Fabrication of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/ZnO Nanocomposite Films for Active Packaging Applications: Impact of ZnO Type on Structure–Property Dynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. ZnO NP Characterization
2.2.2. PHBHHx/ZnO Film Fabrication
2.2.3. Cross-Sectional Film Morphology
2.2.4. Chemical Properties
2.2.5. Thermal Properties
2.2.6. Crystallographic Properties
2.2.7. Mechanical Properties
2.2.8. Wetting Properties
2.2.9. Gas Permeability Properties
2.2.10. UV Barrier Properties
2.2.11. Optical Properties
2.2.12. Antibacterial Activity
2.2.13. Statistical Analysis
3. Results and Discussion
3.1. Morphological Characteristics of ZnO NPs and PHBHHx/ZnO Nanocomposites
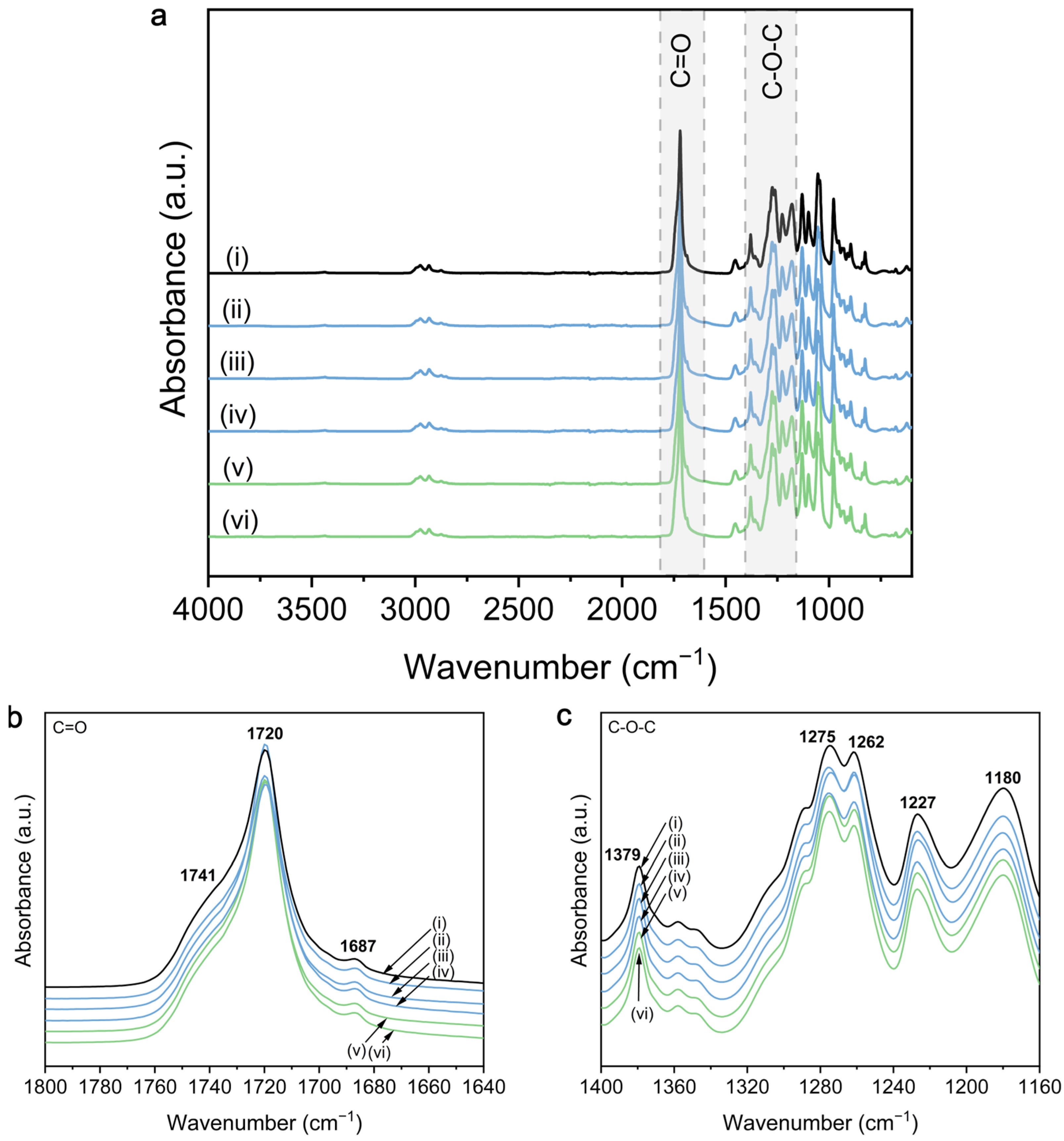
3.2. Chemical Properties
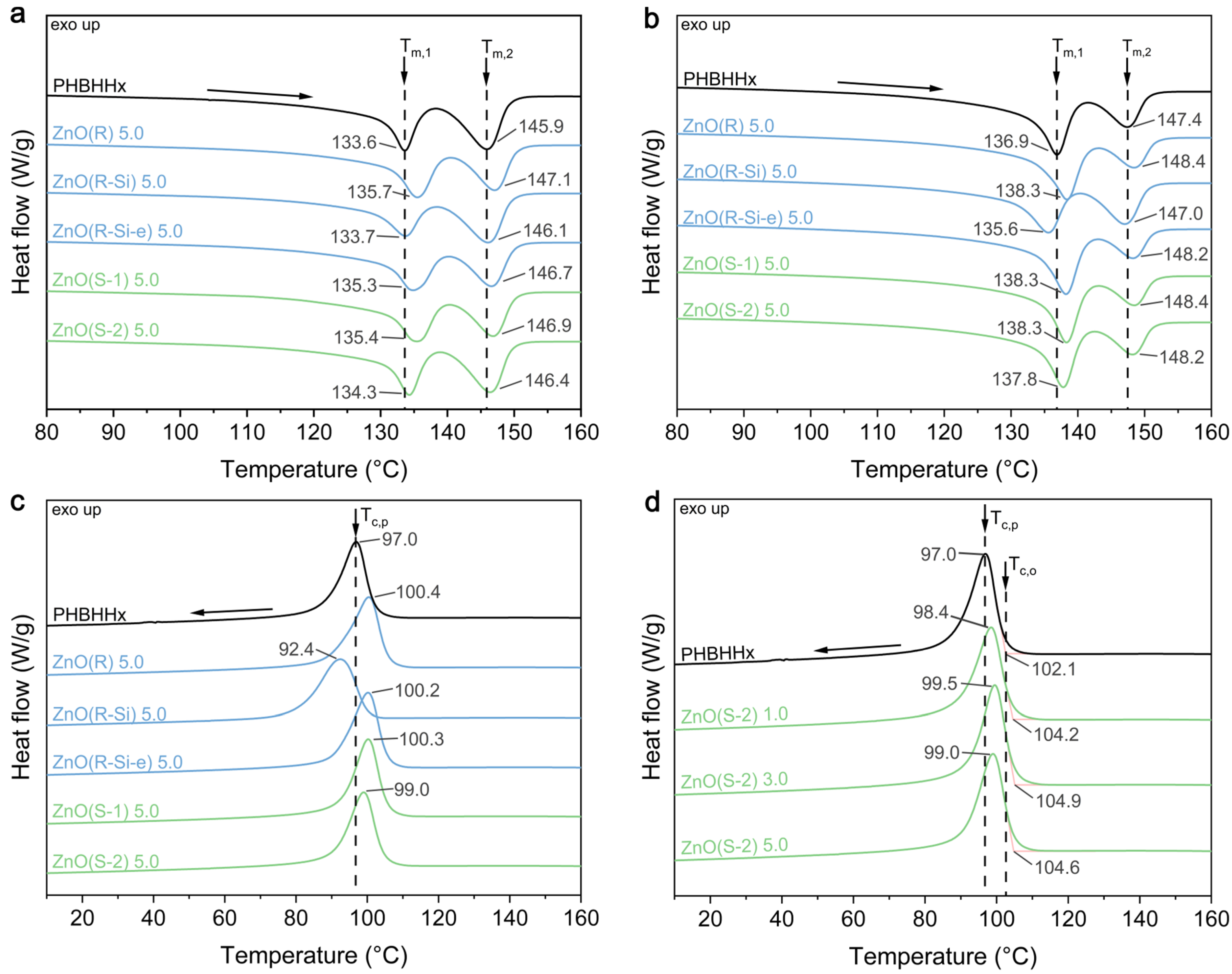
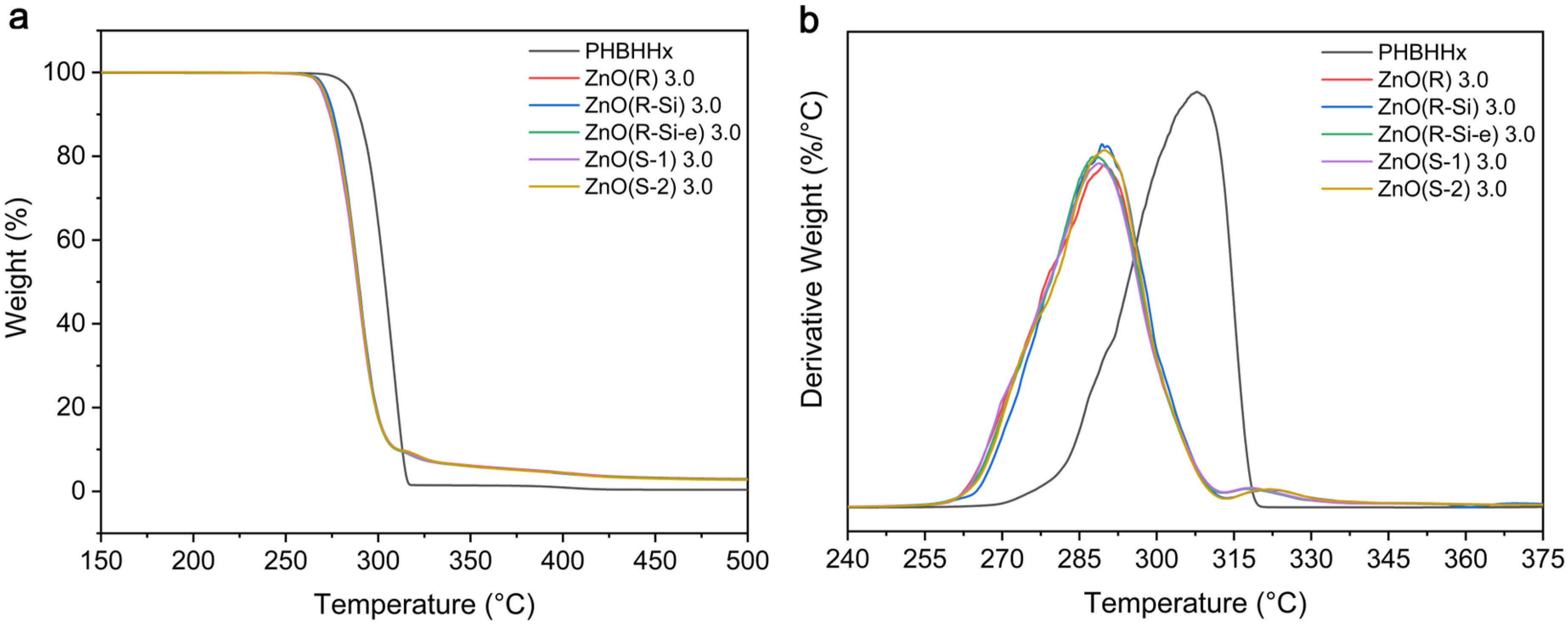
3.3. Thermal Properties
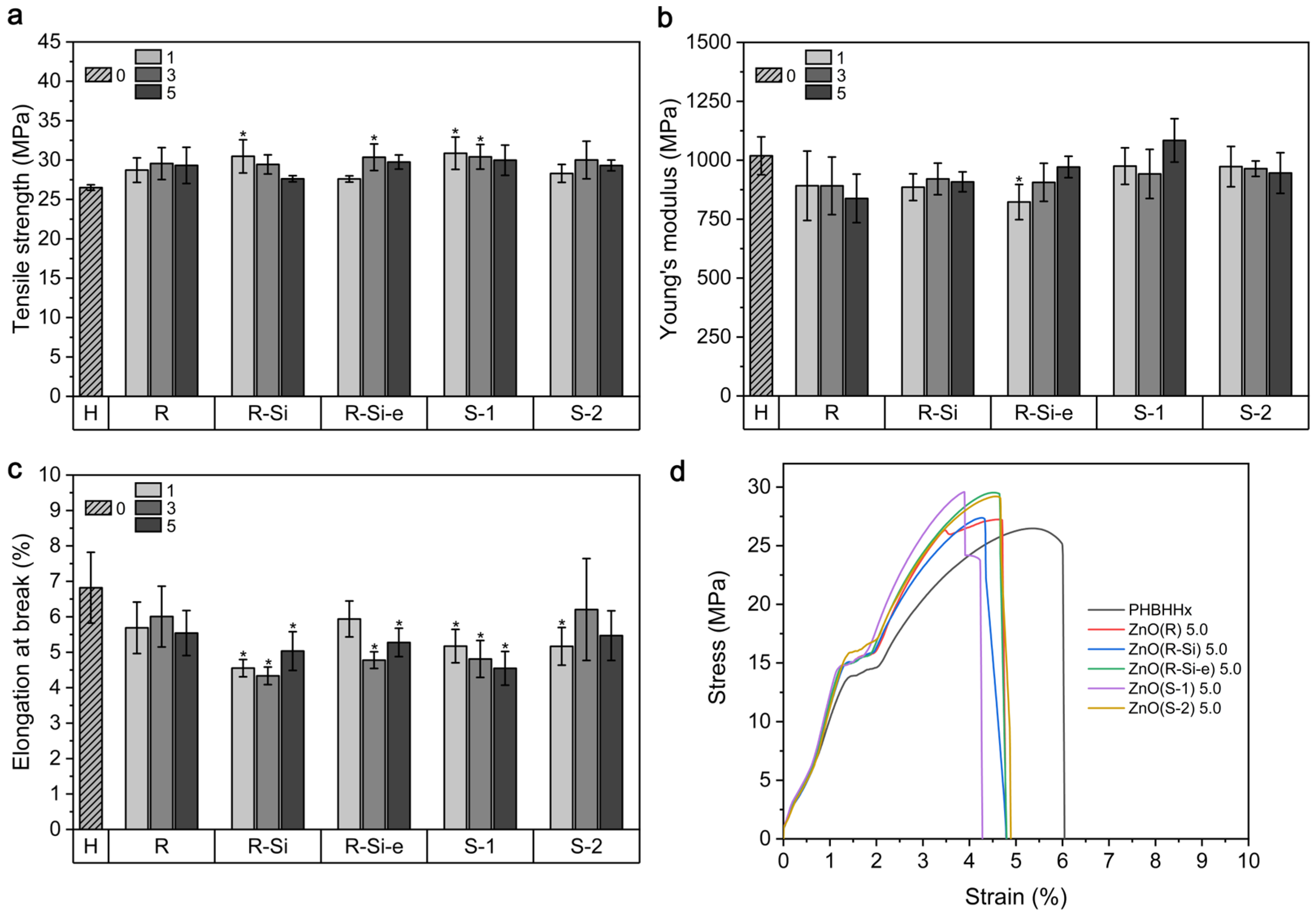
3.4. Mechanical Properties
3.5. UV Barrier Properties
3.6. Colorimetric Properties and Opacity
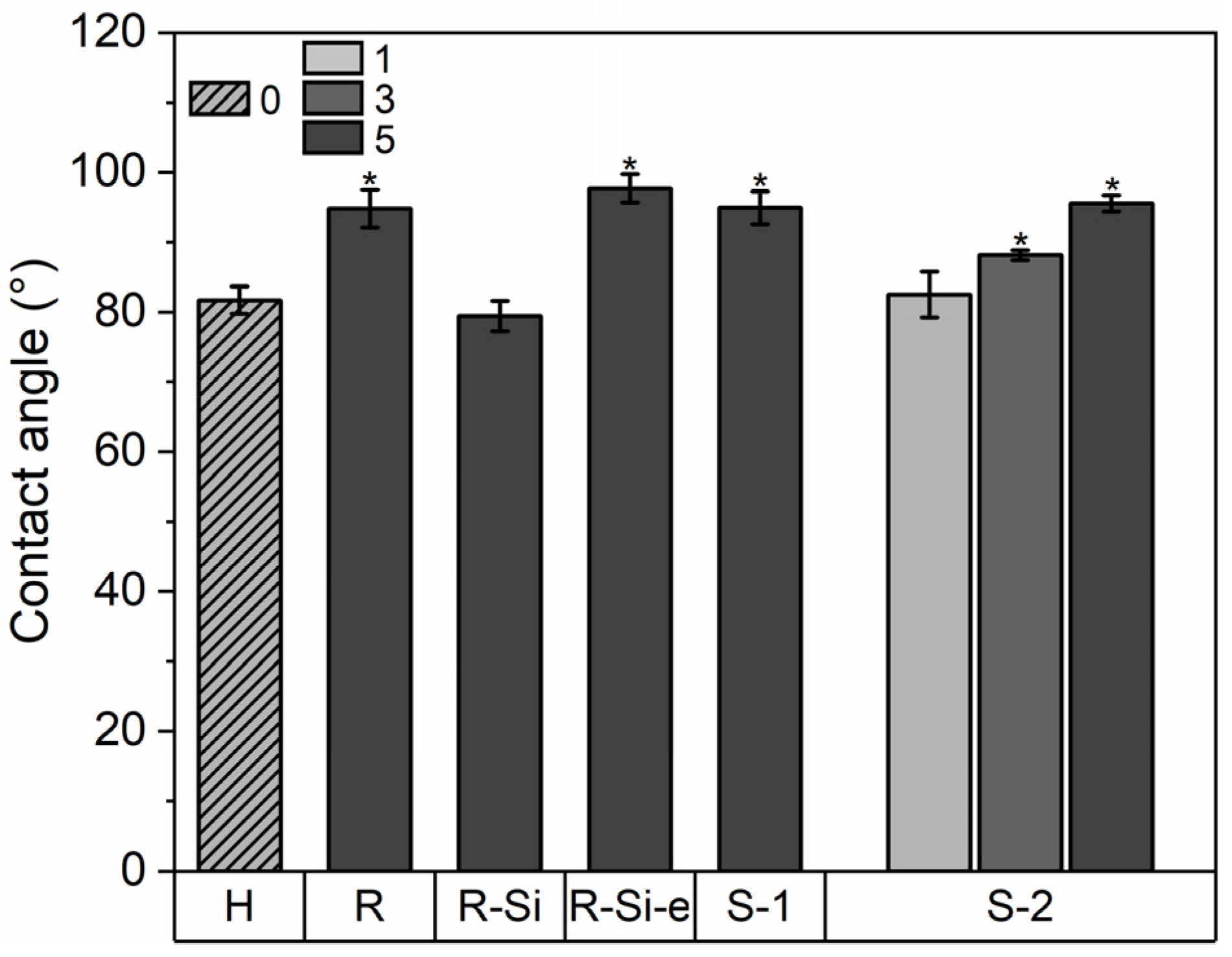
3.7. Wetting Properties
3.8. Gas Permeability Properties
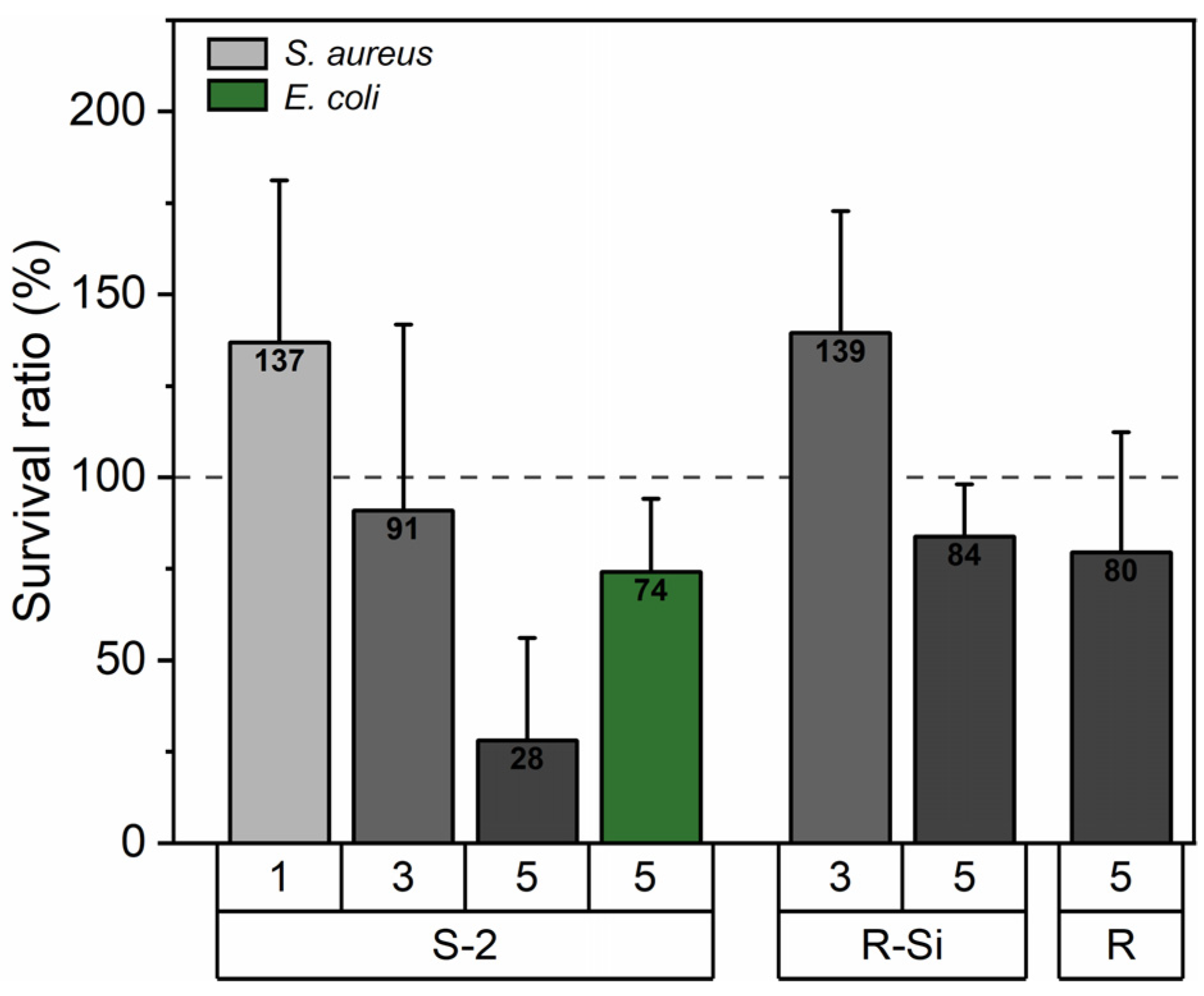
3.9. Antibacterial Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, H.; Samborska, V.; Roser, M. (Eds.) Data Page: Global Plastics Production. In Plastic Pollution; Data adapted from Geyer et al. (2017), OECD (2022); 2023; Available online: https://ourworldindata.org/grapher/global-plastics-production (accessed on 30 May 2024).
- Steilemann, M. It’s Time to Shift to Net-Zero Emissions Plastics; World Economic Forum: Cologny, Switzerland, 2022. [Google Scholar]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in Applications and Prospects of Bioplastics and Biopolymers: A Review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent Advances in Bioplastics: Application and Biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef]
- Bioplastics Market Development Update 2023; European Bioplastics e.V: Berlin, Germany, 2023.
- The Circular Economy for Plastics—A European Analysis 2024; Plastics Europe: Brussels, Belgium, 2024.
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in Research and Development of Bioplastic for Food Packaging: Advances in Research and Development of Bioplastic for Food Packaging. J. Sci. Food Agric. 2019, 100, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Corrado, I.; Vastano, M.; Cascelli, N.; Sannia, G.; Pezzella, C. Turning Wastes into Resources: Exploiting Microbial Potential for the Conversion of Food Wastes into Polyhydroxyalkanoates. In Bio-Valorization of Waste; Shah, S., Venkatramanan, V., Prasad, R., Eds.; Environmental and Microbial Biotechnology; Springer Singapore: Singapore, 2021; pp. 133–168. ISBN 9789811596957. [Google Scholar]
- Mahato, R.P.; Kumar, S.; Singh, P. Production of Polyhydroxyalkanoates from Renewable Resources: A Review on Prospects, Challenges and Applications. Arch. Microbiol. 2023, 205, 172. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; He, H.; Yan, X.; Liu, X.; Scrutton, N.S.; Chen, G.-Q. PHA Is Not Just a Bioplastic! Biotechnol. Adv. 2024, 71, 108320. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Ragaert, P.; Buntinx, M.; Maes, C.; Vanheusden, C.; Peeters, R.; Wang, S.; D’hooge, D.R.; Cardon, L. Polyhydroxyalkanoates for Food Packaging Applications. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-08-100596-5. [Google Scholar]
- Vanheusden, C.; Samyn, P.; Goderis, B.; Hamid, M.; Reddy, N.; Ethirajan, A.; Peeters, R.; Buntinx, M. Extrusion and Injection Molding of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) (PHBHHx): Influence of Processing Conditions on Mechanical Properties and Microstructure. Polymers 2021, 13, 4012. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Qiu, Z. Effect of Comonomer Content on the Crystallization Kinetics and Morphology of Biodegradable Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). Phys. Chem. Chem. Phys. 2009, 11, 9569. [Google Scholar] [CrossRef] [PubMed]
- Dash, K.K.; Deka, P.; Bangar, S.P.; Chaudhary, V.; Trif, M.; Rusu, A. Applications of Inorganic Nanoparticles in Food Packaging: A Comprehensive Review. Polymers 2022, 14, 521. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- GRAS Substances (SCOGS) Database. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database (accessed on 14 May 2024).
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Safety Assessment of the Substance Zinc Oxide, Nanoparticles, for Use in Food Contact Materials. EFSA J. 2016, 14, 4408. [Google Scholar] [CrossRef]
- Lizundia, E.; Ruiz-Rubio, L.; Vilas, J.L.; León, L.M. Towards the Development of Eco-Friendly Disposable Polymers: ZnO-Initiated Thermal and Hydrolytic Degradation in Poly(l-Lactide)/ZnO Nanocomposites. RSC Adv. 2016, 6, 15660–15669. [Google Scholar] [CrossRef]
- Qu, M.; Tu, H.; Amarante, M.; Song, Y.; Zhu, S.S. Zinc Oxide Nanoparticles Catalyze Rapid Hydrolysis of Poly(Lactic Acid) at Low Temperatures. J. Appl. Polym. Sci. 2014, 131, app.40287. [Google Scholar] [CrossRef]
- Del Campo, A.; De Lucas-Gil, E.; Rubio-Marcos, F.; Arrieta, M.P.; Fernández-García, M.; Fernández, J.F.; Muñoz-Bonilla, A. Accelerated Disintegration of Compostable Ecovio Polymer by Using ZnO Particles as Filler. Polym. Degrad. Stab. 2021, 185, 109501. [Google Scholar] [CrossRef]
- Padilla-Gainza, V.; Rodríguez-Tobías, H.; Morales, G.; Ledezma-Pérez, A.; Alvarado-Canché, C.; Rodríguez, C.; Gilkerson, R.; Lozano, K. Processing-structure-property Relationships of Biopolyester/Zinc Oxide Fibrous Scaffolds Engineered by Centrifugal Spinning. Polym. Adv. Technol. 2020, 31, 2601–2614. [Google Scholar] [CrossRef]
- dos Santos Silva, I.D.; Guimarães Jaques, N.; da Cruz Barbosa Neto, M.; Agrawal, P.; Ries, A.; Ramos Wellen, R.M.; Canedo, E.L. Melting and Crystallization of PHB/ZnO Compounds: Effect of Heating and Cooling Cycles on Phase Transition. J. Therm. Anal. Calorim. 2018, 132, 571–580. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Ledezma, A.; Romero, J.; Saldívar, R.; Langlois, V.; Renard, E.; Grande, D. Electrospinning and Electrospraying Techniques for Designing Novel Antibacterial Poly(3-Hydroxybutyrate)/Zinc Oxide Nanofibrous Composites. J. Mater. Sci. 2016, 51, 8593–8609. [Google Scholar] [CrossRef]
- Yu, W.; Lan, C.-H.; Wang, S.-J.; Fang, P.-F.; Sun, Y.-M. Influence of Zinc Oxide Nanoparticles on the Crystallization Behavior of Electrospun Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Nanofibers. Polymer 2010, 51, 2403–2409. [Google Scholar] [CrossRef]
- Bekat, T.; Oner, M. Effects of ZnO Crystals Synthesized in Presence of CMI Biopolymer on PHBV Properties. Pure Appl. Chem. 2017, 89, 89–96. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Fabra, M.J.; Pourrahimi, A.M.; Olsson, R.T.; Lagaron, J.M. The Impact of Zinc Oxide Particle Morphology as an Antimicrobial and When Incorporated in Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Films for Food Packaging and Food Contact Surfaces Applications. Food Bioprod. Process. 2017, 101, 32–44. [Google Scholar] [CrossRef]
- Anžlovar, A.; Kržan, A.; Žagar, E. Degradation of PLA/ZnO and PHBV/ZnO Composites Prepared by Melt Processing. Arab. J. Chem. 2018, 11, 343–352. [Google Scholar] [CrossRef]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Patel, P.R.; Dhanavade, M.J.; Badiger, M.V.; Marathe, Y.N.; Bohara, R.A. Electrospun Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Polyethylene Oxide (PEO) Microfibers Reinforced with ZnO Nanocrystals for Antibacterial and Antibiofilm Wound Dressing Applications. New J. Chem. 2020, 44, 9754–9766. [Google Scholar] [CrossRef]
- Chandar, J.V.; Shanmugan, S.; Mutharasu, D.; Azlan, A.A. Poly (3-Hydroxybutyrate-Co-15 Mol% 3hydroxyhexanoate)/ZnO Nanocomposites by Solvent Casting Method: A. Mater. Res. Express 2017, 4, 015301. [Google Scholar] [CrossRef]
- Silva, N.G.S.; Zanini, N.C.; Barbosa, R.F.S.; De Souza, A.G.; Medeiros, S.F.; Rosa, D.S.; Mulinari, D.R. A Promising Sustainable PHB-ZnO Composite for Development of Biodegradable Filaments. Polym. Compos. 2022, 43, 144–159. [Google Scholar] [CrossRef]
- Jaques, N.G.; Silva, I.D.D.S.; Barbosa Neto, M.D.C.; Diniz, R.K.M.; Wellen, R.M.R.; Canedo, E.L. Comparative Study of the Effect of TiO2 and ZnO on the Crystallization of PHB. Matéria (Rio J.) 2017, 22, e-11880. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Torres-Giner, S.; Enescu, D.; Cabedo, L.; Cerqueira, M.A.; Pastrana, L.M.; Lagaron, J.M. Electrospun Active Biopapers of Food Waste Derived Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Short-Term and Long-Term Antimicrobial Performance. Nanomaterials 2020, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.; Canedo, E.L.; Souto, C.R.; Wellen, R.M.R. Non-Isothermal Cold Crystallization Kinetics of Poly(3-Hydoxybutyrate) Filled with Zinc Oxide. Thermochim. Acta 2016, 637, 74–81. [Google Scholar] [CrossRef]
- Díez-Pascual, A.; Díez-Vicente, A. Poly(3-Hydroxybutyrate)/ZnO Bionanocomposites with Improved Mechanical, Barrier and Antibacterial Properties. Int. J. Mol. Sci. 2014, 15, 10950–10973. [Google Scholar] [CrossRef] [PubMed]
- Naphade, R.; Jog, J. Electrospinning of PHBV/ZnO Membranes: Structure and Properties. Fibers Polym. 2012, 13, 692–697. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. ZnO-Reinforced Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Bionanocomposites with Antimicrobial Function for Food Packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef]
- Shuai, C.; Wang, C.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. Enhanced Crystallinity and Antibacterial of PHBV Scaffolds Incorporated with Zinc Oxide. J. Nanomater. 2020, 2020, 6014816. [Google Scholar] [CrossRef]
- Berrabah, I.; Dehouche, N.; Kaci, M.; Bruzaud, S.; Deguines, C.H.; Delaite, C. Morphological, Crystallinity and Thermal Stability Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate)/Zinc Oxide Nanoparticles Bionanocomposites: Effect of Filler Content. Mater. Today Proc. 2022, 53, 223–227. [Google Scholar] [CrossRef]
- Berrabah, I.; Dehouche, N.; Kaci, M.; Bruzaud, S.; Delaite, C.; Deguines, C.H.; Bououdina, M. A Bionanocomposite of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate)/ZnO-Nanoparticles Intended for Food Packaging. Int. J. Biol. Macromol. 2023, 238, 124162. [Google Scholar] [CrossRef] [PubMed]
- Bekat, T.; Öner, M. Effects of Surface Modification and Ultrasonic Agitation on the Properties of PHBV/ZnO Nanocomposites. Pure Appl. Chem. 2016, 88, 1027–1035. [Google Scholar] [CrossRef]
- Janakiraman, V.C.; Subramani, S.; Devarajan, M.; Abdul Aziz, A. Impact of ZnO Nanoparticles on Dielectric and Optical Properties of Poly (3-Hydroxybutyrate) for Electronics Applications. Polym.-Plast. Technol. Eng. 2017, 56, 1495–1504. [Google Scholar] [CrossRef]
- E Silva, M.B.R.; Tavares, M.I.B.; Junior, A.W.M.; Neto, R.P.C. Evaluation of Intermolecular Interactions in the PHB/ZnO Nanostructured Materials. J. Nanosci. Nanotechnol. 2016, 16, 7606–7610. [Google Scholar] [CrossRef]
- Vishnu Chandar, J.; Shanmugan, S.; Murugan, P.; Mutharasu, D.; Sudesh, K. Structural Analysis of ZnO Nanoparticles Reinforced P(3HB-Co-15 Mol% 3HHx) Bioplastic Composite. J. Polym. Environ. 2017, 25, 1251–1261. [Google Scholar] [CrossRef]
- Râpă, M.; Stefan, M.; Popa, P.A.; Toloman, D.; Leostean, C.; Borodi, G.; Vodnar, D.C.; Wrona, M.; Salafranca, J.; Nerín, C.; et al. Electrospun Nanosystems Based on PHBV and ZnO for Ecological Food Packaging. Polymers 2021, 13, 2123. [Google Scholar] [CrossRef]
- Champa-Bujaico, E.; Díez-Pascual, A.M.; Garcia-Diaz, P. Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Bionanocomposites with Crystalline Nanocellulose and Graphene Oxide: Experimental Results and Support Vector Machine Modeling. Polymers 2023, 15, 3746. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Maldonado-Textle, H.; Grande, D. Long-Term Photo-Degradation of Nanofibrous Composites Based on Poly(3-Hydroxybutyrate) Electrospun Fibers Loaded with Zinc Oxide Nanoparticles. Fibers Polym. 2022, 23, 2717–2724. [Google Scholar] [CrossRef]
- Bressy, C.; Ngo, V.G.; Ziarelli, F.; Margaillan, A. New Insights into the Adsorption of 3-(Trimethoxysilyl)Propylmethacrylate on Hydroxylated ZnO Nanopowders. Langmuir 2012, 28, 3290–3297. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) with Zinc Oxide Nanoparticles for Food Packaging. J. Food Process Eng. 2021, 45, e13814. [Google Scholar] [CrossRef]
- Anžlovar, A.; Primožič, M.; Švab, I.; Leitgeb, M.; Knez, Ž.; Žagar, E. Polyolefin/ZnO Composites Prepared by Melt Processing. Molecules 2019, 24, 2432. [Google Scholar] [CrossRef]
- Murariu, M.; Doumbia, A.; Bonnaud, L.; Dechief, A.; Paint, Y.; Ferreira, M.; Campagne, C.; Devaux, E.; Dubois, P. High-Performance Polylactide/ZnO Nanocomposites Designed for Films and Fibers with Special End-Use Properties. Biomacromolecules 2011, 12, 1762–1771. [Google Scholar] [CrossRef]
- Xie, Y.; Noda, I.; Akpalu, Y.A. Influence of Cooling Rate on the Thermal Behavior and Solid-State Morphologies of Polyhydroxyalkanoates. J. Appl. Polym. Sci. 2008, 109, 2259–2268. [Google Scholar] [CrossRef]
- Vandewijngaarden, J.; Murariu, M.; Dubois, P.; Carleer, R.; Yperman, J.; Adriaensens, P.; Schreurs, S.; Lepot, N.; Peeters, R.; Buntinx, M. Gas Permeability Properties of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). J. Polym. Environ. 2014, 22, 501–507. [Google Scholar] [CrossRef]
- Vanheusden, C.; Vanminsel, J.; Reddy, N.; Samyn, P.; D’Haen, J.; Peeters, R.; Ethirajan, A.; Buntinx, M. Fabrication of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Fibers Using Centrifugal Fiber Spinning: Structure, Properties and Application Potential. Polymers 2023, 15, 1181. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing, (EUCAST). MIC Determination of Non-Fastidious and Fastidious Organisms. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 17 May 2024).
- Lim, J.S.; Noda, I.; Im, S.S. Effect of Hydrogen Bonding on the Crystallization Behavior of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate)/Silica Hybrid Composites. Polymer 2007, 48, 2745–2754. [Google Scholar] [CrossRef]
- Van Ngo, G.; Margaillan, A.; Villain, S.; Leroux, C.; Bressy, C. Synthesis of ZnO Nanoparticles with Tunable Size and Surface Hydroxylation. J. Nanopart. Res. 2013, 15, 1332. [Google Scholar] [CrossRef]
- Berrabah, I.; Kaci, M.; Dehouche, N.; Delaite, C.; Deguines, C.-H.; Bououdina, M. Advancing Food Packaging: Enhancing Stability and Performance of Biodegradable PHBHHx with ZnO Nanofillers. Polym. Bull. 2024, 1–19. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Effect of Graphene Oxide on the Properties of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). Polymers 2021, 13, 2233. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bin Hussein, M.Z.; Taufiq-Yap, Y.H. Hydrothermal Synthesis of Zinc Oxide Nanoparticles Using Rice as Soft Biotemplate. Chem. Cent. J. 2013, 7, 136. [Google Scholar] [CrossRef]
- Lee, P.; Saion, E.; Al-Hada, N.; Soltani, N. A Simple Up-Scalable Thermal Treatment Method for Synthesis of ZnO Nanoparticles. Metals 2015, 5, 2383–2392. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Liu, D.; Ström, V.; Hedenqvist, M.S.; Olsson, R.T.; Gedde, U.W. Heat Treatment of ZnO Nanoparticles: New Methods to Achieve High-Purity Nanoparticles for High-Voltage Applications. J. Mater. Chem. A 2015, 3, 17190–17200. [Google Scholar] [CrossRef]
- Chen, C.; Yu, P.H.F.; Cheung, M.K. Hydrogen Bonding, Miscibility, Crystallization, and Thermal Stability in Blends of Biodegradable Polyhydroxyalkanoates and Polar Small Molecules of 4-Tert-butylphenol. J. Appl. Polym. Sci. 2005, 98, 736–745. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Samyn, P. Novel Processing of Polyhydroxybutyrate with Micro- to Nanofibrillated Cellulose and Effect of Fiber Morphology on Crystallization Behaviour of Composites. Express Polym. Lett. 2020, 14, 115–133. [Google Scholar] [CrossRef]
- Kansiz, M.; Domínguez-Vidal, A.; McNaughton, D.; Lendl, B. Fourier-Transform Infrared (FTIR) Spectroscopy for Monitoring and Determining the Degree of Crystallisation of Polyhydroxyalkanoates (PHAs). Anal. Bioanal. Chem. 2007, 388, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Sato, H.; Noda, I.; Ozaki, Y. Multiple Melting Behavior of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Investigated by Differential Scanning Calorimetry and Infrared Spectroscopy. Polymer 2007, 48, 4777–4785. [Google Scholar] [CrossRef]
- Chen, C.; Cheung, M.K.; Yu, P.H. Crystallization Kinetics and Melting Behaviour of Microbial Poly(3-hydroxybutyrate-Co-3-hydroxyhexanoate). Polym. Int. 2005, 54, 1055–1064. [Google Scholar] [CrossRef]
- Yoshie, N.; Menju, H.; Sato, H.; Inoue, Y. Complex Composition Distribution of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate). Macromolecules 1995, 28, 6516–6521. [Google Scholar] [CrossRef]
- Ding, C.; Cheng, B.; Wu, Q. DSC Analysis of Isothermally Melt-Crystallized Bacterial Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Films. J. Therm. Anal. Calorim. 2011, 103, 1001–1006. [Google Scholar] [CrossRef]
- Vasile, O.R.; Serdaru, I.; Andronescu, E.; Truşcă, R.; Surdu, V.A.; Oprea, O.; Ilie, A.; Vasile, B.Ş. Influence of the Size and the Morphology of ZnO Nanoparticles on Cell Viability. Comptes Rendus Chim. 2015, 18, 1335–1343. [Google Scholar] [CrossRef]
- Vandewijngaarden, J.; Murariu, M.; Dubois, P.; Carleer, R.; Yperman, J.; D’Haen, J.; Peeters, R.; Buntinx, M. Effect of Ultrafine Talc on Crystallization and End-Use Properties of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Bussiere, P.O.; Therias, S.; Gardette, J.-L.; Murariu, M.; Dubois, P.; Baba, M. Effect of ZnO Nanofillers Treated with Triethoxy Caprylylsilane on the Isothermal and Non-Isothermal Crystallization of Poly(Lactic Acid). Phys. Chem. Chem. Phys. 2012, 14, 12301. [Google Scholar] [CrossRef]
- Nguyen, S.; Yu, G.; Marchessault, R.H. Thermal Degradation of Poly(3-Hydroxyalkanoates): Preparation of Well-Defined Oligomers. Biomacromolecules 2002, 3, 219–224. [Google Scholar] [CrossRef]
- Kunioka, M.; Doi, Y. Thermal Degradation of Microbial Copolyesters: Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate). Macromolecules 1990, 23, 1933–1936. [Google Scholar] [CrossRef]
- Daly, P.A.; Bruce, D.A.; Melik, D.H.; Harrison, G.M. Thermal Degradation Kinetics of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). J. Appl. Polym. Sci. 2005, 98, 66–74. [Google Scholar] [CrossRef]
- Vishnu Chandar, J.; Shanmugan, S.; Mutharasu, D.; Azlan, A.A. Impact of ZnO Nanoparticles on Thermal Properties of Poly(3-Hydroxybutyrate-Co-10 Mol % 3-Hydroxyhexanoate) Copolymer. Polym. Sci. Ser. A 2019, 61, 504–513. [Google Scholar] [CrossRef]
- Majerczak, K.; Wadkin-Snaith, D.; Magueijo, V.; Mulheran, P.; Liggat, J.; Johnston, K. Polyhydroxybutyrate: A Review of Experimental and Simulation Studies of the Effect of Fillers on Crystallinity and Mechanical Properties. Polym. Int. 2022, 71, 1398–1408. [Google Scholar] [CrossRef]
- Abbas, M.; Buntinx, M.; Deferme, W.; Reddy, N.; Peeters, R. Oxygen Gas and UV Barrier Properties of Nano-ZnO-Coated PET and PHBHHx Materials Fabricated by Ultrasonic Spray-Coating Technique. Nanomaterials 2021, 14, 449. [Google Scholar] [CrossRef]
- Kwon, S.; Orsuwan, A.; Bumbudsanpharoke, N.; Yoon, C.; Choi, J.; Ko, S. A Short Review of Light Barrier Materials for Food and Beverage Packaging. Korean. J. Packag. Sci. Technol. 2018, 24, 141–148. [Google Scholar] [CrossRef]
- Althues, H.; Henle, J.; Kaskel, S. Functional Inorganic Nanofillers for Transparent Polymers. Chem. Soc. Rev. 2007, 36, 1454. [Google Scholar] [CrossRef] [PubMed]
- Vasi, S.; Ceccio, G.; Cannavò, A.; Pleskunov, P.; Vacík, J. Study of Wettability of Polyethylene Membranes for Food Packaging. Sustainability 2022, 14, 5863. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Sadeghi, K.; Thanakkasaranee, S.; Seo, J. Poly(Lactic Acid)/ZnO Bionanocomposite Films with Positively Charged ZnO as Potential Antimicrobial Food Packaging Materials. Polymers 2019, 11, 1427. [Google Scholar] [CrossRef]
- Follain, N.; Chappey, C.; Dargent, E.; Chivrac, F.; Crétois, R.; Marais, S. Structure and Barrier Properties of Biodegradable Polyhydroxyalkanoate Films. J. Phys. Chem. C 2014, 118, 6165–6177. [Google Scholar] [CrossRef]
- Lallo Da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship Between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview. IJN 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Pasquet, J.; Chevalier, Y.; Couval, E.; Bouvier, D.; Noizet, G.; Morlière, C.; Bolzinger, M.-A. Antimicrobial Activity of Zinc Oxide Particles on Five Micro-Organisms of the Challenge Tests Related to Their Physicochemical Properties. Int. J. Pharm. 2014, 460, 92–100. [Google Scholar] [CrossRef]
- Doumbia, A.S.; Vezin, H.; Ferreira, M.; Campagne, C.; Devaux, E. Studies of Polylactide/Zinc Oxide Nanocomposites: Influence of Surface Treatment on Zinc Oxide Antibacterial Activities in Textile Nanocomposites. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
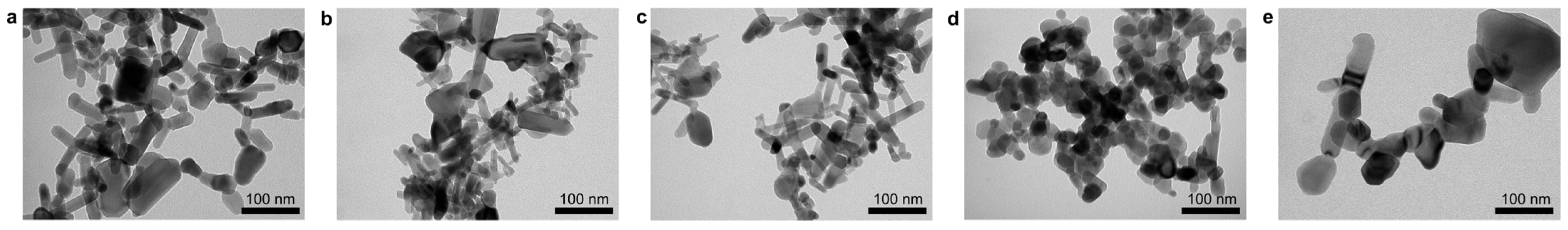
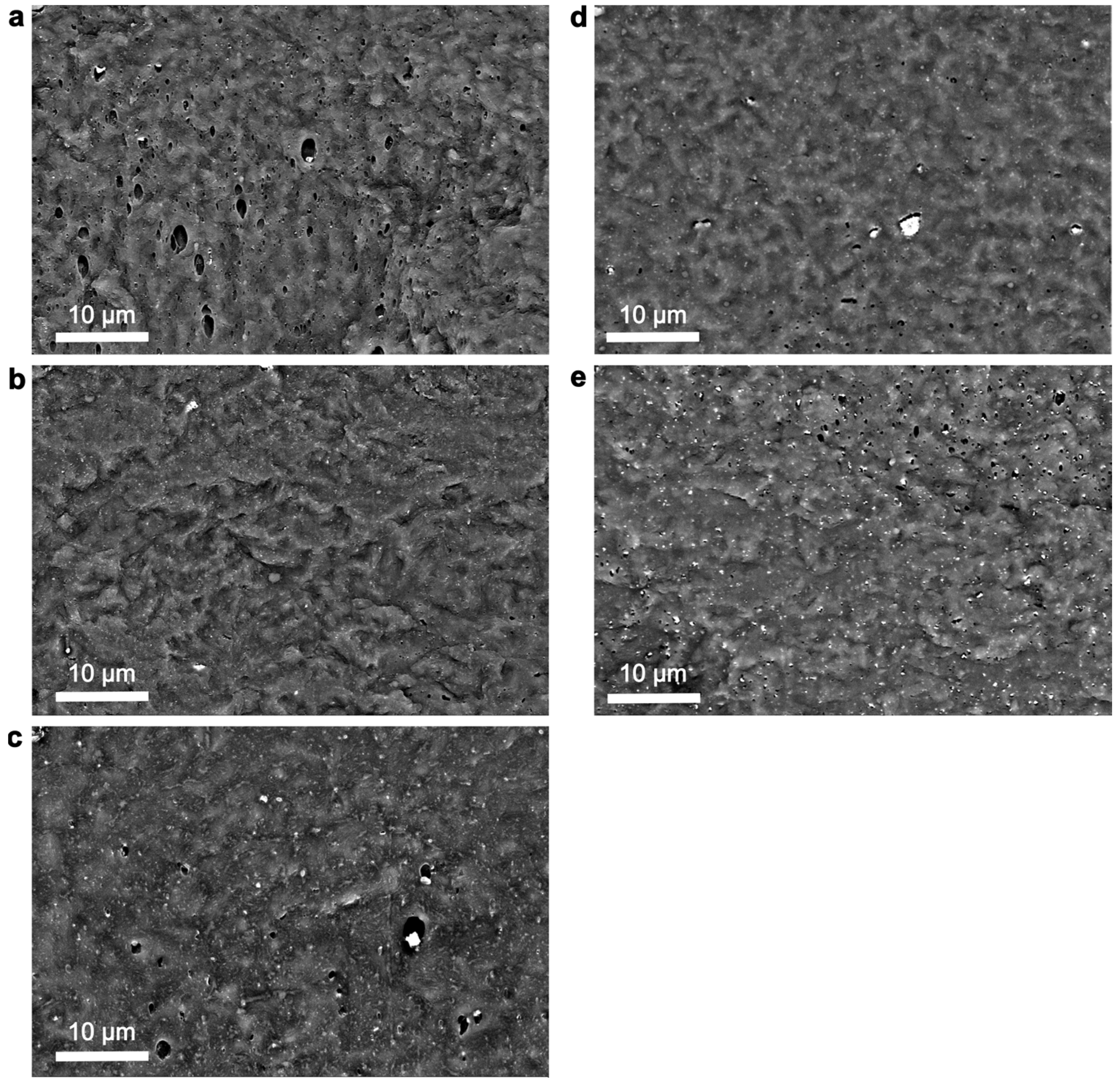


| Abbreviation | Commercial Name | Morphology | Surface Modification |
|---|---|---|---|
| ZnO(R) | Tenray Z2 | Rod | None |
| ZnO(R-Si) | Tenray Z2P | Rod | triethoxy caprylyl silane (TEOS) |
| ZnO(R-Si-e) | Tenray Z2E | Rod | 3-(methacryloyloxy)propyltrimethoxy-silane (MPS) |
| ZnO(S-1) | Nanosun P99/30, NM-112 | Spherical | None |
| ZnO(S-2) | ZnO no. 544906 | Spherical | None |
| Parameter | ZnO(R) | ZnO(R-Si) | ZnO(R-Si-e) | ZnO(S-1) | ZnO(S-2) |
|---|---|---|---|---|---|
| Shape | Rod | Rod | Rod | Spherical | Spherical |
| TEM size L/D (SD) or D (SD) (nm) | 61(20)/24(11) | 60(20)/15(6) | 60(21)/18(8) | 36(9) | 79(62) |
| Surface area (BET *, m2/g) | 34 | 42 | 41 | 30 | 17 |
| Volume of pores (BJH **, cm3/g) | 0.065 | 0.060 | 0.060 | 0.054 | 0.229 |
| Pore size (BJH, 4 V/A, nm) | 5.5 | 5.3 | 5.4 | 5.9 | 1.9 |
| Hydrophobicity (CA, °) | 11 | >160 | 15 | <10 | 15 |
| Sample | CZnO (wt.%) | First Heating | Second Heating | ||||
|---|---|---|---|---|---|---|---|
| Tm,1 (°C) | Tm,2 (°C) | XC,DSC (%) | Tm,1 (°C) | Tm,2 (°C) | ΔHm (J/g) | ||
| PHBHHx | 0 | 133.6 ± 0.2 | 145.9 ± 0.1 | 55.1 ± 0.0 | 136.9 ± 0.2 | 147.4 ± 0.0 | 51.4 ± 0.1 |
| ZnO(R) | 1 | 134.7 ± 1.2 | 146.6 ± 0.5 | 56.0 ± 0.0 | 137.7 ± 0.0 | 148.1 ± 0.0 | 50.4 ± 0.6 |
| 3 | 135.1 ± 1.4 | 146.8 ± 0.7 | 56.2 ± 1.2 | 138.0 ± 0.4 | 148.2 ± 0.1 | 51.4 ± 0.8 | |
| 5 | 135.7 ± 1.0 | 147.1 ± 0.1 | 56.1 ± 0.1 | 138.3 ± 0.1 | 148.4 ± 0.2 | 50.4 ± 0.5 | |
| ZnO(R-Si) | 1 | 132.8 ± 1.0 | 145.7 ± 0.4 | 53.9 ± 0.0 | 135.7 ± 0.0 | 147.1 ± 0.0 | 50.8 ± 0.2 |
| 3 | 133.1 ± 1.1 | 146.0 ± 0.5 | 54.1 ± 0.5 | 136.2 ± 0.1 | 147.5 ± 0.1 | 50.2 ± 0.8 | |
| 5 | 133.7 ± 0.3 | 146.1 ± 0.2 | 54.3 ± 0.3 | 135.6 ± 0.5 | 147.0 ± 0.1 | 51.1 ± 0.1 | |
| ZnO(R-Si-e) | 1 | 134.7 ± 1.3 | 146.7 ± 0.4 | 54.5 ± 1.5 | 137.9 ± 0.2 | 148.3 ± 0.2 | 50.7 ± 0.5 |
| 3 | 135.0 ± 1.4 | 146.8 ± 0.5 | 53.4 ± 0.7 | 138.2 ± 0.2 | 148.3 ± 0.2 | 49.6 ± 1.6 | |
| 5 | 135.3 ± 1.4 | 146.7 ± 0.7 | 55.5 ± 0.4 | 138.3 ± 0.0 | 148.2 ± 0.0 | 51.5 ± 0.4 | |
| ZnO(S-1) | 1 | 134.1 ± 0.2 | 146.3 ± 0.0 | 55.8 ± 0.5 | 137.8 ± 0.0 | 148.1 ± 0.0 | 51.1 ± 0.2 |
| 3 | 135.2 ± 1.2 | 146.8 ± 0.6 | 55.9 ± 0.0 | 137.9 ± 0.1 | 148.3 ± 0.1 | 50.6 ± 0.2 | |
| 5 | 135.4 ± 1.3 | 146.9 ± 0.5 | 54.4 ± 2.1 | 138.3 ± 0.0 | 148.4 ± 0.0 | 51.1 ± 0.3 | |
| ZnO(S-2) | 1 | 135.5 ± 1.4 | 146.8 ± 0.4 | 56.2 ± 0.5 | 137.7 ± 0.0 | 148.0 ± 0.1 | 49.7 ± 0.7 |
| 3 | 135.3 ± 1.5 | 146.8 ± 0.6 | 55.4 ± 0.7 | 138.0 ± 0.1 | 148.2 ± 0.0 | 50.3 ± 0.1 | |
| 5 | 134.3 ± 0.5 | 146.4 ± 0.2 | 55.7 ± 0.5 | 137.8 ± 0.1 | 148.2 ± 0.0 | 51.5 ± 0.2 | |
| Sample | CZnO (wt.%) | First Cooling | ||||
|---|---|---|---|---|---|---|
| Tc,o (°C) | Tc,p (°C) | ΔHc (J/g) | 1/2 (min) | R (min−1) | ||
| PHBHHx | 0 | 102.1 ± 0.8 | 97.0 ± 0.5 | 47.0 ± 0.4 | 0.990 ± 0.024 | 1.01 ± 0.02 |
| ZnO(R) | 1 | 104.3 ± 0.3 | 98.7 ± 0.0 | 47.5 ± 0.0 | 0.903 ± 0.014 | 1.11 ± 0.01 |
| 3 | 105.2 ± 0.7 | 99.7 ± 1.1 | 48.5 ± 0.7 | 0.858 ± 0.087 | 1.17 ± 0.06 | |
| 5 | 105.9 ± 0.2 | 100.4 ± 0.2 | 48.9 ± 0.3 | 0.832 ± 0.031 | 1.20 ± 0.02 | |
| ZnO(R-Si) | 1 | 99.1 ± 0.0 | 93.6 ± 0.2 | 47.7 ± 0.5 | 1.177 ± 0.014 | 0.85 ± 0.01 |
| 3 | 100.3 ± 0.3 | 94.6 ± 0.2 | 48.2 ± 0.4 | 1.102 ± 0.012 | 0.91 ± 0.00 | |
| 5 | 99.8 ± 0.8 | 92.4 ± 1.8 | 48.3 ± 0.4 | 1.208 ± 0.064 | 0.83 ± 0.02 | |
| ZnO(R-Si-e) | 1 | 104.0 ± 0.3 | 98.7 ± 0.3 | 47.2 ± 0.5 | 0.902 ± 0.017 | 1.11 ± 0.01 |
| 3 | 104.9 ± 0.2 | 99.3 ± 0.4 | 46.8 ± 0.2 | 0.907 ± 0.071 | 1.11 ± 0.04 | |
| 5 | 105.7 ± 0.1 | 100.2 ± 0.3 | 49.2 ± 0.2 | 0.830 ± 0.024 | 1.21 ± 0.02 | |
| ZnO(S-1) | 1 | 104.5 ± 0.4 | 99.0 ± 0.4 | 47.4 ± 0.7 | 0.868 ± 0.031 | 1.15 ± 0.02 |
| 3 | 104.9 ± 0.4 | 99.4 ± 0.3 | 48.1 ± 0.5 | 0.873 ± 0.014 | 1.15 ± 0.01 | |
| 5 | 105.7 ± 0.2 | 100.3 ± 0.2 | 48.0 ± 0.1 | 0.812 ± 0.021 | 1.23 ± 0.02 | |
| ZnO(S-2) | 1 | 104.2 ± 0.0 | 98.4 ± 0.1 | 46.3 ± 0.5 | 0.915 ± 0.017 | 1.09 ± 0.01 |
| 3 | 104.9 ± 0.3 | 99.5 ± 0.2 | 46.7 ± 0.2 | 0.853 ± 0.014 | 1.17 ± 0.01 | |
| 5 | 104.6 ± 0.5 | 99.0 ± 0.4 | 47.6 ± 0.5 | 0.875 ± 0.031 | 1.14 ± 0.02 |
| Sample | CZnO (wt.%) | L* | a* | b* | |
|---|---|---|---|---|---|
| PHBHHx | 0 | 94.4 ± 0.1 | −0.9 ± 0.1 | 4.8 ± 0.4 | / |
| ZnO(R) | 1 | 94.8 ± 0.1 | −1.0 ± 0.1 | 4.9 ± 0.3 | 0.37 |
| 3 | 94.8 ± 0.1 | −1.0 ± 0.0 | 5.9 ± 0.2 | 1.10 | |
| 5 | 94.5 ± 0.1 | −1.1 ± 0.0 | 7.8 ± 0.3 | 3.01 | |
| ZnO(R-Si) | 1 | 95.0 ± 0.1 | −0.8 ± 0.1 | 3.7 ± 0.3 | 1.25 |
| 3 | 95.4 ± 0.1 | −0.9 ± 0.0 | 3.8 ± 0.2 | 1.43 | |
| 5 | 95.3 ± 0.1 | −1.2 ± 0.0 | 5.6 ± 0.3 | 1.13 | |
| ZnO(R-Si-e) | 1 | 94.8 ± 0.1 | −1.0 ± 0.0 | 5.2 ± 0.2 | 0.51 |
| 3 | 95.0 ± 0.1 | −1.1 ± 0.1 | 5.5 ± 0.3 | 0.87 | |
| 5 | 95.0 ± 0.1 | −1.1 ± 0.0 | 5.8 ± 0.2 | 1.15 | |
| ZnO(S-1) | 1 | 94.8 ± 0.1 | −1.1 ± 0.1 | 5.2 ± 0.2 | 0.54 |
| 3 | 94.7 ± 0.1 | −1.1 ± 0.0 | 6.5 ± 0.2 | 1.74 | |
| 5 | 94.6 ± 0.0 | −1.1 ± 0.0 | 7.2 ± 0.2 | 2.36 | |
| ZnO(S-2) | 1 | 95.1 ± 0.1 | −1.1 ± 0.0 | 5.3 ± 0.2 | 0.85 |
| 3 | 95.3 ± 0.1 | −1.1 ± 0.0 | 5.8 ± 0.2 | 1.31 | |
| 5 | 94.7 ± 0.9 | −1.2 ± 0.1 | 7.9 ± 0.4 | 3.06 |
| Sample | ZnO Concentration (wt.%) | PO2 | |
|---|---|---|---|
| (cm³ mm/m² day) | % Change | ||
| PHBHHx | 0 | 3.67 ± 0.11 | Reference |
| ZnO(R) | 5 | 3.20 ± 0.06 | −13 |
| ZnO(R-Si) | 5 | 4.30 ± 0.23 | +17 |
| ZnO(S-2) | 1 | 2.35 ± 0.15 | −36 |
| 3 | 2.84 ± 0.25 | −23 | |
| 5 | 2.71 ± 0.01 | −26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanheusden, C.; Samyn, P.; Vackier, T.; Steenackers, H.; D’Haen, J.; Peeters, R.; Buntinx, M. Fabrication of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/ZnO Nanocomposite Films for Active Packaging Applications: Impact of ZnO Type on Structure–Property Dynamics. Polymers 2024, 16, 1861. https://doi.org/10.3390/polym16131861
Vanheusden C, Samyn P, Vackier T, Steenackers H, D’Haen J, Peeters R, Buntinx M. Fabrication of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/ZnO Nanocomposite Films for Active Packaging Applications: Impact of ZnO Type on Structure–Property Dynamics. Polymers. 2024; 16(13):1861. https://doi.org/10.3390/polym16131861
Chicago/Turabian StyleVanheusden, Chris, Pieter Samyn, Thijs Vackier, Hans Steenackers, Jan D’Haen, Roos Peeters, and Mieke Buntinx. 2024. "Fabrication of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/ZnO Nanocomposite Films for Active Packaging Applications: Impact of ZnO Type on Structure–Property Dynamics" Polymers 16, no. 13: 1861. https://doi.org/10.3390/polym16131861






