-H.; Liu, H.; Susman, G. Development of Facial-Skin Temperature Driven Thermal Comfort and Sensation Modeling for a Futuristic Application. Build. Environ. 2022, 207, 108479. [Google Scholar] [CrossRef]
- Desai, J.; Mallya, R. A Review on Novel Topical Formulations of Vitamins. J. Rep. Pharm. Sci. 2021, 10, 159. [Google Scholar] [CrossRef]
- Flament, F.; Francois, G.; Qiu, H.; Ye, C.; Hanaya, T.; Batisse, D.; Cointereau-Chardon, S.; Seixas, M.D.G.; Dal Belo, S.E.; Bazin, R. Facial Skin Pores: A Multiethnic Study. Clin. Cosmet. Investig. Dermatol. 2015, 8, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Spierings, N.M.K. Evidence for the Efficacy of Over-the-Counter Vitamin A Cosmetic Products in the Improvement of Facial Skin Aging: A Systematic Review. J. Clin. Aesthet. Dermatol. 2021, 14, 33–40. [Google Scholar] [PubMed]
- Al-Niaimi, F.; Chiang, N.Y.Z. Topical Vitamin C and the Skin: Mechanisms of Action and Clinical Applications. J. Clin. Aesthet. Dermatol. 2017, 10, 14–17. [Google Scholar] [PubMed]
- Thiele, J.J.; Hsieh, S.N.; Ekanayake-Mudiyanselage, S. Vitamin E: Critical Review of Its Current Use in Cosmetic and Clinical Dermatology. Dermatol. Surg. 2005, 31, 805. [Google Scholar] [CrossRef]
- Nabarretti, B.H.; Rigon, R.B.; Burga-Sánchez, J.; Leonardi, G.R. A Review of Alternative Methods to the Use of Animals in Safety Evaluation of Cosmetics. Einstein 2022, 20, eRB5578. [Google Scholar] [CrossRef]
- Singh, P.; Bhat, S.S.; Singh, N.; Venkanna, B.U.; Mohamed, R.; Rao, R.P. Cell-Based Model Systems for Validation of Various Efficacy-Based Claims for Cosmetic Ingredients. Cosmetics 2022, 9, 107. [Google Scholar] [CrossRef]
- Corrêa, G.D.O.P.; Marcato, D.C.; Ramos, W.S.; Corrêa, M.A.; Cicarelli, R.M.B.; Isaac, V.L.B. In Vitro Evaluation of the Cytotoxicity and Eye Irritation Potential of Preservatives Widely Used in Cosmetics. Braz. J. Pharm. Sci. 2022, 58, e20039. [Google Scholar] [CrossRef]
- Macário, I.P.E.; Oliveira, H.; Menezes, A.C.; Ventura, S.P.M.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.A.P.; Gonçalves, F.J.M. Cytotoxicity Profiling of Deep Eutectic Solvents to Human Skin Cells. Sci. Rep. 2019, 9, 3932. [Google Scholar] [CrossRef]
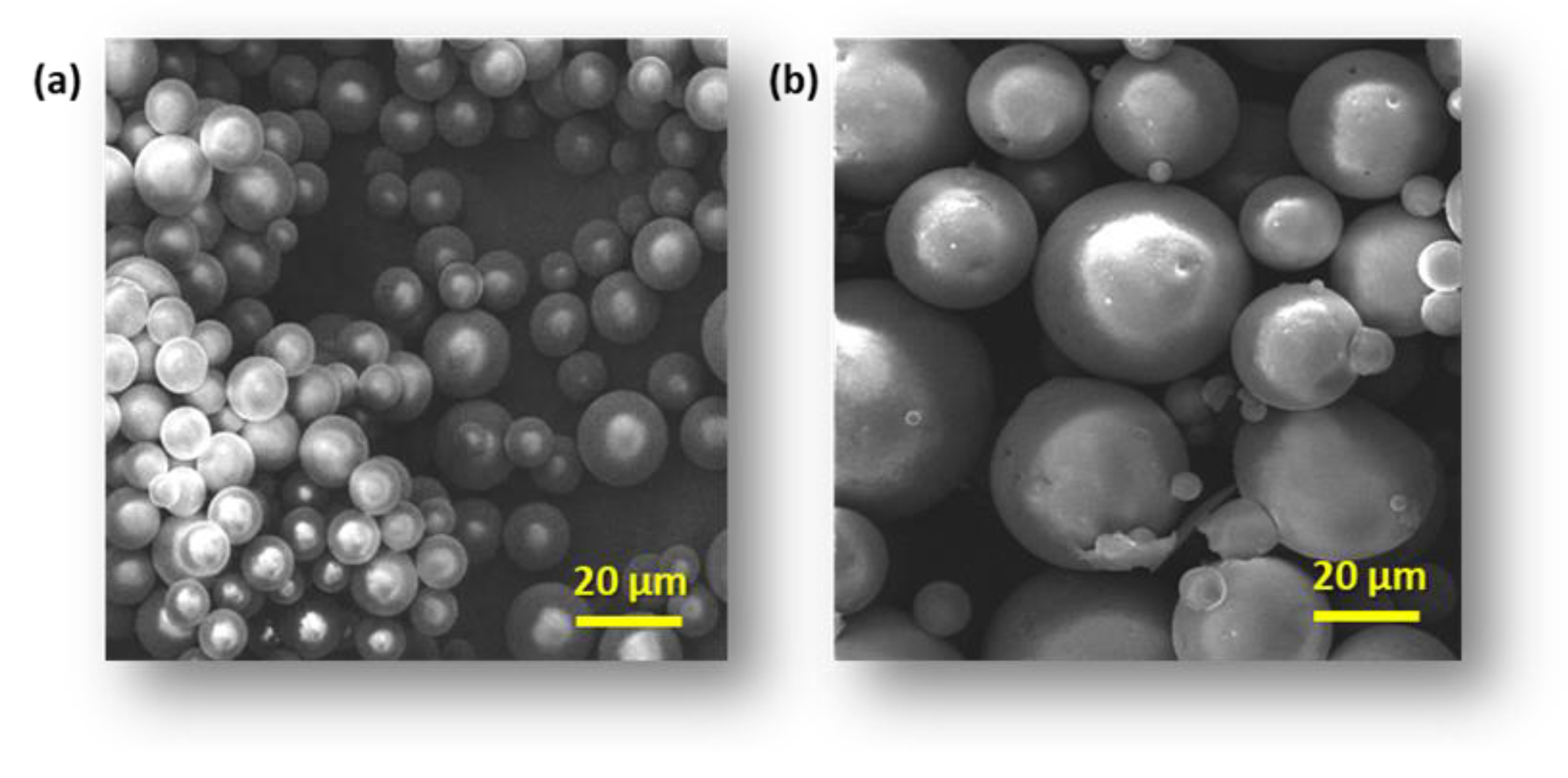
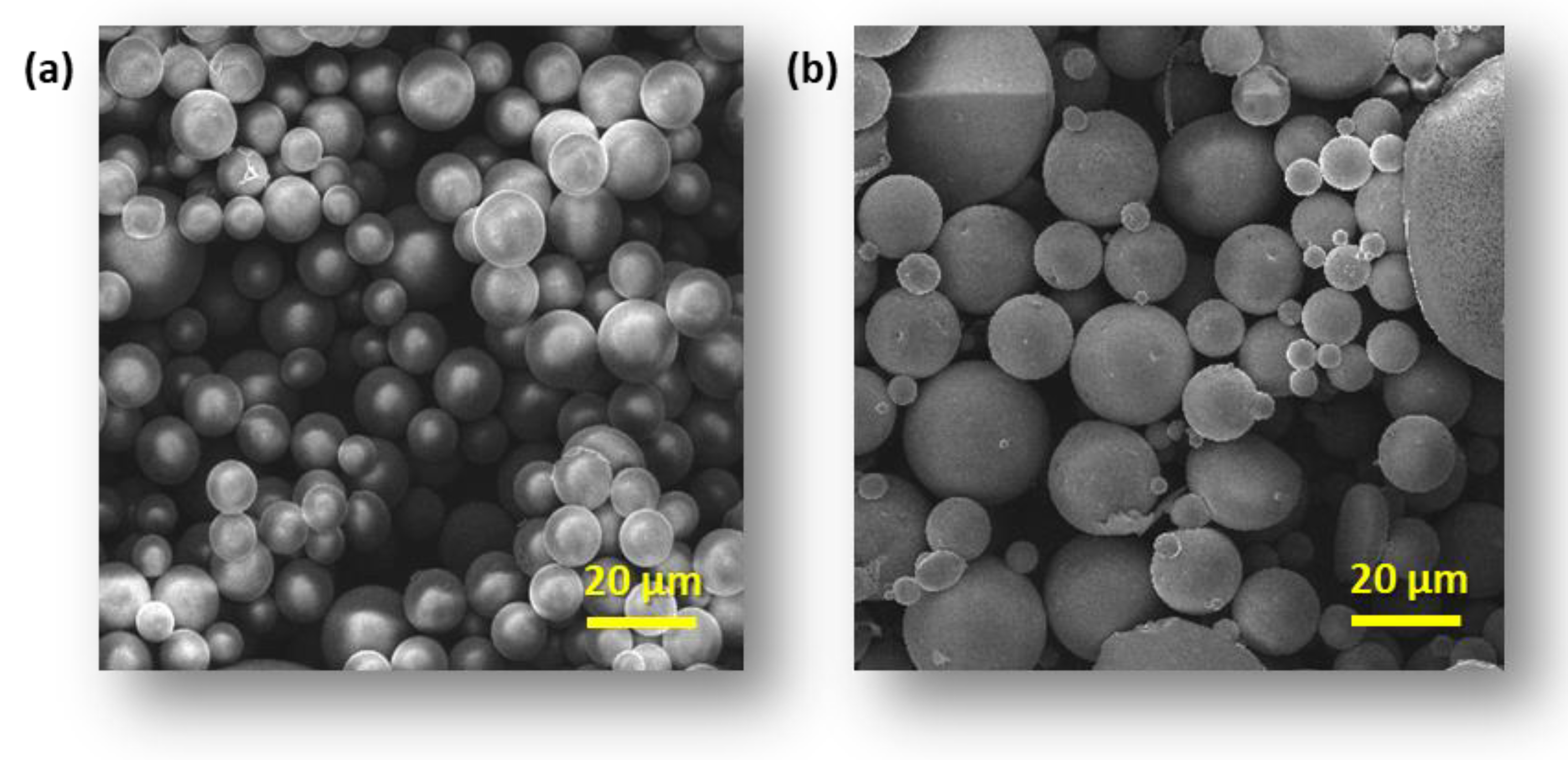

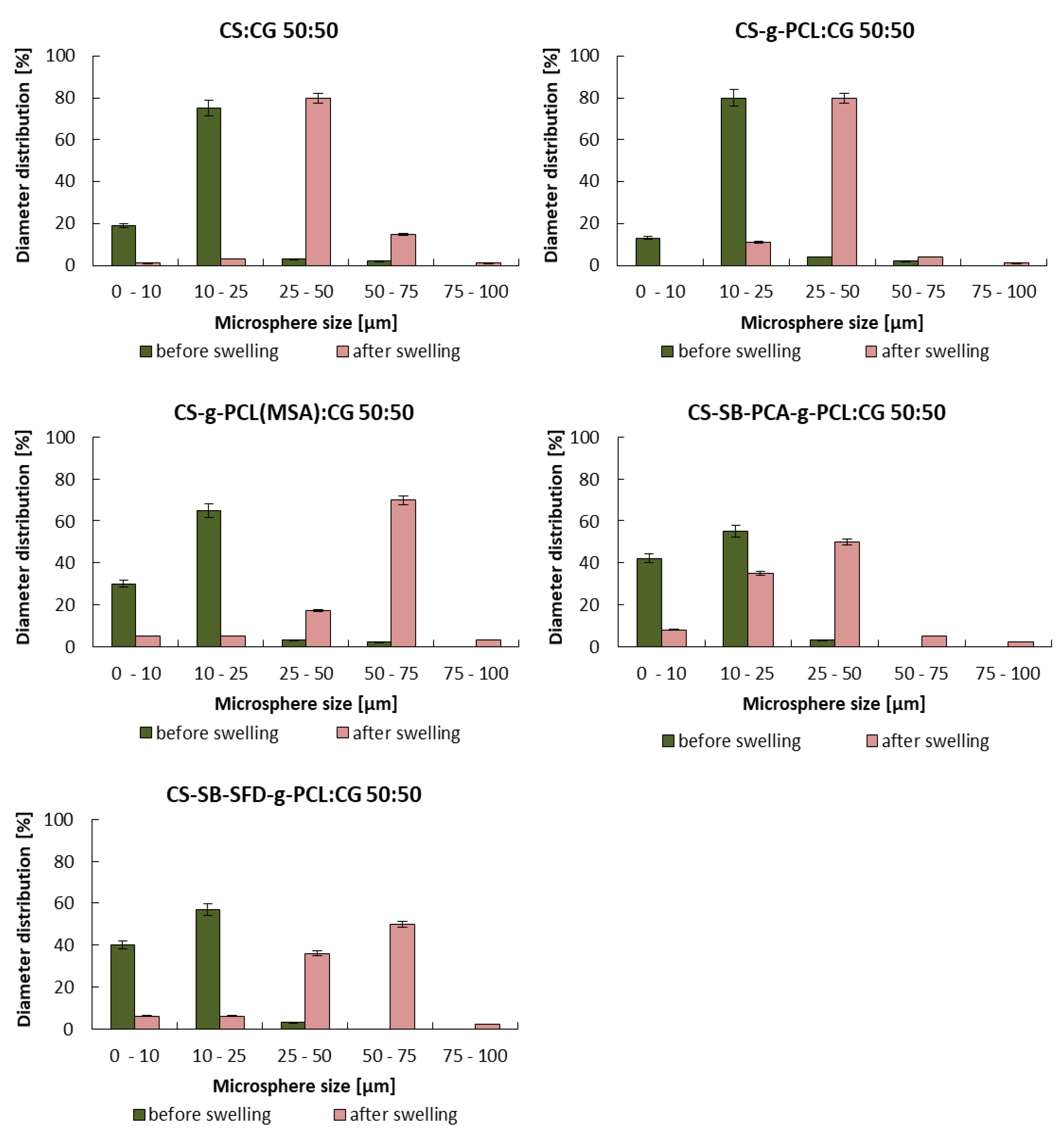
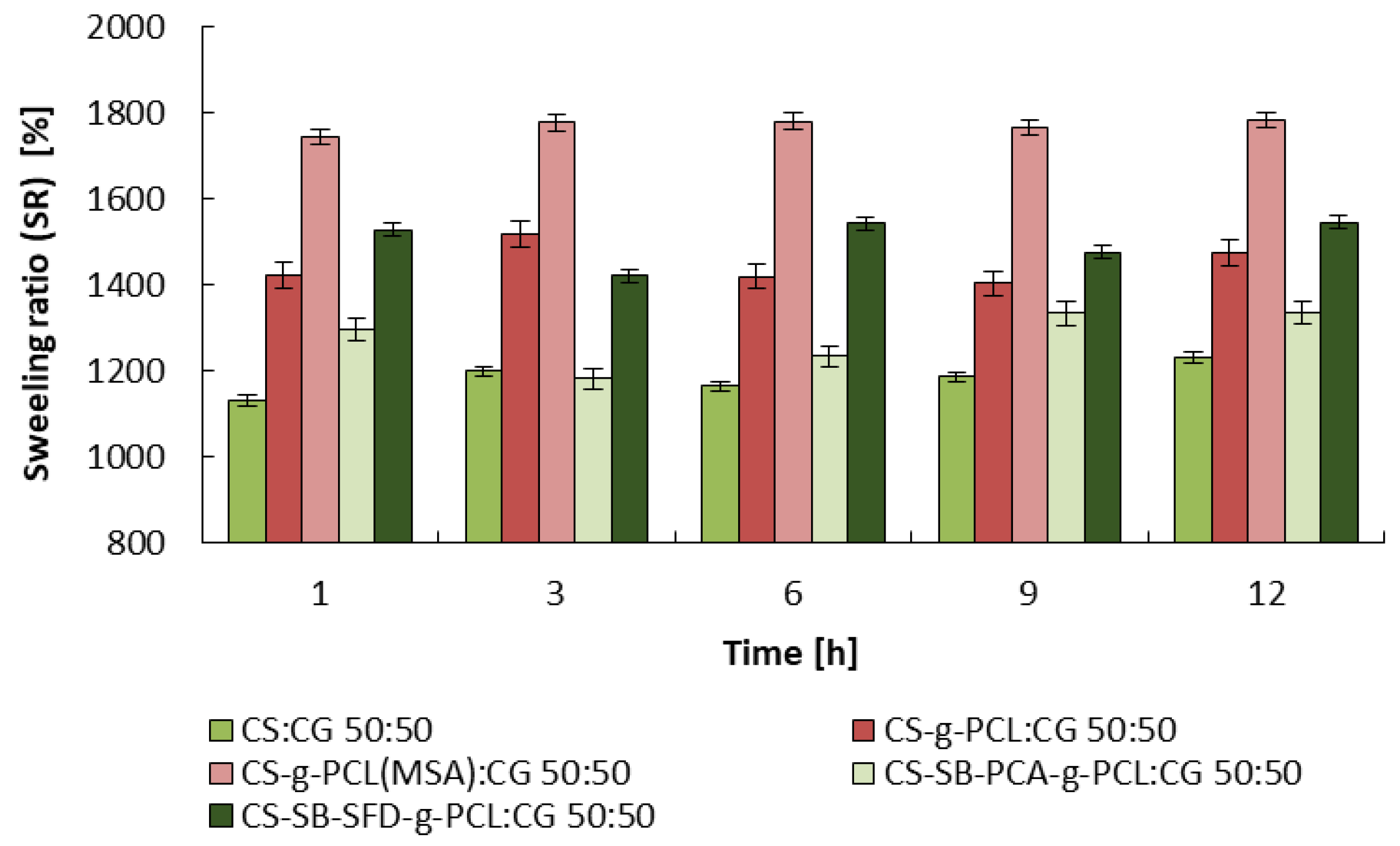
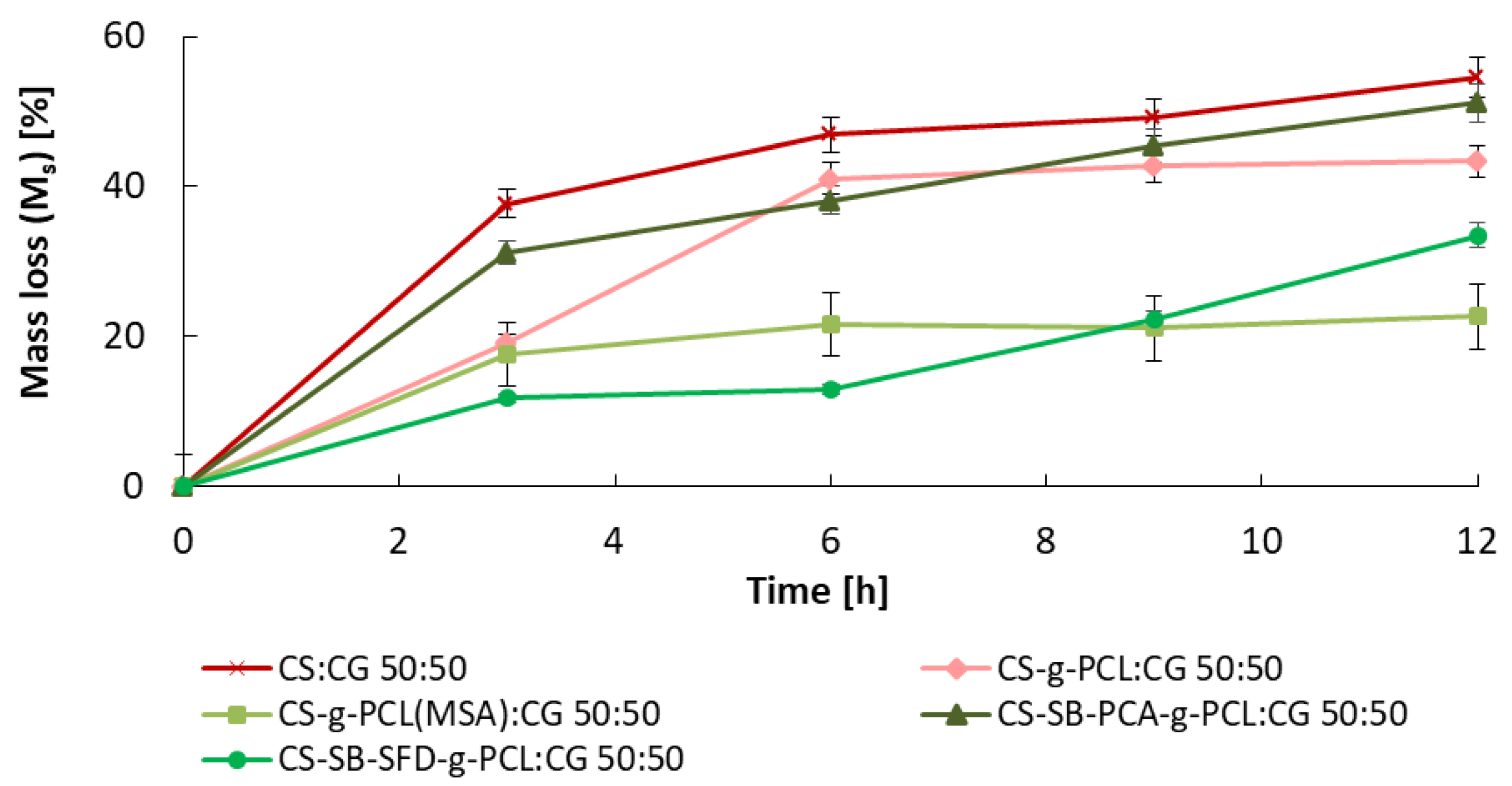
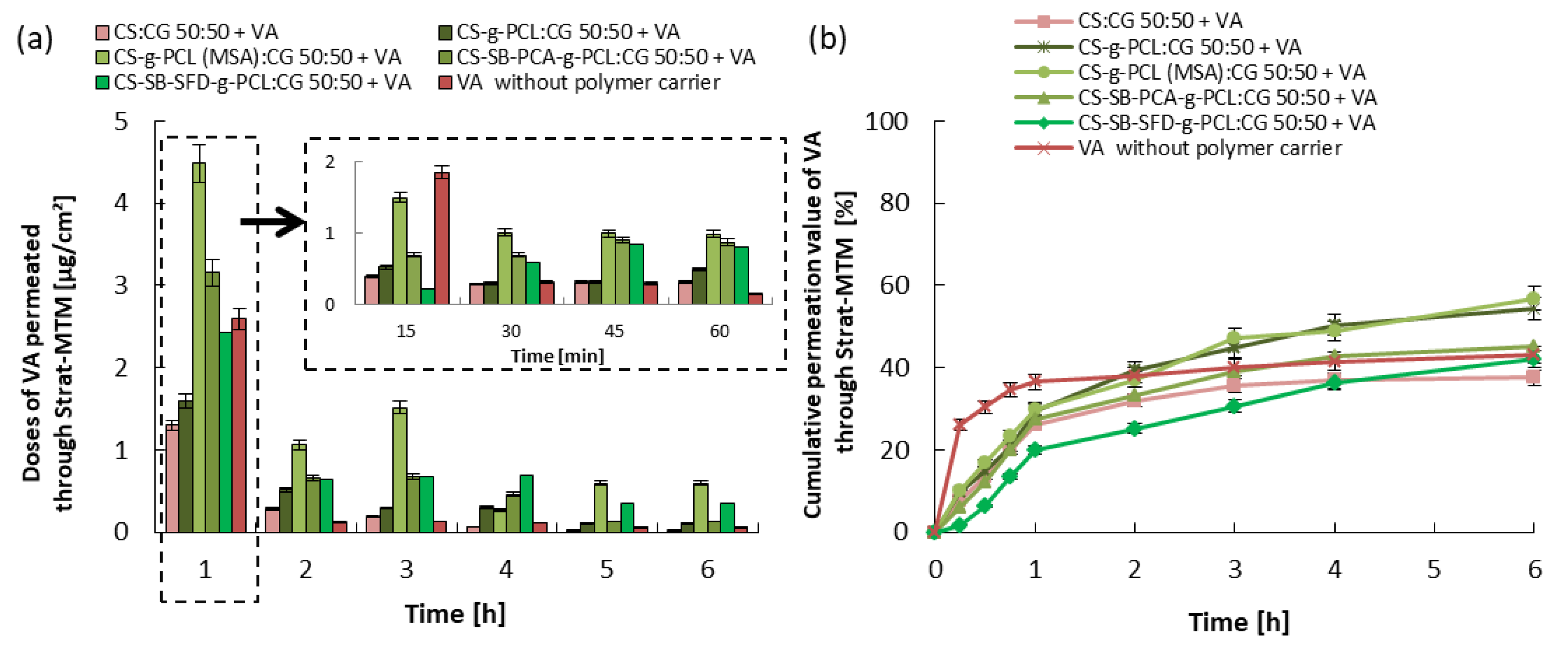
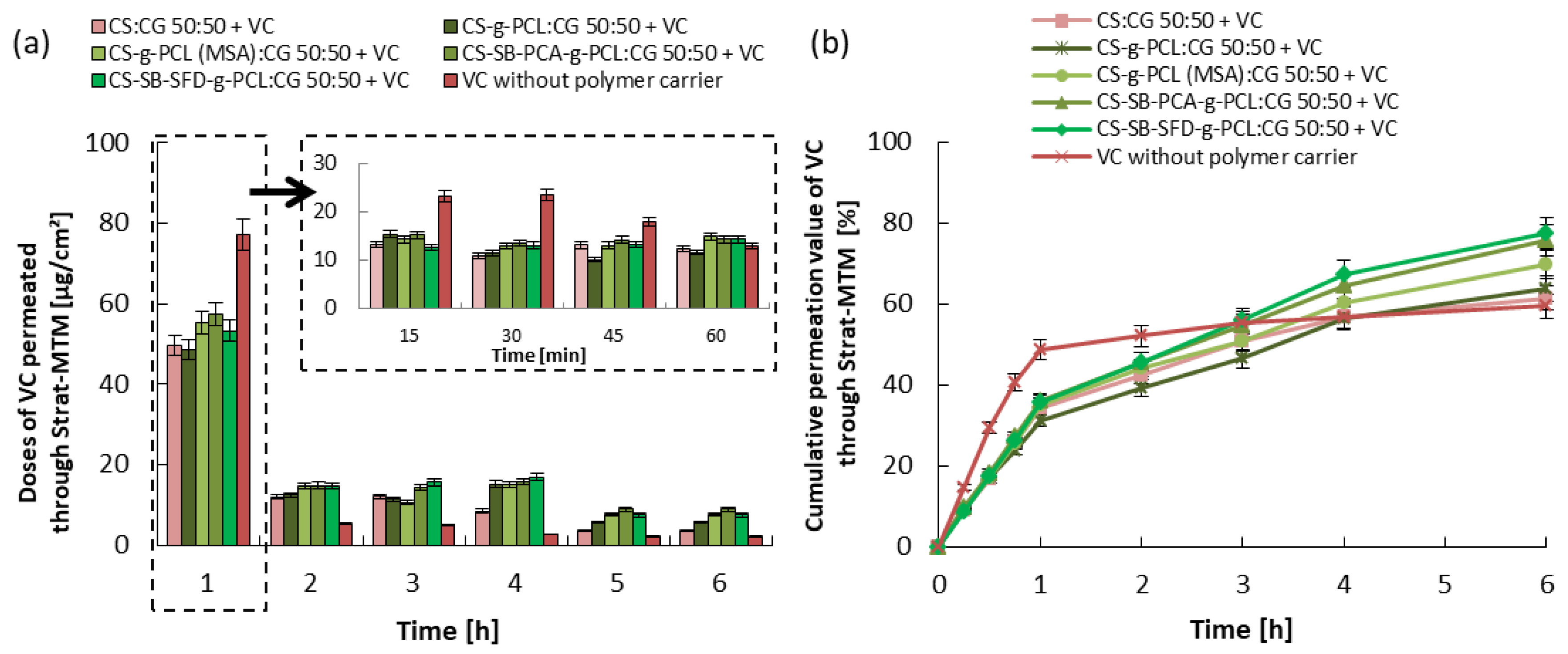
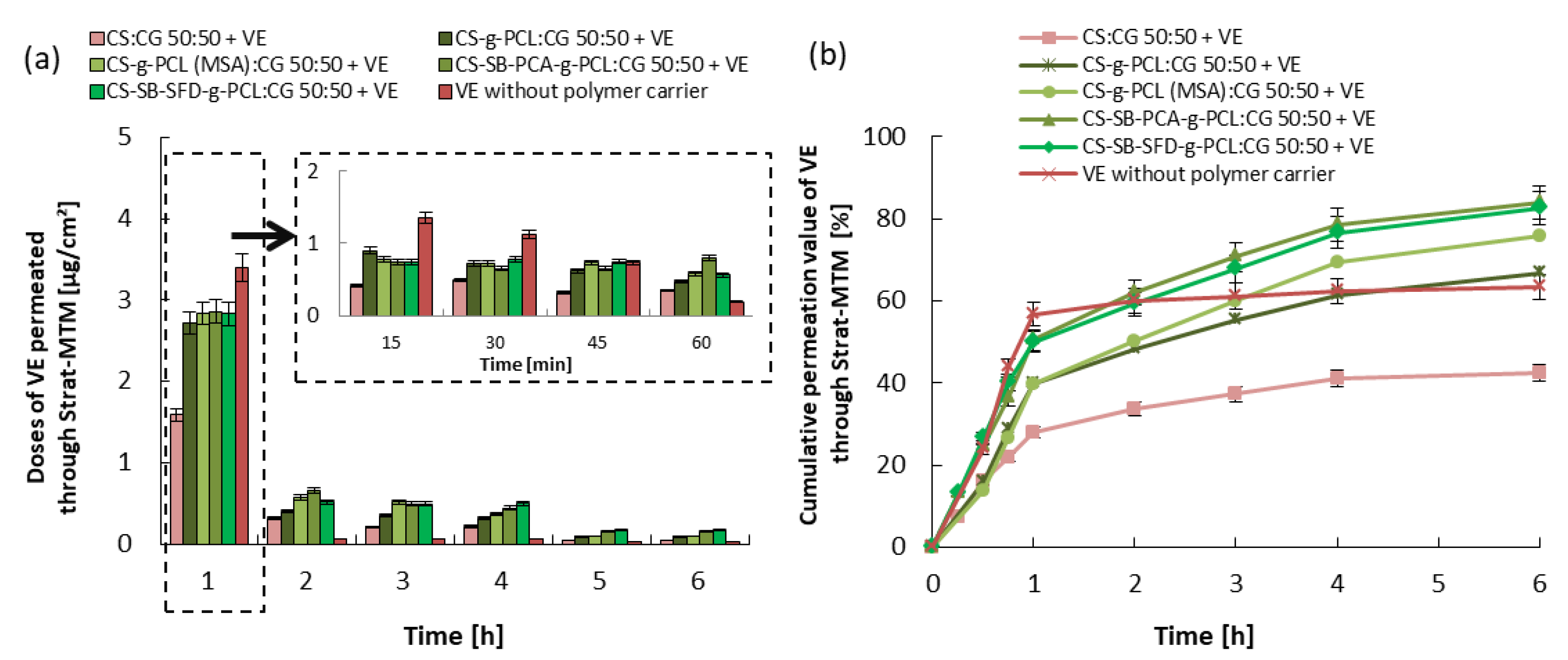


| Blends | |
|---|---|
| CS:CG 50:50 | chitosan-blend-(ĸ-carrageenan), 50:50 (w/w) |
| CS-g-PCL:CG 50:50 | [chitosan-graft-poly(ε-caprolactone)]-blend-(ĸ-carrageenan), 50:50 (w/w) |
| CS-g-PCL(MSA):CG 50:50 | [chitosan-graft-poly(ε-caprolactone) obtained with the presence of methanesulfonic acid]-blend-(ĸ-carrageenan), 50:50 (w/w) |
| CS-SB-PCA-g-PCL:CG 50:50 | [chitosan-2-pyridinecarboxaldehyde-graft-poly(ε-caprolactone)]-blend-(ĸ-carrageenan), 50:50 (w/w) |
| CS-SB-SFD-gPCL: CG 50:50 | [chitosan-sodium-4-formylbenzene-1,3-disulfonate-graft-poly(ε-caprolactone)]-blend-(ĸ-carrageenan), 50:50 (w/w) |
| Sample | Vitamins | LE [%] |
|---|---|---|
| CS:CG 50:50 | A | 96.5 |
| C | 70.6 | |
| E | 96.7 | |
| CS-g-PCL:CG 50:50 | A | 93.6 |
| C | 71.5 | |
| E | 91.0 | |
| CS-g-PCL(MSA):CG 50:50 | A | 93.4 |
| C | 76.3 | |
| E | 92.2 | |
| CS-SB-PCA-g-PCL:CG 50:50 | A | 96.9 |
| C | 72.6 | |
| E | 96.6 | |
| CS-SB-SFD-g-PCL:CG 50:50 | A | 97.4 |
| C | 74.8 | |
| E | 97.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewicka, K.; Smola-Dmochowska, A.; Dobrzyński, P.; Śmigiel-Gac, N.; Jelonek, K.; Musiał-Kulik, M.; Rychter, P. Microspheres Based on Blends of Chitosan Derivatives with Carrageenan as Vitamin Carriers in Cosmeceuticals. Polymers 2024, 16, 1815. https://doi.org/10.3390/polym16131815
Lewicka K, Smola-Dmochowska A, Dobrzyński P, Śmigiel-Gac N, Jelonek K, Musiał-Kulik M, Rychter P. Microspheres Based on Blends of Chitosan Derivatives with Carrageenan as Vitamin Carriers in Cosmeceuticals. Polymers. 2024; 16(13):1815. https://doi.org/10.3390/polym16131815
Chicago/Turabian StyleLewicka, Kamila, Anna Smola-Dmochowska, Piotr Dobrzyński, Natalia Śmigiel-Gac, Katarzyna Jelonek, Monika Musiał-Kulik, and Piotr Rychter. 2024. "Microspheres Based on Blends of Chitosan Derivatives with Carrageenan as Vitamin Carriers in Cosmeceuticals" Polymers 16, no. 13: 1815. https://doi.org/10.3390/polym16131815
APA StyleLewicka, K., Smola-Dmochowska, A., Dobrzyński, P., Śmigiel-Gac, N., Jelonek, K., Musiał-Kulik, M., & Rychter, P. (2024). Microspheres Based on Blends of Chitosan Derivatives with Carrageenan as Vitamin Carriers in Cosmeceuticals. Polymers, 16(13), 1815. https://doi.org/10.3390/polym16131815








