Formation of Olive-like TiO2 Nanospheres in a Polymeric Mesh by Sol-Gel Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Olive-like TiO2 Nanospheres
2.2. Characterization of Olive-like TiO2 Nanospheres by FESEM
2.3. Characterization of Olive-like TiO2 Nanospheres by X-ray
2.4. Characterization of Olive-like TiO2 Nanospheres by TGA-DTA-DTG Analysis
2.5. Characterization of Olive-like TiO2 Nanospheres by STM
2.6. Characterization of Olive-like TiO2 Nanospheres by Mountains Lab Software
3. Results and Discussion
3.1. Characterization of Olive-like TiO2 Nanospheres by SEM
3.2. Particle Size Distribution
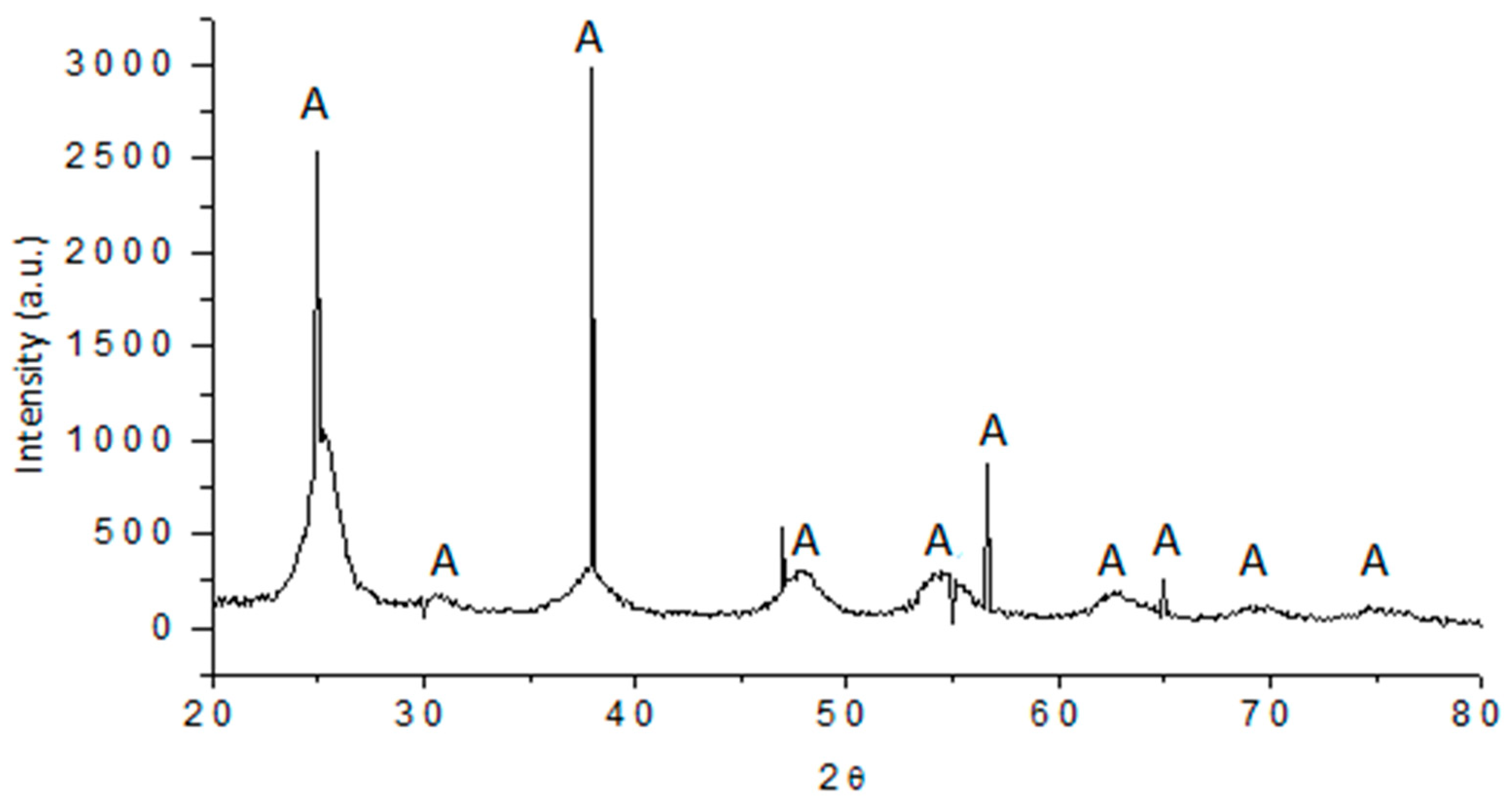
3.3. X-ray Diffraction Pattern
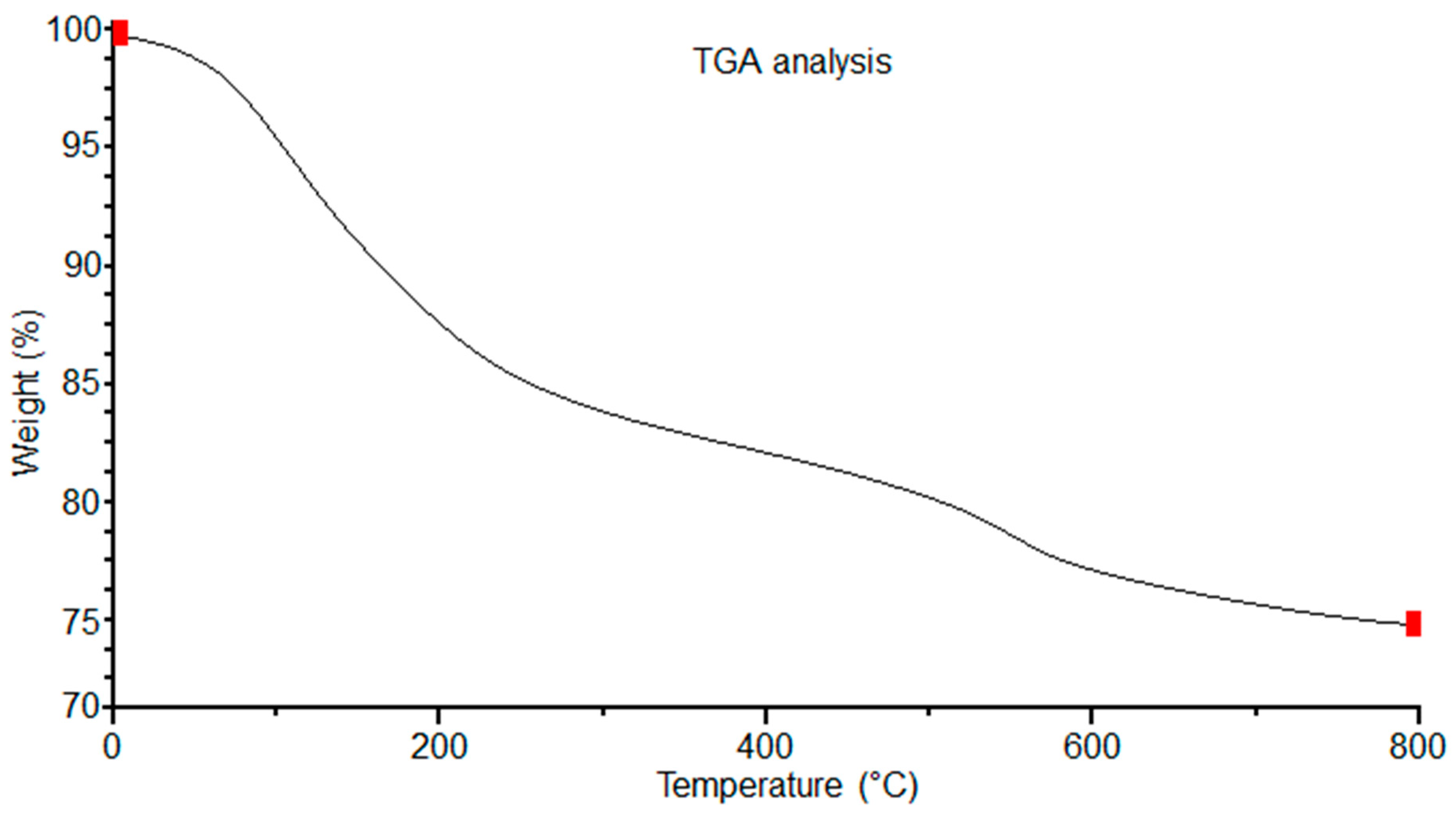
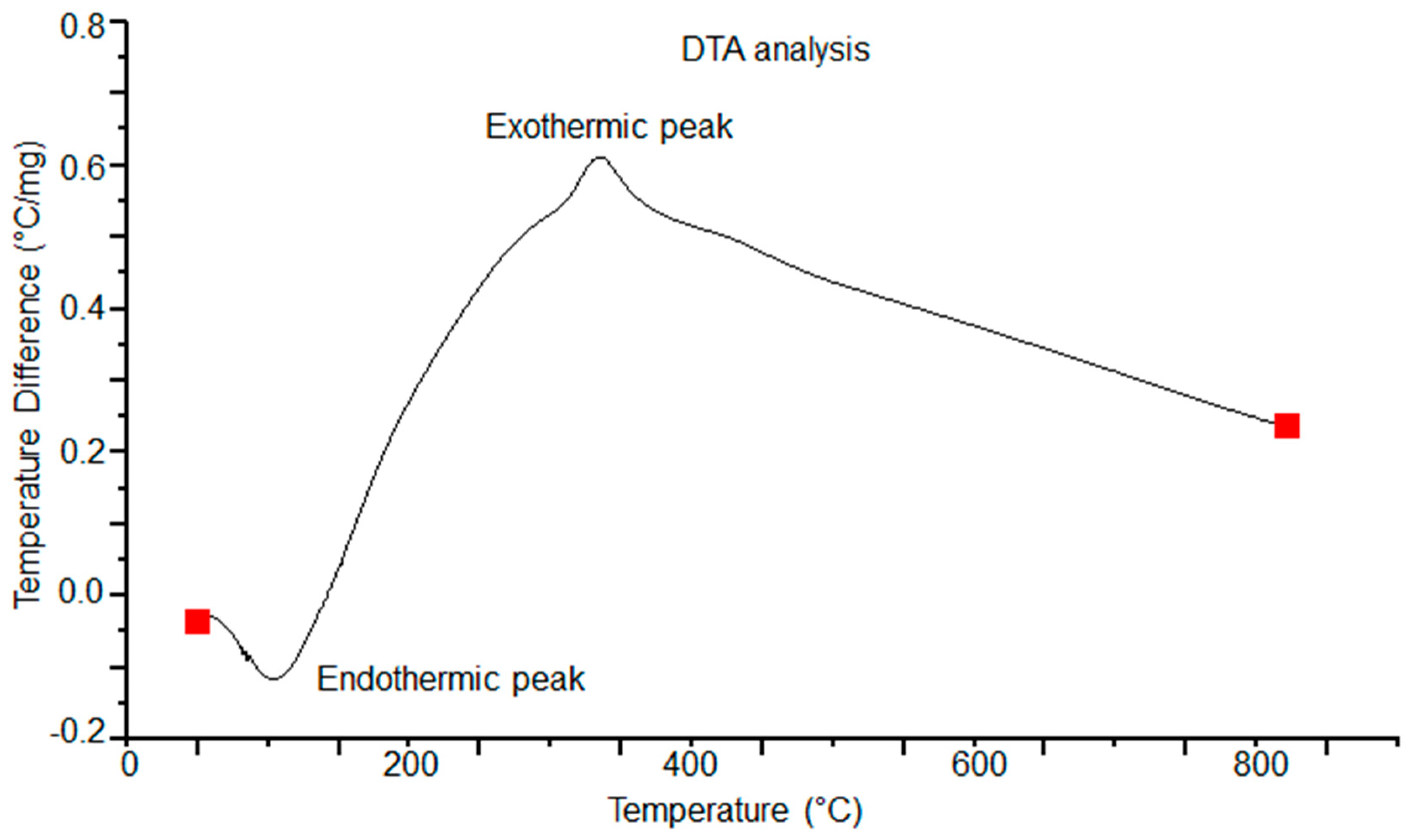
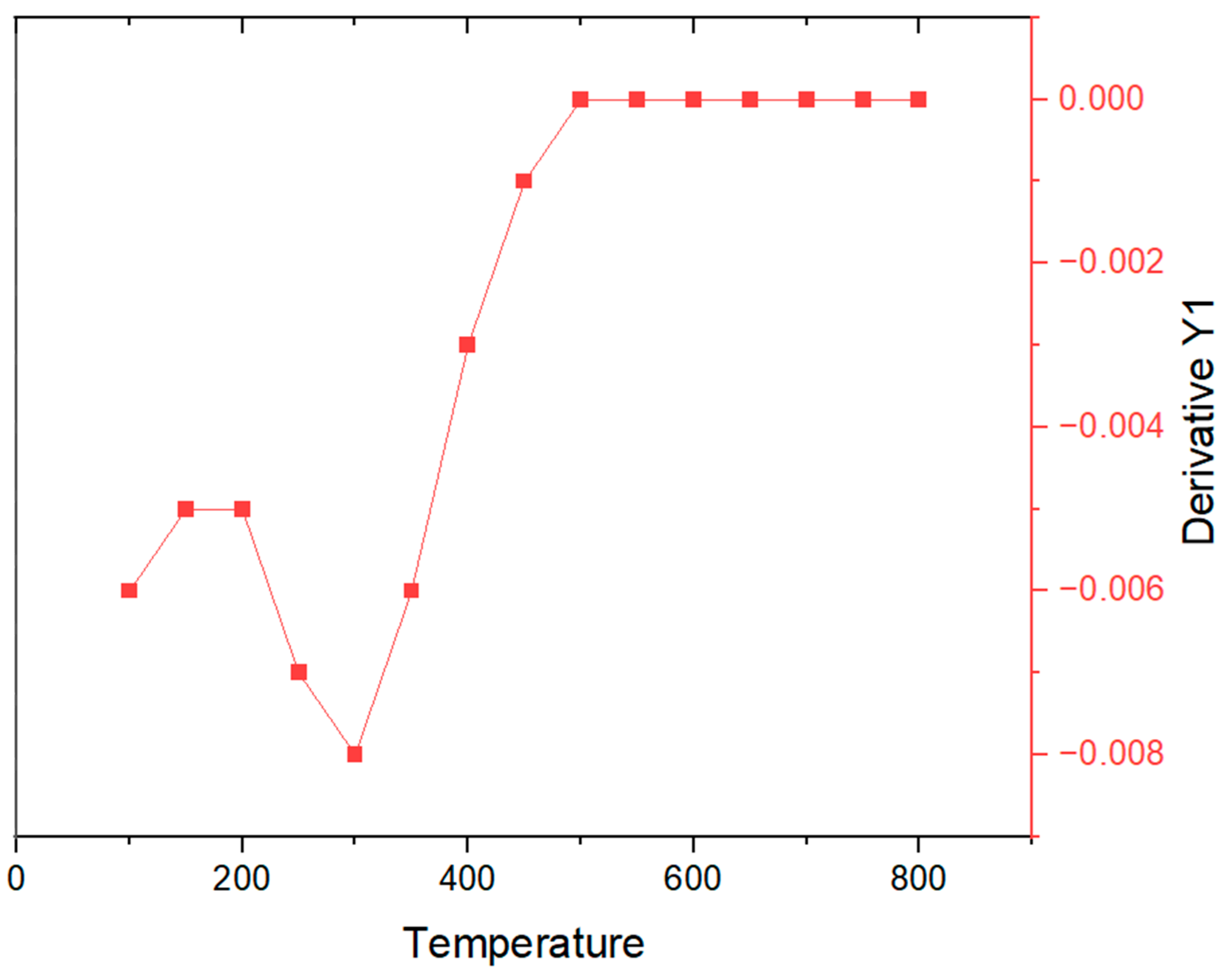
3.4. Thermo-Gravimetric and Differential Thermal Analysis
3.5. Characterization of Olive-like TiO2 Nanospheres by Scanning Tunneling Microscopy (STM)
3.6. Characterization of Olive-like TiO2 Nanospheres by FFT
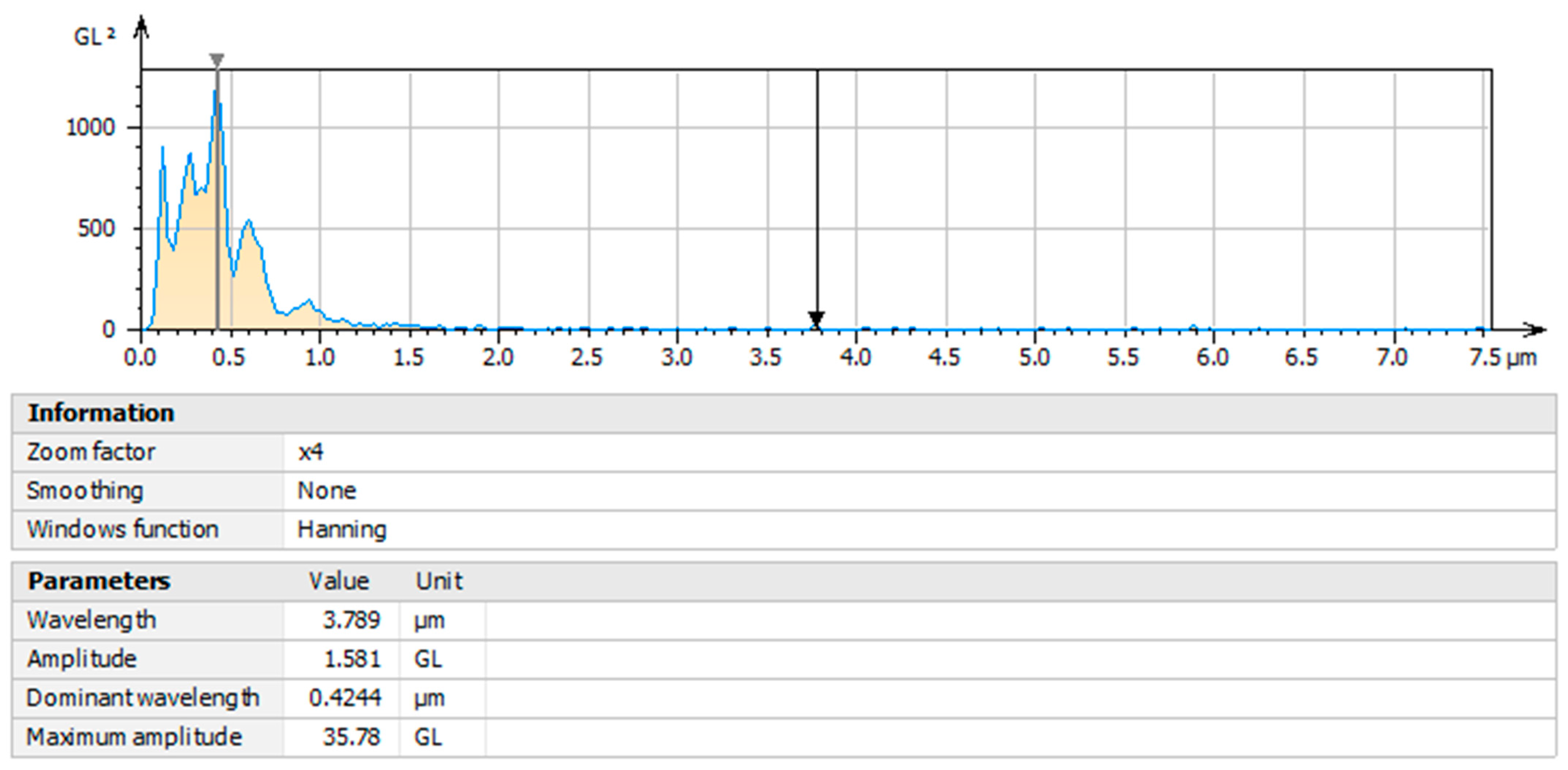
3.7. Characterization by Power Spectral Density (PSD) Analysis of Olive-like TiO2 Nanospheres
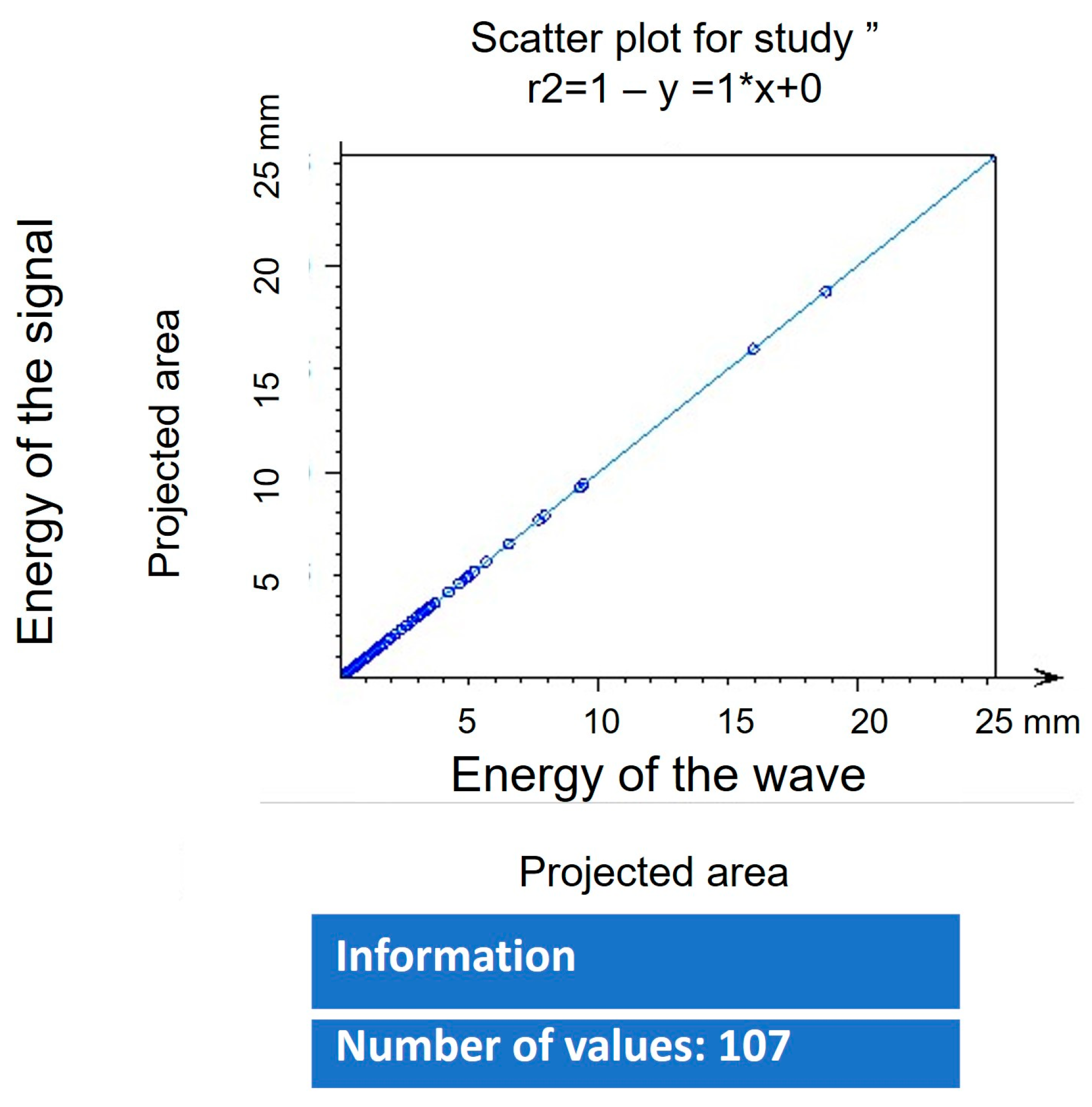
3.8. Fractal Dimension Analysis
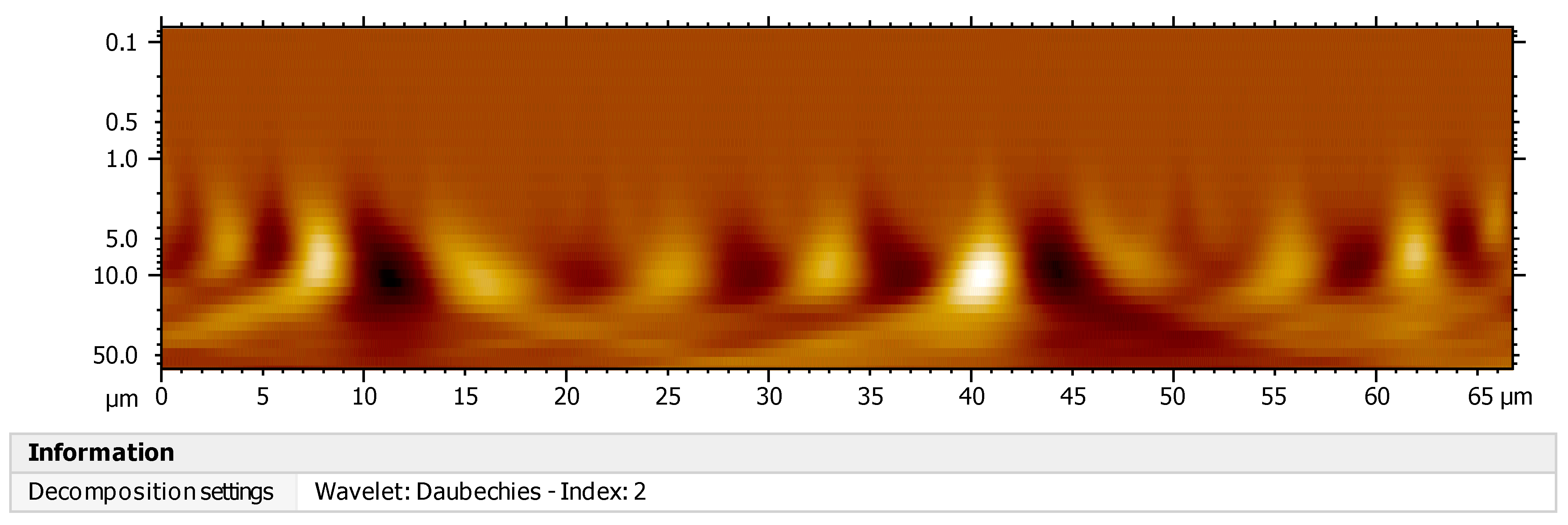
3.9. Characterization by Continuous Wavelet Transform (CWT)
3.10. Characterization of the Profile of the Holes of Olive-like TiO2 Nanospheres
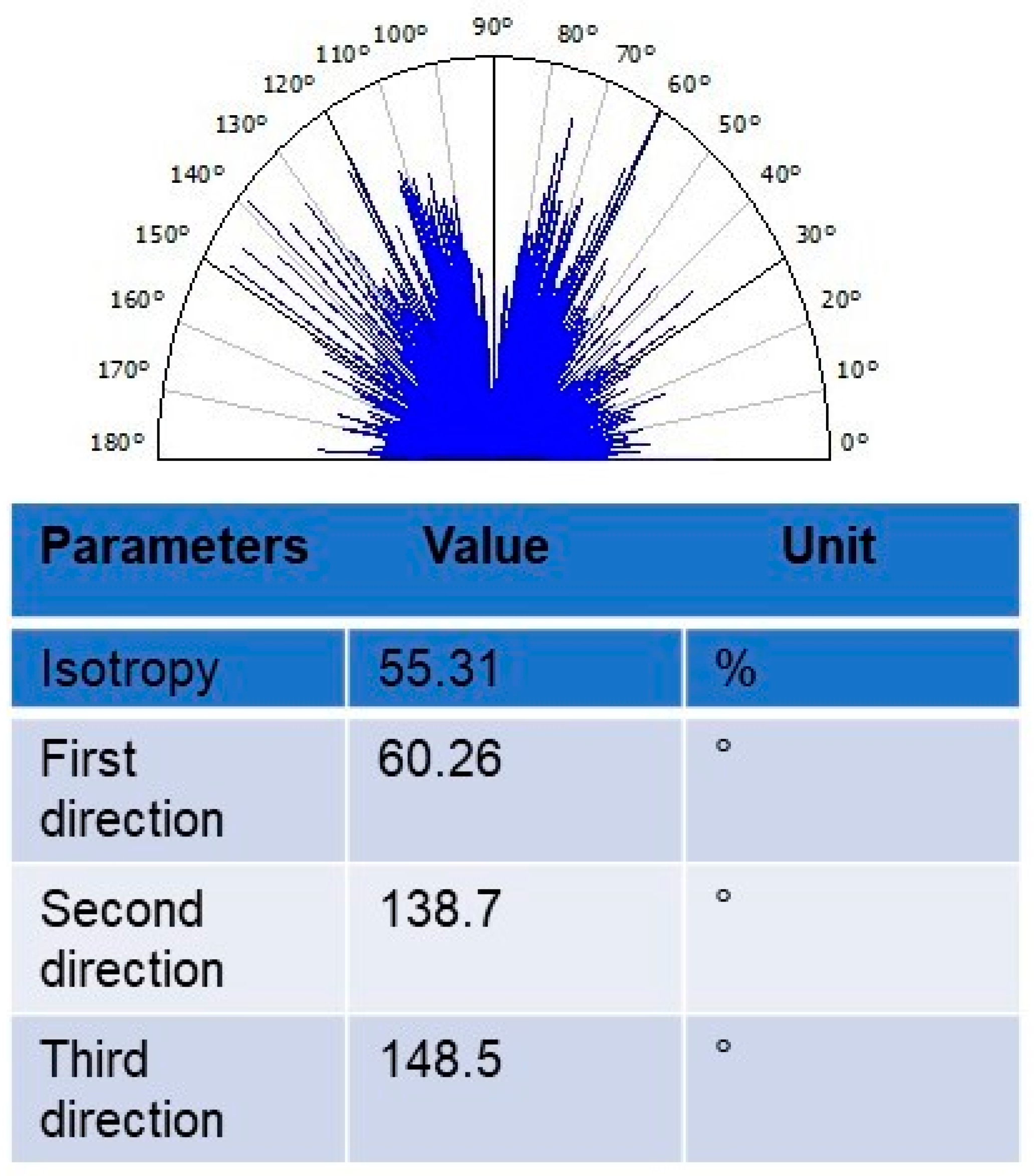
3.11. Texture Isotropy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaison, J.; Ahmed, B.; Yen, S.; Alain, D.; Michael, D. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar]
- Artur, M.; Natalia, Ł.; Maciej, S.; Izabela, M.; Wojciech, S. Electrodeposition of Copper and Brass Coatings with Olive-Like Structure. Materials 2021, 14, 1762. [Google Scholar] [CrossRef]
- Reghunath, S.; Pinheiro, D.; KR, S.D. A review of hierarchical nanostructures of TiO2: Advances and applications. Appl. Surf. Sci. Adv. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Lu, Y.; Gan, Z.; Xia, J.; Du, K.; Peng, Z.; Cao, Y.; Hu, G.; Xiao, J. Hydrothermal Synthesis of Tunable Olive-Like Ni0.8Co0.1Mn0.1CO3 and its Transformation to LiNi0.8Co0.1Mn0.1O2 Cathode Materials for Li-Ion Batteries. ChemElectroChem 2019, 6, 5661–5670. [Google Scholar] [CrossRef]
- Fröschl, T.; Hörmann, U.; Kubiak, P.; Kucerová, G.; Pfanzelt, M.; Weiss, C.; Behm, R.; Hüsing, N.; Kaiser, U.; Landfester, K.; et al. High surface area crystalline titanium dioxide: Potential and limits in electrochemical energy storage and catalysis. Chem. Soc. Rev. 2012, 41, 5313–5360. [Google Scholar] [CrossRef]
- Ye, L.; Zhenping, Q.; Hongxia, G.; Hanxiao, Y.; Guojun, Z.; Shulan, J.; Tingying, Z. Low-temperature synthesis of anatase TiO2 nanoparticles with tunable surface charges for enhancing photocatalytic activity. PLoS ONE 2014, 9, 114638. [Google Scholar]
- Satheesh, J.; Goud, D.; Narsimlu, N. Synthesis and Characterization of TiO2 nanoparticles in PVA Polymer Matrix. J. Appl. Phys. 2021, 13, 50–55. [Google Scholar]
- Yufan, Z.; Fan, F.; Yuzhou, L.; Desuo, Z.; Yuyue, C. One-Step Synthesis of Ag@TiO2 Nanoparticles for Enhanced Photocatalytic Performance. Nanomaterials 2018, 12, 1032. [Google Scholar] [CrossRef]
- Alamgir, M.; Ashis, M.; Nayak, S.; Tiwari, K. Development of PMMA/TiO2 nanocomposites as excellent dental materials. J. Mech. Sci. Technol. 2019, 33, 4755–4760. [Google Scholar] [CrossRef]
- Shalini, A.; Vibhav, S. Synthesis and Thermal Characterization of PMMA-TiO2 Nanocomposites. Mater. Sci. Res. 2014, 11, 168–172. [Google Scholar]
- Tian, Y.; Yang, D.; Wang, Y.; Jiang, Z. Template-free synthesis of TiO2 microcages in agarose gels with improved photocatalytic activity. J. Nanoparticle Res. 2013, 15, 2141. [Google Scholar] [CrossRef]
- Venkatraman, M.; Rama, K.; Muthukumara, S.; Natarajana, A.; Santhanama, V.; Asokan, S.; Dhayalan, V. Size controlled synthesis of TiO2 nanoparticles by modified solvothermal method towards effective photo catalytic and photovoltaic applications. Mater. Res. Bull. 2018, 97, 351–360. [Google Scholar]
- Aravind, M.; Amalanathan, M.; Sony, M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Mohammad, H.; Riccardo, C. Sol-Gel Synthesis of TiO2 Nanocrystalline Particles with Enhanced Surface Area through the Reverse Micelle Approach. Adv. Mater. Sci. Eng. 2019, 2019, 1567824. [Google Scholar]
- Takeshi, S.; Takafumi, O.; Hiroki, N.; Koji, N.; Masato, M.; Bartolome, S.; Ken, K.; Masafumi, Y.; Hiroki, T.; Takao, S.; et al. Development of Nanostructured Water Treatment Membranes Based on Thermotropic Liquid Crystals: Molecular Design of Sub-Nanoporous Materials. Adv. Sci. 2018, 5, 1700405. [Google Scholar]
- Meagan, S.; Menachem, E.; Chinedum, O. Nanocomposites of vertically aligned single-walled carbon nanotubes by magnetic alignment and polymerization of a lyotropic precursor. ACS Nano 2010, 4, 6651–6658. [Google Scholar]
- Zeynep, A.; Iva, C.; Gabriella, D.; Sabine, P.; Soccorso, G.; Ibtisam, E. Development of functionalized nanostructured polymeric membranes for water purification. Chem. Eng. J. 2016, 300, 358–366. [Google Scholar]
- Antonio, G.; Mani, D.; Monique, K.; John, A.; Jeroen, J.; Fang, Y. Development of a PCL-silica nanoparticles composite membrane for Guided Bone Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 85, 154–161. [Google Scholar]
- Dina, E.; Norhan, A.; MhdAmmar, H.; Abdelbaki, B.; Alaa, H. Nanoparticles functionalized ceramic membranes: Fabrication, surface modification, and performance. Environ. Sci. Pollut. Res. 2021, 10, 12256–12281. [Google Scholar]
- Meyers, M.; McKittrick, P.Y.; Chen, P. Structural biological materials: Critical mechanics-materials connections. Science 2013, 339, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Wegst, U.; Bai, H.; Saiz, E.; Tomsia, A.; Ritchie, R. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Eder, M.; Amini, S.; Fratzl, S. Biological composites-complex structures for functional diversity. Science 2018, 362, 543–547. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, D.; Shi, Y.; Jiang, Z. Bio-inspired synthesis of TiO2 hollow nanospheres in agarose gels. J. Alloys Compd. 2013, 560, 42–48. [Google Scholar] [CrossRef]
- Saba, H.; Masoud, J. Synthesis of TiO2 nanoparticles coated on cellulose nanofibers with different morphologies: Effect of the template and sol-gel parameters. Mater. Sci. Semicond. Process. 2020, 109, 104927. [Google Scholar]
- Gao, M.; Li, L.; Liu, Q.; Xue, Y.; Wang, W.; Zhao, Y.; Zou, X. Synthesis of multifunctional silica composite nanospheres and their application in separation of MBP-tagged protein. Mater. Lett. 2022, 318, 132–222. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Cao, L.; Yonghui, W.; Zhao, Y. Synthesis of silica nanospheres with Ni2+-iminodiacetic acid for protein separation. J. Sol-Gel Sci. Technol. 2014, 71, 80–384. [Google Scholar]
- Gonzalez-Reyna, M.; Rodriguez-Lopez, A.; Pérez-Robles, J.F. One-step synthesis of carbon nanospheres with an encapsulated iron-nickel nanoalloy and its potential use as an electrocatalyst. Nanotechnology 2020, 32, 095706. [Google Scholar] [CrossRef]
- Feng, Y.; Liao, J.; Chen, X.; Wang, H.; Guo, B.; Li, H.; Zhou, L.; Huang, J.; Li, H. Synthesis of rattle-structured CuCo2O4 nanospheres with tunable sizes based on heterogeneous contraction and their ultrahigh performance toward ammonia borane hydrolysis. J. Alloys Compd. 2021, 863, 158089. [Google Scholar] [CrossRef]
- Paques, J.P.; van der Linden, E.; van Rijn, C.J.; Sagis, L.M. Preparation methods of alginate nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Xu, H.; Meng, X.; Han, Q. Two-phase synthesis of olive-like NiS particles and chain-like Bi2S3 nanowires. Mater. Sci.-Pol. 2015, 33, 1–5. [Google Scholar] [CrossRef]
- Ghosh, O.S.N.; Gayathri, S.; Alagarasan, D.; Kumar, K.V.P.; Viswanath, A.K. One pot transparent sol-gel synthesis of TiO2 nanospheres with pristine anatase phase and their physicochemical characteristics. Appl. Sci. Lett. 2016, 2, 23–30. [Google Scholar] [CrossRef]
- Urbano, M.A.V.; Vargas, A.; Muñoz, Y.H.O.; Ochoa, H.; Fernandez, Y.O.; Mosquera, P.; Paez, J.E.R.; Rodríguez, E.; Amado, R.J.C.; Camargo, J. Nanoparticles of TiO2, anatase phase, synthesized by chemical methods. Ing. Des. 2011, 29, 186–201. [Google Scholar]
- Gowthambabu, V.; Deshpande, M.; Govindaraj, R.; Nithesh Krishna, V.K.; Leela Charumathi, M.; Manish Kumar, J.; Dhilip Vignesh, M.S.; Isaac Daniel, R.; Ramasamy, P. Synthesis of anatase TiO2 microspheres and their efficient performance in dye-sensitized solar cell. J. Mater. Sci. Mater. Electron. 2021, 32, 26306–26317. [Google Scholar] [CrossRef]
- Chen, H.; Wu, T.; Li, X.; Lu, S.; Zhang, F.; Wang, Y.; Zhao, H.; Liu, Q.; Luo, Y.; Asiri, A.M.; et al. Modulating Oxygen Vacancies of TiO2 Nanospheres by Mn-Doping to Boost Electrocatalytic N2 Reduction. ACS Sustain. Chem. Eng. 2021, 9, 1512–1517. [Google Scholar] [CrossRef]
- Scheiber, P.; Fidler, M.; Dulub, O.; Schmid, M.; Diebold, U.; Hou, W.; Aschauer, U.; Selloni, A. (Sub)surface mobility of oxygen vacancies at the TiO2 anatase (101) surface. Phys. Rev. Lett. 2012, 109, 136103. [Google Scholar] [CrossRef]
- Arenas-Hernandez, A.; Zuñiga Islas, C.; Moreno, M.; Calleja Arriaga, W.; Mendoza-Cervantes, J.C.; Carlos, N.; Ascencio-Hurtado, C.R.; Heredia Jiménez, A. Study of Oxygen Vacancies in TiO2 Nanostructures and Their Relationship with Photocatalytic Activity. Appl. Sci. 2022, 12, 3690. [Google Scholar] [CrossRef]
- Emanuele, C.; Giulio, B.; Giuseppe, C.; Damiano, A.; Pasqualantonio, P.; Gabriele, F.; Luca, G. Aggregation and fractal formation of Au and TiO2 nanostructures obtained by fs-pulsed laser deposition: Experiment and simulation. J. Nanopart. Res. 2017, 19, 311. [Google Scholar]
- Cavaliere, E.; Ferrini, G.; Pingue, P.; Gavioli, L. Fractal TiO2 nanostructures by non-thermal laser ablation at ambient pressure. J. Phys. Chem. C 2013, 117, 23305–23312. [Google Scholar] [CrossRef]
- Celardo, G.; Archetti, D.; Ferrini, G.; Gavioli, L.; Pingue, P.; Cavaliere, E. Evidence of diffusive fractal aggregation of TiO2 nanoparticles by femtosecond laser ablation at ambient conditions. Mater. Res. Express 2017, 4, 015013. [Google Scholar] [CrossRef]
- Lavisse, L.; Le, G.; Hallo, L.; Jouvard, J.; Carles, S.; Perez, J.; Mitchell, J.; Decloux, J.; Girault, M.; Potin, V.; et al. In-situ small-angle X-ray scattering study of nanoparticles in the plasma plume induced by pulsed laser irradiation of metallic targets. Appl. Phys. Lett. 2012, 16, 164103. [Google Scholar] [CrossRef]
- Dobrescu, G.; Papa, F.; State, R.; Ballint, I. Characterization of bimetallic nanoparticles by fractal analysis. Powder Technol. 2018, 338, 905–914. [Google Scholar] [CrossRef]
- Lin, Y.; Rui, X.; Xue, X.; Li, P.; Wen, X. Wavelet analysis of the surface morphologic of nanocrystalline TiO2 thin films. Surf. Sci. 2005, 579, 37–46. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, E. Effectiveness of the continuous wavelet transform in the analysis of some dispersive elastic waves. J. Acoust. Soc. Am. 2001, 110, 86–94. [Google Scholar] [CrossRef]
- Tkachenko, I.; Kozhanova, E.; Belyaev, E.; Yazbeck, H. Comparative analysis of application of wavelet analysis for the recognition of element composition nanostructures. J. Phys. Conf. Ser. 2019, 1309, 012020. [Google Scholar] [CrossRef]
- Justh, N.; Bakos, L.P.; Hernádi, K.; Kiss, G.; Réti, B.; Erdélyi, Z.; Parditka, B.; Szilágyi, I.M. Photocatalytic hollow TiO2 and ZnO nanospheres prepared by atomic layer deposition. Sci. Rep. 2017, 7, 4337. [Google Scholar] [CrossRef]
- Ghaderi, A.; Shafiekhani, A.; Solaymani, S.; Ţălu, Ş.; da Fonseca Filho, H.D.; Ferreira, N.S.; Matos, R.S.; Zahrabi, H.; Dejam, L. Advanced microstructure, morphology and CO gas sensor properties of Cu/Ni bilayers at nanoscale. Sci. Rep. 2022, 12, 12002. [Google Scholar] [CrossRef]
- Josso, B.; Burton, D.R.; Lalor, M.J. Texture orientation and anisotropy calculation by Fourier transform and Principal Component Analysis. Mech. Syst. Signal Process. 2005, 19, 1152–1161. [Google Scholar] [CrossRef]
- Hu, W.; Li, H.; Wang, C.; Gou, S.; Fu, L. Characterization of collagen fibers by means of texture analysis of second harmonic generation images using orientation-dependent gray level co-occurrence matrix method. J. Biomed. Opt. 2012, 17, 026007. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Velay-Lizancos, M.; Weaver, C.J.; Athamneh, A.I.; Zavattieri, P.D.; Suter, D.M.; Raman, A. Anisotropy vs isotropy in living cell indentation with AFM. Sci. Rep. 2019, 9, 5757. [Google Scholar] [CrossRef]
- Jiang, H.; He, X.; Ma, Y.; Fu, B.; Xu, X.; Subramanian, B.; Hu, C. Isotropic Hedgehog-Shaped-TiO2/Functional-Multiwall-Carbon Nanotube Micromotors with Phototactic Motility in Fuel-Free Environments. ACS Appl. Mater. Interfaces 2021, 13, 5406–5417. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Melendez, C.; Monreal Romero, H.A.; Carreño-Gallardo, C.; Martinez Mata, G.; Pacheco Santiesteban, R.; Pérez Piñon, T.; Pérez Piñon, D.; López Aguilar, H.A.; Estrada Macias, M.E.; Chacón-Nava, J.G. Formation of Olive-like TiO2 Nanospheres in a Polymeric Mesh by Sol-Gel Method. Polymers 2024, 16, 1875. https://doi.org/10.3390/polym16131875
López Melendez C, Monreal Romero HA, Carreño-Gallardo C, Martinez Mata G, Pacheco Santiesteban R, Pérez Piñon T, Pérez Piñon D, López Aguilar HA, Estrada Macias ME, Chacón-Nava JG. Formation of Olive-like TiO2 Nanospheres in a Polymeric Mesh by Sol-Gel Method. Polymers. 2024; 16(13):1875. https://doi.org/10.3390/polym16131875
Chicago/Turabian StyleLópez Melendez, Claudia, Humberto Alejandro Monreal Romero, Caleb Carreño-Gallardo, Guillermo Martinez Mata, Rosaura Pacheco Santiesteban, Teresa Pérez Piñon, Dagoberto Pérez Piñon, Héctor Alfredo López Aguilar, Marvin Elco Estrada Macias, and José Guadalupe Chacón-Nava. 2024. "Formation of Olive-like TiO2 Nanospheres in a Polymeric Mesh by Sol-Gel Method" Polymers 16, no. 13: 1875. https://doi.org/10.3390/polym16131875
APA StyleLópez Melendez, C., Monreal Romero, H. A., Carreño-Gallardo, C., Martinez Mata, G., Pacheco Santiesteban, R., Pérez Piñon, T., Pérez Piñon, D., López Aguilar, H. A., Estrada Macias, M. E., & Chacón-Nava, J. G. (2024). Formation of Olive-like TiO2 Nanospheres in a Polymeric Mesh by Sol-Gel Method. Polymers, 16(13), 1875. https://doi.org/10.3390/polym16131875












