The Use of Polyimide as a Bonding Material to Improve the Mechanical Stability, Magnetic and Acoustic Properties of the Transformer Core Based on Amorphous Steel
Abstract
1. Introduction
2. Materials and Methods
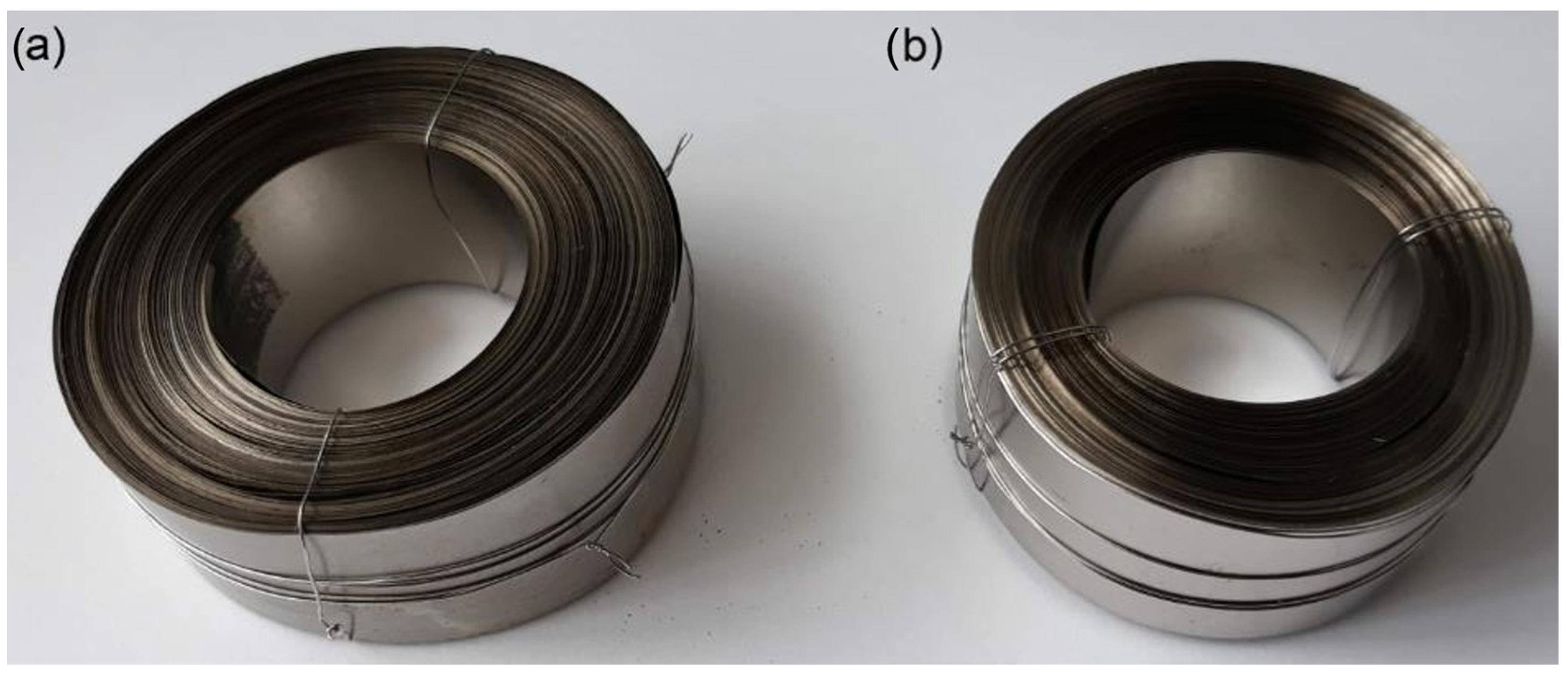
2.1. Amorphous Metal Ribbon
2.2. Preparation of Polyimide Layers
2.3. Confocal Microscopy
2.4. Scanning Electron Microscopy
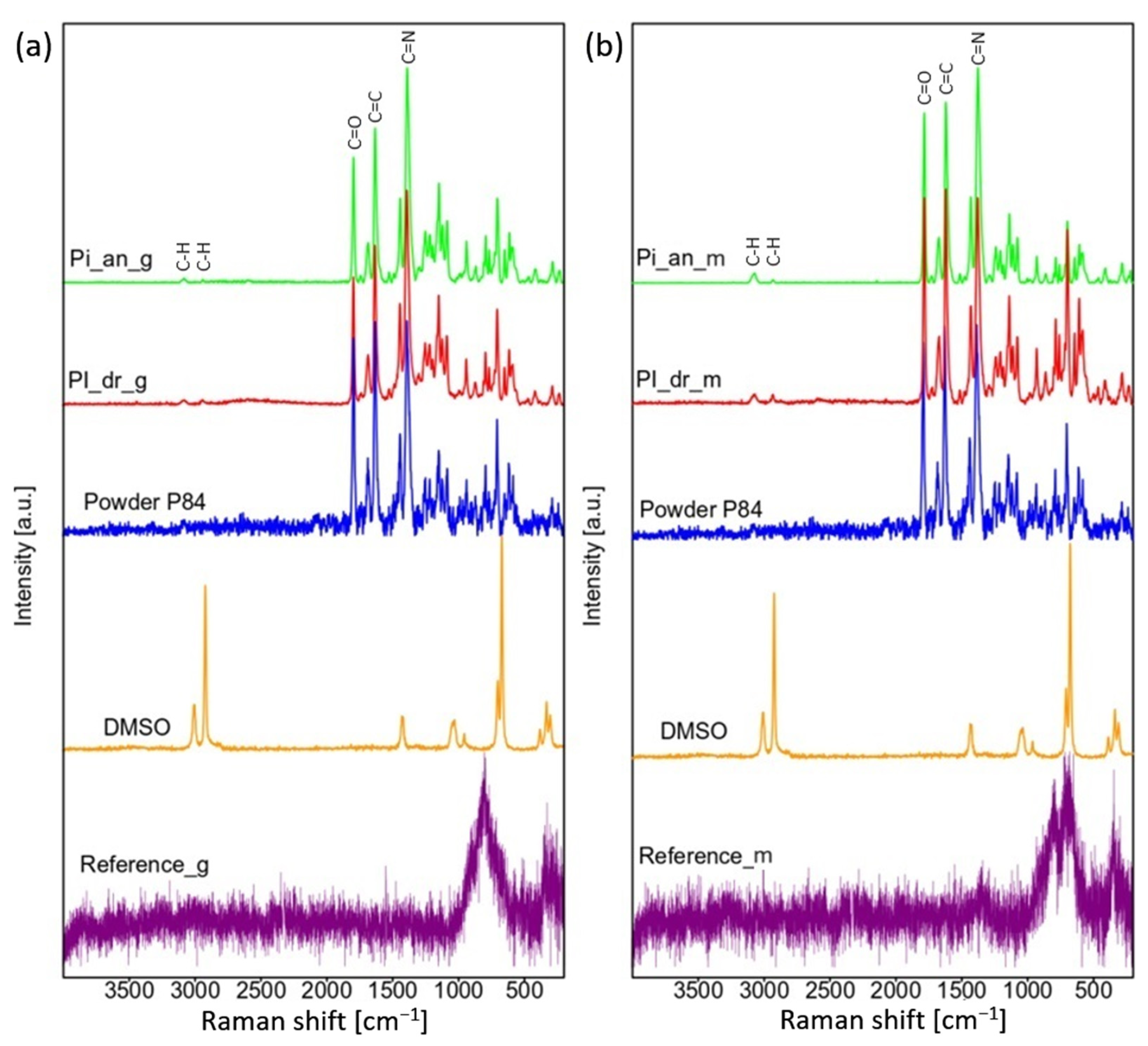
2.5. Raman Spectroscopy
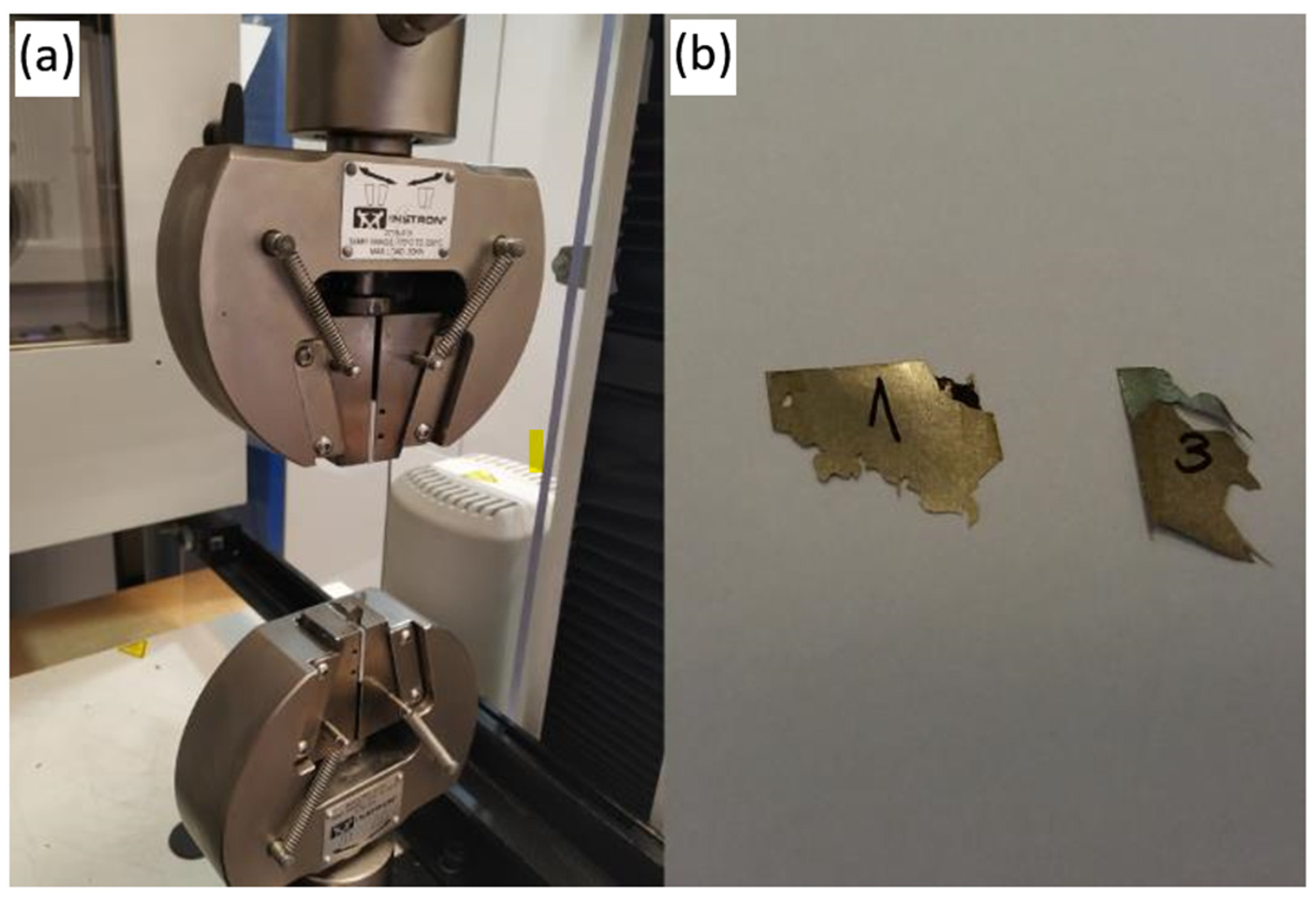
2.6. Mechanical Examination
2.7. Corrosion Resistance
2.8. Dielectric Measurements
- ε*(f)—complex permittivity [F/m],
- ε′(f)—real part of complex permittivity [F/m],
- ε″(f)—imaginary part of complex permittivity [F/m],
2.9. Preparation of Toroidal Magnetic Core
2.10. Magnetic Measurements
2.11. Noise Test Setup
3. Results and Discussion
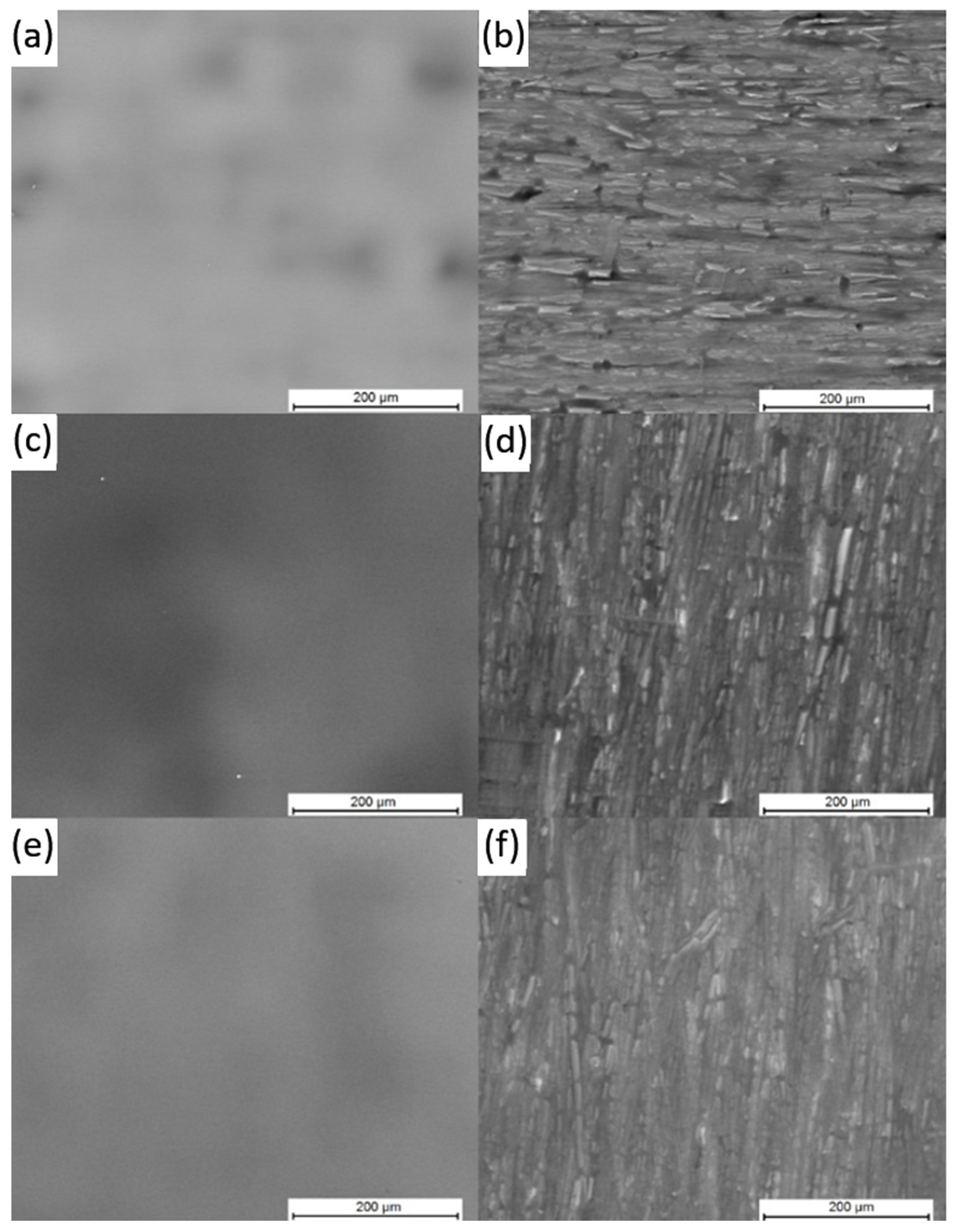
3.1. Confocal Microscopy
3.2. Scanning Electron Microscopy
3.3. Raman Spectroscopy
3.4. Mechanical Examination
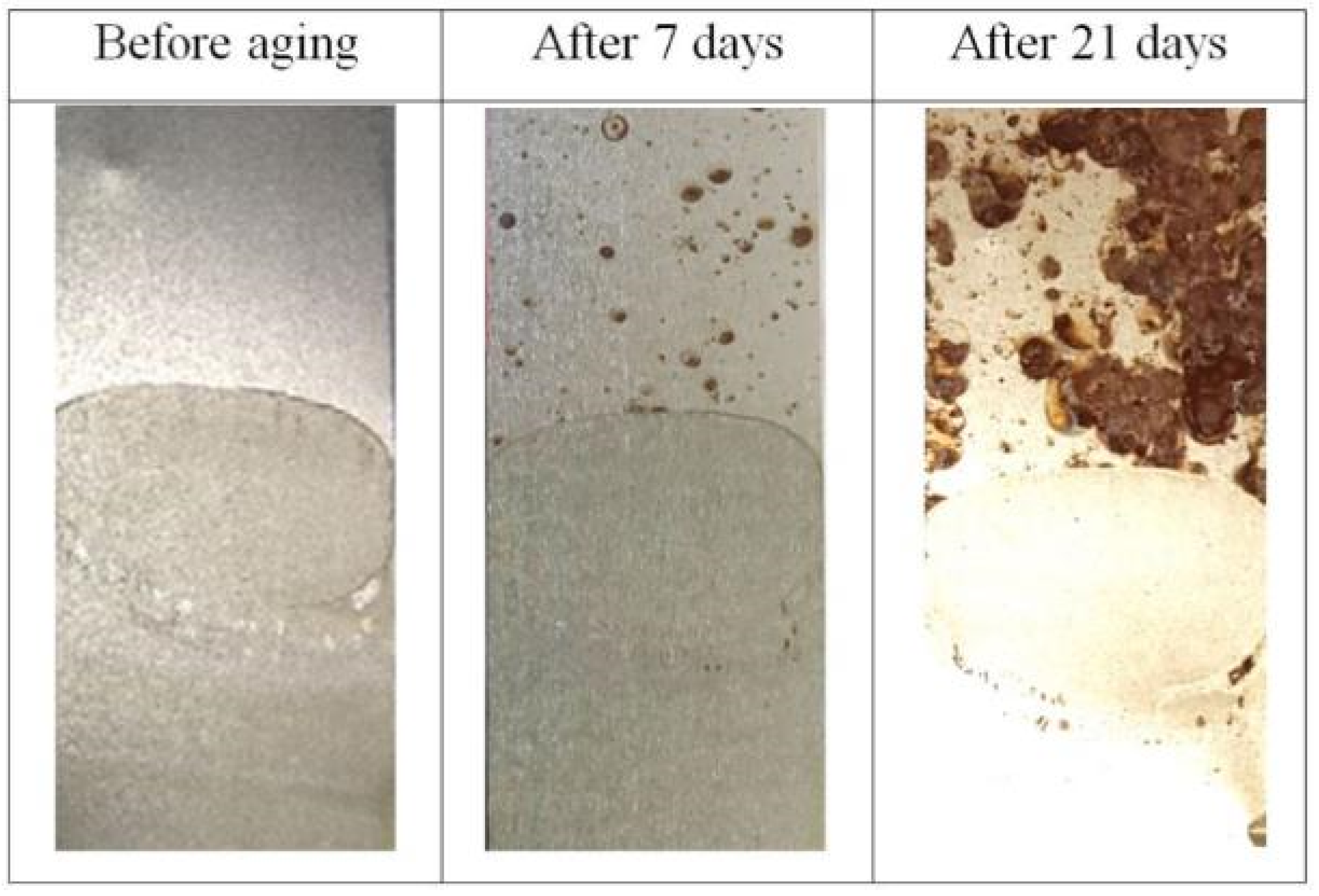
3.5. Corrosion Resistance
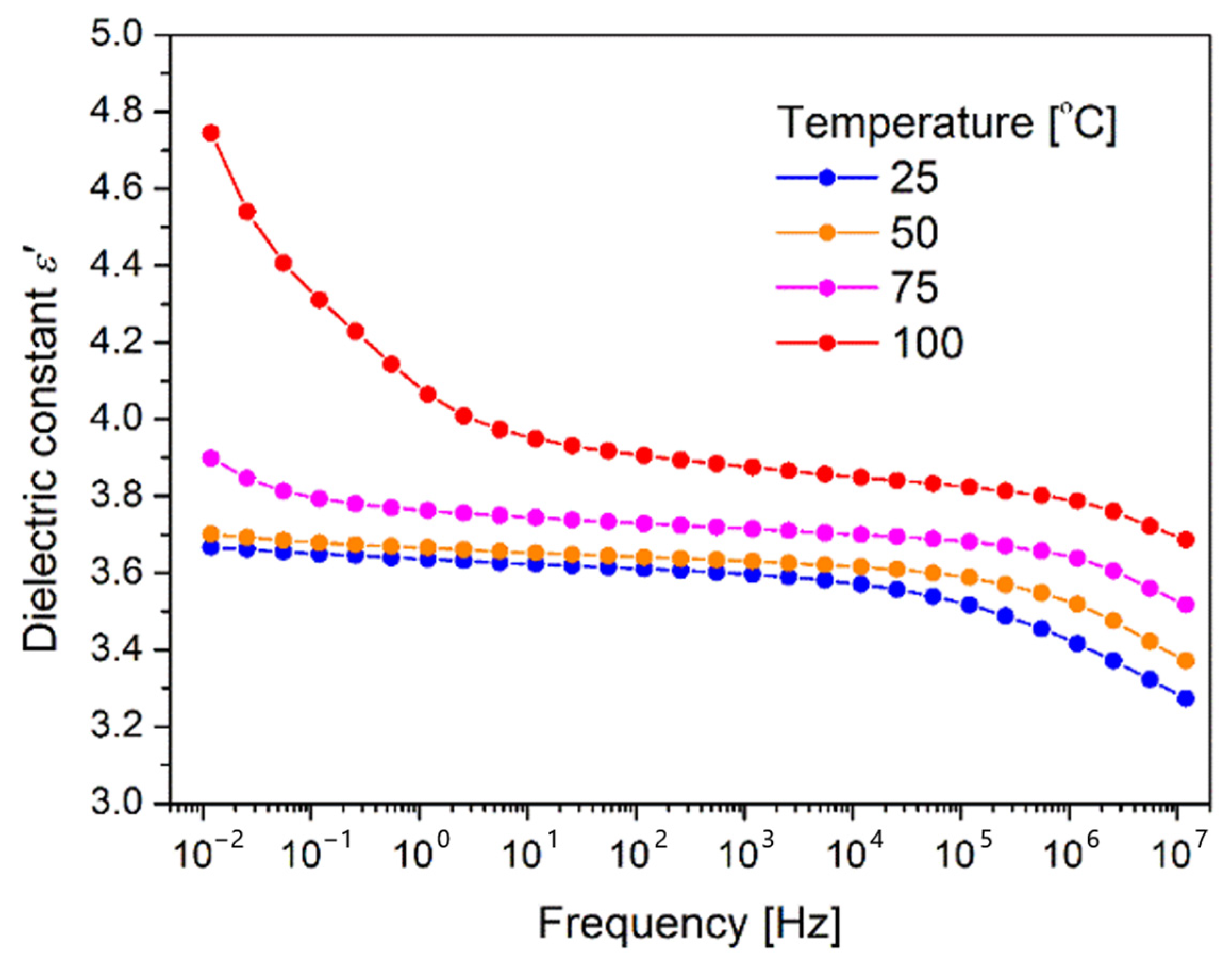
3.6. Dielectric Measurements
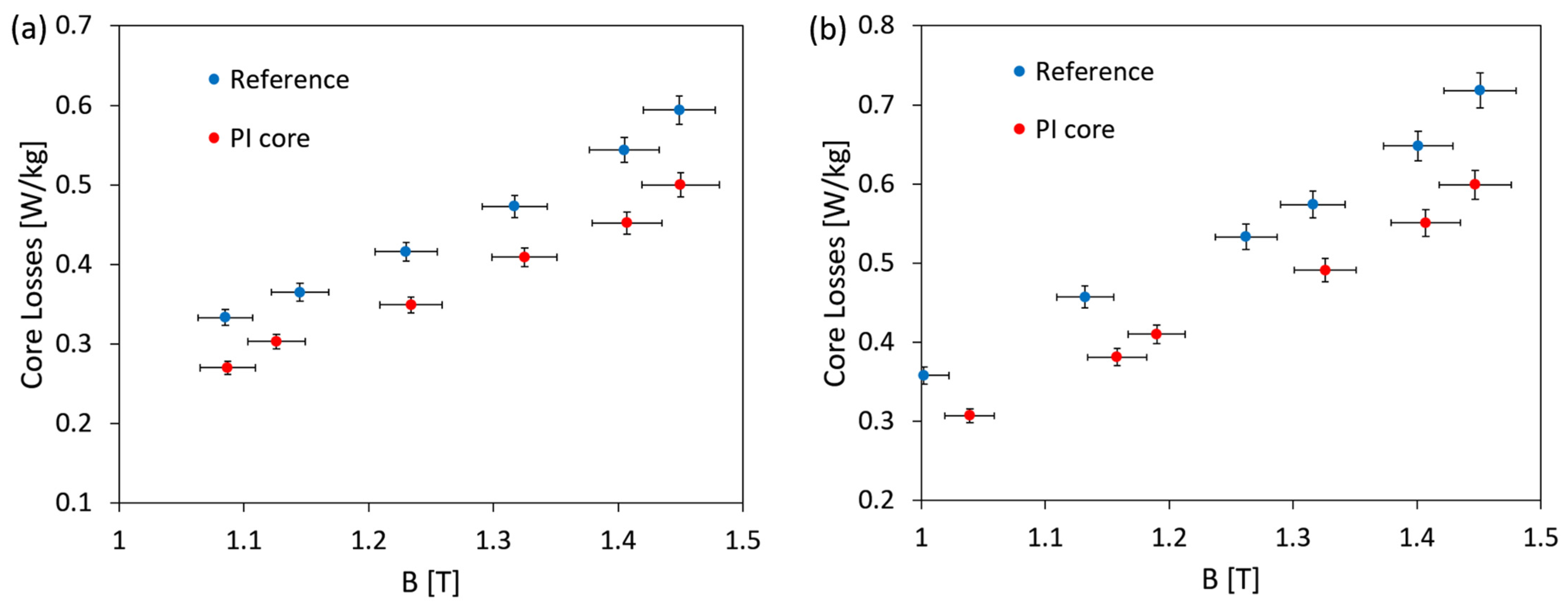
3.7. Magnetic Measurements
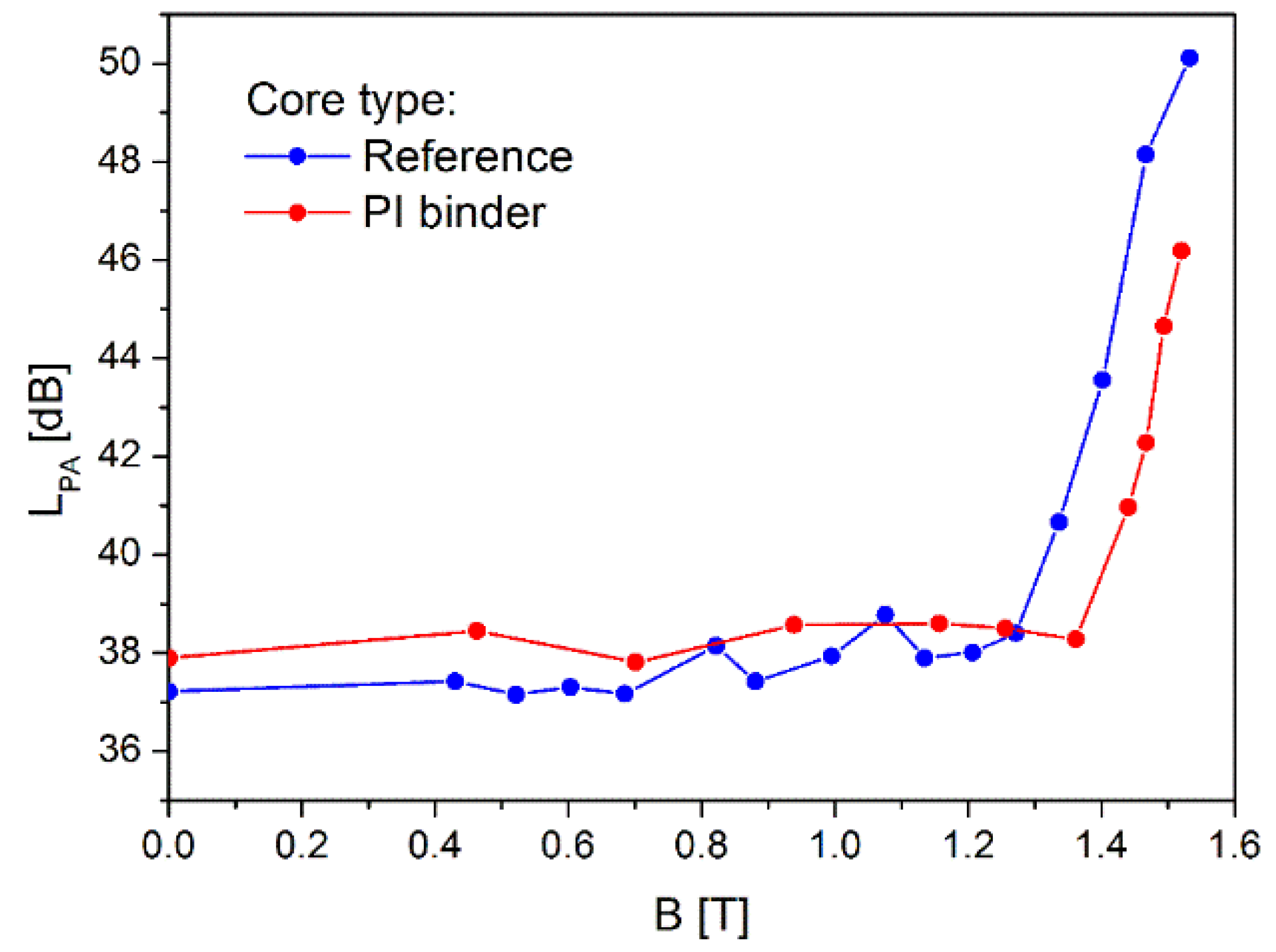
3.8. Noise Analysis
4. Conclusions
- PI binder resulted in high bonding strength (average value of 1520 kPa).
- Corrosion resistance was enhanced in relation to the raw amorphous metal.
- Applied deposition method resulted in formation of good dielectric layer (dielectric constant from 3.6–4.0 depending on temperature).
- Transformer core bonded with PI exhibited much lower core losses in relation to the reference (core losses of PI bonded core are 17% lower than losses of reference core).
- Core bonded with PI becomes noisy at noticeably higher flux density and is two times less noisy than the reference core.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Piska, M.; Sliwkowa, P.; Vnukowa, Z.; Petrenec, M.; Sedlakova-Valaskova, E. Exquisite Energy Savings at Cold Metal Forming of Threads through the Application of Polymers. Polymers 2022, 14, 1084. [Google Scholar] [CrossRef]
- Hu, M.; Wang, Y.; Xia, B.; Huang, G. What is the relationship between energy consumption and economic development? New evidence from a rapidly growing economic development region. Environ. Dev. Sustain. 2023, 25, 3601–3626. [Google Scholar] [CrossRef]
- Kurt, E.; Hatem, S. Design and Implementation of a MHz Frequency Transformer with a Ferromagnetic Fluid Core. Sustainability 2023, 15, 23. [Google Scholar] [CrossRef]
- Chen, M.; Yu, Y.J.; Xiao, L.Y.; Wang QLChung, W.; Kim, K.; Baang, S. The Magnetic Properties of the Ferromagmetic Materials Used for HTS Transformers at 77K. IEEE Trans. Appl. Supercond. 2003, 13, 2313–2316. [Google Scholar] [CrossRef]
- Heathcote, M.J. J&P Transformer Book, 12th ed.; Newnes: Oxford, UK, 1998; pp. 40–102+422–444. [Google Scholar]
- Sima, W.; Liu, Y.; Sun, P.; Zhou, Y.; Peng, D.; Yang, M. The Effect of Different Core Materials on Transformer Inrush Currentsm. In Proceedings of the 2018 IEEE International Magnetics Conference (INTERMAG), Singapore, 23–27 April 2018. [Google Scholar]
- McLyman, C.W.T. Transformer and Inductor Design Handbook, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011; Volume 1, p. 11. [Google Scholar]
- Molnar, A.; Smith, G.V.; Bartok, M. New Catalytic Materials from Amorphous Metal Alloys. Adv. Catal. 1989, 36, 329–383. [Google Scholar]
- Ramanan, V.R.V.; Smith, C.H.; Fish, G.E. Metallic glasses in magnetic applications. Key Eng. Mater. 1987, 13–15, 849–861. [Google Scholar] [CrossRef]
- Kronmuller, H.; Fahnle, M.; Domann, M.; Grimm, H.; Grimm, R.; Groger, B. Magnetic properties of amorphous ferromagnetic alloys. J. Magn. Magn. Mater. 1979, 13, 53–70. [Google Scholar] [CrossRef]
- Sato, T.; Yamada, T. Improvement of core loss by chemical thinning in a thick amorphous alloy ribbon. IEEE Trans. Magn. 1972, 28, 2775–2777. [Google Scholar] [CrossRef]
- Azuma, D.; Hasegawa, R. Core Loss in Toroidal Cores Based on Fe-Based Amorphous Metglas 2605HB1 Alloy. IEEE Trans. Magn. 2011, 47, 3460–3462. [Google Scholar] [CrossRef]
- Distribution Goes Green. Available online: https://library.e.abb.com/public/4b6d4b3cfd353a88c1257a25002696a8/ABB%20Review%202-2012_72dpi.pdf (accessed on 11 May 2024).
- Luborsky, F.; Becker, J.; McCary, R. Magnetic annealing of amorphous alloys. IEEE Trans. Magn. 1975, 11, 1644–1649. [Google Scholar] [CrossRef]
- Ramanan, V.R.V.; Pasquale, M.; Bertotti, G. The influence of microstructure on power losses in Fe-B-Si metallic glasses. J. Magn. Magn. Mater. 1994, 133, 362–365. [Google Scholar] [CrossRef]
- Kmita, G.; Sekula, R.; Rybak, A.; Kozupa, M.; Klys, P. Method of Manufacturing an Amorphous Magnetic Core and Amorphous Magnetic Core. European Patent Application No. EP3035351B1, 20 February 2019. [Google Scholar]
- Chisca, S.; Musteata, V.E.; Sava, I.; Bruma, M. Dielectric behavior of some aromatic polyimide films. Eur. Polym. J. 2011, 47, 1186–1197. [Google Scholar] [CrossRef]
- Sava, I.; Chisca, S. Surface properties of aromatic polymer film during thermal treatment. Mater. Chem. Phys. 2012, 134, 116–121. [Google Scholar] [CrossRef]
- Okada, T.; Ando, S. Conformational characterization of imide compounds and polyimides using far-infrared spectroscopy and DFT calculations. Polymer 2016, 86, 83–90. [Google Scholar] [CrossRef]
- Chen, W.; Chen, W.; Zhang, B.; Yang, S.; Liu, C.Y. Thermal imidization process of polyimide film: Interplay between solvent evaporation and imidization. Polymer 2017, 109, 205–215. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Z.; He, Z.; Yang, C.; Qi, Y.; Han, S.; Chen, S.; Yu, H.; Liu, J. Synthesis and Characterization of Organo-Soluble Polyimides Based on Polycondensation Chemistry of Fluorene-Containing Dianhydride and Amide-Bridged Diamines with Good Optical Transparency and Glass Transition Temperatures over 400 °C. Polymers 2023, 15, 3549. [Google Scholar] [CrossRef]
- Jiang, G.-L.; Wang, D.-Y.; Du, H.-P.; Wu, X.; Zhang, Y.; Tan, Y.-Y.; Wu, L.; Liu, J.-G.; Zhang, X.-M. Reduced Coefficients of Linear Thermal Expansion of Colorless and Transparent Semi-Alicyclic Polyimide Films via Incorporation of Rigid-Rod Amide Moiety: Preparation and Properties. Polymers 2020, 12, 413. [Google Scholar] [CrossRef]
- Lee, J.S.; Yan, Y.-Z.; Park, S.S.; Ahn, S.-K.; Ha, C.-S. A Novel Diamine Containing Ester and Diphenylethane Groups for Colorless Polyimide with a Low Dielectric Constant and Low Water Absorption. Polymers 2022, 14, 4504. [Google Scholar] [CrossRef]
- Soroko, I.; Bhole, Y.; Livingstone, A.G. Environmentally friendly route for the preparation of solvent resistant polyimide nanofiltration membranes. Green. Chem. 2011, 13, 162–168. [Google Scholar] [CrossRef]
- Goldman, I.; Grant, T.W.; Grote, J.K.; Collins, R.L.; Houser, K. Edge Bonding for Amorphous Metal Transformer. U.S. Patent Application No. US6413351B1, 2 July 2002. [Google Scholar]
- Grimes, F.H.; Krause, R.F. Methods of Consolidating a Magnetic Core. U.S. Patent Application No. US4615106A, 7 October 1986. [Google Scholar]
- Lupinski, J.H. Bonded Amorphous Metal Electromagnetic Components. U.S. Patent Application No. US4201837A, 6 May 1980. [Google Scholar]
- Verbunt, J.P.M. Lamellar Magnetic Core Utilizing Low Viscosity Epoxy Adhesive. U.S. Patent Application No. US4713297A, 15 December 1987. [Google Scholar]
- Columbus, M.R.; Brown, R.; Takahashi, K.; Hasegawa, R. Method of Reducing Audible Noise in Magnetic Cores and Magnetic Cores Having Reduced Audible Noise. U.S. Patent Application No. US8427272B1, 23 April 2013. [Google Scholar]
- Distribution Transformer Electrical Steel from Metglas. Available online: https://metglas.com/distribution-transformer-electrical-steel (accessed on 10 May 2024).
- ISO 21920-2:2021; Geometrical Product Specifications (GPS)—Surface Texture: Profile, Part 2: Terms, Definitions and Surface Texture Parameters. International Organization for Standardization: Geneva, Switzerland, 2021.
- Martens, W.N.; Frost, R.L.; Kristof, J.; Kloprogge, T. Raman spectroscopy of dimethyl sulphoxide and deuterated dimethyl sulphoxide at 298 and 77 K. J. Raman Spectrosc. 2002, 33, 84. [Google Scholar] [CrossRef]
- Harito, C.; Bavykin, D.V.; Yuliarto, B.; Dipojono, H.K.; Walsh, F.C. Inhibition of Polyimide Photodegradation by Incorporation of Titanate Nanotubes into a Composite. J. Polym. Environ. 2019, 27, 1501–1515. [Google Scholar] [CrossRef]
- Nieroda, J.; Rybak, A.; Kmita, G.; Niziol, J.; Gondek, E.; Sitarz, M. Synthesis and characterization of silane based binder for the amorphous metal ribbon. Thin Solid Films 2020, 716, 138433. [Google Scholar] [CrossRef]
- Nieroda, J.; Mastalska-Popławska, J.; Rybak, A.; Sitarz, M. Spectroscopic and rheological investigation of candidates for the double-layered binder for amorphous metal ribbon. J. Mol. Struct. 2020, 1207, 127763. [Google Scholar] [CrossRef]
- Butnaru, I.; Bruma, M.; Gaan, S. Phosphine oxide based polyimides: Structure–property relationships. RSC Adv. 2017, 7, 50508–50518. [Google Scholar] [CrossRef]



| Sample | Ra |
|---|---|
| Reference_g | 0.248 ± 0.065 |
| Reference_m | 1.046 ± 0.213 |
| PI_dr_g | 0.642 ± 0.116 |
| PI_dr_m | 1.559 ± 0.422 |
| PI_an_g | 0.392 ± 0.050 |
| PI_an_m | 1.571 ± 0.386 |
| Sample | Element [At %] | ||||
|---|---|---|---|---|---|
| Fe | Si | O | C | S | |
| Reference_g | 90 | 6 | 4 | ||
| Reference_m | 92 | 4 | 4 | ||
| PI_dr_g | 10 | 1 | 6 | 82 | 1 |
| PI_dr_m | 12 | 1 | 6 | 80 | 1 |
| PI_an_g | 11 | 1 | 5 | 83 | |
| PI_an_m | 12 | 1 | 5 | 82 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieroda, J.; Kmita, G.; Kozupa, M.; Piela, S.; Rybak, A. The Use of Polyimide as a Bonding Material to Improve the Mechanical Stability, Magnetic and Acoustic Properties of the Transformer Core Based on Amorphous Steel. Polymers 2024, 16, 1840. https://doi.org/10.3390/polym16131840
Nieroda J, Kmita G, Kozupa M, Piela S, Rybak A. The Use of Polyimide as a Bonding Material to Improve the Mechanical Stability, Magnetic and Acoustic Properties of the Transformer Core Based on Amorphous Steel. Polymers. 2024; 16(13):1840. https://doi.org/10.3390/polym16131840
Chicago/Turabian StyleNieroda, Jolanta, Grzegorz Kmita, Michal Kozupa, Szymon Piela, and Andrzej Rybak. 2024. "The Use of Polyimide as a Bonding Material to Improve the Mechanical Stability, Magnetic and Acoustic Properties of the Transformer Core Based on Amorphous Steel" Polymers 16, no. 13: 1840. https://doi.org/10.3390/polym16131840
APA StyleNieroda, J., Kmita, G., Kozupa, M., Piela, S., & Rybak, A. (2024). The Use of Polyimide as a Bonding Material to Improve the Mechanical Stability, Magnetic and Acoustic Properties of the Transformer Core Based on Amorphous Steel. Polymers, 16(13), 1840. https://doi.org/10.3390/polym16131840








