Recent Advances in Environment-Friendly Polyurethanes from Polyols Recovered from the Recycling and Renewable Resources: A Review
Abstract
:1. Introduction
2. Waste-Based Feedstocks for Polyurethanes
2.1. Waste Polyethylene Terephthalate for PU
2.1.1. Glycolysis
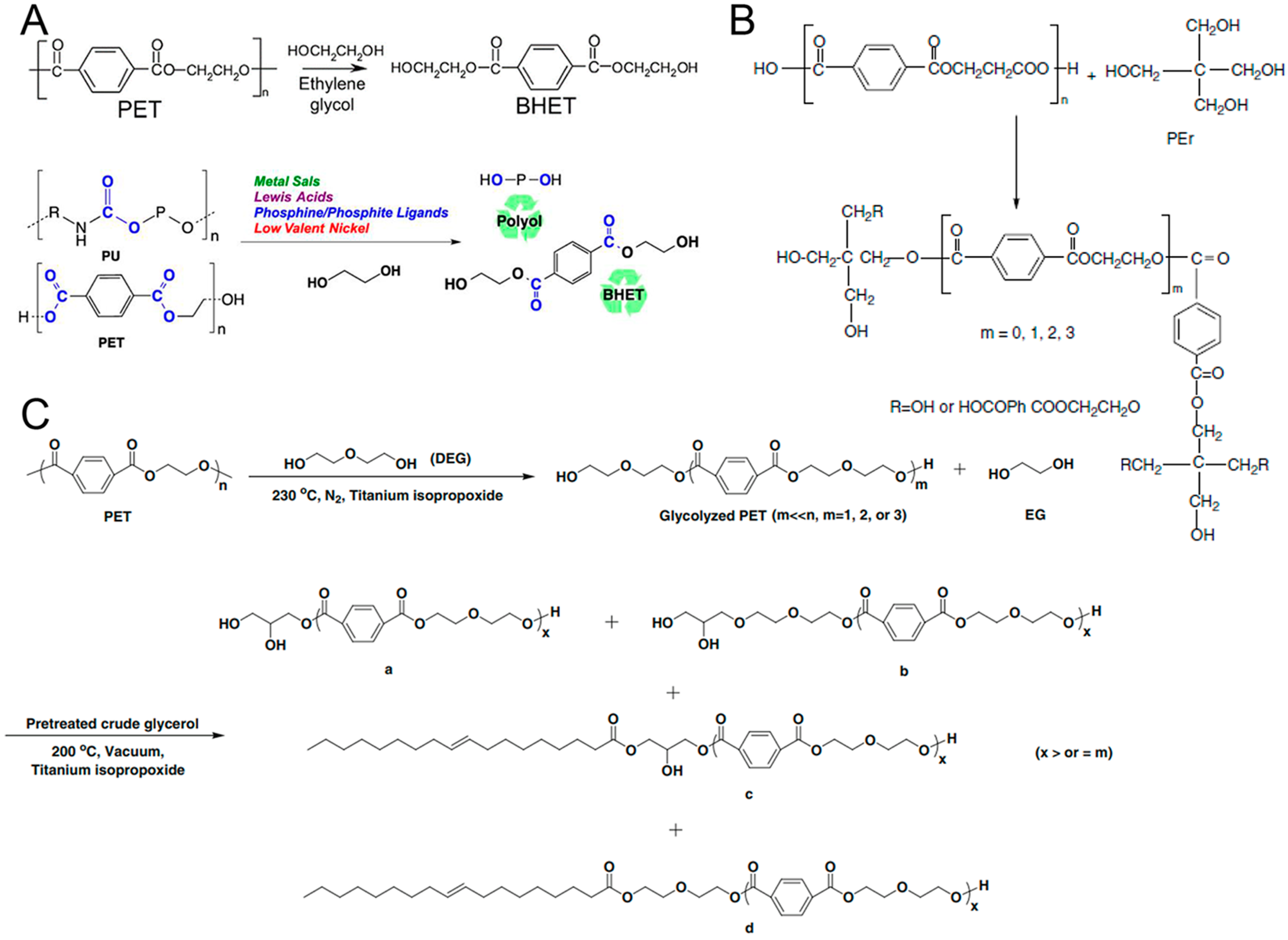
2.1.2. Hydrolysis
2.1.3. Alcoholysis
2.1.4. Biodegradation
2.2. Waste Polyurethane for PU
2.3. Waste Polycarbonate for PU
3. Bio-Based Feedstocks for Polyurethanes
3.1. PU from Renewable Monomers
3.1.1. Vegetable Oil

3.1.2. Cashew Nut Shell Liquid
3.1.3. Terpene
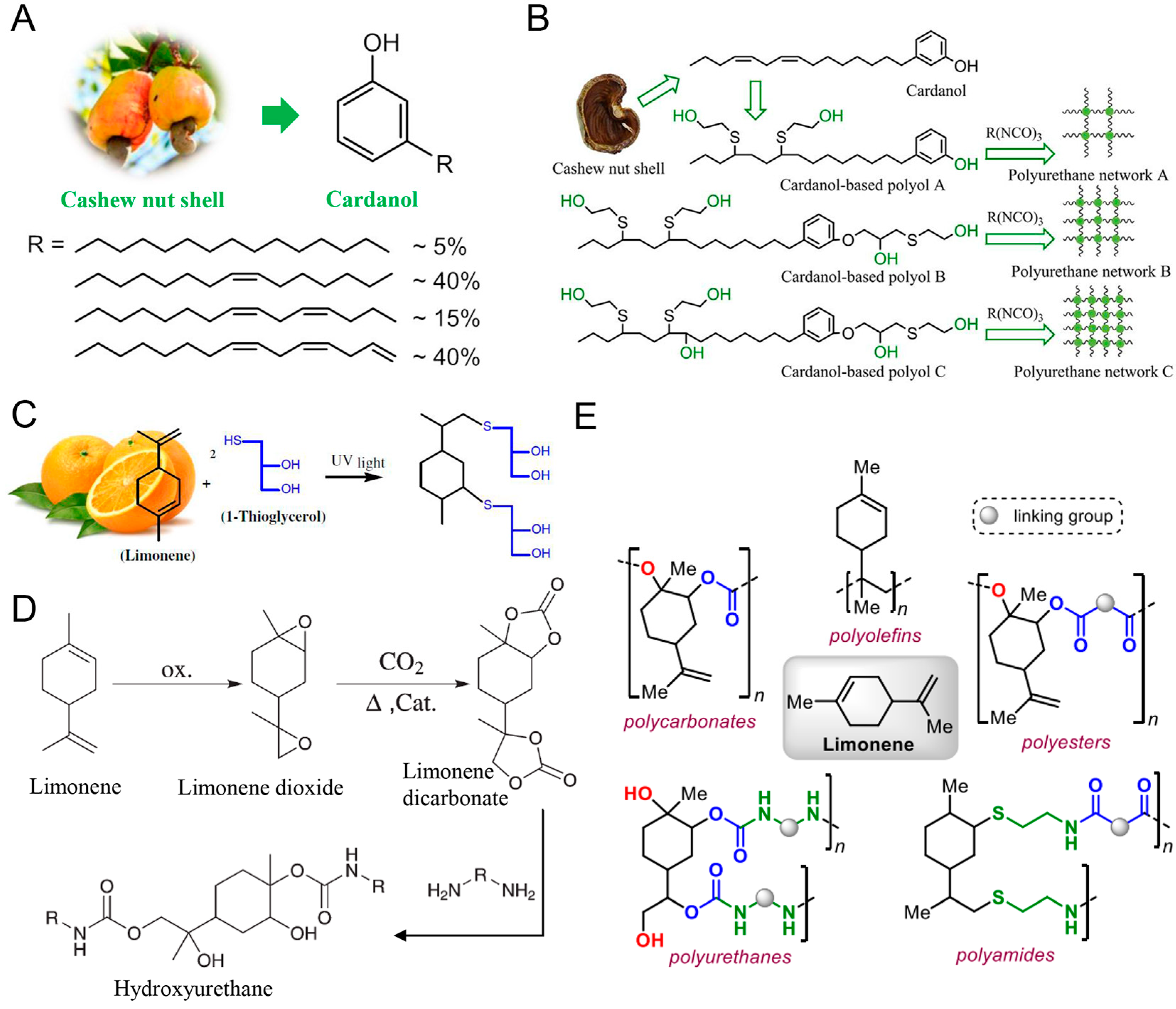
3.1.4. Rosin
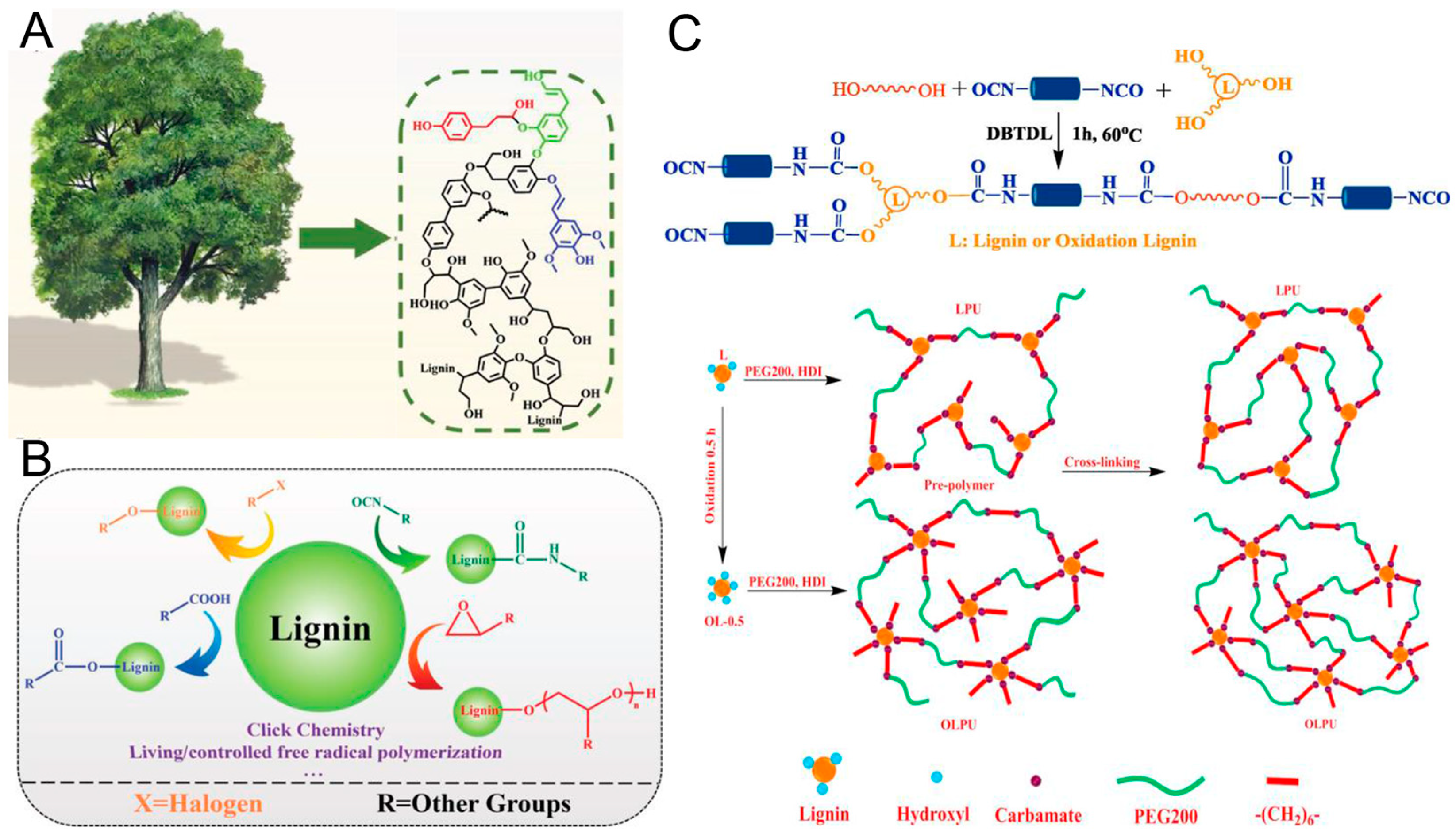
3.2. PU from Lignin
4. Biomacromolecules for Polyurethane
4.1. PU from Polysaccharides
4.1.1. Cellulose for PU
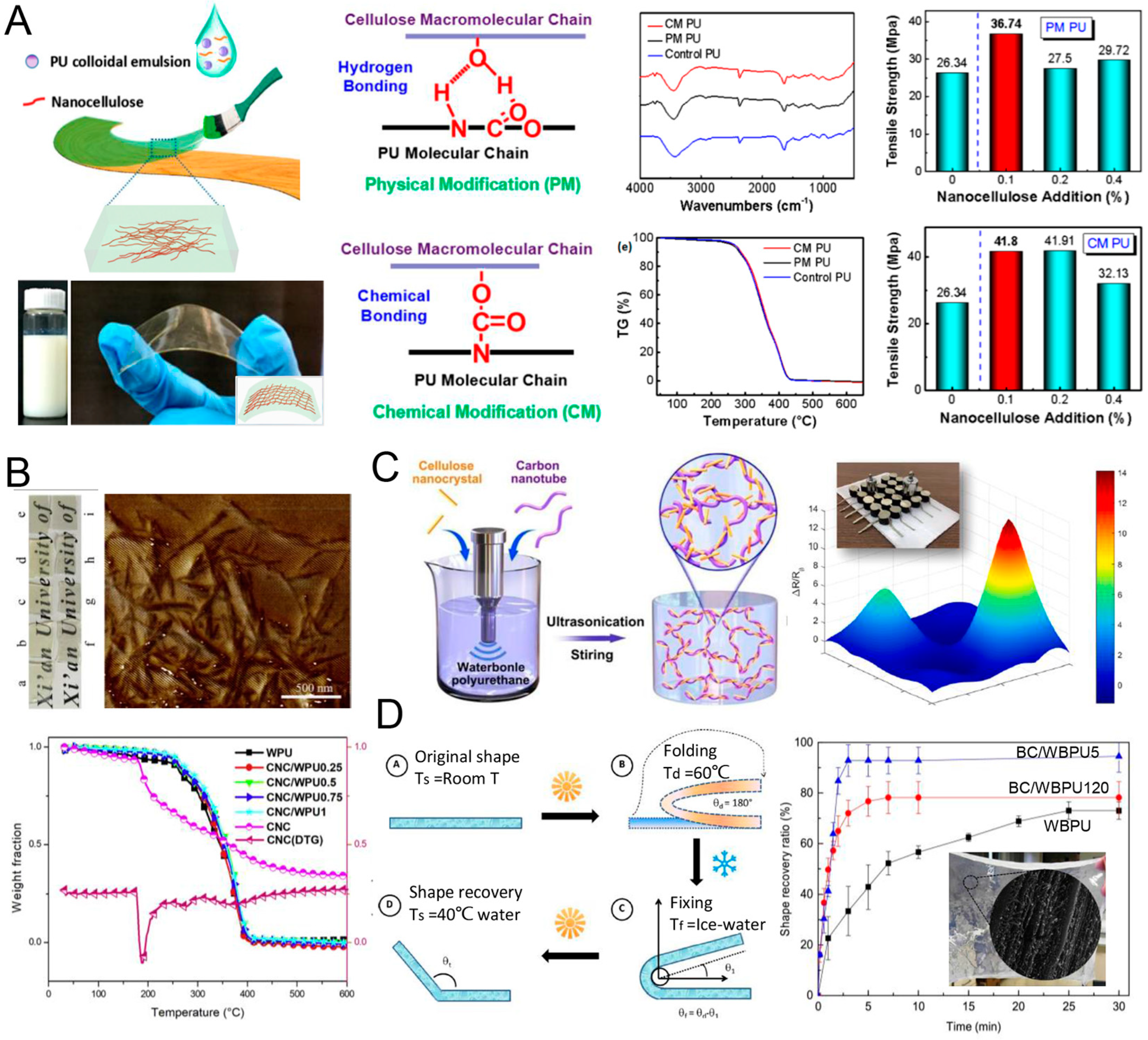
4.1.2. Starch for PU
4.1.3. Chitosan for PU
4.1.4. Sodium Alginate for PU

4.1.5. Glucomannan
4.2. PU from Protein
5. Sustainability for Polyurethane
| Feedstock | Yield (t/y) | Price ($/t) | Products | Applications | Ref. |
|---|---|---|---|---|---|
| PET | 19.9 × 106 | 418–448 | PU Foams | Cushion packaging Oil sorption Fireproof materials | [42,43,259] |
| PU | 12 × 106 | 150–300 | PU Foams | Foaming agent | [89,260] |
| PC | 9.8 × 106 | 700–900 | PU films WPU | Biomimetic anti-fouling Coatings | [119,121] |
| VOs | 90 × 106 | 1800–2000 | PU Foams | Mattress Ink | [3,261,262] |
| CNSL | 0.03 × 106 | 450–700 | PU Foams coatings | Building materials Anticorrosive coatings | [153,263] |
| Terpene | 0.45 × 106 | 3000–3500 | PU Foams | Flame retardancy | [264] |
| Rosin | 0.65 × 106 | 1800–2000 | Elastomer | Shape memory material | [174] |
| Lignin | 50 × 106 | 50–200 | Elastomer WPU | Anti-bacterial coatings Shoe Sole | [157,189] |
| Cellulose | 100 × 106 | 20–45 | WPU Coatings | Wood antibacterial | [215] |
| Starch | 30 × 106 | 450–600 | PU films | Biomedical materials | [239] |
| Chitosan | 100 × 109 | 2500–3000 | PU gels Elastomer | Shape memory material Biomedical materials | [247,248] |
| Sodium alginate | 0.23 × 106 | 600–800 | WPU | Biomedical materials | [252] |
| Glucomannan | 0.25 × 106 | 500–1000 | WPU | Food packaging | [252] |
| Protein | 0.2 × 106 | 200–350 | Elastomer | Biomedical materials | [256] |
6. Conclusions and Prospects
- An important challenge for recycling resources is improved extraction, degradation and transformation, which are valuable building blocks that will be optimized at the cost of both the performance and monomers. At the same time, the purity and stability of the monomers prepared from recycling resources will also be an important consideration in the future research of PU.
- From the upstream design, collection and classification, to the last part of the regeneration process, the regeneration process is developing rapidly at present, but the real expansion of this market requires concerted action across the value chain. Upstream classification and sorting systems tend to be more efficient, and downstream access to high-quality raw materials will be less efficient.
- A crucial, and sometimes underestimated, synthesis route is the need to ensure the raw materials are compatible with current equipment condition. Although it is still early days to directly quantify and compare traditional petrochemical PU, there is enough research to show that recyclable materials have gradually become part of the view of the PU production, particularly regarding environmental pollution and fossil resource depletion.
- Strengthen the connection between the solid waste classification and recovery system and the renewable resource recovery system, connect the renewable resource industry chain with the waste industry chain, improve the resource utilization rate, and strive to explore the industrialization development of the circular economy of polyurethane.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.R.; Li, T.; Li, J.; Zhao, R.H.; Ding, A.; Xu, F.J. Structural Engineering of Polyurethanes for Biomedical Applications. Prog. Polym. Sci. 2024, 151, 101803. [Google Scholar] [CrossRef]
- Poopalam, K.D.; Ismail, T.N.M.T.; Hanzah, N.A.; Alias, A.H.; Wahab, N.A.; Ibrahim, Z.; Subramaniam, V.; Armylisas, A.N.; Idris, Z. Utilization of oil palm biomass and Polyurethanes as sustainable construction materials: A review. Dev. Built. Environ. 2024, 17, 100380. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Dandeniyage, L.S.; Adhikari, R.; Bown, M.; Shanks, R.; Adhikari, B. Advancements in the Development of Biostable Polyurethanes. Polym. Rev. 2019, 59, 391–417. [Google Scholar] [CrossRef]
- Cevher, D.; Surdem, S. Polyurethane Adhesive Based on Polyol Monomers BHET and BHETA Depolymerised from PET Waste. Int. J. Adhes. 2021, 105, 102799. [Google Scholar] [CrossRef]
- Li, L.; Yu, T.L. Curing comparison and performance investigation of polyurethane concrete with retarders. Constr. Build. Mater. 2022, 326, 126883. [Google Scholar] [CrossRef]
- Jiang, R.J.; Zheng, X.Y.; Zhu, S.S.; Li, W.Y.; Zhang, H.W.; Liu, Z.H.; Zhou, X. Recent Advances in Functional Polyurethane Chemistry: From Structural Design to Applications. Chemistryselect 2023, 8, e202204132. [Google Scholar] [CrossRef]
- Stadler, B.M.; Hinze, S.; Tin, S.; de Vries, J.G. Hydrogenation of Polyesters to Polyether Polyols. Chemsuschem 2019, 12, 4082–4087. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.P.; Yu, X.H.; Kan, S.Y.; Luo, Y.; Han, K.B.; Liang, Y.Z.; Gao, J.P. Preparation of Polyol From Waste Polyethylene Terephthalate (PET) and Its Application to Polyurethane (PU) Modified Asphalt. Constr. Build Mater. 2024, 427, 136286. [Google Scholar] [CrossRef]
- Grdadolnik, M.; Drincic, A.; Oreski, A.; Onder, O.C.; Utrosa, P.; Pahovnik, D.; Zagar, E. Insight into Chemical Recycling of Flexible Polyurethane Foams by Acidolysis. ACS Sustain. Chem. Eng. 2022, 10, 1323–1332. [Google Scholar] [CrossRef]
- Alshabebi, A.S.; Alrashed, M.M.; El Blidi, L.; Haider, S. Preparation of Bio-Based Polyurethane Coating from Citrullus colocynthis Seed Oil: Characterization and Corrosion Performance. Polymers 2024, 16, 214. [Google Scholar] [CrossRef]
- Stuhr, R.; Bayer, P.; Stark, C.B.W.; Jacobi von Wangelin, A. Light-Driven Waste-To-Value Upcycling: Bio-Based Polyols and Polyurethanes from the Photo-Oxygenation of Cardanols. Chemsuschem 2021, 14, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Han, J.W.; Sun, J.; Chen, Y.; Wang, X.G.; Gu, X.Y.; Zhang, S. Preparation of Aliphatic Bio-Based Polyester Polyols for Water-Blown and Halogen-Free Flame Retardant Rigid Polyurethane Foam. Polym. Degrad. Stab. 2024, 226, 110832. [Google Scholar] [CrossRef]
- Kazemi, M.; Kabir, S.F.; Fini, E.H. State of the Art in Recycling Waste Thermoplastics and Thermosets and Their Applications in Construction. Resour. Conserv. Recycl. 2021, 174, 105776. [Google Scholar] [CrossRef]
- Caki, S.M.; Risti, I.S.; Cincovi, M.M.; Nikolic, N.C.; Nikolic, L.B.; Cvetinov, M.J. Synthesis And Properties Biobased Waterborne Polyurethanes from Glycolysis Product of PET Waste and Poly(Caprolactone) Diol. Prog. Org. Coat. 2017, 105, 111–122. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Fang, C.; Yu, R.; Li, Y.; Lei, W. Structure and thermal properties of various alcoholysis products from waste poly(ethylene terephthalate). Waste Manag. 2019, 85, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Simón, D.; Borreguero, A.M.; Lucas, A.D.; Gutiérrez, C.; Rodríguez, J.F. Sustainable Polyurethanes: Chemical Recycling to Get It; Springer: Cham, Switzerland, 2014; Volume 32, pp. 229–260. [Google Scholar] [CrossRef]
- Lee, D.K.; Tsai, H.B.; Tsai, R.S.; Chen, P.H. Preparation and Properties of Transparent Thermoplastic Segmented Polyurethanes Derived from Different Polyols. Polym. Eng. Sci. 2007, 47, 695–701. [Google Scholar] [CrossRef]
- Savani, N.G.; Naveen, T.; Dholakiya, B.Z. A review on the synthesis of maleic anhydride based polyurethanes from renewable feedstock for different industrial applications. J. Polym. Res. 2023, 30, 175. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Zahoor, A.F. Bio-Based Polyurethane: An Efficient and Environment Friendly Coating Systems: A Review. Prog. Org. Coat. 2016, 91, 25–32. [Google Scholar] [CrossRef]
- Su, Y.; Ma, S.Q.; Wang, B.B.; Xu, X.W.; Feng, H.Z.; Hu, K.Z.; Zhang, W.Q.; Zhou, S.C.; Weng, G.S.; Zhu, J. High-Performance Castor Oil-Based Polyurethane Thermosets: Facile Synthesis and Properties. React. Funct. Polym. 2023, 183, 105496. [Google Scholar] [CrossRef]
- Petrovic, Z.S. Polyurethanes from Vegetable Oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Sarim, M.; Alavi Nikje, M.M.; Dargahi, M. Synthesis and characterization of polyurethane rigid foam by using feedstocks received from renewable and recyclable resources. J. Porous Mater. 2023, 30, 1337–1356. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for Polyurethane and Polyurethane Composites, Recycling and Recovery: A Review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Yuan, L.Y.; Zhou, W.; Shen, Y.; Li, Z.B. Chemically Recyclable Polyurethanes Based on Bio-Renewable Gamma-Butyrolactone: From Thermoplastics to Elastomers. Polym. Degrad. Stabil. 2022, 204, 110116. [Google Scholar] [CrossRef]
- Peng, Y.; Yuan, X.; Jiang, L.; Yang, J.; Liu, Z.; Zhao, Y.; Chen, H. The Fabricating Methods, Properties and Engineering Applications of Foamed Concrete with Polyurethane: A Review. Int. J. Environ. Sci. Technol. 2022, 20, 2293–2312. [Google Scholar] [CrossRef]
- Pfister, D.P.; Xia, Y.; Larock, R.C. Recent Advances in Vegetable Oil-Based Polyurethanes. Chemsuschem 2011, 4, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.P.; Paone, E.; Rodriguez-Padron, D.; Luque, R.; Mauriello, F. Reductive Catalytic Routes towards Sustainable Production of Hydrogen, Fuels and Chemicals from Biomass Derived Polyols. Renew. Sustain. Energy Rev. 2020, 127, 109852. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.J.; Lu, J.M.; Wang, F. Photo Splitting of Bio-Polyols and Sugars to Methanol and Syngas. Nat. Commun. 2020, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M. New Poly(lactide-urethane-isocyanurate) Foams Based on Bio-Polylactide Waste. Polymers 2019, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, F.H.; Najafi, S.K.; Najafi, F.; Pizzi, A.; Behrooz, R.; Sandberg, D. The Extraction of Polyol for The Synthesis of Lignin-Based Polyurethane Coatings—A Review. Wood Mater. Sci. Eng. 2024, 19, 794–802. [Google Scholar] [CrossRef]
- Klempner, D.; Frisch, K.C. Advances in Urethane: Science and Technology; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Sonnenschein, M.F. Polyurethanes; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Kazemi, M.; Fini, E.H. State of the art in the application of functionalized waste polymers in the built environment. Resour. Conserv. Recycl. 2021, 177, 105967. [Google Scholar] [CrossRef]
- Zhou, X.; Deng, J.; Fang, C.; Yu, R.; Lei, W.; He, X.; Zhang, C. Preparation and characterization of lysozyme@carbon nanotubes/waterborne polyurethane composite and the potential application in printing inks. Prog. Org. Coat. 2020, 142, 105600. [Google Scholar] [CrossRef]
- Karpati, L.; Szarka, G.; Hartman, M.; Vargha, V. Oligoester and Polyester Production via Acido-Alcoholysis of PET Waste. Period. Polytech. Chem. Eng. 2018, 62, 336–344. [Google Scholar] [CrossRef]
- Bicerano, J. Crystallization of Polypropylene and Poly(Ethylene Terephthalate). Polym. Rev. 2008, 38, 391–479. [Google Scholar] [CrossRef]
- Smith, R.L.; Takkellapati, S.; Riegerix, R. Recycling of Plastics in the United States: Plastic Material Flows and Polyethylene Terephthalate (PET) Recycling Processes. ACS Sustain. Chem. Eng. 2022, 10, 2084–2096. [Google Scholar] [CrossRef] [PubMed]
- Welle, F. Twenty Years of PET Bottle to Bottle Recycling-an Overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Caputto, M.D.D.; Navarro, R.; Valentin, J.L.; Marcos-Fernandez, A. Chemical Upcycling of Poly(Ethylene Terephthalate) Waste: Moving to A Circular Model. J. Polym. Sci. 2022, 60, 3269–3283. [Google Scholar] [CrossRef]
- Duldner, M.; Dolana, S.; Bartha, E.; Teodorescu, F.; Slabu, A.; Tincu, R.; Sarbu, A.; Ghiurea, M.; Gavrila, A.M.; Ghebaur, A.; et al. Nano-composite semi-flexible polyurethane foams containing montmorillonite intercalated/exfoliated in situ during the synthesis of the intermediate polyester-polyols obtained from PET wastes. Eur. Polym. J. 2023, 196, 112280. [Google Scholar] [CrossRef]
- Senra, E.M.; Silva, A.L.N.; Pacheco, E.B.A.V. A Review of Waterborne Polyurethane Coatings and Adhesives with Polyester Polyol from Poly(Ethylene Terephthalate) Waste. J. Polym. Environ. 2023, 31, 3719–3739. [Google Scholar] [CrossRef]
- Luo, X.L.; Li, Y.B. Synthesis and Characterization of Polyols and Polyurethane Foams from PET Waste and Crude Glycerol. J. Polym. Environ. 2014, 22, 318–328. [Google Scholar] [CrossRef]
- Atta, A.M.; Brostow, W.; Datashvili, T.; El-Ghazawy, R.A.; Lobland, H.E.H.; Hasan, A.R.M.; Perez, J.M. Porous Polyurethane Foams Based on Recycled Poly(Ethylene Terephthalate) for Oil Sorption. Polym. Int. 2013, 62, 116–126. [Google Scholar] [CrossRef]
- Sangalang, A.; Bartolome, L.; Kim, D.H. Generalized Kinetic Analysis of Heterogeneous PET Glycolysis: Nucleation-Controlled Depolymerization. Polym. Degrad. Stab. 2015, 115, 45–53. [Google Scholar] [CrossRef]
- Fang, P.T.; Liu, B.; Xu, J.L.; Zhou, Q.; Zhang, S.J.; Ma, J.Y.; Lu, X.M. High-Efficiency Glycolysis of Poly(Ethylene Terephthalate) by Sandwich-Structure Polyoxometalate Catalyst with Two Active Sites. Polym. Degrad. Stab. 2018, 156, 22–31. [Google Scholar] [CrossRef]
- Kim, H.T.; Kim, J.K.; Cha, H.G.; Kang, M.J.; Lee, H.S.; Khang, T.U.; Yun, E.J.; Lee, D.H.; Song, B.K.; Park, S.J.; et al. Biological Valorization of Poly(ethylene terephthalate) Monomers for Upcycling Waste PET. ACS Sustain. Chem. Eng. 2019, 7, 19396–19406. [Google Scholar] [CrossRef]
- Imek, B.; Uygunolu, T.; Korucu, H.; Kocakerim, M.M. Analysis of the Effects of Dioctyl Terephthalate Obtained from Polyethylene Terephthalate Wastes on Concrete Mortar: A Response Surface Methodology Based Desirability Function Approach Application. J. Clean. Prod. 2017, 170, 437–445. [Google Scholar] [CrossRef]
- Liu, L.; Hao, P.P.; Zhang, R.Q.; Zhang, Y.J.; Zhou, Q.; Lu, X.M.; Yan, D.X.; Li, Y.; Shi, C.Y. Preparation of High-Performance Polyurethane Elastomers Via Direct Use of Alcoholyzed Waste PET by High Molecular Weight Polyester Diols. J. Appl. Polym. Sci. 2023, 140, e54238. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K.A. Bacterium that Degrades and Assimilates Poly(Ethylene Terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Breite, D.; Song, C.; Grasing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic Degradation Efficiency of Postconsumer Polyethylene Terephthalate Packaging Determined by Their Polymer Microstructures. Adv. Sci. 2019, 6, 1900491. [Google Scholar] [CrossRef] [PubMed]
- Esquer, R.; Garcia, J.J. Metal-Catalysed Poly(Ethylene) Terephthalate and Polyurethane Degradations by Glycolysis. J. Organomet. Chem. 2019, 902, 120972. [Google Scholar] [CrossRef]
- Scremin, D.M.; Miyazaki, D.Y.; Lunelli, C.E.; Silva, S.A.; Zawadzki, S.F. Pet Recycling by Alcoholysis Using a New Heterogeneous Catalyst: Study and Its Use in Polyurethane Adhesives Preparation. Macromol. Symp. 2019, 383, 1800027. [Google Scholar] [CrossRef]
- Kathalewar, M.; Dhopatkar, N.; Pacharane, B.; Sabnis, A.; Raut, P.; Bhave, V. Chemical Recycling of PET Using Neopentyl Glycol: Reaction Kinetics and Preparation of Polyurethane Coatings. Prog. Org. Coat. 2013, 76, 147–156. [Google Scholar] [CrossRef]
- Nunes, S.; da Silva, M.J.V.; da Silva, D.C.; Freitas, A.R.; Rosa, F.A.; Rubira, A.F.; Muniz, E.C. PET Depolymerisation In Supercritical Ethanol Catalysed by [Bmim][BF4]. Rsc Adv. 2014, 4, 20308–20316. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Abd Hamid, M.K.; Samsudin, S.A.; Sabeen, A.H. Current Developments in Chemical Recycling of Post-Consumer Polyethylene Terephthalate Wastes for New Materials Production: A Review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Pham, C.T.; Nguyen, B.T.; Nguyen, M.T.; Nguyen, T.H.; Hoang, C.N.; Nguyen, N.N.; Lee, P.C.; Kim, J.; Hoang, D. The Advancement of Bis(2-Hydroxyethyl)Terephthalate Recovered from Post-Consumer Poly(Ethylene Terephthalate) Bottles Compared to Commercial Polyol for Preparation of High Performance Polyurethane. J. Ind. Eng. Chem. 2021, 93, 196–209. [Google Scholar] [CrossRef]
- Mendiburu-Valor, E.; Larraza, I.; Echeverria-Altuna, O.; Harismendy, I.; Pena-Rodriguez, C.; Eceiza, A. Thermoset polyurethanes from biobased and recycled components. J. Polym. Environ. 2023, 31, 4946–4959. [Google Scholar] [CrossRef]
- Shamsi, R.; Sadeghi, G.M.M.; Vahabi, H.; Seyfi, J.; Sheibani, R.; Zarrintaj, P.; Laoutid, F.; Saeb, M.R. Hopes Beyond PET Recycling: Environmentally Clean and Engineeringly Applicable. J. Polym. Environ. 2019, 27, 2490–2508. [Google Scholar] [CrossRef]
- Shamsi, R.; Sadeghi, G.M.M. Novel Polyester Diol Obtained From PET Waste and Its Application in The Synthesis of Polyurethane and Carbon Nanotube-Based Composites: Swelling Behavior and Characteristic Properties. RSC Adv. 2016, 6, 38399–38415. [Google Scholar] [CrossRef]
- Scheirs, J.; Kaminsky, W. Feedstock Recycling and Pyrolysis of Waste Plastics: Converting Waste Plastics into Diesel and Other Fuels; Focus on Catalysts; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 161–192. [Google Scholar] [CrossRef]
- Azeem, M.; Attallah, O.A.; Tas, C.E.; Fournet, M.B. All Green Microwave Assisted 99% Depolymerisation of Polyethylene Terephthalate into Value Added Products via Glycerol Pre-treatment and Hydrolysis Reaction. J. Polym. Environ. 2023, 32, 303–315. [Google Scholar] [CrossRef]
- Strukil, V. Highly Efficient Solid-State Hydrolysis of Waste Polyethylene Terephthalate by Mechanochemical Milling and Vapor-Assisted Aging. Chemsuschem 2020, 14, 330–338. [Google Scholar] [CrossRef]
- Kang, M.J.; Yu, H.J.; Jegal, J.; Kim, H.S.; Cha, H.G. Depolymerization of PET into Terephthalic Acid in Neutral Media Catalyzed by the ZSM-5 Acidic Catalyst. Chem. Eng. J. 2020, 398, 125655. [Google Scholar] [CrossRef]
- Liu, Q.L.; Li, R.S.; Fang, T. Investigating and Modeling PET Methanolysis under Supercritical Conditions by Response Surface Methodology Approach. Chem. Eng. J. 2015, 270, 535–541. [Google Scholar] [CrossRef]
- Jadhav, A.L.; Malkar, R.S.; Yadav, G.D. Zn-and Ti-Modified Hydrotalcites for Transesterification of Dimethyl Terephthalate with Ethylene Glycol: Effect of the Metal Oxide and Catalyst Synthesis Method. Acs Omega 2020, 5, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, G.M.M.; Shamsi, R.; Sayaf, M. From Aminolysis Product of PET Waste to Novel Biodegradable Polyurethanes. J. Polym. Environ. 2011, 19, 522–534. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Delavardea, A.; Savin, G.; Derkenne, P.; Boursier, M.; Morales-Cerrada, R.; Nottelet, B.; Pinaud, J.; Caillol, S. Sustainable polyurethanes: Toward new cutting-edge opportunities. Prog. Polym. Sci. 2024, 151, 101805. [Google Scholar] [CrossRef]
- Shaaban, A.; Elsabbagh, A.M. Crashworthiness Optimization of Impact Attenuators Constructed of Polyurethane Foam. Int. J. Automot. Technol. 2022, 23, 389–401. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, D.; Xu, W. Effect of Polyurethane Non-Transparent Coating Process on Paint Film Performance Applied on Modified Poplar. Coatings 2022, 12, 39. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J.; Zo, S.M.; Rao, K.M.; Seok, Y.J.; Won, S.Y.; Han, S.S. Revised Manuscript with Corrections: Polyurethane-Based Conductive Composites: From Synthesis to Applications. Int. J. Mol. Sci. 2022, 23, 1938. [Google Scholar] [CrossRef] [PubMed]
- Nering, K.; Kowalska-Koczwara, A. Determination of Vibroacoustic Parameters of Polyurethane Mats for Residential Building Purposes. Polymers 2022, 14, 314. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.H.; Zhu, S.W.; Liu, S.W.; Liu, Y. Analysis of the Influencing Factors of the Efficient Degradation of Waste Polyurethane and Its Scheme Optimization. Polymers 2023, 15, 2337. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Y.; Westman, Z.; Richardson, K.; Lim, D.; Stottlemyer, A.L.; Farmer, T.; Gillis, P.; Hooshyar, N.; Vlcek, V.; Christopher, P.; et al. Polyurethane Foam Chemical Recycling: Fast Acidolysis with Maleic Acid and Full Recovery of Polyol. ACS Sustain. Chem. Eng. 2024, 12, 4435–4443. [Google Scholar] [CrossRef]
- Gu, X.; Wang, X.; Guo, X.; Liu, S.; Lou, C.; Liu, Y. Study on Efficient Degradation of Waste PU Foam. Polymers 2023, 15, 2359. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Choi, J.W.; Choi, N.E.; Chun, B.C. Endurance of linear and cross-linked shape memory polyurethane under rigorous hydrolysis conditions. Fiber. Polym. 2009, 10, 576–582. [Google Scholar] [CrossRef]
- Gu, X.H.; Wang, X.Y.; Guo, X.Y.; Liu, S.W.; Li, Q.; Liu, Y. Study and Characterization of Regenerated Hard Foam Prepared by Polyol Hydrolysis of Waste Polyurethane. Polymers 2023, 15, 1445. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.Y.; Hatano, B.; Kadokawa, J.; Tagaya, H. Effect of Diaminotoluene on the Decomposition of Polyurethane Foam Waste in Superheated Water. Polym. Degrada. Stab. 2002, 76, 179–184. [Google Scholar] [CrossRef]
- Trzebiatowska, P.J.; Benešb, H.; Datta, J. Evaluation of the Glycerolysis Process and Valorisation of Recovered Polyol in Polyurethane Synthesis. React. Funct. Polym. 2019, 139, 25–33. [Google Scholar] [CrossRef]
- Najafi-Shoa, S.; Barikani, M.; Ehsani, M.; Ghaffari, M.; Vandalvand, M. Efficient and Eco-Friendly UV-Cured Polyurethane Coating: Harnessing Thiol-Yne Systems for Corrosion Protection. Mater. Today Commun. 2024, 39, 109037. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Czupryński, B.; Liszkowska, J. Glycolysis of Rigid Polyurethane-Polyisocyanurate Foams. J. Elastomers Plast. 2016, 48, 340–353. [Google Scholar] [CrossRef]
- Beneš, H.; Roesner, J.; Holler, P.; Synkova, H.; Kotek, J.; Horak, Z. Glycolysis of Flexible Polyurethane Foam in Recycling of Car Seats. Polym. Adv. Technol. 2007, 18, 149–156. [Google Scholar] [CrossRef]
- Motokucho, S.; Nakayama, Y.; Morikawa, H.; Nakatani, H. Environment-Friendly Chemical Recycling of Aliphatic Polyurethanes by Hydrolysis in a CO2-Water System. J. Appl. Polym. Sci. 2018, 135, 45897. [Google Scholar] [CrossRef]
- Mahoney, L.R.; Weiner, S.A.; Ferris, F.C. Hydrolysis of Polyurethane Foam Waste. Environ. Sci. Technol. 1974, 8, 135–139. [Google Scholar] [CrossRef]
- Hung, C.S.; Barlow, D.E.; Varaljay, V.A.; Drake, C.A.; Crouch, A.L.; Russell, J.N.; Nadeau, L.J.; Crookes-Goodson, W.J.; Biffinger, J.C. The Biodegradation of Polyester and Polyester Polyurethane Coatings Using Papiliotrema Laurentii. Int. Biodeterior. Biodegrad. 2019, 139, 34–43. [Google Scholar] [CrossRef]
- Yamane, S.; Watanabe, R.; Ata, S.; Mizukado, J.; Shinzawa, H. Solvent-Induced Degradation of Polyurethane Studied by Two-Dimensional (2D) Infrared (IR) Correlation Spectroscopy. Vib. Spectrosc. 2020, 108, 103062. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; De Lucas, A.; Rodríguez, J.F. Glycolysis of Viscoelastic Flexible Polyurethane Foam Wastes. Polym. Degrad. Stab. 2015, 116, 23–35. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane Recycling and Disposal: Methods and Prospects. Ploymers 2020, 12, 1752. [Google Scholar] [CrossRef]
- Simón, D.; de Lucas, A.; Rodríguez, J.F.; Borreguero, A.M. Flexible Polyurethane Foams Synthesized Employing Recovered Polyols from Glycolysis: Physical and Structural Properties. J. Appl. Polym. Sci. 2017, 134, 45087. [Google Scholar] [CrossRef]
- Datta, J.; Haponiuk, J.T. Advanced Coating of Interior of Tanks for Rising Environmental Safety-Novel Applications of Polyurethanes. Pol. Marit. Res. 2008, 15, 8–13. [Google Scholar] [CrossRef]
- Ko, J.Y.; Zarei, M.; Lee, S.G.; Cho, K.L. Single-Phase Recycling of Flexible Polyurethane Foam by Glycolysis and Oxyalkylation: Large-Scale Industrial Evaluation. ACS Sustain. Chem. Eng. 2023, 11, 10074–10082. [Google Scholar] [CrossRef]
- Shin, S.R.; Maim, V.D.; Lee, D.S. Chemical Recycling of Used Printed Circuit Board Scraps: Recovery and Utilization of Organic Products. Processes 2019, 7, 22. [Google Scholar] [CrossRef]
- Ionescu, M. Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology Limited: Shawbury, UK, 2005. [Google Scholar]
- Shin, S.R.; Kim, H.N.; Liang, J.Y.; Lee, S.H.; Lee, D.S. Sustainable Rigid Polyurethane Foams Based on Recycled Polyols from Chemical Recycling of Waste Polyurethane Foams. J. Appl. Polym. Sci. 2019, 136, 47916. [Google Scholar] [CrossRef]
- Vanbergen, T.; Verlent, I.; De Geeter, J.; Haelterman, B.; Claes, L.; De Vos, D. Recycling of Flexible Polyurethane Foam by Split-Phase Alcoholysis: Identification of Additives and Alcoholyzing Agents to Reach Higher Efficiencies. Chemsuschem 2020, 13, 3835–3843. [Google Scholar] [CrossRef]
- Zhu, P.; Cao, Z.B.; Chen, Y.; Zhang, X.J.; Qian, G.R.; Chu, Y.L.; Zhou, M. Glycolysis Recycling of Rigid Waste Polyurethane Foam from Refrigerators. Environ. Technol. 2014, 35, 2676–2684. [Google Scholar] [CrossRef]
- Simon, D.; Rodriguez, J.F.; Carmona, M.; Serrano, A.; Borreguero, A.M. Glycolysis of Advanced Polyurethanes Composites Containing Thermoregulating Microcapsules. Chem. Eng. J. 2018, 350, 300–311. [Google Scholar] [CrossRef]
- Kanaya, K.; Takahashi, S. Decomposition of Polyurethane Foams by Alkanolamines. J. Appl. Polym. Sci. 2010, 51, 675–682. [Google Scholar] [CrossRef]
- Modesti, M.; Simioni, F. Chemical Recycling of Reinforced Polyurethane from the Automotive Industry. Polym. Eng. Sci. 1996, 36, 2173–2178. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Garmarudi, A.B.; Idris, A.B. Polyurethane Waste Reduction and Recycling: From Bench to Pilot Scales. Des. Monomers Polym. 2011, 14, 395–421. [Google Scholar] [CrossRef]
- Troev, K.; Atanasov, V.I.; Tsevi, R. Chemical Degradation of Polyurethanes I: Degradation of Microporous Polyurethane Elastomer by Dimethyl Phosphonate. Polym. Degrad. Stab. 2000, 67, 159–165. [Google Scholar] [CrossRef]
- Troev, K.; Grancharov, G.; Tsevi, R.; Tsekova, A. A Novel Approach to Recycling of Polyurethanes: Chemical Degradation of Flexible Polyurethane Foams by Triethyl Phosphate. Polymer 2000, 41, 7017–7022. [Google Scholar] [CrossRef]
- Troev, K.; Tsekova, A.; Tsevi, R. Chemical Degradation of Polyurethanes II: Degradation of Microporous Polyurethane Elastomer by Phosphoric Acid Esters. J. Appl. Polym. Sci. 2000, 76, 886–893. [Google Scholar] [CrossRef]
- Mitova, V.; Grancharov, G.; Molero, C.; Borreguero, A.M.; Troev, K.; Rodriguez, J.F. Chemical Degradation of Polymers (Polyurethanes, Polycarbonate and Polyamide) by Esters of H-phosphonic and Phosphoric Acids. J. Macromol. Sci. Part A 2013, 50, 774–795. [Google Scholar] [CrossRef]
- Abolins, A.; Yakushin, V.; Vilsone, D. Properties of Polyurethane Coatings Based on Linseed Oil Phosphate Ester Polyol. J. Renew. Mater. 2018, 6, 737–745. [Google Scholar] [CrossRef]
- Heinen, M.; Gerbase, A.E.; Petzhold, C.L. Vegetable Oil-Based Rigid Polyurethanes and Phosphorylated Flame-Retardants Derived from Epoxydized Soybean Oil. Polym. Degrad. Stab. 2014, 108, 76–86. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Nikrah, M.; Mohammadi, F.H.A. Microwave-Assisted Polyurethane Bond Cleavage via Hydroglycolysis Process at Atmospheric Pressure. J. Cell. Plast. 2008, 44, 367–380. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of Polyurethanes from Laboratory to Industry, a Journey towards the Sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef]
- Gerlock, J.; Braslaw, J.; Zinbo, M. Polyurethane Waste Recycling; Glycolysis and Hydroglycolysis of Water-Blown Foams. Ind. Eng. Chem. Process. Des. Dev. 1984, 23, 545–552. [Google Scholar] [CrossRef]
- Ansari, I.; Singh, P.; Mittal, A.; Mahato, R.I.; Chitkara, D. 2,2-Bis(Hydroxymethyl) Propionic Acid Based Cyclic Carbonate Monomers and Their (Co)Polymers as Advanced Materials for Biomedical Applications. Biomaterials 2021, 275, 120953. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.R.; Bhat, G.A.; Darensbourg, D.J. Enabling New Approaches: Recent Advances in Processing Aliphatic Polycarbonate-Based Materials. Angew. Chem. Int. Ed. 2023, 62, e202307507. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Yoo, K.; Borchardt, L.; Kim, J.G. Chemical Recycling of Polycarbonate and Polyester without Solvent and Catalyst: Mechanochemical Methanolysis. Green Chem. 2024, 26, 2087–2093. [Google Scholar] [CrossRef]
- Fehér, Z.; Németh, R.; Kiss, J.; Balterer, B.; Verebélyi, K.; Iván, B.; Kupai, J. A Silica-Supported Organocatalyst for Polycarbonate Methanolysis Under Mild and Economic Conditions. Chem. Eng. J. 2024, 485, 149832. [Google Scholar] [CrossRef]
- Kim, D.; Kim, B.K.; Cho, Y.; Han, M.; Kim, B.S. Kinetics of Polycarbonate Glycolysis in Ethylene Glycol. Ind. Eng. Chem. Res. 2009, 48, 685–691. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Askarzadeh, M. Chemical Recycling of Polycarbonate Wastes into Bisphenol a by Using Green Solvent Composition. Polimery 2013, 58, 292–294. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Yeung, A.D. A Concise Review of Computational Studies of The Carbon Dioxide–Epoxide Copolymerization Reactions. Polym. Chem. 2014, 5, 3949–3962. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, Y.H.; Chen, L.Y.; Dai, C.A.; Dai, S.H.A.; Chen, Y.H.; Wu, C.H.; Jeng, R.J. Robust Thermoplastic Polyurethane Elastomers Prepared from Recycling Polycarbonate. Polymer 2021, 212, 123296. [Google Scholar] [CrossRef]
- Jang, T.; Kim, H.J.; Jang, J.B.; Kim, T.H.; Lee, W.; Seo, B.; Ko, W.B.; Lim, C.S. Synthesis of Waterborne Polyurethane Using Phosphorus-Modified Rigid Polyol and its Physical Properties. Polymer 2021, 13, 432. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Cai, W.H.; Li, X.; Wang, K.B.; Zhou, L.; You, T.X.; Wang, R.; Chen, H.; Zhao, Y.C.; Wang, J. Preparation of Phospholipid-Based Polycarbonate Urethanes for Potential Applications of Blood-Contacting Implants. Regen. Biomater. 2020, 7, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Orgiles-Calpena, E.; Aran-Ais, F.; Torro-Palau, A.M.; Orgiles-Barcelo, C. Novel Polyurethane Reactive Hot Melt Adhesives Based on Polycarbonate Polyols Derived from CO2 For the Footwear Industry. Int. J. Adhes. Adhes. 2016, 70, 218–224. [Google Scholar] [CrossRef]
- Garcia-Pacios, V.; Costa, V.; Colera, M.; Martin-Martinez, J.M. Waterborne Polyurethane Dispersions Obtained with Polycarbonate of Hexanediol Intended for Use as Coatings. Prog. Org. Coat. 2011, 71, 136–146. [Google Scholar] [CrossRef]
- Benes, H.; Paruzel, A.; Hodan, J.; Trhlikova, O. Impact of Natural Oil-Based Recycled Polyols on Properties of Cast Polyurethanes. J. Renew. Mater. 2018, 6, 697–706. [Google Scholar] [CrossRef]
- Lambeth, R.H.; Henderson, T.J. Organocatalytic Synthesis of (Poly) Hydroxyurethanes from Cyclic Carbonates and Amines. Polymer 2013, 54, 5568–5573. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Isocyanate, and Non-isocyanate Polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Yang, X.X.; Wang, S.B.; Liu, X.X.; Huang, Z.; Huang, X.J.; Xu, X.; Liu, H.; Wang, D.; Shang, S.B. Preparation of Non-isocyanate Polyurethanes from Epoxy Soybean Oil: Dual Dynamic Networks to Realize Self-healing and Reprocessing under Mild Conditions. Green Chem. 2021, 23, 6349–6355. [Google Scholar] [CrossRef]
- Tran, M.H.; Lee, E.Y. Production of polyols and polyurethane from biomass: A review. Environ. Chem. Lett. 2023, 21, 2199–2223. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Q.; Li, J.; Jiang, L.; Wang, S.H.; Fan, H.J.; Chen, Y.; Yan, J.; Xiang, J. Tuning the Hydrophobicity of Bio-Based Waterborne Polyurethane by Leveraging a Diol Derived from Oleic Acid. Ind. Crops Prod. 2022, 187, 115400. [Google Scholar] [CrossRef]
- Kaikade, D.S.; Sabnis, A.S. Polyurethane foams from vegetable oil-based polyols: A review. Polym. Bull. 2023, 80, 2239–2261. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, H.; Rastin, H.; Movahedifar, E.; Antoun, K.; Brosse, N.; Saeb, M.R. Flame Retardancyof Bio-Based Polyurethanes: Opportunities and Challenges. Polymers 2020, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Hormaiztegui, M.E.V.; Aranguren, M.I.; Mucci, V.L. Synthesis and Characterization of a Waterborne Polyurethane Made from Castor Oil and Tartaric Acid. Eur. Polym. J. 2018, 102, 151–160. [Google Scholar] [CrossRef]
- Gurunathan, T.; Chung, J.S. Physicochemical Properties of Amino-Silane-Terminated Vegetable Oil-Based Waterborne Polyurethane Nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 4645–4653. [Google Scholar] [CrossRef]
- Liang, H.Y.; Wang, S.W.; He, H.; Wang, M.Q.; Liu, L.X.; Lu, J.Y.; Zhang, Y.; Zhang, C.Q. Aqueous Anionic Polyurethane Dispersions from Castor Oil. Ind. Crops Prod. 2018, 122, 182–189. [Google Scholar] [CrossRef]
- Luong, N.D.; Sinh, L.H.; Minna, M.; Jurgen, W.; Torsten, W.; Matthias, S.; Jukka, S. Synthesis and Characterization of Castor Oil-Segmented Thermoplastic Polyurethane with Controlled Mechanical Properties. Eur. Polym. J. 2016, 81, 129–137. [Google Scholar] [CrossRef]
- Dodangeh, F.; Dorraji, M.S.S.; Rasoulifard, M.H.; Ashjari, H.R. Synthesis and Characterization of Alkoxy Silane Modified Polyurethane Wood Adhesive Based on Epoxidized Soybean Oil Polyester Polyol. Compos. Part B 2020, 187, 107857. [Google Scholar] [CrossRef]
- Liu, L.X.; Lu, J.Y.; Zhang, Y.; Liang, H.Y.; Liang, D.S.; Jiang, J.Z.; Lu, Q.M.; Quirino, R.L.; Zhang, C.Q. Thermosetting Polyurethanes Prepared with The Aid of a Fully Bio-Based Emulsifier with High Bio-Content, High Solid Content, and Superior Mechanical Properties. Green Chem. 2019, 21, 526–537. [Google Scholar] [CrossRef]
- Shendi, H.K.; Omrani, I.; Ahmadi, A.; Farhadian, A.; Babnejad, N.; Nabid, M.R. Synthesis and Characterization of a Novel Internal Emulsifier Derived from Sunflower Oil for the Preparation of Waterborne Polyurethane and Their Application in Coatings. Prog. Org. Coat. 2017, 105, 303–309. [Google Scholar] [CrossRef]
- Babanejad, N.; Farhadian, A.; Omrani, I.; Nabid, M.R. Design, Characterization and in Vitro Evaluation of Novel Amphiphilic Block Sunflower Oil-Based Polyol Nanocarrier as a Potential Delivery System: Raloxifene-Hydrochloride as a Model. Mater. Sci. Eng. C 2017, 78, 59–68. [Google Scholar] [CrossRef]
- Omrani, I.; Farhadian, A.; Babanejad, N.; Shendi, H.K.; Ahmadi, A.; Nabid, M.R. Synthesis of Novel High Primary Hydroxyl Functionality Polyol from Sunflower Oil using Thiol-Ene Reaction and Their Application in Polyurethane Coating. Eur. Polym. J. 2016, 82, 220–231. [Google Scholar] [CrossRef]
- Hajirahimkhan, S.; Xu, C.C.; Ragogna, P.J. UV Curable Coatings of Modified Lignin. ACS Sustain. Chem. Eng. 2018, 6, 14685–14694. [Google Scholar] [CrossRef]
- Guo, L.; Huang, S.; Qu, J.Q. Synthesis and Properties of High-Functionality Hydroxyl-Terminated Polyurethane Dispersions. Prog. Org. Coat. 2018, 119, 214–220. [Google Scholar] [CrossRef]
- Valverde, C.; Lligadas, G.; Ronda, J.C. Hydroxyl Functionalized Renewable Polyesters Derived From 10-Undecenoic Acid: Polymer Structure and Postpolymerization Modification. Eur. Polym. J. 2018, 105, 68–78. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Q.T.; Yan, Z.; Liao, G.F.; Zhang, B.X.; Yu, Y.M.; Yi, C.F.; Xu, Z.S. Design and Synthesis of Novel Aminosiloxane Crosslinked Linseed Oil-Based Waterborne Polyurethane Composites and Its Physicochemical Properties. Prog. Org. Coat. 2019, 127, 194–201. [Google Scholar] [CrossRef]
- Lu, K.T.; Chang, J.P. Synthesis and Antimicrobial Activity of Metal-Containing Linseed Oil-Based Waterborne Urethane Oil Wood Coatings. Polymer 2020, 12, 663. [Google Scholar] [CrossRef]
- Chen, R.Q.; Zhang, C.Q.; Kessler, M.R. Anionic Waterborne Polyurethane Dispersion from a Bio-Based Ionic Segment. RSC Adv. 2014, 4, 35476–35483. [Google Scholar] [CrossRef]
- Openshaw, K. A Review of Jatropha curcas: An Oil Plant of Unfulfilled Promise. Biomass Bioenergy 2000, 19, 1–15. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S. An Evaluation of Multipurpose Oil Seed Crop for Industrial Uses (Jatropha curcas L.): A Review. Ind. Crops Prod. 2008, 28, 1–10. [Google Scholar] [CrossRef]
- Ling, C.K.; Aung, M.M.; Rayung, M.; Abdullah, L.C.; Lim, H.N.; Noor, I.S.M. Performance of Ionic Transport Properties in Vegetable Oil-Based Polyurethane Acrylate Gel Polymer Electrolyte. ACS Omega 2019, 4, 2554–2564. [Google Scholar] [CrossRef]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Biak, D.R.A.; Basri, M.; Jusoh, E.R.; Mamat, S. Physicochemical Properties of Jatropha Oil-Based Polyol Produced by a Two Steps Method. Molecules 2017, 22, 551. [Google Scholar] [CrossRef] [PubMed]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Biak, D.R.A.; Basri, M.; Jusoh, E.R.; Mamat, S. Colloidal Stability and Rheology of Jatropha Oil-Based Waterborne Polyurethane (JPU) Dispersion. Prog. Org. Coat. 2018, 125, 348–357. [Google Scholar] [CrossRef]
- Yusof, N.S.B.; Sapuan, S.M.; Sultan, M.T.H.; Jawaid, M. Manufacturing Process Selection of “Green” Oil Palm Natural Fiber Reinforced Polyurethane Composites Using Hybrid TEA Criteria Requirement and AHP Method for Automotive Crash Box. J. Renew. Mater. 2020, 8, 647–660. [Google Scholar] [CrossRef]
- Su, Y.P.; Lin, H.; Zhang, S.T.; Yang, Z.H.; Yuan, T. One-Step Synthesis of Novel Renewable Vegetable Oil-Based Acrylate Prepolymers and Their Application in UV-Curable Coatings. Polymer 2020, 12, 1165. [Google Scholar] [CrossRef] [PubMed]
- Sittinun, A.; Pisitsak, P.; Manuspiya, H.; Thiangtham, S.; Chang, Y.H.; Ummartyotin, S. Utilization of Palm Olein-Based Polyol for Polyurethane Foam Sponge Synthesis: Potential as A Sorbent Material. J. Polym. Environ. 2020, 28, 3181–3191. [Google Scholar] [CrossRef]
- Ghosh, T.; Karak, N. Cashew Nut Shell Liquid Terminated Self-Healable Polyurethane as an Effective Anticorrosive Coating with Biodegradable Attribute. Prog. Org. Coat. 2020, 139, 105472. [Google Scholar] [CrossRef]
- Kathalewar, M.; Sabnis, A. Preparation of Novel CNSL-Based Urethane Polyol via Nonisocyanate Route: Curing with Melamine-Formaldehyde Resin and Structure-Property Relationship. J. Appl. Polym. Sci. 2014, 132, 41391. [Google Scholar] [CrossRef]
- Shrestha, M.L.; Ionescu, M.; Wan, X.M.; Bilic, N.; Petrovic, Z.S.; Upshaw, T. Biobased Aromatic-Aliphatic Polyols from Cardanol by Thermal Thiol-Ene Reaction. J. Renew. Mater. 2018, 6, 87–101. [Google Scholar] [CrossRef]
- Wang, H.R.; Zhou, Q.X. Synthesis of Cardanol-Based Polyols via Thiol-ene/Thiol-epoxy Dual Click-Reactions and Thermosetting Polyurethanes Therefrom. ACS Sustain. Chem. Eng. 2018, 6, 12088–12095. [Google Scholar] [CrossRef]
- Monica, F.D.; Kleij, A.W. From Terpenes to Sustainable and Functional Polymers. Polym. Chem. 2020, 11, 5109–5127. [Google Scholar] [CrossRef]
- Touaibia, M.; Boutekedjiret, C.; Perino, S. Natural Terpenes as Building Blocks for Green Chemistry; Springer: Singapore, 2019; pp. 171–195. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.F.; Wu, G.M.; Jin, C.; Kong, Z.W. Preparation and Antimicrobial Activity of Terpene-Based Polyurethane Coatings with Carbamate Group-Containing Quaternary Ammonium Salts. Prog. Org. Coat. 2015, 80, 150–155. [Google Scholar] [CrossRef]
- Firdaus, M.; Meier, M.A.R. Renewable Polyamides and Polyurethanes Derived from Limonene. Green Chem. 2013, 15, 370–380. [Google Scholar] [CrossRef]
- Gupta, R.K.; Ionescu, M.; Radojcic, D.; Wan, X.; Petrovic, Z.S. Novel Renewable Polyols Based on Limonene for Rigid Polyurethane Foams. J. Polym. Environ. 2014, 22, 304–309. [Google Scholar] [CrossRef]
- Bhr, M.; Bitto, A.; Mülhaupt, R. Cyclic Limonene Dicarbonate as a New Monomer for Non-Isocyanate Oligo- and Polyurethanes (NIPU) Based upon Terpenes. Green Chem. 2012, 14, 1447–1454. [Google Scholar] [CrossRef]
- Luc, C.; Xavier, F.; Serge, K. Ultrasonic and Catalyst Free Epoxidation of Limonene and Other Terpenes Using Dimethyl Dioxirane in Semi-Batch Conditions. ACS Sustain. Chem. Eng. 2018, 6, 12224–12231. [Google Scholar] [CrossRef]
- Wu, G.M.; Kong, Z.W.; Chen, J.; Huo, S.P.; Liu, G.F. Preparation and Properties of Waterborne Polyurethane/Epoxy Resin Composite Coating from Anionic Terpene-Based Polyol Dispersion. Prog. Org. Coat. 2014, 77, 315–321. [Google Scholar] [CrossRef]
- Maiti, S.; Ray, S.S.; Kundu, A.K. Rosin: A Renewable Resource for Polymers and Polymer Chemicals. Prog. Polym. Sci. 1989, 14, 297–338. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.X.; Tang, C.B. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef]
- Li, T.T.; Liu, X.Q.; Jiang, Y.H.; Ma, S.Q.; Zhu, J. Bio-Based Shape Memory Epoxy Resin Synthesized from Rosin Acid. Iran. Polym. J. 2016, 25, 1–9. [Google Scholar] [CrossRef]
- Xu, X.; Song, Z.Q.; Shang, S.B.; Cui, S.Q.; Rao, X.P. Synthesis and Properties of Novel Rosin-Based Water-Borne Polyurethane. Polym. Int. 2011, 60, 1521–1526. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Chen, Y.C. Synthesis of Bio-Based Polyurethane Foam Modified with Rosin Using an Environmentally-Friendly Process. J. Clean. Prod. 2020, 276, 124203. [Google Scholar] [CrossRef]
- Liu, G.F.; Wu, G.M.; Chen, J.; Kong, Z.W. Synthesis, Modification and Properties of Rosin-Based Non-Isocyanate Polyurethanes Coatings. Prog. Org. Coat. 2016, 101, 461–467. [Google Scholar] [CrossRef]
- Xu, C.A.; Qu, Z.C.; Lu, M.G.; Meng, H.F.; Zhan, Y.J.; Chen, B.; Wu, K.; Shi, J. Effect of Rosin on the Antibacterial Activity against S. aureus and Adhesion Properties of UV-Curable Polyurethane/Polysiloxane Pressure-Sensitive Adhesive. Colloids Surf. A 2021, 614, 126146. [Google Scholar] [CrossRef]
- Vevere, L.; Fridrihsone, A.; Kirpluks, M.; Cabulis, U. A Review of Wood Biomass-Based Fatty Acids and Rosin Acids Use in Polymeric Materials. Polymer 2020, 12, 2706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Jiang, Y.H.; Xiong, Z.; Liu, X.Q.; Na, H.N.; Zhang, R.Y.; Zhu, J. Highly Recoverable Resin-Based Shape Memory Polyurethenes. J. Mater. Chem. A. 2013, 1, 3263. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2013, 110, 3552–3599. [Google Scholar] [CrossRef]
- Rad, E.R.; Vahabi, H.; de Anda, A.R.; Saeb, M.R.; Thomas, S. Bio-epoxy resins with inherent flame retardancy. Prog. Org. Coat. 2020, 135, 608–612. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Zhu, J.; Yan, N. Lignin-Based Polyurethane: Recent Advances and Future Perspectives. Macromol. Rapid Commun. 2020, 42, 2000492. [Google Scholar] [CrossRef]
- Domenek, S.; Louaifi, A.; Guinault, A.; Baumberger, S. Potential of Lignins as Antioxidant Additive in Active Biodegradable Packaging Materials. J. Polym. Environ. 2013, 21, 692–701. [Google Scholar] [CrossRef]
- El Salamouny, S.; Shapiro, M.L.K.S.; Shepard, B.M. Black Tea and Lignin as Ultraviolet Protectants for the Beet Armyworm Nucleopolyhedrovirus. J. Entomol. Sci. 2009, 44, 50–58. [Google Scholar] [CrossRef]
- Liu, L.N.; Qian, M.B.; Song, P.A.; Huang, G.B.; Yu, Y.M.; Fu, S.Y. Fabrication of Green Lignin-Based Flame Retardants for Enhancing the Thermal and Fire Retardancy Properties of Polypropylene/Wood Composites. ACS Sustain. Chem. Eng. 2016, 4, 2422–2431. [Google Scholar] [CrossRef]
- Ren, L.F.; Zhao, Y.X.; Qiang, T.T.; He, Q.Q. Synthesis of a Biobased Waterborne Polyurethane with Epichlorohydrin-Modified Lignin. J. Dispers. Sci. Technol. 2019, 40, 1499–1506. [Google Scholar] [CrossRef]
- Du, J.H.; Wang, H.; Huang, Z.Y.; Liu, X.C.; Yin, X.S.; Wu, J.X.; Lin, W.J.; Lin, X.F.; Yi, G.B. Construction and mechanism study of lignin-based polyurethane with high strength and high self-healing properties. Int. J. Biol. Macromol. 2023, 248, 125925. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhao, Z.; Zhou, T.; Fu, Y.; Tian, G.; Wang, Z. Fabrication of Bio-Based Flexible Polyurethane Foam with Biodegradation by Etherification of Kraft Lignin. ACS Appl. Polym. Mater. 2024, 6, 4441–4448. [Google Scholar] [CrossRef]
- Quan, P.; Kiziltas, A.; Gondaliya, A.; Siahkamari, M.; Nejad, M.; Xie, X. Kraft Lignin with Improved Homogeneity Recovered Directly from Black Liquor and Its Application in Flexible Polyurethane Foams. ACS Omega 2022, 7, 16705–16715. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Ye, Y.; Ying, X.; Huang, J.; Li, X. Introduction of Aminated Sodium Lignosulfonate as a Chain Extender for Preparation of High-Performance Waterborne Polyurethane. Int. J. Adhes. Adhes. 2023, 125, 103415. [Google Scholar] [CrossRef]
- Cheradame, H.; Detoisien, M.; Gandini, A.; Pla, F.; Roux, G. Polyurethane from Kraft Lignin. Br. Polym. J. 1989, 21, 269–275. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Li, P. Conversion of Biomass Lignin to High-Value Polyurethane: A Review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Griffini, G.; Passoni, V.; Suriano, R.; Levi, M.; Turni, S. Polyurethane Coatings Based on Chemically Unmodified Fractionated Lignin. ACS Sustain. Chem. Eng. 2015, 3, 1145–1154. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wyman, C.E.; Cai, C.M.; Ragauskas, A.J. Lignin-Based Polyurethanes from Unmodified Kraft Lignin Fractionated by Sequential Precipitation. ACS Appl. Polym. Mater. 2019, 1, 1672–1679. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Fang, X.; Bai, F.D.; Qiao, K.; Wang, L.M. Renewable High Performance Polyurethane Bioplastics Derived from Lignin-Poly(Ε-Caprolactone). ACS Sustain. Chem. Eng. 2017, 5, 4276–4284. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Cui, C.; Argyropoulos, D.S. Toward Thermoplastic Lignin Polymers. Part I. Selective Masking of Phenolic Hydroxyl Groups in Kraft Lignins via Methylation and Oxypropylation Chemistries. Ind. Eng. Chem. Res. 2012, 51, 16713–16720. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Yan, R.; Ngo, T.D.; Zhao, Q.; Duan, J.C.; Du, X.W.; Wang, Y.L.; Liu, B.J.; Sun, Z.Y.; Hu, W. Ozone Oxidized Lignin-Based Polyurethane with Improved Properties. Eur. Polym. J. 2019, 117, 114–122. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Scheidemantle, B.; Ragauskas, A.J. Polyurethanes Based on Unmodified and Refined Technical Lignins: Correlation between Molecular Structure and Material Properties. Biomacromolecules 2021, 22, 2129–2136. [Google Scholar] [CrossRef]
- Hu, L.S.; Guang, C.Y.; Liu, Y.; Su, Z.Q.; Gong, S.D.; Yao, Y.J.; Wang, Y.P. Adsorption Behavior of Dyes from An Aqueous Solution onto Composite Magnetic Lignin Adsorbent. Chemosphere 2020, 246, 125757. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, T.T.; Lou, C.W.; Lin, J.H. Facile Method for Tent Fabrics with Eco-Friendly/Durable Properties Using Waterborne Polyurethane/Lignin: Preparation and Evaluation. J. Ind. Text. 2022, 51, 4149S–4166S. [Google Scholar] [CrossRef]
- Oveissi, F.; Naficy, S.; Le, T.Y.L.; Fletcher, D.F.; Dehghani, F. Tough and Processable Hydrogels Based on Lignin and Hydrophilic Polyurethane. ACS Appl. Bio Mater. 2018, 1, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Castro, E.D.; Von Pinho, E.V.D.; de Lima, A.E.; Labory, C.R.G.; dos Santos, H.O.; Alves, E.; Guerra, A.F.D. Physiological Quality, Lignin and the Ultrastructural Characterization of Soybean Seeds. Acta Sci. Agron. 2024, 46, e63621. [Google Scholar] [CrossRef]
- Zhang, J.M.; Hori, N.; Takemura, A. Thermal and Time Regularities during Oilseed Rape Straw Liquefaction Process to Produce Bio-Polyol. J. Clean. Prod. 2020, 277, 124015. [Google Scholar] [CrossRef]
- Chen, F.G.; Lu, Z.M. Liquefaction of Wheat Straw and Preparation of Rigid Polyurethane Foam from the Liquefaction Products. J. Appl. Polym. Sci. 2010, 111, 508–516. [Google Scholar] [CrossRef]
- Wang, T.P.; Zhang, L.H.; Li, D.; Yin, J.; Wu, S.; Mao, Z.H. Mechanical Properties of Polyurethane Foams Prepared from Liquefied Corn Stover with PAPI. Bioresour. Technol. 2008, 99, 2265–2268. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.; Yan, N. Producing Bark-based Polyols through Liquefaction: Effect of Liquefaction Temperature. ACS Sustain. Chem. Eng. 2013, 1, 534–540. [Google Scholar] [CrossRef]
- Ye, L.Y.; Zhang, J.M.; Zhao, J.; Tu, S. Liquefaction of Bamboo Shoot Shell for the Production of Polyols. Bioresour. Technol. 2014, 153, 147–153. [Google Scholar] [CrossRef]
- Hu, S.J.; Wan, C.X.; Li, Y.B. Production and Characterization of Biopolyols and Polyurethane Foams from Crude Glycerol Based Liquefaction of Soybean Straw. Bioresour. Technol. 2012, 103, 227–233. [Google Scholar] [CrossRef]
- Serrano, L.; Rincon, E.; Garcia, A.; Rodriguez, J.; Briones, R. Bio-Degradable Polyurethane Foams Produced by Liquefied Polyol from Wheat Straw Biomass. Polymer 2020, 12, 2646. [Google Scholar] [CrossRef]
- Li, Y.Y.; Jing, W.W.; Wang, J.H.; Li, J.F. Elucidating the Relationship Between Structure and Property of Waterborne Polyurethane-Cellulose Nanocrystals Nanocomposite Films. Sci. Adv. Mater. 2020, 12, 1213–1224. [Google Scholar] [CrossRef]
- Mahdieh, A.; Motasadizadeh, H.; Yeganeh, H.; Nyström, B.; Dinarvand, R. Redox-Responsive Waterborne Polyurethane Nanocarriers for Targeted Doxorubicin Delivery. Int. J. Pharm. 2022, 628, 122275. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Murugadoss, V.; Jiang, J.; Gao, X.; Lin, Z.; Huang, M.; Jiang, G.; Alsareii, S.A.; Algadi, H.; Kathiresan, M. Waterborne Polyurethane and Its Nanocomposites: A Mini-Review for Anti-Corrosion Coating, Flame Retardancy, and Biomedical Applications. Adv. Compos. Hybrid Mater. 2022, 5, 641–650. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, L.N. High-Strength Waterborne Polyurethane Reinforced with Waxy Maize Starch Nanocrystals. J. Nanosci. Nanotechnol. 2008, 8, 5831–5838. [Google Scholar] [CrossRef] [PubMed]
- Kashcheyeva, E.I.; Gismatulina, Y.A.; Mironova, G.F.; Gladysheva, E.K.; Budaeva, V.V.; Skiba, E.A.; Zolotuhin, V.N.; Shavyrkina, N.A.; Kortusov, A.N.; Korchagina, A.A. Properties and Hydrolysis Behavior of Celluloses of Different Origin. Polymer 2022, 14, 3899. [Google Scholar] [CrossRef] [PubMed]
- Reis, R.S.; Souza, D.D.H.S.; Marques, M.D.F.V.; da Luz, F.S.; Monteiro, S.N. Novel Bionanocomposite of Polycaprolactone Reinforced with Steam-Exploded Microfibrillated Cellulose Modified with ZnO. J. Mater. Res. Technol. 2021, 13, 1324–1335. [Google Scholar] [CrossRef]
- Stanzione, M.; Oliviero, M.; Cocca, M.; Errico, M.E.; Gentile, G.; Avella, M.; Lavorgna, M.; Buonocore, G.G.; Verdolotti, L. Tuning of polyurethane foam mechanical and thermal properties using ball-milled cellulose. Carbohydr. Polym. 2020, 231, 115772. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Lee, K.Y.; Aitomaki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the Use of Nanocellulose as Reinforcement in Polymer Matrix Composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in Bio-Based Food Packaging Applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent Advances in Nanocellulose for Biomedical Applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Chen, R.D.; Huang, C.F.; Hsu, S.H. Composites of Waterborne Polyurethane and Cellulose Nanofibers for 3D Printing and Bioapplications. Carbohydr. Polym. 2019, 212, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Dutta, G.K.; Karak, N. Waste Brewed Tea Leaf Derived Cellulose Nanofiber Reinforced Fully Bio-Based Waterborne Polyester Nanocomposite as An Environmentally Benign Material. RSC Adv. 2019, 9, 20829–20840. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Lee, M.W.; Shin, E.J. One-Pot Processing of Regenerated Cellulose Nanoparticles/Waterborne Polyurethane Nanocomposite for Eco-Friendly Polyurethane Matrix. Polymers 2019, 11, 356. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.L.; Xu, D.D.; He, Z.X.; Wang, F.Q.; Gui, S.H.; Fan, J.L.; Pan, X.Y.; Dai, X.H.; Dong, X.Y.; Liu, B.X.; et al. Nanocellulose-Reinforced Polyurethane for Waterborne Wood Coating. Molecules 2019, 24, 3151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, X.; Wang, D.; Fang, C.Q.; Lei, W.Q.; Huang, Z.G.; Song, Y.H.; He, X.Y.; Huang, Y.W. Preparation and Characterization of Waterborne Polyurethane/Cellulose Nanocrystal Composite Membrane from Recycling Waste Paper. J. Renew. Mater. 2020, 8, 631–645. [Google Scholar] [CrossRef]
- Lei, W.Q.; Zhou, X.; Fang, C.Q.; Song, Y.H.; Li, Y.G. Eco-Friendly Waterborne Polyurethane Reinforced with Cellulose Nanocrystal from Office Waste Paper by Two Different Methods. Carbohydr. Polym. 2019, 209, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.D.; Sun, K.; Liu, H.; Chen, X.Y.; Zheng, Y.J.; Shi, X.Z.; Zhang, D.B.; Mi, L.W.; Liu, C.T.; Shen, C.Y. Enhanced Piezoresistive Performance of Conductive WPU/CNT Composite Foam Through Incorporating Brittle Cellulose Nanocrystal. Chem. Eng. J. 2020, 387, 124045. [Google Scholar] [CrossRef]
- Zhang, P.B.; Lu, Y.D.; Fan, M.M.; Jiang, P.P.; Dong, Y.M. Modified Cellulose Nanocrystals Enhancement to Mechanical Properties and Water Resistance of Vegetable Oil-Based Waterborne Polyurethane. J. Appl. Polym. Sci. 2019, 136, 48228. [Google Scholar] [CrossRef]
- Cheng, L.S.; Ren, S.B.; Lu, X.N. Application of Eco-Friendlywaterborne Polyurethane Composite Coating Incorporated with Nano Cellulose Crystalline and Silver Nano Particles on Wood Antibacterial Board. Polymers 2020, 12, 407. [Google Scholar] [CrossRef]
- Hormaiztegui, M.E.V.; Daga, B.; Aranguren, M.I.; Mucci, V. Bio-Based Waterborne Polyurethanes Reinforced with Cellulose Nanocrystals as Coating Films. Prog. Org. Coat. 2020, 144, 105649. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Yang, J.; Li, Z.; Wang, H. Functionalized Bacterial Cellulose Derivatives and Nanocomposites. Carbohydr. Polym. 2014, 101, 1043–1060. [Google Scholar] [CrossRef]
- Urbina, L.; Alonso-Varona, A.; Saralegi, A.; Palomares, T.; Eceiza, A.; Corcuera, M.A.; Retegi, A. Hybrid and Biocompatible Cellulose/Polyurethane Nanocomposites with Water-Activated Shape Memory Properties. Carbohydr. Polym. 2019, 216, 86–96. [Google Scholar] [CrossRef]
- Zia, F.; Zia, K.M.; Zuber, M.; Kamal, S.; Aslam, N. Starch Based Polyurethanes: A Critical Review. Carbohydr. Polym. 2015, 134, 784–798. [Google Scholar] [CrossRef]
- Tai, N.L.; Ghasemlou, M.; Adhikari, R.; Adhikari, B. Starch-based isocyanate- and non-isocyanate polyurethane hybrids: A review on synthesis, performance and biodegradation. Carbohydr. Polym. 2021, 265, 118029. [Google Scholar] [CrossRef]
- Chemelli, A.; Gomernik, F.; Thaler, F.; Huber, A.; Hirn, U.; Bauer, W.; Spirk, S. Cationic Starches in Paper-Based Applications—A Review on Analytical Methods. Carbohydr. Polym. 2020, 235, 115964. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.D. Nanocomposites for Food Packaging Applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef]
- Tsupphayakorn-aek, P.; Suwan, A.; Chaisit, T.; Tulyapitak, T.; Phinyocheep, P.; Pilard, J.F.; Saetung, N.; Saetung, A. A new UV-curable biodegradable waterborne polyurethane-acrylate based on natural rubber blended with cassava starch. J. Appl. Polym. 2023, 140, e53787. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, B.K. Covalent Incorporation of Starch Derivative into Waterborne Polyurethane for Biodegradability. Carbohydr. Polym. 2012, 87, 1803–1809. [Google Scholar] [CrossRef]
- Tai, N.L.; Adhikari, R.; Shanks, R.; Halley, P.; Adhikari, B. Flexible Starch-Polyurethane Films: Effect of Mixed Macrodiol Polyurethane Ionomers on Physicochemical Characteristics and Hydrophobicity. Carbohydr. Polym. 2018, 197, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.R.; Ai, F.J.; Chen, Y.; Dufresne, A.; Huang, J. Effects of Starch Nanocrystal-Graft-Polycaprolactone on Mechanical Properties of Waterborne Polyurethane-Based Nanocomposites. J. Appl. Polym. Sci. 2008, 111, 619–627. [Google Scholar] [CrossRef]
- Chen, G.J.; Wei, M.; Chen, J.H.; Huang, J.; Dufresne, A.; Chang, P.R. Simultaneous Reinforcing and Toughening: New Nanocomposites of Waterborne Polyurethane Filled with Low Loading Level of Starch Nanocrystals. Polymer 2008, 49, 1860–1870. [Google Scholar] [CrossRef]
- Lee, D.S.; Hur, P.; Kim, B.K. Chemical Hybridization of Waterborne Polyurethane with Beta-Cyclodextrin by Sol-Gel Reaction. Prog. Org. Coat. 2017, 111, 107–111. [Google Scholar] [CrossRef]
- Konieczny, J.; Loos, K. Bio-Based Polyurethane Films Using White Dextrins. J. Appl. Polym. Sci. 2019, 136, 47454. [Google Scholar] [CrossRef]
- Manna, S.; Seth, A.; Gupta, P.; Nandi, G.; Dutta, R.; Jana, S.; Jana, S. Chitosan Derivatives as Carriers for Drug Delivery and Biomedical Applications. ACS Biomater. Sci. Eng. 2023, 9, 2181–2202. [Google Scholar] [CrossRef]
- Aoun, R.B.; Trabelsi, N.; Abdallah, M.; Mourtzinos, I.; Mhamdi, R. Towards a Greener Future: Exploring the Challenges of Extraction of Chitin and Chitosan as Bioactive Polysaccharides. Mater. Today Commun. 2024, 39, 108761. [Google Scholar] [CrossRef]
- Shaabani, A.; Sedghi, R. Preparation of Chitosan Biguanidine/PANI-Containing Self-Healing Semi-Conductive Waterborne Scaffolds for Bone Tissue Engineering. Carbohydr. Polym. 2021, 264, 118045. [Google Scholar] [CrossRef] [PubMed]
- Bankoti, K.; Rameshbabu, A.P.; Datta, S.; Maity, P.P.; Goswami, P.; Datta, P.; Ghosh, S.K.; Mitra, A.; Dhara, S. Accelerated Healing of Full Thickness Dermal Wounds by Macroporous Waterborne Polyurethane-Chitosan Hydrogel Scaffolds. Mater. Sci. Eng. C 2017, 81, 133–143. [Google Scholar] [CrossRef]
- Lin, L.; Tu, Y.; Li, Z.; Wu, H.; Mao, H.; Wang, C. Synthesis and Application of Multifunctional Lignin-Modified Cationic Waterborne Polyurethane in Textiles. Int. J. Biol. Macromol. 2024, 262, 130063. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zou, Y.L.; Wang, J.; Wang, S.; Liu, X.F. A Novel Cationic Waterborne Polyurethane Coating Modified by Chitosan Biguanide Hydrochloride with Application Potential in Medical Catheters. J. Appl. Polym. Sci. 2020, 138, e50290. [Google Scholar] [CrossRef]
- Lin, T.W.; Hsu, S.H. Self-Healing Hydrogels and Cryogels from Biodegradable Polyurethane Nanoparticle Crosslinked Chitosan. Adv. Sci. 2020, 7, 1901388. [Google Scholar] [CrossRef]
- Gupta, A.; Kim, B.S. Shape Memory Polyurethane Biocomposites Based on Toughened Polycaprolactone Promoted by Nano-Chitosan. Nanomaterials 2019, 9, 225. [Google Scholar] [CrossRef]
- Kabir, I.I.; Sorrell, C.C.; Mofarah, S.S.; Yang, W.; Yuen, A.C.Y.; Nazir, M.T.; Yeoh, G.H. Alginate/Polymer-Based Materials for Fire Retardancy: Synthesis, Structure, Properties and Applications. Polym. Rev. 2021, 61, 357–414. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liang, H.; Zhou, X.; Fang, C.; Zhang, C.; Luo, Y. Synthesis and Properties of Castor Oil-Based Waterborne Polyurethane/Sodium Alginate Composites with Tunable Properties. Carbohydr. Polym. 2019, 208, 391–397. [Google Scholar] [CrossRef]
- Tan, S.H.; Chua, D.A.C.; Tang, J.R.J.; Bonnard, C.; Leavesley, D.; Liang, K. Design of Hydrogel-Based Scaffolds for in Vitro Three-Dimensional Human Skin Model Reconstruction. Acta Biomater. 2022, 153, 13–37. [Google Scholar] [CrossRef]
- Zia, F.; Zia, K.M.; Zuber, M.; Ahmad, H.B.; Muneer, M. Glucomannan Based Polyurethanes: A Critical Short Review of Recent Advances and Future Perspectives. Int. J. Biol. Macromol. 2016, 87, 229–236. [Google Scholar] [CrossRef]
- Alonso-Sande, M.; Teijeiro-Osorio, D.; Remunan-Lopez, C.; Alonso, M.J. Glucomannan, a Promising Polysaccharide for Biopharmaceutical Purposes. Eur. J. Pharm. Biopharm. 2009, 72, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Huang, Q.; Zhang, L.; Zhou, J.; Gao, S. Miscibility and Properties of Blend Materials from Waterborne Polyurethane and Carboxymethyl Konjac Glucomannan. J. Appl. Polym. Sci. 2010, 92, 77–83. [Google Scholar] [CrossRef]
- Zhang, S.; Xiang, A.M.; Tian, H.F.; Rajulu, A.V. Water-Blown Castor Oil-Based Polyurethane Foams with Soy Protein as a Reactive Reinforcing Filler. J. Polym. Environ. 2018, 26, 15–22. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Lendlein, A. Degradable Polyurethane/Soy Protein Shape-Memory Polymer Blends Prepared Via Environmentally-Friendly Aqueous Dispersions. Macromol. Mater. Eng. 2012, 297, 1213–1224. [Google Scholar] [CrossRef]
- Chan-Chan, L.H.; Gonzalez-Garcia, G.; Vargas-Coronado, R.F.; Cervantes-Uc, J.M.; Hernandez-Sanchez, F.; Marcos-Femandez, A.; Cauich-Rodriguez, J.V. Characterization of model compounds and poly(amide-urea) urethanes based on amino acids by FTIR, NMR and other analytical techniques. Eur. Polym. J. 2017, 92, 27–39. [Google Scholar] [CrossRef]
- Liang, C.; Gracida-Alvarez, U.R.; Gallant, E.T.; Gillis, P.A.; Marques, Y.A.; Abramo, G.P.; Hawkins, T.R.; Dunn, J.B. Material Flows of Polyurethane in the United States. Environ. Sci. Technol. 2021, 55, 14215–14224. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.T.; Nguyen, B.T.; Nguyen, H.T.T.; Kang, S.J.; Kim, J.; Lee, P.C.; Hoang, D.Q. Comprehensive Investigation of the Behavior of Polyurethane Foams Based on Conventional Polyol and Oligo-Ester-Ether-Diol from Waste Poly(ethylene terephthalate): Fireproof Performances, Thermal Stabilities, and Physicomechanical Properties. ACS Omega 2021, 5, 33053–33063. [Google Scholar] [CrossRef] [PubMed]
- Molero, C.; De Lucas, A.; Romero, F.; Rodríguez, J.F. Influence of the Use of Recycled Polyols Obtained by Glycolysis on the Preparation and Physical Properties of Flexible Polyurethane. J. Appl. Polym. Sci. 2008, 109, 617–626. [Google Scholar] [CrossRef]
- Tan, S.Q.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid Polyurethane Foams from a Soybean Oil-Based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Lu, Y.S.; Larock, R.C. Soybean Oil-Based, Aqueous Cationic Polyurethane Dispersions: Synthesis and Properties. Prog. Org. Coat. 2010, 69, 31–37. [Google Scholar] [CrossRef]
- Gandhi, T.S.; Patel, M.R.; Dholakiya, B.Z. Synthesis of Cashew Mannich Polyol Via a Three Step Continuous Route and Development of PU Rigid Foams with Mechanical, Thermal and Fire Studies. J. Polym. Eng. 2015, 35, 533–544. [Google Scholar] [CrossRef]
- Zhang, C.; Bhoyate, S.; Ionescu, M.; Kahol, P.K.; Gupta, R.K. Highly Flame Retardant and Bio-Based Rigid Polyurethane Foams Derived from Orange Peel Oil. Polym. Eng. Sci. 2018, 58, 2078–2087. [Google Scholar] [CrossRef]
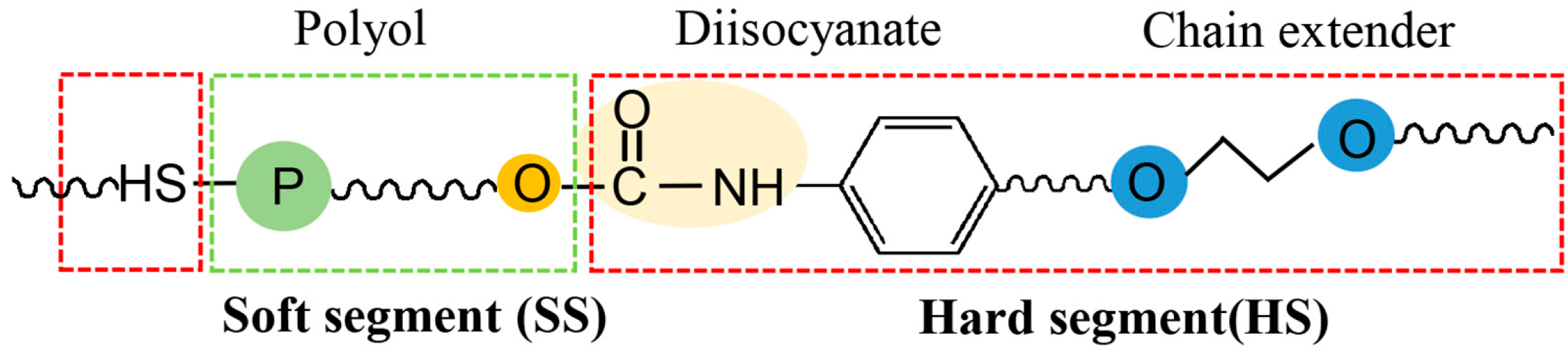
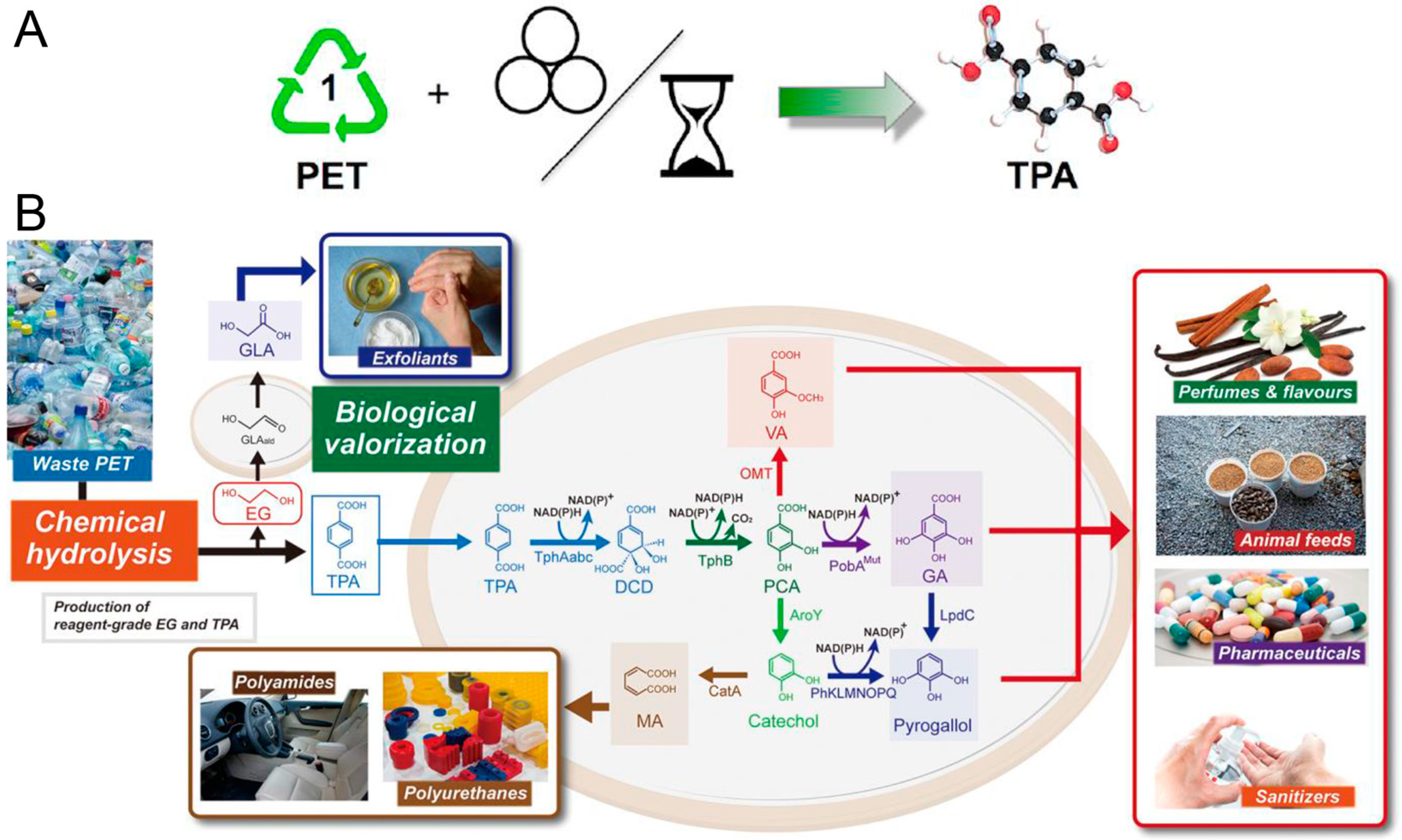

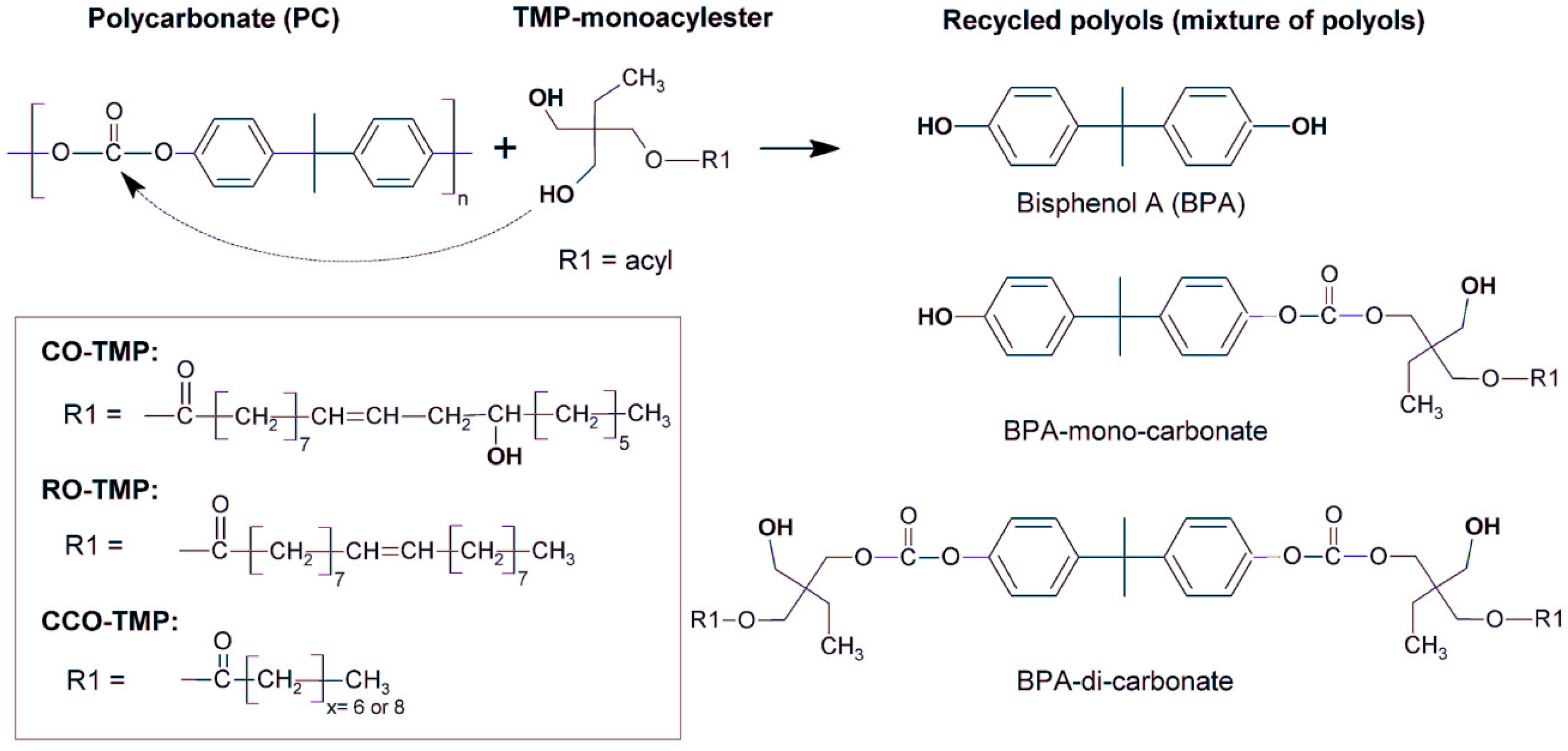
| Methods | Degradation Reagents | Products | Potential Applications | Ref. |
|---|---|---|---|---|
| Glycolysis | Diethylene glycol Crude glycero Pentaerytheritol Trimethyloylpropane Ethylene glycol Neopentyl glycol Supercritical ethanol-ionic liquid | Polyol BHET DET Oligomer | PU foams for oil sorption PU adhesive Polyester-polyol PU coatings | [42,43,44,45,51,52,53,54] |
| Hydrolysis | Deionized water NaOH | TPA, EG | Aromatics Aromatic-derived compounds | [46] |
| Alcoholysis | Isooctyl alcohol Methanol | DMT EG | Polyvinyl chloride | [47,48] |
| Biodegradation | Sakaiensis bacterium Hydrolases | MHET, BHET TPA, EG | PU foams | [49,50] |
| Methods | Degradation Reagents | Products | Potential Applications | Refs. |
|---|---|---|---|---|
| Hydrolysis | CO2-water KOH-water | Polyol Diamines | Synthesis of PU Translated into isocyanate | [76,77,78] |
| Glycolysis | Diethylene glycol Ethylene glycol Glycerol Diglycerol Pentaerythritol Crude glycerine | Polyol Aromatic-carbamates | Synthesis of new flexible PU foam Thermoplastic PU and toluenediamines | [79,80,81,82,87] |
| Ammonolysis | Alkanolamines Dibutylamine ethanolamine | Polyol Aromatic amines Alkanolamine derivatives | Synthesis of new PUs melamine resins, epoxy resins, polyester, polycarbonates | [99,100] |
| Phosphorolysis | Phosphonic acids Phosphate Phosphoric acid Ethyl ester | Phosphorus Chlorine element oligomer | Flame retardant polyurethane or PVC materials | [104,105,106] |
| Hydroglycolysis | Water and glycols | Polyol Intermediate chemicals | Synthesis of new PUs | [107,108,109] |
| Bio-Based Feedstocks | Molecular Structure | Products | Properties | Ref. |
|---|---|---|---|---|
| Vegetable oil | 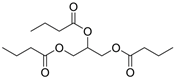 | PU elastomer WPU | Mechanical properties Thermal resistance Physicomechanical stability Good adhesion | [128,129,134] |
| Cashew nut shell liquid | 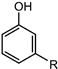 | PU foams | Good mechanical, thermal and fire properties | [110,156] |
| Terpene | 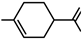 | PU elastomer | Good mechanical properties | [161,162] |
| Rosin |  | PU elastomer | Excellent mechanical rigidity, Heat resistance, shape memory property | [155,168,172] |
| Lignin |  | PU foams | Antibacterial, oxidation and ultraviolet resistance Flame retardant | [183,184,185] |
| Cellulose | 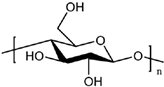 | WPU | Biodegradability, mechanical properties, renewability Thermal properties | [221,229] |
| Starch | 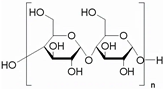 | PU films | Good transparency, thermal properties Mechanical properties | [165,237,238] |
| Chitosan |  | WPU PU films | Self-healing properties Good shape recovery | [197,247] |
| Sodium alginate | 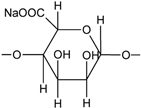 | WPU | Hydrophobicity Mechanical properties Thermal stability | [250] |
| Glucomannan |  | WPU | Outstanding mechanical properties Good compatibility | [253,254] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, M.; Fang, C.; Zhou, X.; Wang, D.; Lin, Y.; Lei, W.; Li, L. Recent Advances in Environment-Friendly Polyurethanes from Polyols Recovered from the Recycling and Renewable Resources: A Review. Polymers 2024, 16, 1889. https://doi.org/10.3390/polym16131889
Pu M, Fang C, Zhou X, Wang D, Lin Y, Lei W, Li L. Recent Advances in Environment-Friendly Polyurethanes from Polyols Recovered from the Recycling and Renewable Resources: A Review. Polymers. 2024; 16(13):1889. https://doi.org/10.3390/polym16131889
Chicago/Turabian StylePu, Mengyuan, Changqing Fang, Xing Zhou, Dong Wang, Yangyang Lin, Wanqing Lei, and Lu Li. 2024. "Recent Advances in Environment-Friendly Polyurethanes from Polyols Recovered from the Recycling and Renewable Resources: A Review" Polymers 16, no. 13: 1889. https://doi.org/10.3390/polym16131889
APA StylePu, M., Fang, C., Zhou, X., Wang, D., Lin, Y., Lei, W., & Li, L. (2024). Recent Advances in Environment-Friendly Polyurethanes from Polyols Recovered from the Recycling and Renewable Resources: A Review. Polymers, 16(13), 1889. https://doi.org/10.3390/polym16131889











