Influence of Reduced Graphene Oxide and Carbon Nanotubes on the Structural, Electrical, and Photoluminescent Properties of Chitosan Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Films Preparation
2.3. UV–Vis Spectroscopy
2.4. Films Transparency
2.5. Fourier Transform Infrared Spectroscopy
2.6. Films Thickness
2.7. Color Analysis
2.8. Ionic Exchange Capacity (IEC)
2.9. Swelling and Solubility
2.10. XRD Analysis
2.11. Mechanical Characterization
2.12. X-ray Photoelectron Spectroscopy
2.13. SEM Analysis
2.14. Resistivity Measurements
2.15. Photoluminescence
3. Results
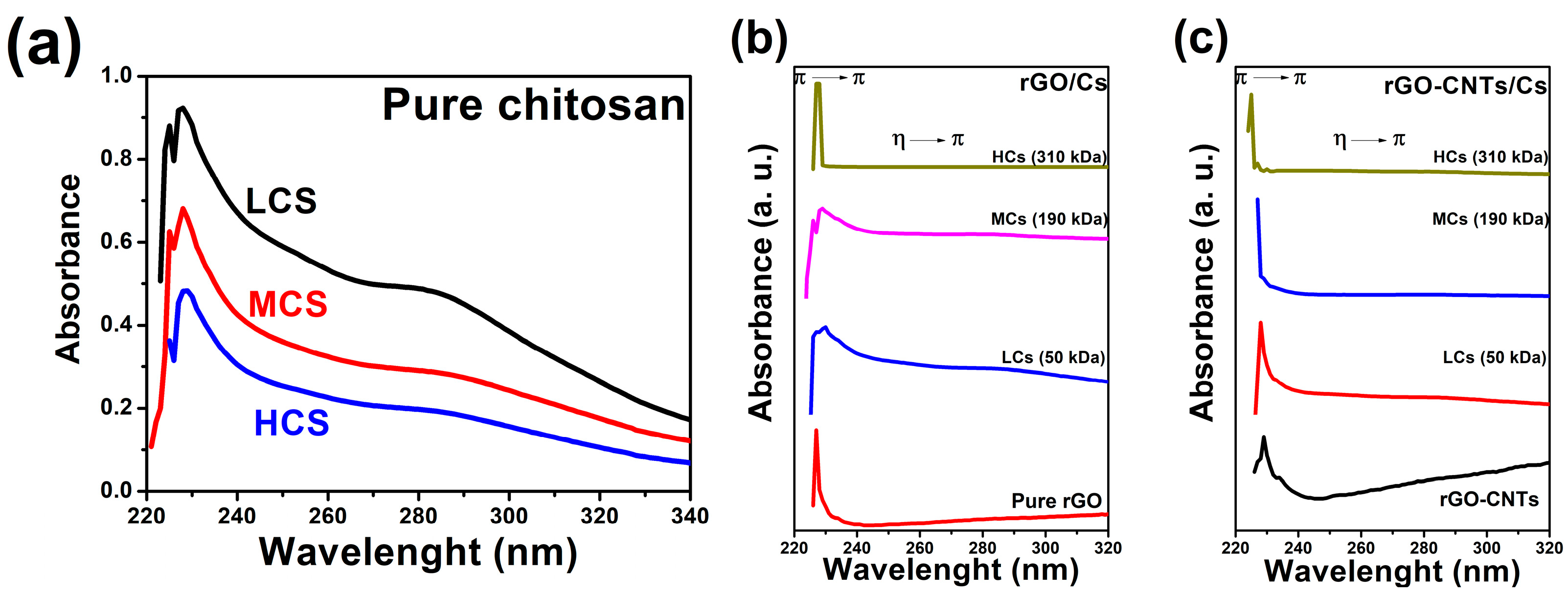
3.1. UV–Vis Analysis
3.2. FTIR Spectroscopy
3.3. Films Thickness
3.4. Color and Opacity
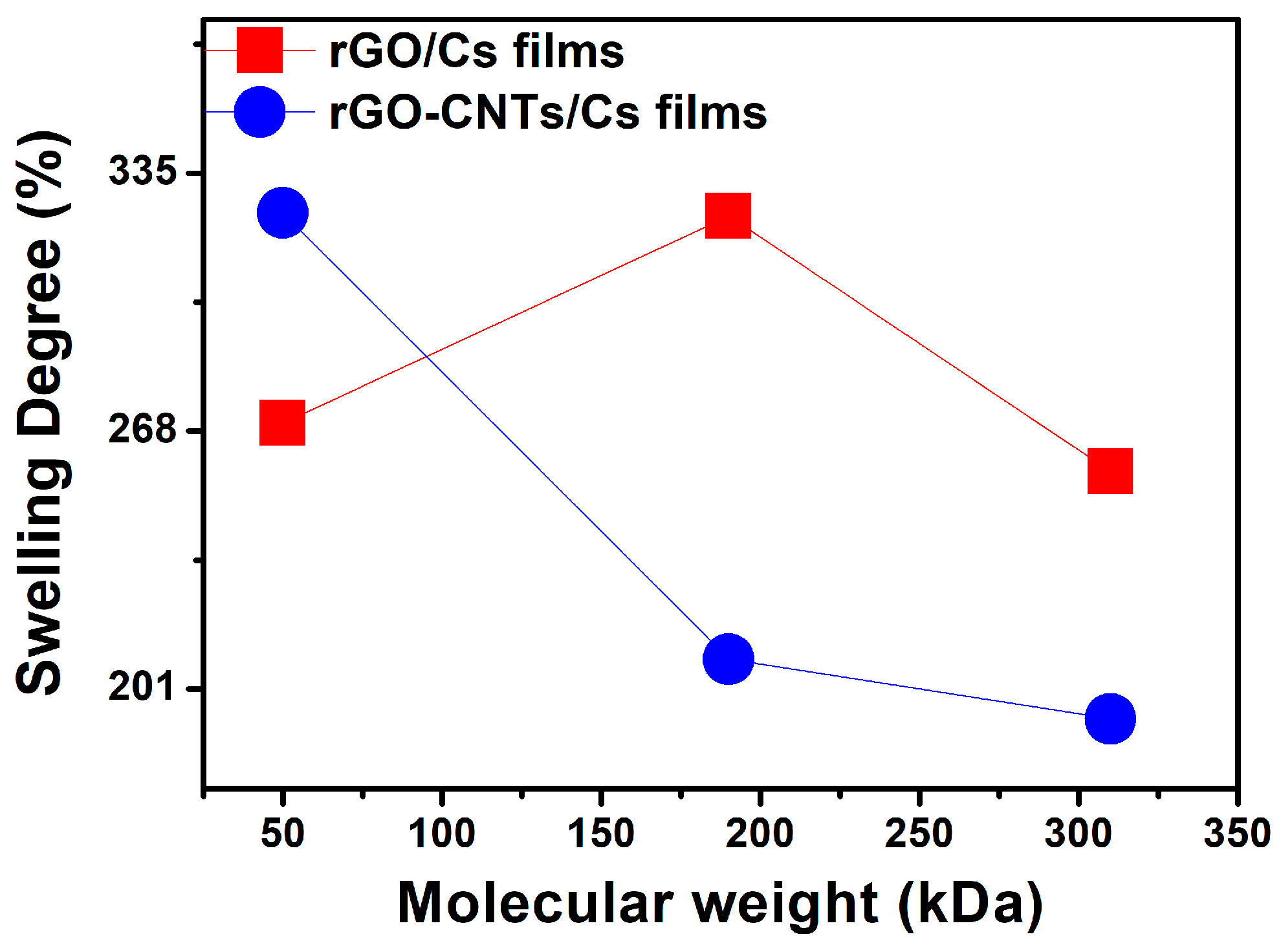
3.5. Ion Exchange Capacity and Swelling Degree
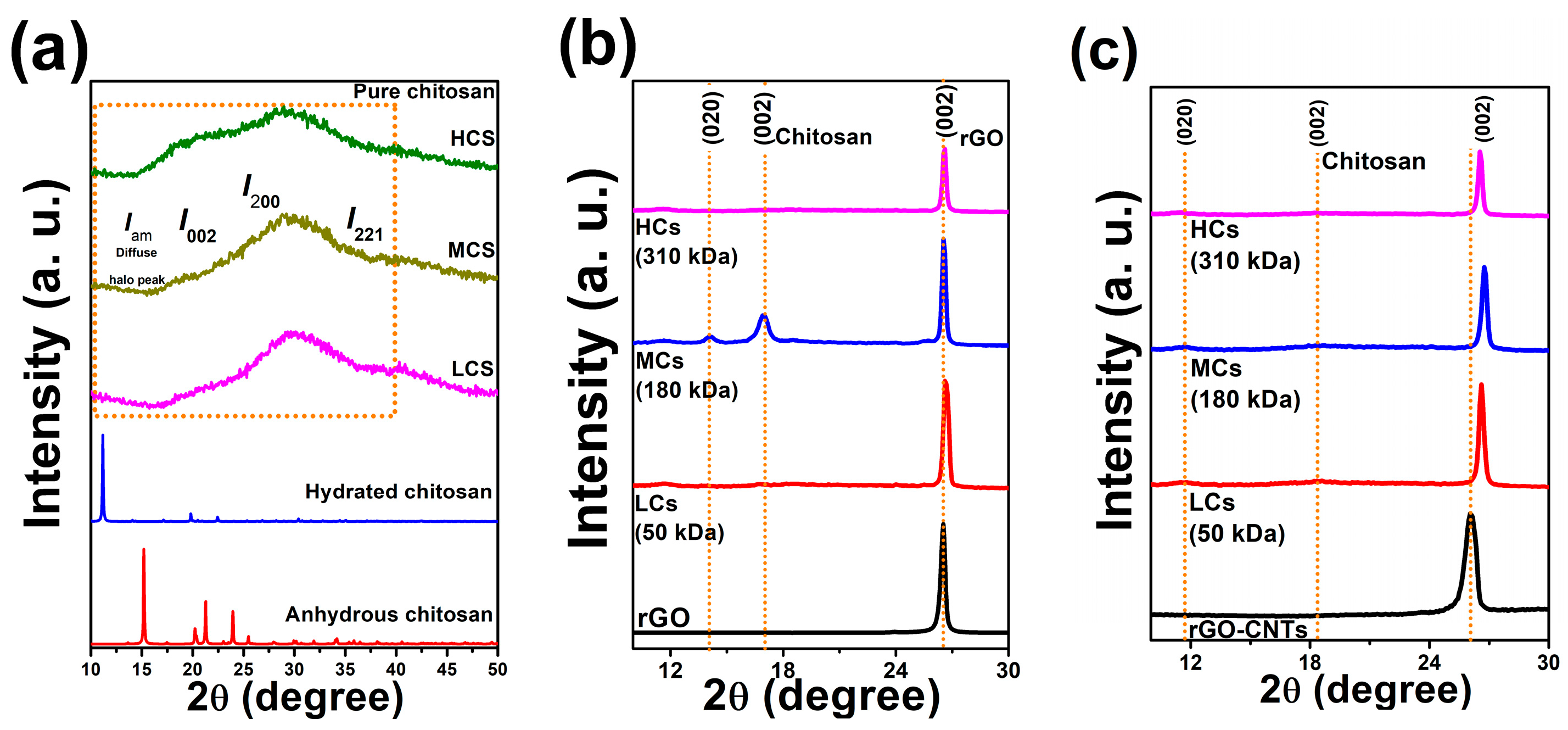
3.6. XRD Diffraction Analysis
3.7. Mechanical Properties
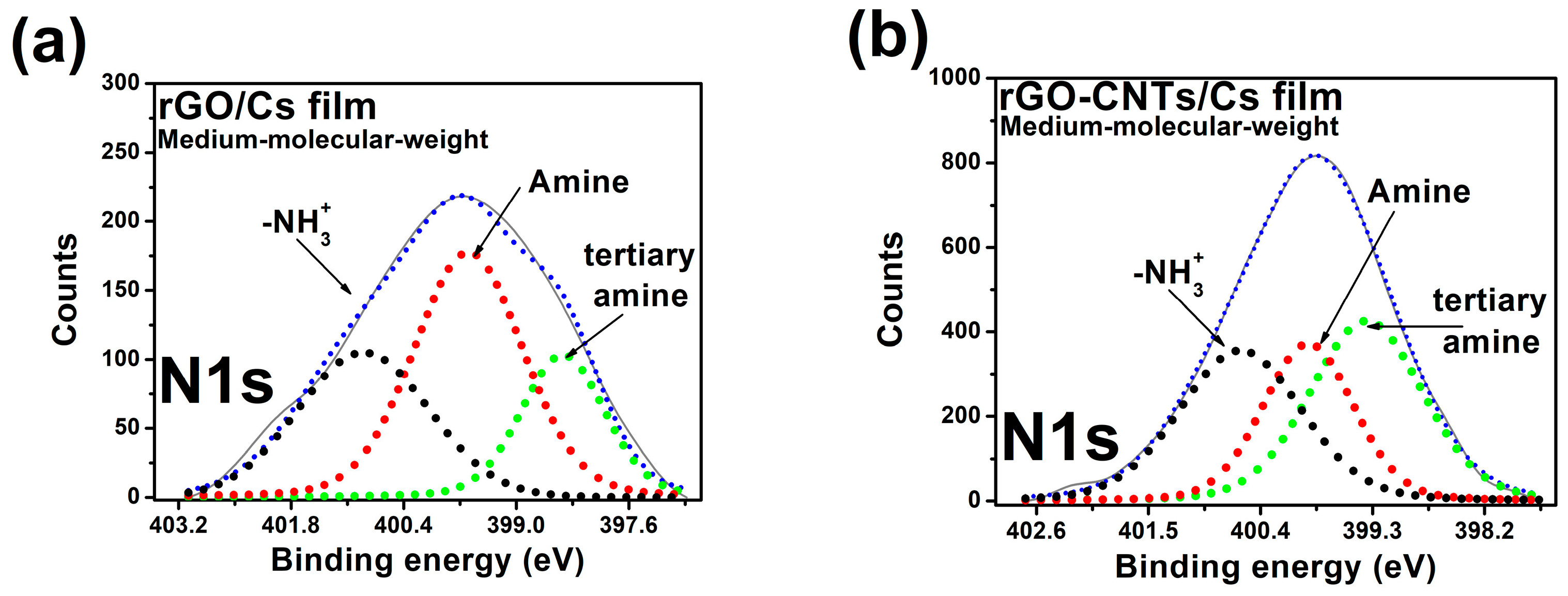
3.8. X-ray Photoelectron Spectroscopy (XPS)
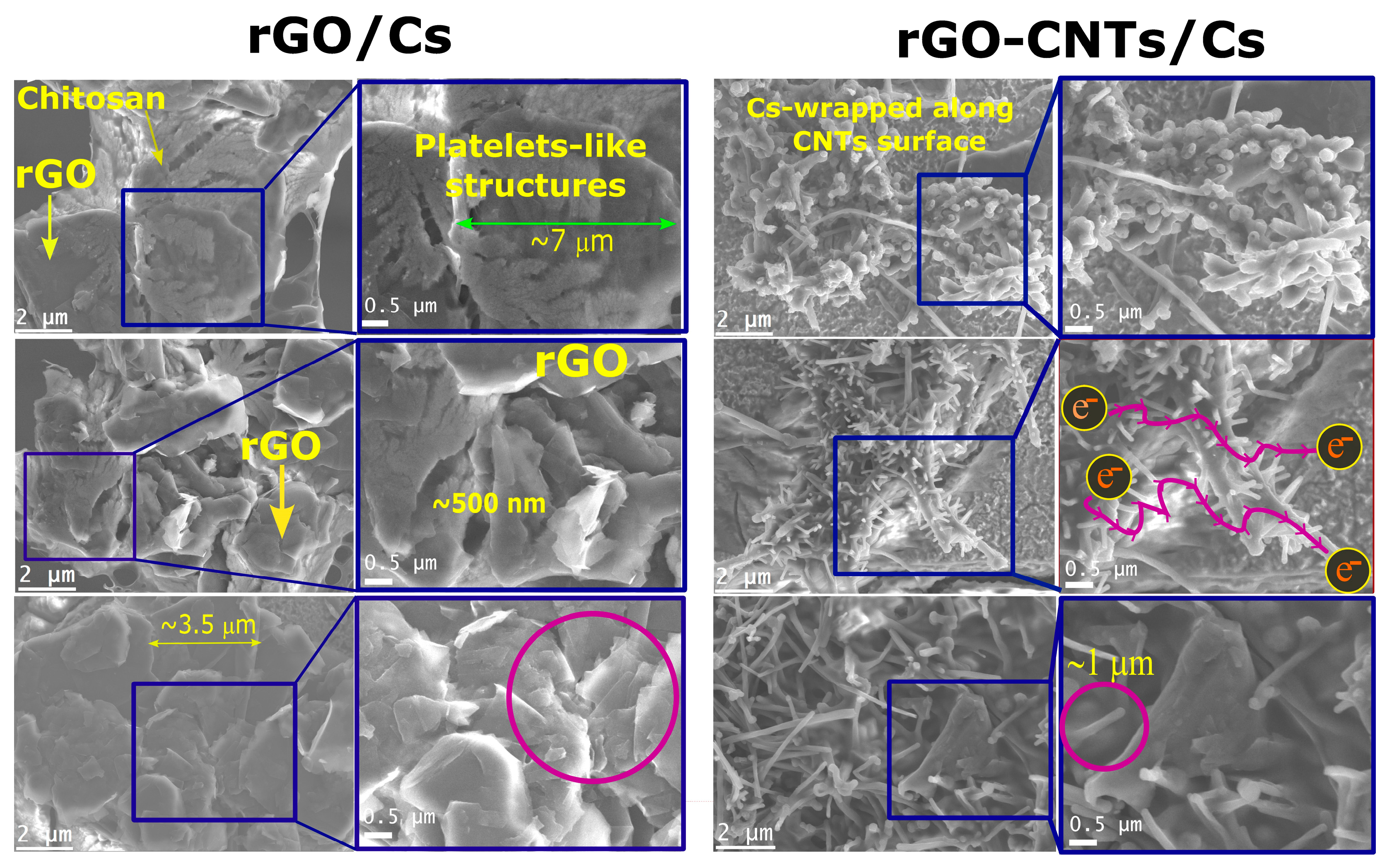
3.9. SEM Analysis
3.10. Resistivity Measurements
3.11. Photoluminescence Measurement (PL)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santiago-Castillo, K.; Del Angel-López, D.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Willcock, H.; Brachetti-Sibaja, S.B. Effect on the processability, structure and mechanical properties of highly dispersed in situ ZnO:CS nanoparticles into PVA electrospun fibers. J. Mater. Res. Technol. 2021, 11, 929–945. [Google Scholar] [CrossRef]
- Santiago-Castillo, K.; Torres-Huerta, A.M.; del Ángel-López, D.; Domínguez-Crespo, M.A.; Dorantes-Rosales, H.; Palma-Ramírez, D.; Willcock, H. In Situ Growth of Silver Nanoparticles on Chitosan Matrix for the Synthesis of Hybrid Electrospun Fibers: Analysis of Microstructural and Mechanical Properties. Polymers 2022, 14, 674. [Google Scholar] [CrossRef] [PubMed]
- Nisha, U.M.; Meeran, M.N.; Sethupathi, R.; Subha, V.; Rajeswaran, P.; Sivakarthik, P. Studies on Chitosan modified ZrO2–CeO2 metal oxide and their Photo catalytic activity under solar light irradiation for water remediation. J. Indian Chem. Soc. 2023, 100, 101061. [Google Scholar] [CrossRef]
- Dinakaran, K.; Senthamilselvi, S.; Gayathri, T.; Kavitha, N.; Roy, D. Enhanced electromagnetic interference shielding in nickel oxide and graphene composite nanostructures loaded polyvinylidene difluoride thin films. J. Indian Chem. Soc. 2023, 100, 101091. [Google Scholar] [CrossRef]
- Buinov, A.S.; Gafarova, E.R.; Grebenik, E.A.; Bardakova, K.N.; Kholkhoev, B.C.; Veryasova, N.N.; Nikitin, P.V.; Kosheleva, N.V.; Shavkuta, B.S.; Kuryanova, A.S.; et al. Fabrication of Conductive Tissue Engineering Nanocomposite Films Based on Chitosan and Surfactant-Stabilized Graphene Dispersions. Polymers 2022, 14, 3792. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Labbaf, S.; Karimzadeh, F. A conductive film of chitosan-polycaprolcatone-polypyrrole with potential in heart patch application. Polym. Test. 2019, 75, 254–261. [Google Scholar] [CrossRef]
- Macias-Almazan, A.; Lois-Correa, J.A.; Dominguez-Crespo, M.A.; Lopez-Oyama, A.B.; Torres-Huerta, A.M.; Brachetti-Sibaja, S.B.; Rodriguez-Salazar, A.E. Influence of operating conditions on proton conductivity of nanocellulose films using two agroindustrial wastes: Sugarcane bagasse and pinewood sawdust. Carbohydr. Polym. 2020, 238, 116171. [Google Scholar] [CrossRef]
- Pulido-Barragán, E.U.; Morales-Cepeda, A.B.; Castro-Guerrero, C.F.; Koschella, A.; Heinze, T. Upgrading Euphorbia Antisyphilitica fiber compost: A waste material turned into nanocrystalline cellulose. Ind. Crops Prod. 2020, 160, 113111. [Google Scholar] [CrossRef]
- Barragán, E.U.P.; Guerrero, C.F.C.; Zamudio, A.M.; Cepeda, A.B.M.; Heinze, T.; Koschella, A. Isolation of Cellulose Nanocrystals from Typha domingensis Named Southern Cattail Using a Batch Reactor. Fibers Polym. 2019, 20, 1136–1144. [Google Scholar] [CrossRef]
- Umnyakova, E.S.; Zharkova, M.S.; Berlov, M.N.; Shamova, O.V.; Kokryakov, V.N. Human antimicrobial peptides in autoimmunity. Autoimmunity 2020, 53, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Jiang, Y.; Ren, H.; Deng, S.; Sun, J.; Cheng, F.; Jing, J.; Chen, Y. 3D-Printed Carbon-Based Conformal Electromagnetic Interference Shielding Module for Integrated Electronics. Nano-Micro Lett. 2024, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Crespo, M.A.; Rodriguez, E.; Torres-Huerta, A.M.; Soni-Castro, I.J.; Brachetti-Sibaja, S.B.; Narro-Garcia, R.; Lopez-Oyama, A.B. Production of BN nanostructures by pulsed laser ablation in liquids: Influence of the applied Nd:YAG harmonics on the structural, optical and photoluminescence properties. Ceram. Int. 2020, 46, 21667–21680. [Google Scholar] [CrossRef]
- López-Oyama, A.B.; Silva-Molina, R.A.; Ruíz-García, J.; Gámez-Corrales, R.; Guirado-López, R.A. Structure, electronic properties, and aggregation behavior of hydroxylated carbon nanotubes. J. Chem. Phys. 2014, 141, 174703. [Google Scholar] [CrossRef] [PubMed]
- López-Oyama, A.B.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Onofre-Bustamante, E.; Gámez-Corrales, R.; Cayetano-Castro, N. Electrochemical alternative to obtain reduced graphene oxide by pulse potential: Effect of synthesis parameters and study of corrosion properties. Diam. Relat. Mater. 2018, 88, 167–188. [Google Scholar] [CrossRef]
- López-Oyama, A.B.; Domínguez-Crespo, M.A.; Torres-Huerta, A.M.; Onofre-Bustamante, E.; Gámez-Corrales, R.; Cayetano-Castro, N.; Ferrel-Álvarez, A.C. Dataset on electrochemical reduced graphene oxide production: Effect of synthesis parameters. Data Brief 2018, 21, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Barra, A.; Ferreira, N.M.; Martins, M.A.; Lazar, O.; Pantazi, A.; Jderu, A.A.; Neumayer, S.M.; Rodriguez, B.J.; Enăchescu, M.; Ferreira, P.; et al. Eco-friendly preparation of electrically conductive chitosan-reduced graphene oxide flexible bionanocomposites for food packaging and biological applications. Compos. Sci. Technol. 2019, 173, 53–60. [Google Scholar] [CrossRef]
- Du, X.; Qin, Z.; Li, Z. Free-Standing rGO-CNT Nanocomposites with Excellent Rate Capability and Cycling Stability for Na2SO4 Aqueous Electrolyte Supercapacitors. Nanomaterials 2021, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Yun, J.-H.; Back, S.Y.; Lee, J.-S.; Kang, N.; Jang, Y.-I.; Lim, J.; Son, J.-H.; Park, J.-Y.; Kim, J.; et al. Superior thermoelectric cooling performance by suppressing bipolar diffusion effect and enhancing anisotropic texture in p-/n-type Bi2Te3 based compounds. J. Alloys Compd. 2021, 888, 161572. [Google Scholar] [CrossRef]
- Sadiq, M.; Khan, M.A.; Sarvar, M.; Raza, M.M.H.; Aalam, S.M.; Zulfequar, M.; Ali, J. High electrochemical performance of rGO-CNTs composites as an electrode material for supercapacitors applications. Hybrid Adv. 2023, 3, 100051. [Google Scholar] [CrossRef]
- Kong, L.; Yin, X.; Xu, H.; Yuan, X.; Wang, T.; Xu, Z.; Huang, J.; Yang, R.; Fan, H. Powerful absorbing and lightweight electromagnetic shielding CNTs/RGO composite. Carbon 2019, 145, 61–66. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Yu, Y.; Huang, H. Influence of CNT-rGO composite structures on their permeability and selectivity for membrane water treatment. J. Membr. Sci. 2018, 551, 326–332. [Google Scholar] [CrossRef]
- Pan, Y.; Bao, H.; Li, L. Noncovalently Functionalized Multiwalled Carbon Nanotubes by Chitosan-Grafted Reduced Graphene Oxide and Their Synergistic Reinforcing Effects in Chitosan Films. ACS Appl. Mater. Interfaces 2011, 3, 4819–4830. [Google Scholar] [CrossRef] [PubMed]
- Mergen, Ö.B.; Arda, E.; Evingür, G.A. Electrical, optical and mechanical properties of chitosan biocomposites. J. Compos. Mater. 2019, 54, 1497–1510. [Google Scholar] [CrossRef]
- Vega-Cázarez, C.A.; López-Cervantes, J.; Sánchez-Machado, D.I.; Madera-Santana, T.J.; Soto-Cota, A.; Ramírez-Wong, B. Preparation and Properties of Chitosan–PVA Fibers Produced by Wet Spinning. J. Polym. Environ. 2018, 26, 946–958. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting Antimicrobial Packaging Films and Measuring Their Physical Properties and Antimicrobial Activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Escárcega-Galaz, A.A.; Sánchez-Machado, D.I.; López-Cervantes, J.; Sanches-Silva, A.; Madera-Santana, T.J.; Paseiro-Losada, P. Mechanical, structural and physical aspects of chitosan-based films as antimicrobial dressings. Int. J. Biol. Macromol. 2018, 116, 472–481. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: Conshohocken, PA, USA, 2018.
- Shipovskaya, A.B.; Fomina, V.I.; Kazmicheva, O.F.; Rudenko, D.A.; Malinkina, O.N. Optical activity of films based on chitosan of various molecular masses and modifications. Polym. Sci. Ser. A 2017, 59, 330–341. [Google Scholar] [CrossRef]
- Miao, J.; Liu, H.; Li, Y.; Zhang, X. Biodegradable Transparent Substrate Based on Edible Starch–Chitosan Embedded with Nature-Inspired Three-Dimensionally Interconnected Conductive Nanocomposites for Wearable Green Electronics. ACS Appl. Mater. Interfaces 2018, 10, 23037–23047. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wu, T.; Bao, H.; Li, L. Green fabrication of chitosan films reinforced with parallel aligned graphene oxide. Carbohydr. Polym. 2011, 83, 1908–1915. [Google Scholar] [CrossRef]
- Xia, Y.; Zuo, H.; Lv, J.; Wei, S.; Yao, Y.; Liu, Z.; Lin, Q.; Yu, Y.; Yu, W.; Huang, Y. Preparation of multi-layered microcapsule-shaped activated biomass carbon with ultrahigh surface area from bamboo parenchyma cells for energy storage and cationic dyes removal. J. Clean. Prod. 2023, 396, 136517. [Google Scholar] [CrossRef]
- Aztatzi-Pluma, D.; Castrejón-González, E.O.; Almendarez-Camarillo, A.; Alvarado, J.F.J.; Durán-Morales, Y. Study of the Molecular Interactions between Functionalized Carbon Nanotubes and Chitosan. J. Phys. Chem. C 2016, 120, 2371–2378. [Google Scholar] [CrossRef]
- Carson, L.; Kelly-Brown, C.; Stewart, M.; Oki, A.; Regisford, G.; Luo, Z.; Bakhmutov, V.I. Synthesis and characterization of chitosan–carbon nanotube composites. Mater. Lett. 2009, 63, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Marroquin, J.B.; Rhee, K.Y.; Park, S.J. Chitosan nanocomposite films: Enhanced electrical conductivity, thermal stability, and mechanical properties. Carbohydr. Polym. 2013, 92, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. Mechanical and biological properties of chitosan/carbon nanotube nanocomposite films. J. Biomed. Mater. Res. Part A 2014, 102, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.M.A.; Kuo, H.-W.; Den, W.; Sultan, M.; Rasool, K.; Bilal, M. Chapter 10—Recent trends of carbon nanotubes and chitosan composites for hexavalent chromium removal from aqueous samples. In Separation Science and Technology; Ahuja, S., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 15, pp. 177–207. [Google Scholar]
- Nhung, L.T.T.; Kim, I.Y.; Yoon, Y.S. Quaternized Chitosan-Based Anion Exchange Membrane Composited with Quaternized Poly(vinylbenzyl chloride)/Polysulfone Blend. Polymers 2020, 12, 2714. [Google Scholar] [CrossRef]
- Lewandowska, K.; Szulc, M. Rheological and Film-Forming Properties of Chitosan Composites. Int. J. Mol. Sci. 2022, 23, 8763. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, E.P.; Saldanha, T.D.P.; Navarro, M.V.M.; Medeiros, A.C.; Ginani, M.F.; Raffin, F.N. Mechanical properties and release studies of chitosan films impregnated with silver sulfadiazine. J. Appl. Polym. Sci. 2006, 102, 3462–3470. [Google Scholar] [CrossRef]
- González, J.A.; Mazzobre, M.F.; Villanueva, M.E.; Díaz, L.E.; Copello, G.J. Chitin hybrid materials reinforced with graphene oxide nanosheets: Chemical and mechanical characterisation. RSC Adv. 2014, 4, 16480–16488. [Google Scholar] [CrossRef]
- Podgorbunskikh, E.; Kuskov, T.; Rychkov, D.; Lomovskii, O.; Bychkov, A. Mechanical Amorphization of Chitosan with Different Molecular Weights. Polymers 2022, 14, 4438. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, G.P.; Debone, H.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Design and characterization of chitosan/zeolite composite films—Effect of zeolite type and zeolite dose on the film properties. Mater. Sci. Eng. C 2016, 60, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. Characterization on the mechanical and physical properties of chicken skin gelatin films in comparison to mammalian gelatin films. IOP Conf. Ser. Mater. Sci. Eng. 2018, 440, 012033. [Google Scholar] [CrossRef]
- Ma, J.; Liu, C.; Li, R.; Wang, J. Properties and structural characterization of chitosan/graphene oxide biocomposites. Bio-Med. Mater. Eng. 2012, 22, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Lin, Y.; Chen, S.; Shi, S.; Cai, Y.; Li, L.; Peng, X. Superior strength and toughness of graphene/chitosan fibers reinforced by interfacial complexation. Compos. Sci. Technol. 2020, 194, 108174. [Google Scholar] [CrossRef]
- Rodríguez Llanos, J.H.; De Oliveira Vercik, L.C.; Vercik, A. Physical Properties of Chitosan Films Obtained after Neutralization of Polycation by Slow Drip Method. J. Biomater. Nanobiotechnol. 2015, 6, 15. [Google Scholar] [CrossRef]
- Güldiken, Ç.G.; Karaosmanoğlu, O.; Sivas, H.; Gerçel, H.F. ZnO microparticle-loaded chitosan/poly(vinyl alcohol)/acacia gum nanosphere-based nanocomposite thin film wound dressings for accelerated wound healing. J. Appl. Polym. Sci. 2020, 137, 48445. [Google Scholar] [CrossRef]
- Valencia-Sullca, C.; Ben Messaoud, G.; Sánchez-González, L.; Tehrany, E.A.; Vargas, M.; Atarés, L.; Chiralt, A. Chitosan films containing encapsulated eugenol in alginate microspheres. Food Hydrocoll. 2024, 151, 109791. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; de la Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.; Wei, P.; Zhong, Y.; Chen, C.; Cai, J. Extremely strong and tough chitosan films mediated by unique hydrated chitosan crystal structures. Mater. Today 2021, 51, 27–38. [Google Scholar] [CrossRef]
- Miles, K.B.; Ball, R.L.; Matthew, H.W.T. Chitosan films with improved tensile strength and toughness from N-acetyl-cysteine mediated disulfide bonds. Carbohydr. Polym. 2016, 139, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X.M. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-C.; Liao, G.M.; Kumar, S.R.; Shih, C.-M.; Yang, C.-C.; Wang, D.-M.; Lue, S.J. Fabrication and Characterization of Chitosan Nanoparticle-Incorporated Quaternized Poly(Vinyl Alcohol) Composite Membranes as Solid Electrolytes for Direct Methanol Alkaline Fuel Cells. Electrochim. Acta 2016, 187, 616–628. [Google Scholar] [CrossRef]
- Gong, Y.; Yu, Y.; Kang, H.; Chen, X.; Liu, H.; Zhang, Y.; Sun, Y.; Song, H. Synthesis and Characterization of Graphene Oxide/Chitosan Composite Aerogels with High Mechanical Performance. Polymers 2019, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.-T.R.; Yamaguchi, M.; Li, X.; Bando, Y.; Golberg, D.; Winnik, F.M. Facile and Mild Strategy Toward Biopolymer-Coated Boron Nitride Nanotubes via a Glycine-Assisted Interfacial Process. J. Phys. Chem. C 2013, 117, 19568–19576. [Google Scholar] [CrossRef]
- Molla-Abbasi, P.; Ghaffarian, S.R. Decoration of carbon nanotubes by chitosan in a nanohybrid conductive polymer composite for detection of polar vapours. RSC Adv. 2014, 4, 30906–30913. [Google Scholar] [CrossRef]
- Tallury, S.S.; Pasquinelli, M.A. Molecular Dynamics Simulations of Polymers with Stiff Backbones Interacting with Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2010, 114, 9349–9355. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, J.; Chen, X.; Xin, J.H. Decoration of carbon nanotubes with chitosan. Carbon 2005, 43, 3178–3180. [Google Scholar] [CrossRef]
- Falamarzpour, P.; Ghaffarian Anbaran, S.R. Synergistic effect of carbon nanotubes on chitosan-graphene oxide supramolecular structure. J. Polym. Res. 2022, 29, 211. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Sanchez, G.; López-Rubio, A. Impact of molecular weight on the formation of electrosprayed chitosan microcapsules as delivery vehicles for bioactive compounds. Carbohydr. Polym. 2016, 150, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, J. Ammonia adsorption on graphene and graphene oxide: A first-principles study. Front. Environ. Sci. Eng. 2013, 7, 403–411. [Google Scholar] [CrossRef]
- Oliva, C.; Strodel, P.; Goldbeck, G.; Maiti, A. Unravelling the Interaction of Ammonia with Carbon Nanotubes. 2008. Available online: https://www.researchgate.net/publication/220002326_Unravelling_the_interaction_of_ammonia_with_carbon_nanotubes (accessed on 14 June 2024).
- Wang, S.F.; Shen, L.; Zhang, W.D.; Tong, Y.J. Preparation and mechanical properties of chitosan/carbon nanotubes composites. Biomacromolecules 2005, 6, 3067–3072. [Google Scholar] [CrossRef] [PubMed]
- Charlier, J.C. Defects in carbon nanotubes. Acc. Chem. Res. 2002, 35, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Omoto, Y.; Morita, H.; Sato, Y.; Nishida, T.; Motomiya, K.; Katsui, H.; Goto, T. Are Non-Six-Membered Ring Defects Formed in Single-Walled Carbon Nanotubes Treated by a Fluorination–Defluorination Process? Nanomaterials 2023, 13, 1086. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Araby, S.; Gibson, C.T.; Meng, Q.; Zhu, S.; Ma, J. Graphene Platelets and Their Polymer Composites: Fabrication, Structure, Properties, and Applications. Adv. Funct. Mater. 2018, 28, 1706705. [Google Scholar] [CrossRef]
- Shimamoto, D.; Muramatsu, H.; Hayashi, T.; Kim, Y.A.; Endo, M.; Park, J.S.; Saito, R.; Terrones, M.; Dresselhaus, M.S. Strong and stable photoluminescence from the semiconducting inner tubes within double walled carbon nanotubes. Appl. Phys. Lett. 2009, 94, 083106. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed]
- Sedky, A.; Ali, A.M.; Somaily, H.H.; Algarni, H. Electrical, photoluminescence and optical investigation of ZnO nanoparticles sintered at different temperatures. Opt. Quantum Electron. 2021, 53, 243. [Google Scholar] [CrossRef]
- Cao, L.; Meziani, M.J.; Sahu, S.; Sun, Y.-P. Photoluminescence Properties of Graphene versus Other Carbon Nanomaterials. Acc. Chem. Res. 2013, 46, 171–180. [Google Scholar] [CrossRef] [PubMed]


| Films | Color Attributes | ΔE | Opacity (A ∙ mm−1) | Thickness (µm) | ||
|---|---|---|---|---|---|---|
| L* | a* | b* | ||||
| rGO/LCs | 34.82 | −0.07 | 1.39 | 62.84 | 3.31 | 48 |
| rGO-CNTs/LCs | 34.53 | 0.05 | −0.11 | 63.16 | 0.85 | 106 |
| rGO/MCs | 38.54 | 0.08 | 0.88 | 59.12 | 2.4 | 52 |
| rGO-CNTs/MCs | 31.14 | 0.02 | 0.23 | 66.53 | 1.18 | 70 |
| rGO/HCs | 36.41 | 0.26 | 0.94 | 61.26 | 1.9 | 76 |
| rGO-CNTs/HCs | 32.88 | −0.11 | −0.09 | 64.80 | 1.04 | 60 |
| Films | DS (%) | SW (%) | IEC (meq g−1) |
|---|---|---|---|
| rGO/LCs | 270.1 | 21.1 | 0.0077 |
| rGO-CNTs/LCs | 324.7 | 34.6 | 0.0073 |
| rGO/MCs | 323.9 | 29.1 | 0.0074 |
| rGO-CNTs/MCs | 208.7 | 21.4 | 0.0076 |
| rGO/HCs | 208.7 | 21.4 | 0.0063 |
| rGO-CNTs/HCs | 193.2 | 15.0 | 0.0076 |
| Cs Molecular Weight | CrI (%) | Crystallite Size (nm) | ||
|---|---|---|---|---|
| rGO | rGO/CNTs | rGO | rGO/CNTs | |
| LCs | 95 | 94 | 31.57 | 42.08 |
| MCs | 56 | 93 | 42.08 | 31.58 |
| HCs | 92 | 97 | 42.08 | 42.08 |
| Films | Tensile Strength (MPa) | Elongation at Break (%) | Young Modulus (MPa) | Modulus of Toughness (MJ/m3) |
|---|---|---|---|---|
| LCs | 72.13 ± 20.03 | 4.75 ± 1.47 | 3189.59 ± 524.34 | 2.06 ± 0.99 |
| rGO/LCs | 83.76 ± 20.94 | 4.39 ± 1.16 | 4241.22 ± 1344.57 | 1.92 ± 0.80 |
| rGO-CNTs/LCs | 39.88 ± 10.35 | 2.71 ± 0.68 | 2225.41 ± 292.00 | 0.66 ± 0.26 |
| MCs | 35.75 ± 2.88 | 4.75 ± 0.86 | 1620.60 ± 232.64 | 1.17 ± 0.32 |
| rGO/MCs | 80.43 ± 14.31 | 5.30 ± 1.81 | 3897.96 ± 920.32 | 2.58 ± 1.04 |
| rGO-CNTs/MCs | 45.77 ± 5.86 | 6.33 ± 2.68 | 2405.75 ± 559.05 | 1.64 ± 0.59 |
| HCs | 49.60 ± 1.55 | 4.02 ± 0.81 | 2344.19 ± 466.55 | 1.21 ± 0.65 |
| rGO/HCs | 51.02 ± 9.58 | 5.22 ± 0.65 | 2447.75 ± 249.02 | 1.94 ± 0.39 |
| rGO-CNTs/HCs | 74.38 ± 13.65 | 8.57 ± 2.43 | 3729.1 ± 487.65 | 4.93 ± 1.96 |
| XPS Core Level | Films | |
|---|---|---|
| rGO/MCs | rGO-CNTs/MCs | |
| C 1s | 285.05 | 284.91 |
| 285.83 | 286.02 | |
| 286.3 | 287.05 | |
| 287.01 | 288.65 | |
| N 1s | 398.32 | 399.37 |
| 399.63 | 399.93 | |
| 400.93 | 400.60 | |
| Films | C (%) | O (%) | N (%) | C/O Ratio | C/N Ratio |
|---|---|---|---|---|---|
| rGO/MCs | 78.2 | 14.2 | 6.2 | 5.50 | 12.61 |
| rGO-CNTs/MCs | 68.1 | 25.5 | 5.0 | 2.67 | 13.62 |
| Films | σ (S/cm) |
|---|---|
| rGO/LCs | 1.55 × 10−9 |
| rGO-CNTs/LCs | 1.1 × 10−4 |
| rGO/MCs | 7.45 × 10−8 |
| rGO-CNTs/MCs | 1 × 10−9 |
| rGO/HCs | 7.6 × 10−10 |
| rGO-CNTs/HCs | 1.03 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Martínez, J.R.; López-Oyama, A.B.; Del Ángel-López, D.; García-Guendulain, C.; Rodríguez-González, E.; Pulido-Barragan, E.U.; Barffuson-Domínguez, F.; Magallanes-Vallejo, A.G.; Mogica-Cantú, P.J. Influence of Reduced Graphene Oxide and Carbon Nanotubes on the Structural, Electrical, and Photoluminescent Properties of Chitosan Films. Polymers 2024, 16, 1827. https://doi.org/10.3390/polym16131827
González-Martínez JR, López-Oyama AB, Del Ángel-López D, García-Guendulain C, Rodríguez-González E, Pulido-Barragan EU, Barffuson-Domínguez F, Magallanes-Vallejo AG, Mogica-Cantú PJ. Influence of Reduced Graphene Oxide and Carbon Nanotubes on the Structural, Electrical, and Photoluminescent Properties of Chitosan Films. Polymers. 2024; 16(13):1827. https://doi.org/10.3390/polym16131827
Chicago/Turabian StyleGonzález-Martínez, Jesús R., Ana B. López-Oyama, Deyanira Del Ángel-López, Crescencio García-Guendulain, Eugenio Rodríguez-González, Eder U. Pulido-Barragan, Felipe Barffuson-Domínguez, Aurora G. Magallanes-Vallejo, and Pablo J. Mogica-Cantú. 2024. "Influence of Reduced Graphene Oxide and Carbon Nanotubes on the Structural, Electrical, and Photoluminescent Properties of Chitosan Films" Polymers 16, no. 13: 1827. https://doi.org/10.3390/polym16131827
APA StyleGonzález-Martínez, J. R., López-Oyama, A. B., Del Ángel-López, D., García-Guendulain, C., Rodríguez-González, E., Pulido-Barragan, E. U., Barffuson-Domínguez, F., Magallanes-Vallejo, A. G., & Mogica-Cantú, P. J. (2024). Influence of Reduced Graphene Oxide and Carbon Nanotubes on the Structural, Electrical, and Photoluminescent Properties of Chitosan Films. Polymers, 16(13), 1827. https://doi.org/10.3390/polym16131827







