Transparent Superhydrophobic and Self-Cleaning Coating
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
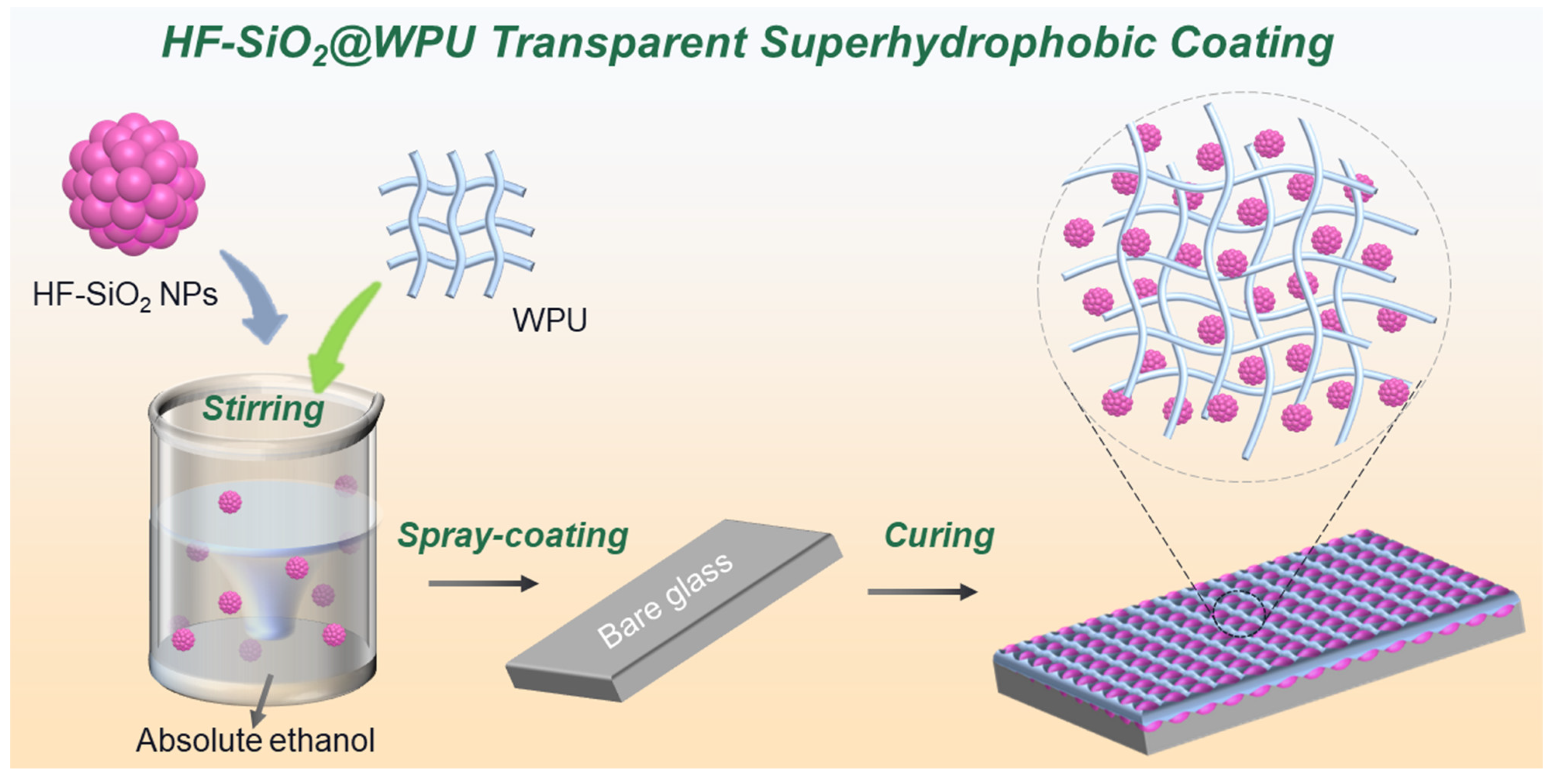
2.2. Fabrication of Transparent Superhydrophobic Coating
2.3. Characterizations
3. Results and Discussion
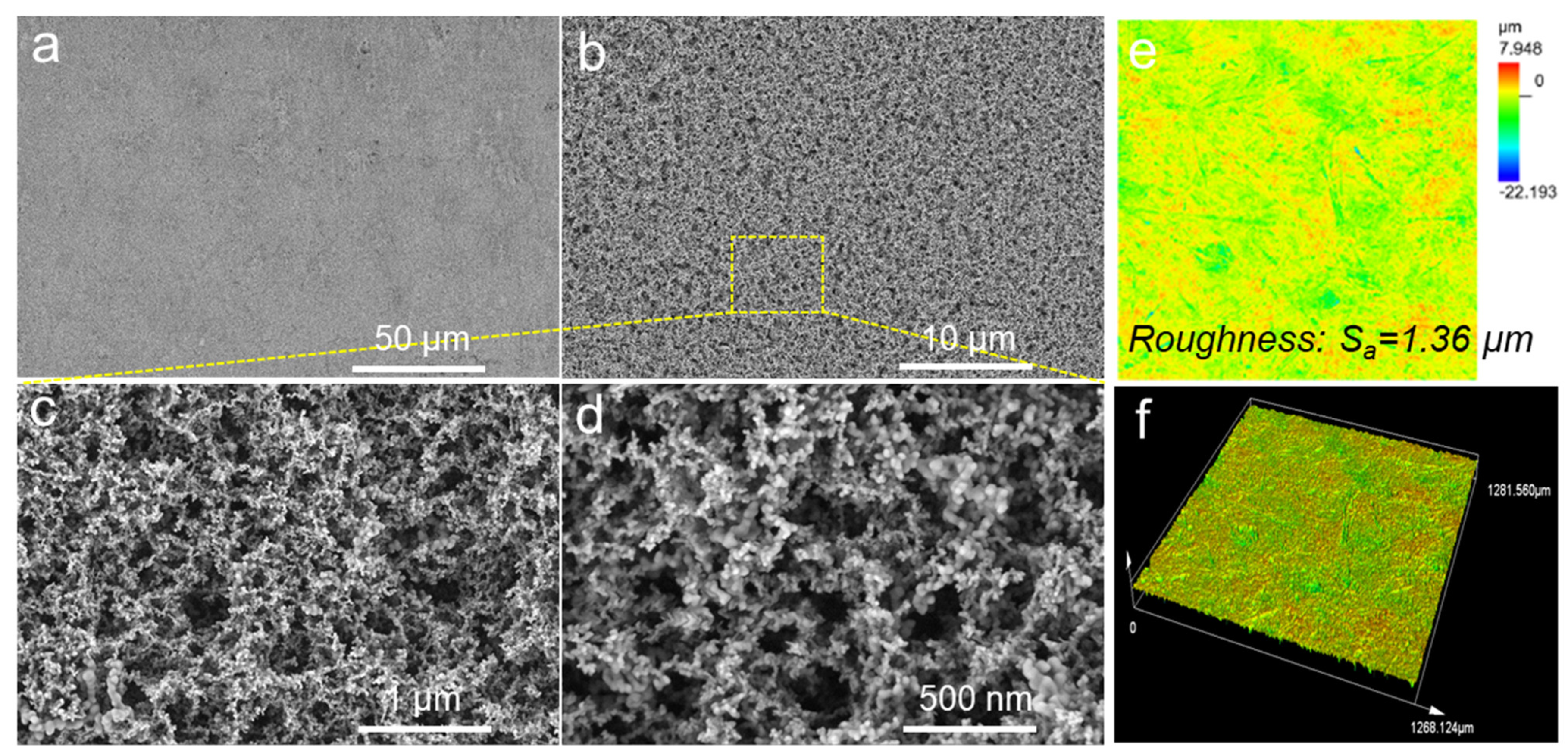
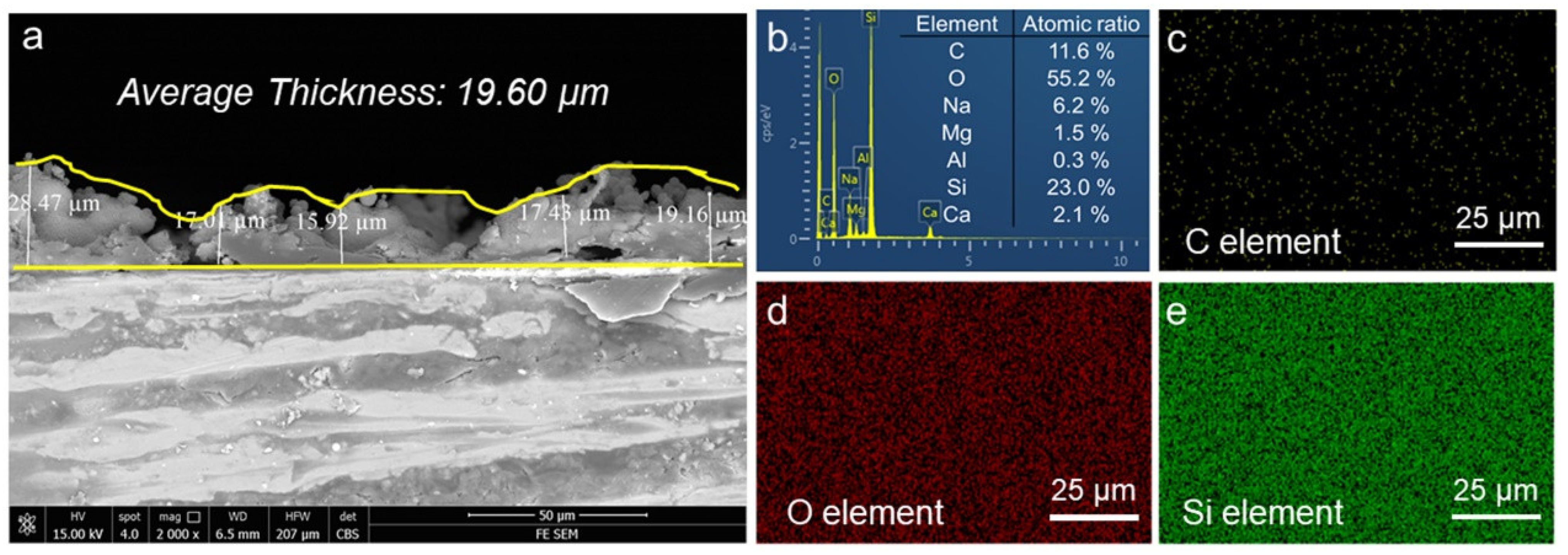
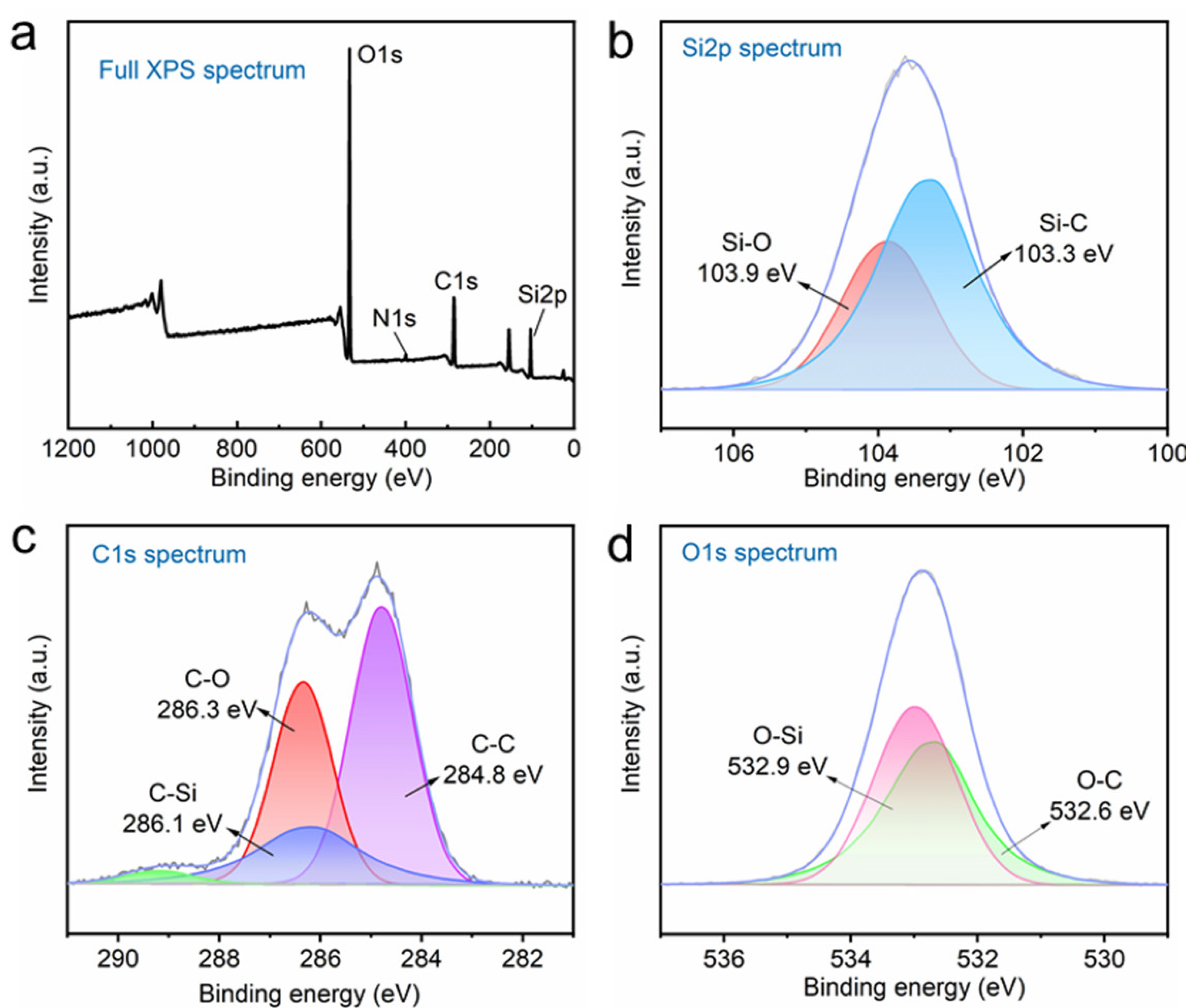
3.1. Surface Morphologies and Chemical Compositions
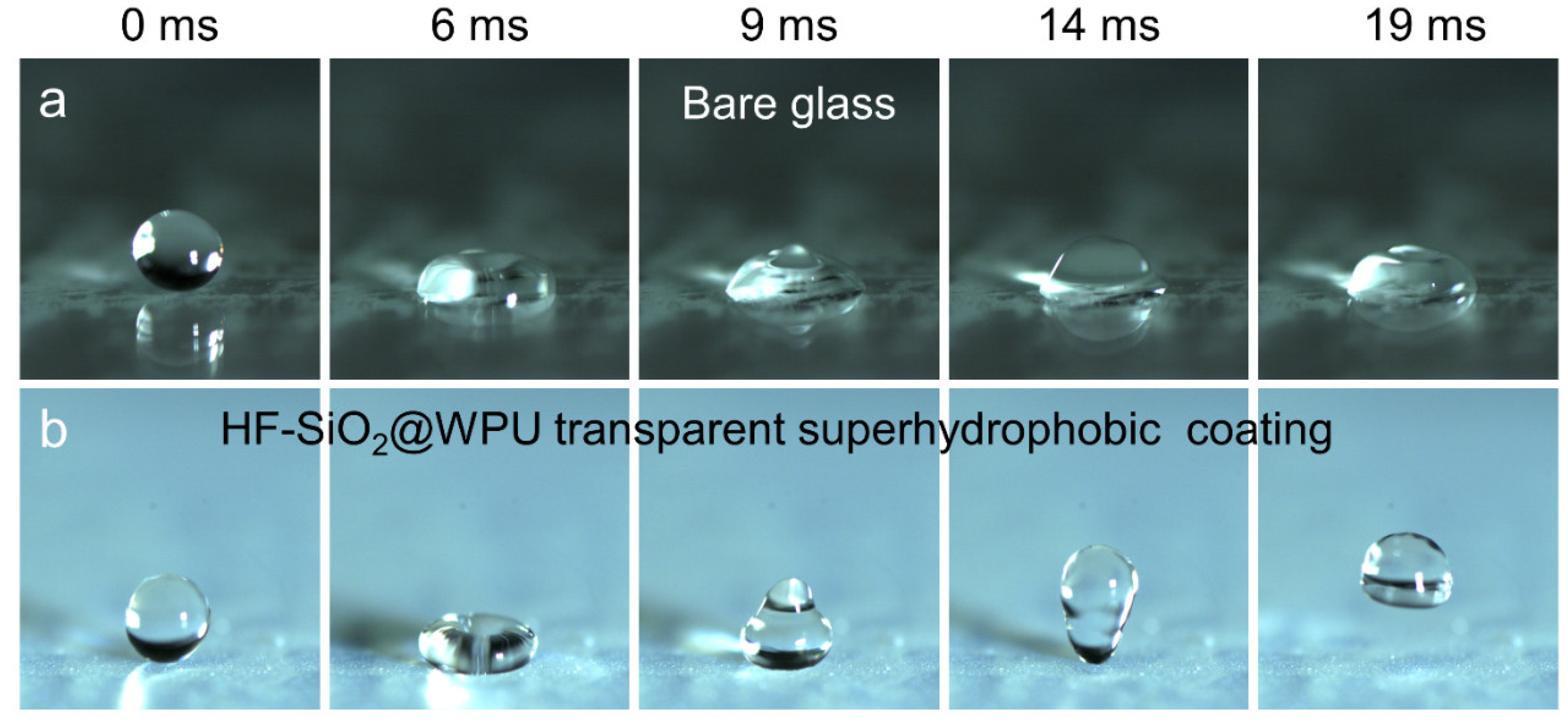
3.2. Surface Wettability
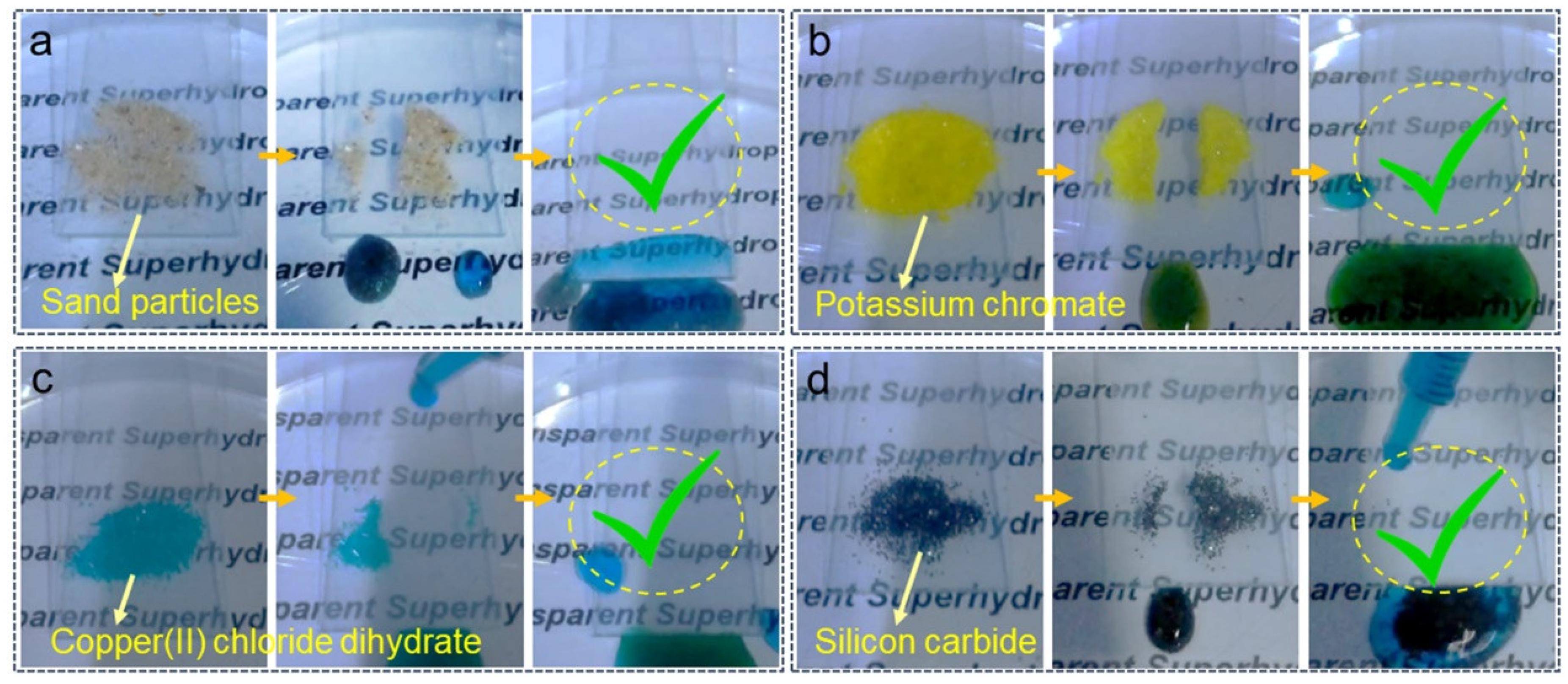
3.3. Optical Transparency and Self-Cleaning Ability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerhard, C.; Taleb, A.; Pelascini, F.; Hermann, J. Quantification of surface contamination on optical glass via sensitivity-improved calibration-free laser-induced breakdown spectroscopy. Appl. Surf. Sci. 2021, 537, 147984. [Google Scholar] [CrossRef]
- Kang, Z.; He, Z.; Wen, Y.; Liao, M.; Li, X.; Chen, H.; Zhang, Q. Smart COD sensor using UV–Vis spectroscopy against optical window surface contamination. Measurement 2022, 187, 110125. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Cai, S.; Wang, J.; He, H. Single-Side Superhydrophobicity in Si3N4-Doped and SiO2-Treated Polypropylene Nonwoven Webs with Antibacterial Activity. Polymers 2022, 14, 2952. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Xu, D.; Wei, J.; Li, B.; Zhang, J. Long-lasting anti-bacterial face masks enabled by combining anti-bacterial materials and superhydrophobic coating. Surf. Coat. Technol. 2024, 476, 130229. [Google Scholar] [CrossRef]
- Wang, D.; Ma, C.; Liu, J.; Li, W.; Shang, W.; Peng, N.; Wen, Y. Corrosion resistance and anti-soiling performance of micro-arc oxidation/graphene oxide/stearic acid superhydrophobic composite coating on magnesium alloys. Int. J. Miner. Metall. Mater. 2023, 30, 1128–1139. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Zhou, K.; Liu, H.; Wang, J.; Zhang, B. Enhanced mechanical stability and corrosion resistance of superhydrophobic coating reinforced with inorganic binder. J. Cent. South Univ. 2024. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, G.; Xu, W.; Duan, J.; Hou, B. Hybrid superamphiphobic anti-corrosion coating with integrated functionalities of liquid repellency, self-cleaning, and anti-icing. J. Mater. Sci. Technol. 2024, 184, 256–268. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, H.; Zheng, B.; Guan, X.; Sun, B.; Liao, Y.; Yue, Y.; Duan, W.; Ding, H. A Super-Robust Armoured Superhydrophobic Surface with Excellent Anti-icing Ability. J. Bionic Eng. 2023, 20, 1891–1904. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Xie, Q.; Duan, W.; Zhang, X.; Han, H. A Passive Anti-icing Strategy Based on a Superhydrophobic Mesh with Extremely Low Ice Adhesion Strength. J. Bionic Eng. 2021, 18, 55–64. [Google Scholar] [CrossRef]
- Cao, K.; Huang, X.; Pan, J. Application of Superhydrophobic Mesh Coated by PDMS/TiO2 Nanocomposites for Oil/Water Separation. Polymers 2022, 14, 5431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, X.; Chao, Y.; Chen, D.; Liao, Y. Super-Hydrophobic Magnetic Fly Ash Coated Polydimethylsiloxane (MFA@PDMS) Sponge as an Absorbent for Rapid and Efficient Oil/Water Separation. Polymers 2022, 14, 3726. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, J.; Xu, W.; Zhang, B. A mechanically robust superhydrophobic corrosion resistant coating with self-healing capability. Mater. Des. 2024, 240, 112881. [Google Scholar] [CrossRef]
- Kim, T.; Song, M.G.; Kim, K.; Jeon, H.; Kim, G.H. Recyclable Superhydrophobic Surface Prepared via Electrospinning and Electrospraying Using Waste Polyethylene Terephthalate for Self-Cleaning Applications. Polymers 2023, 15, 3810. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, J.; Wang, G.; Hao, X.; Li, A. Construction of a durable superhydrophobic flame-retardant coating on the PET fabrics. Mater. Des. 2023, 233, 112258. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Miao, X.; Deng, W. Fabrication of polydopamine-boehmite modified superhydrophobic coating for self-cleaning, oil-water separation, oil sorption and flame retardancy. Surf. Interfaces 2023, 38, 102775. [Google Scholar] [CrossRef]
- Ma, J.; Qing, Y.; Song, H.; Yao, Y.; Xu, X.; Long, C.; Liu, N.; Li, H.; Liu, C. Micro-/nanofiber-coupled superhydrophobic and conductive textile for underwater wearable strain sensors with full-scale human motion detection ability. J. Mater. Chem. C 2023, 11, 9539–9551. [Google Scholar] [CrossRef]
- Su, C.; Huang, X.; Zhang, L.; Zhang, Y.; Yu, Z.; Chen, C.; Ye, Y.; Guo, S. Robust superhydrophobic wearable piezoelectric nanogenerators for self-powered body motion sensors. Nano Energy 2023, 107, 108095. [Google Scholar] [CrossRef]
- Park, S.; Jung, S.; Heo, J.; Hong, J. Facile synthesis of polysilsesquioxane toward durable superhydrophilic/superhydrophobic coatings for medical devices. J. Ind. Eng. Chem. 2019, 77, 97–104. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W.; Du, G.; Fu, Q.; Mo, J.; Nie, S. Superhydrophobic cellulosic triboelectric materials for distributed energy harvesting. Chem. Eng. J. 2023, 452, 139259. [Google Scholar] [CrossRef]
- Jung, S.; Woo, S.; Heo, D.; Park, S.; Cho, S.; Choi, M.; Cho, Y.; Lee, S.; Hong, J. Droplet-based energy harvesting superhydrophobic membrane with bacterial sensing ability for continuous monitoring. Chem. Eng. J. 2023, 457, 141066. [Google Scholar] [CrossRef]
- Gao, W.; Ma, F.; Yin, Y.; Li, J. Robust and durable transparent superhydrophobic F-TNTs/TiN coating fabricated by structure tuning on surface of TiN hard coating. Appl. Surf. Sci. 2023, 613, 155967. [Google Scholar] [CrossRef]
- Nomeir, B.; Lakhouil, S.; Boukheir, S.; Ali, M.A.; Naamane, S. Durable and transparent superhydrophobic coating with temperature-controlled multi-scale roughness for self-cleaning and anti-icing applications. Prog. Org. Coat. 2024, 189, 108338. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, J.; Xu, W.; Zhang, Y.; Duan, J.; Hou, B. Robust, scalable and fluorine-free superhydrophobic anti-corrosion coating with shielding functions in marine submerged and atmospheric zones. Mater. Des. 2022, 223, 111246. [Google Scholar] [CrossRef]
- Moghadam, S.G.; Parsimehr, H.; Ehsani, A. Multifunctional superhydrophobic surfaces. Adv. Colloid Interface Sci. 2021, 290, 102397. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ji, G.; Lu, Y.; Zhang, B. Sustainable corrosion-resistant superhydrophobic composite coating with strengthened robustness. J. Ind. Eng. Chem. 2023, 121, 215–227. [Google Scholar] [CrossRef]
- Yu, S.; Guo, Z.; Liu, W. Biomimetic transparent and superhydrophobic coatings: From nature and beyond nature. Chem. Commun. 2015, 51, 1775–1794. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Du, W.; Lei, W.; Si, X.; Zhou, T.; Lin, J.; Peng, L. Superhydrophobic silica antireflective coatings with high transmittance via one-step sol-gel process. Thin Solid Film. 2017, 631, 193–199. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Q.; Lyons, A.M. Durable, optically transparent, superhydrophobic polymer films. Appl. Surf. Sci. 2019, 470, 187–195. [Google Scholar] [CrossRef]
- Ebert, D.; Bhushan, B. Transparent, superhydrophobic, and wear-resistant surfaces using deep reactive ion etching on PDMS substrates. J. Colloid Interface Sci. 2016, 481, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Bhushan, B. Transparent wear-resistant, superhydrophobic and superoleophobic poly(dimethylsiloxane) (PDMS) surfaces. J. Colloid Interface Sci. 2017, 488, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Latthe, S.S.; Nadargi, D.Y.; Hirashima, H.; Ganesan, V. Preparation of MTMS based transparent superhydrophobic silica films by sol–gel method. J. Colloid Interface Sci. 2009, 332, 484–490. [Google Scholar]
- Mates, J.E.; Ibrahim, R.; Vera, A.; Guggenheim, S.; Qin, J.; Calewarts, D.; Waldroup, D.E.; Megaridis, C.M. Environmentally-safe and transparent superhydrophobic coatings. Green Chem. 2016, 18, 2185–2192. [Google Scholar] [CrossRef]
- Li, F.; Du, M.; Zheng, Q. Transparent and durable SiO2-containing superhydrophobic coatings on glass. J. Appl. Polym. Sci. 2015, 132, 41500. [Google Scholar] [CrossRef]
- De Vos, R.M.; Maier, W.F.; Verweij, H. Hydrophobic silica membranes for gas separation. J. Membr. Sci. 1999, 158, 277–288. [Google Scholar] [CrossRef]
- Xu, R.; Wang, B.; Cai, Y. Preparation and structures of PEBA gas separation membrane modified by fumed silica for oil vapor separation. Sci. Rep. 2022, 12, 1025. [Google Scholar] [CrossRef] [PubMed]
- Pipatchanchai, T.; Srikulkit, K. Hydrophobicity modification of woven cotton fabric by hydrophobic fumed silica coating. J. Sol-Gel Sci. Technol. 2007, 44, 119–123. [Google Scholar] [CrossRef]
- Pramualkijja, W.; Jiratumnukul, N. The preparation of hydrophobic hybrid film coatings from siloxane-modified polyacrylate associated with nano-fumed silica and organo-modified clay. J. Coat. Technol. Res. 2022, 19, 1467–1492. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Rheological and adhesive properties of nanocomposite bitumen binders based on hydrophilic or hydrophobic silica and modified with bio-oil. Constr. Build. Mater. 2022, 342, 127946. [Google Scholar] [CrossRef]
- Bahattab, M.A.; Pacios, V.G.; Robles, J.D.; Martinez, J.M.M. Comparative Properties of Hydrophilic and Hydrophobic Fumed Silica Filled Two-Component Polyurethane Adhesives. J. Adhes. Sci. Technol. 2012, 26, 303–315. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, C.; Lv, J.; Zhang, J.; Feng, J. Robust fluorine-free superhydrophobic coating on polyester fabrics by spraying commercial adhesive and hydrophobic fumed SiO2 nanoparticles. Prog. Org. Coat. 2020, 138, 105342. [Google Scholar] [CrossRef]
- Baba, E.M.; Cansoy, C.E.; Zayim, E.O. Investigation of wettability and optical properties of superhydrophobic polystyrene-SiO2 composite surfaces. Prog. Org. Coat. 2016, 99, 378–385. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Wang, Q.; Wang, Y.; Ji, X.; Yang, G.; Chen, J.; Ni, Y. Superhydrophobic, strong and transparent paper made from cellulosic fibers. Cellulose 2022, 29, 1993–2003. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Zhang, S.; Zhang, D.; Liu, X.; He, Y.; Mi, L.; Zhang, J.; Liu, C.; Shen, C.; et al. Superhydrophobic Electrically Conductive Paper for Ultrasensitive Strain Sensor with Excellent Anticorrosion and Self-Cleaning Property. ACS Appl. Mater. Interfaces 2019, 11, 21904–21914. [Google Scholar] [CrossRef] [PubMed]
- Do, V.T.; Chun, D.M. Fabrication of large-scale, flexible, and robust superhydrophobic composite films using hydrophobic fumed silica nanoparticles and polydimethylsiloxane. Polymer 2022, 244, 124630. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Chen, S.; Mahmood, A.; Wang, J. Development of Durable, Fluorine-free, and Transparent Superhydrophobic Surfaces for Oil/Water Separation. ACS Omega 2019, 4, 6947–6954. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, M.; Wen, J.; Yuan, J.; Zhu, L.; Yu, H. Robust, Transparent, and Superhydrophobic Coating Fabricated with Waterborne Polyurethane and Inorganic Nanoparticle Composites. Ind. Eng. Chem. Res. 2019, 58, 8050–8060. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Xue, X.; Zhao, L.; Hou, B. Transparent Superhydrophobic and Self-Cleaning Coating. Polymers 2024, 16, 1876. https://doi.org/10.3390/polym16131876
Zhang B, Xue X, Zhao L, Hou B. Transparent Superhydrophobic and Self-Cleaning Coating. Polymers. 2024; 16(13):1876. https://doi.org/10.3390/polym16131876
Chicago/Turabian StyleZhang, Binbin, Xiaochen Xue, Lixia Zhao, and Baorong Hou. 2024. "Transparent Superhydrophobic and Self-Cleaning Coating" Polymers 16, no. 13: 1876. https://doi.org/10.3390/polym16131876
APA StyleZhang, B., Xue, X., Zhao, L., & Hou, B. (2024). Transparent Superhydrophobic and Self-Cleaning Coating. Polymers, 16(13), 1876. https://doi.org/10.3390/polym16131876






