The Impact of Lignin Biopolymer Sources, Isolation, and Size Reduction from the Macro- to Nanoscale on the Performances of Next-Generation Sunscreen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Biomass Chemical Characterization
2.3. Lignin Macroparticles (LMPs) Extraction
2.4. Lignin Nanoparticles (LNPs) Preparation
2.5. Elemental Analysis
2.6. Size Exclusion Chromatography (SEC)
2.7. Nuclear Magnetic Resonance (NMR)
2.8. Dynamic Light Scattering (DLS)
2.9. Transmission Electron Microscopy (TEM)
2.10. Lignin-Based Sunscreen Preparation and Study
3. Results
3.1. LMPs Extraction and Characterization
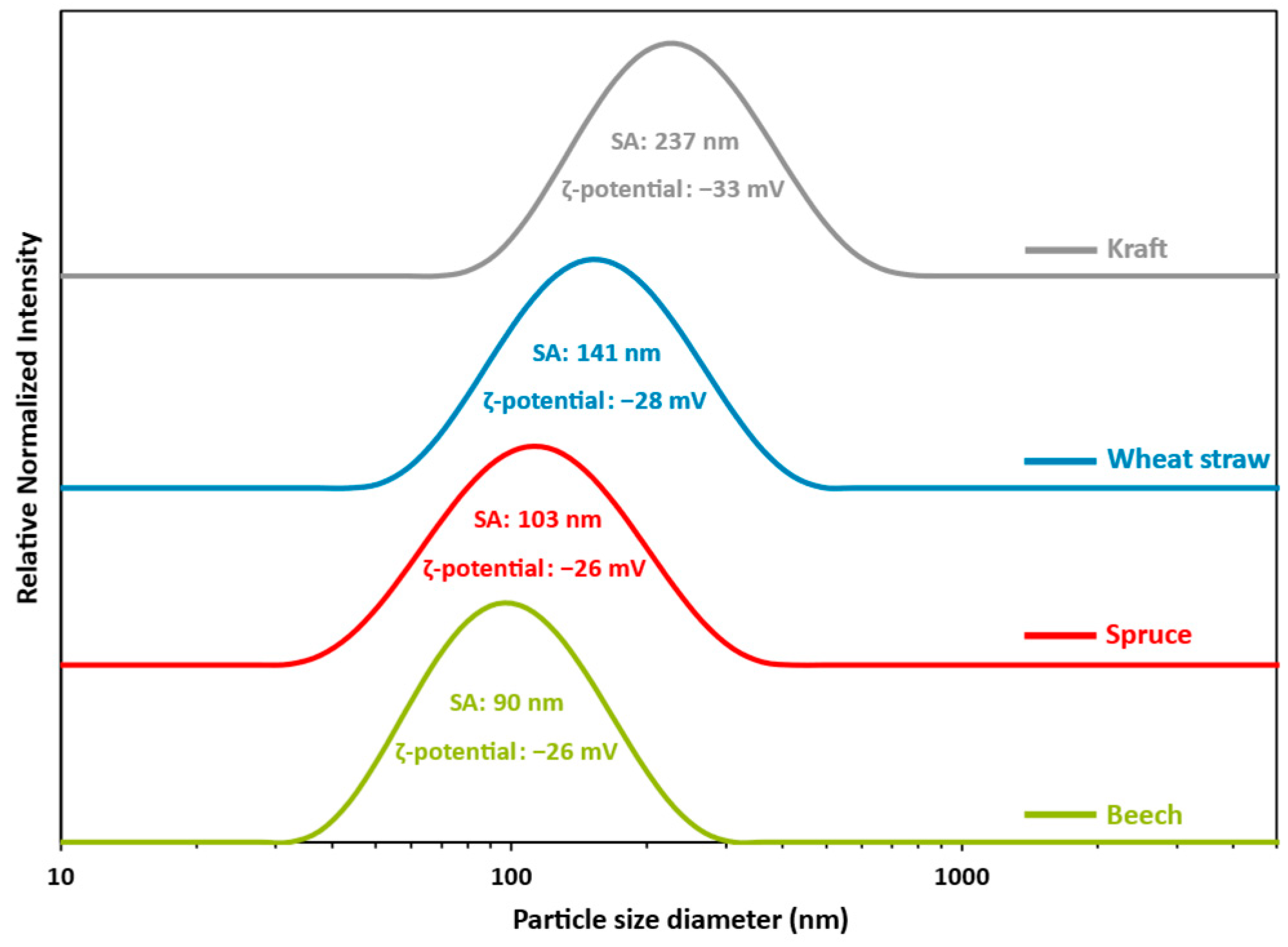
3.2. LNPs Production and Characterization
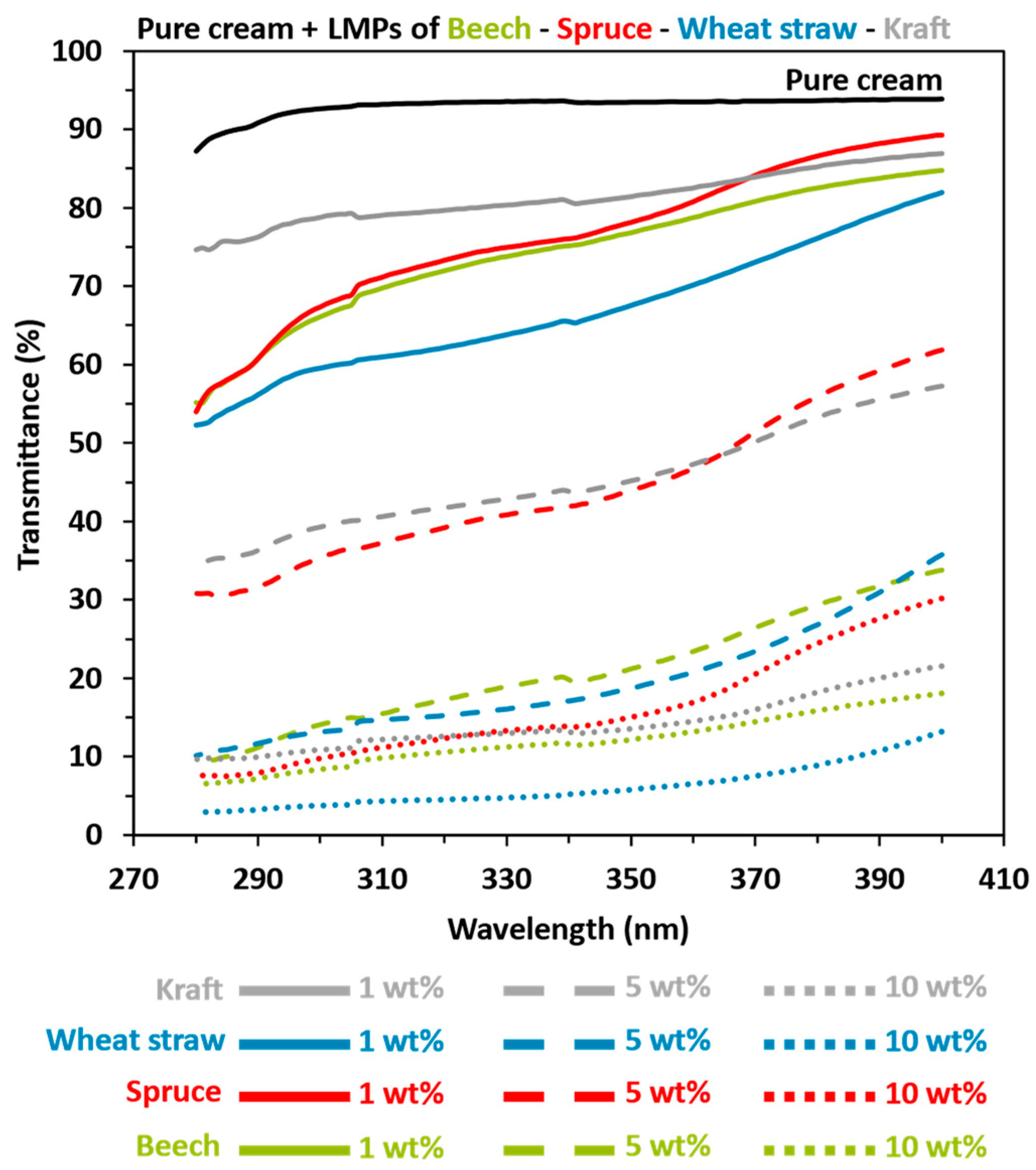
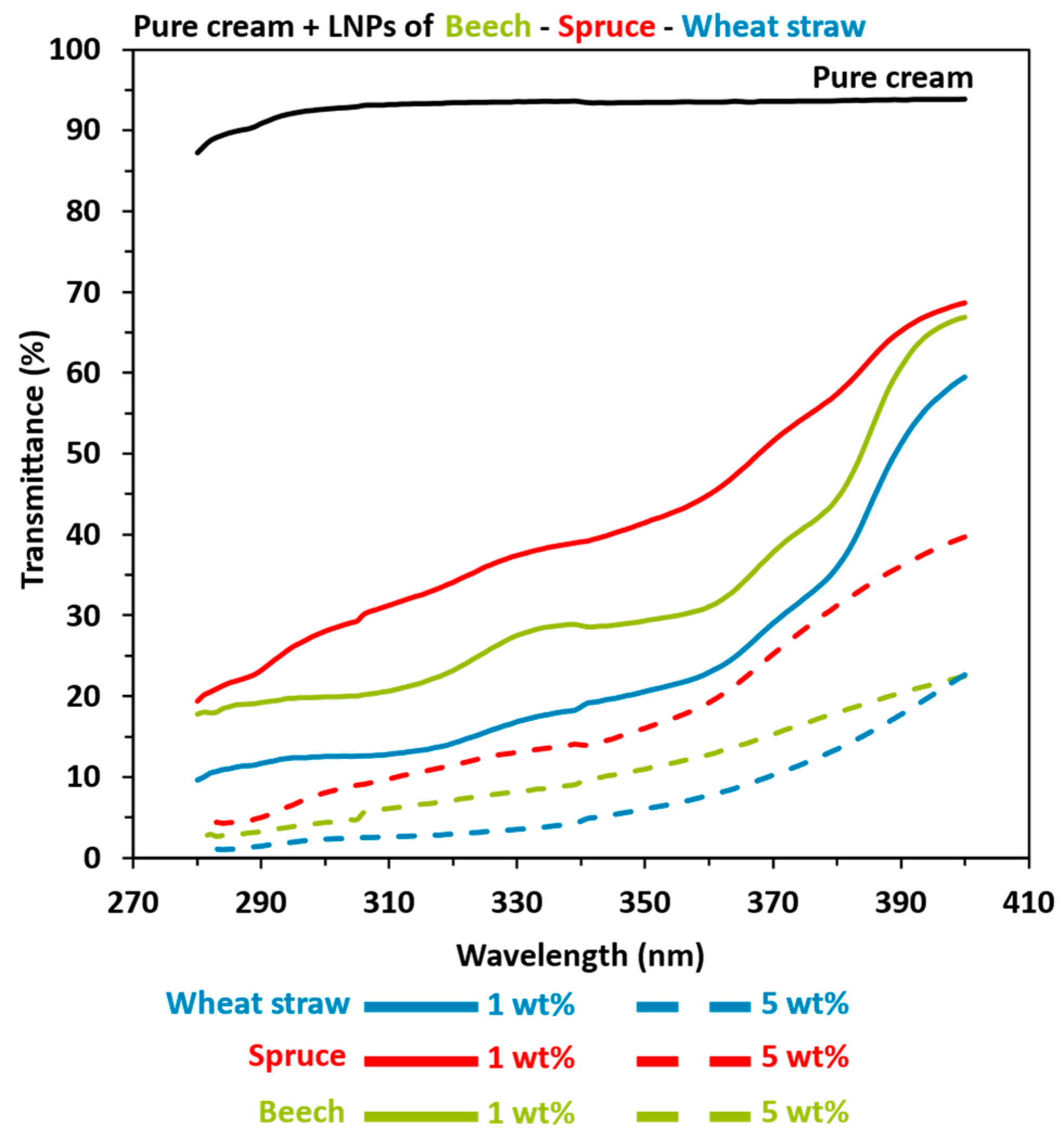
3.3. Lignin-Based Sunscreen Analysis
3.4. Factors Affecting Lignin UV-Shielding Properties
- (1)
- Lignin UV-shielding properties are related to chromophore functional groups and especially phenolic hydroxyl groups [22,27,31,75,84]. Guo et al. [85] previously demonstrated that the S phenolic group exhibited stronger UV-absorbing properties due to the additional methoxyl group compared to the G and H units (Figure 1). Consequently, this enhanced the abundance of free electron pairs from oxygen atoms. Part of the results aligns with these explanations, such as beech and wheat straw biomass, which present advantageous chemical structures (high S/G phenolic hydroxyl content, i.e., high methoxy content and darker [24] color) and had greater UV-shielding properties compared to spruce lignin. It was previously demonstrated by Wang et al. [26] that the lignin chemical structure remains unchanged with respect to the nanoprecipitation process, which explains why the same trends can be observed between sunscreens prepared with both LMPs and LNPs. However, the observation that wheat straw lignin exhibited superior, stronger UV-shielding properties over beech lignin suggests that while methoxyl groups and phenolic units may be predominant, they do not fully account for all aspects of lignin-enhanced UV protection. The complex and interconnected structure of wheat straw lignin with proven tricin units [71] and other phenolic end groups, such as aryl acetic acid, can also contribute to the enhanced UV-shielding properties observed in herbaceous lignins [86]. Other chromophores in lignin, such as quinones or -CH=CH-, -C=C-, and -C=O bonds [84] and Mw [87], can contribute to UVB and UVA absorbance.
- (2)
- Although the extrinsic properties of lignin are often given less consideration, particle size, shape, and purity can be modified by processes and are also important factors that can explain UV absorption properties. First, the relatively highest level of impurities (7%) was found in beech lignin compared to other organosolv-extracted lignin (3.7 and 3.9% for wheat straw and spruce, respectively), which may reduce UV-shielding properties to a greater extent. In the case of kraft, 9.5% of the impurities coupled with 1.8% sulfur presence can also contribute to lower UV absorption despite a large number of aromatic rings. Regarding lignin particle size and morphology, recent studies [22,88] showed that lignin nanoparticles compared to macroparticles have a higher specific surface area, higher transparency, better dispersibility, and, consequently, better UV-shielding properties per weight (Figure S1). Reduced particle size induces a higher specific surface area, thereby permitting the greater availability of chromophores per unit weight. This results in enhanced UV-shielding properties, lighter coloration, and improved dispersion within the sunscreen formulation and ultimately leads to a reduced reliance on organic UV filter (Figure S1). The results from this study have indeed proven previous observations with respect to a general decrease in UVB average transmittance between 2.5 and 6.5 with the same lignin types but different size scales. With respect to particle shape, Tan et al. [70] showed that the spherical (better A/V ratio) design provided a larger surface area because of the minimum packing density compared with other forms, thus allowing higher chromophore availability and concentration, which can boost UV absorption properties.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaz, J.H.; Nesbitt, L.T. Sun Exposure Behavior and Protection: Recommendations for Travelers. J. Travel Med. 2013, 20, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Narbutt, J.; Harrison, G.I.; Lawrence, K.P.; Bell, M.; O’Connor, C.; Olsen, P.; Grys, K.; Baczynska, K.A.; Rogowski-Tylman, M.; et al. Optimal Sunscreen Use, during a Sun Holiday with a Very High Ultraviolet Index, Allows Vitamin D Synthesis without Sunburn. Br. J. Dermatol. 2019, 181, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.; Pirot, F.; Bertholle, V.; Roussel, L.; Falson, F.; Padois, K. Commonly Used UV Filter Toxicity on Biological Functions: Review of Last Decade Studies. Int. J. Cosmet. Sci. 2013, 35, 208–219. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. A Review of Inorganic UV Filters Zinc Oxide and Titanium Dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef]
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV Filters: From Sunscreens to Human Body and the Environment. TrAC Trends Anal. Chem. 2007, 26, 360–374. [Google Scholar] [CrossRef]
- Damiani, E.; Astolfi, P.; Giesinger, J.; Ehlis, T.; Herzog, B.; Greci, L.; Baschong, W. Assessment of the Photo-Degradation of UV-Filters and Radical-Induced Peroxidation in Cosmetic Sunscreen Formulations. Free Radic. Res. 2010, 44, 304–312. [Google Scholar] [CrossRef]
- Levine, A. Sunscreen Use and Awareness of Chemical Toxicity among Beach Goers in Hawaii Prior to a Ban on the Sale of Sunscreens Containing Ingredients Found to Be Toxic to Coral Reef Ecosystems. Mar. Policy 2020, 117, 103875. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Fauth, J.E.; Segal, R.; Bronstein, O.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; Pennington, P.; Kushmaro, A.; et al. Toxicological Effects of the Sunscreen UV Filter, Benzophenone-2, on Planulae and in Vitro Cells of the Coral, Stylophora Pistillata. Ecotoxicology 2014, 23, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of Inorganic UV Filters Contained in Sunscreen Products on Tropical Stony Corals (Acropora Spp.). Sci. Total Environ. 2018, 637–638, 1279–1285. [Google Scholar] [CrossRef]
- Ouchene, L.; Litvinov, I.V.; Netchiporouk, E. Hawaii and Other Jurisdictions Ban Oxybenzone or Octinoxate Sunscreens Based on the Confirmed Adverse Environmental Effects of Sunscreen Ingredients on Aquatic Environments. J. Cutan. Med. Surg. 2019, 23, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Adler, B.L.; DeLeo, V.A. Sunscreen Safety: A Review of Recent Studies on Humans and the Environment. Curr Derm Rep 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Widsten, P. Lignin-Based Sunscreens—State-of-the-Art, Prospects and Challenges. Cosmetics 2020, 7, 85. [Google Scholar] [CrossRef]
- Tan, S.S.Y.; MacFarlane, D.R.; Upfal, J.; Edye, L.A.; Doherty, W.O.S.; Patti, A.F.; Pringle, J.M.; Scott, J.L. Extraction of Lignin from Lignocellulose at Atmospheric Pressure Using Alkylbenzenesulfonate Ionic Liquid. Green Chem. 2009, 11, 339. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, Y.; Yang, R.; Wang, C. Alkaline Lignin Extracted from Furfural Residues for pH-Responsive Pickering Emulsions and Their Recyclable Polymerization. Green Chem. 2012, 14, 3230. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Pourhashem, G.; Ullah, A.H.; Bajwa, S.G. A Concise Review of Current Lignin Production, Applications, Products and Their Environmental Impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A Nature-Inspired Sun Blocker for Broad-Spectrum Sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Sunscreen Performance of Lignin from Different Technical Resources and Their General Synergistic Effect with Synthetic Sunscreens. ACS Sustain. Chem. Eng. 2016, 4, 4029–4035. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of Lignin Color via One-Step UV Irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Qian, Y.; Zhong, X.; Li, Y.; Qiu, X. Fabrication of Uniform Lignin Colloidal Spheres for Developing Natural Broad-Spectrum Sunscreens with High Sun Protection Factor. Ind. Crops Prod. 2017, 101, 54–60. [Google Scholar] [CrossRef]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; İlk, S.; Kaya, M.; Labidi, J. Potential Use of Kraft and Organosolv Lignins as a Natural Additive for Healthcare Products. RSC Adv. 2018, 8, 24525–24533. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Tran, T.M.T.; Choi, J.W.; Won, K. Lignin for White Natural Sunscreens. Int. J. Biol. Macromol. 2019, 122, 549–554. [Google Scholar] [CrossRef]
- Lee, S.C.; Yoo, E.; Lee, S.H.; Won, K. Preparation and Application of Light-Colored Lignin Nanoparticles for Broad-Spectrum Sunscreens. Polymers 2020, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, D.; Wang, H.-M.; Yuan, T.-Q.; Sun, R.-C. Green and Facile Preparation of Regular Lignin Nanoparticles with High Yield and Their Natural Broad-Spectrum Sunscreens. ACS Sustain. Chem. Eng. 2019, 7, 2658–2666. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Fu, S.; Chen, Y. Fabrication of Light-Colored Lignin Microspheres for Developing Natural Sunscreens with Favorable UV Absorbability and Staining Resistance. Ind. Eng. Chem. Res. 2019, 58, 13858–13867. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Fu, S.; Chen, Y. High-Value Utilization of Kraft Lignin: Color Reduction and Evaluation as Sunscreen Ingredient. Int. J. Biol. Macromol. 2019, 133, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, H.; Rezende, C.A. Pure, Stable and Highly Antioxidant Lignin Nanoparticles from Elephant Grass. Ind. Crops Prod. 2020, 145, 112105. [Google Scholar] [CrossRef]
- Ratanasumarn, N.; Chitprasert, P. Cosmetic Potential of Lignin Extracts from Alkaline-Treated Sugarcane Bagasse: Optimization of Extraction Conditions Using Response Surface Methodology. Int. J. Biol. Macromol. 2020, 153, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Widsten, P.; Tamminen, T.; Liitiä, T. Natural Sunscreens Based on Nanoparticles of Modified Kraft Lignin (CatLignin). ACS Omega 2020, 5, 13438–13446. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M. Comparative Antioxidant and Cytotoxic Effects of Lignins from Different Sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef] [PubMed]
- Tortora, M.; Cavalieri, F.; Mosesso, P.; Ciaffardini, F.; Melone, F.; Crestini, C. Ultrasound Driven Assembly of Lignin into Microcapsules for Storage and Delivery of Hydrophobic Molecules. Biomacromolecules 2014, 15, 1634–1643. [Google Scholar] [CrossRef] [PubMed]
- Gil-Chávez, G.J.; Padhi, S.S.P.; Pereira, C.V.; Guerreiro, J.N.; Matias, A.A.; Smirnova, I. Cytotoxicity and Biological Capacity of Sulfur-Free Lignins Obtained in Novel Biorefining Process. Int. J. Biol. Macromol. 2019, 136, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Gordobil, O.; Oberemko, A.; Saulis, G.; Baublys, V.; Labidi, J. In Vitro Cytotoxicity Studies of Industrial Eucalyptus Kraft Lignins on Mouse Hepatoma, Melanoma and Chinese Hamster Ovary Cells. Int. J. Biol. Macromol. 2019, 135, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.M.C.; Cerqueira, M.A.; Gonçalves, C.; Azinheiro, S.; Garrido-Maestu, A.; Vicente, A.A.; Pastrana, L.M.; Teixeira, J.A.; Michelin, M. Green Synthesis of Lignin Nano- and Micro-Particles: Physicochemical Characterization, Bioactive Properties and Cytotoxicity Assessment. Int. J. Biol. Macromol. 2020, 163, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Menima-Medzogo, J.A.; Walz, K.; Lauer, J.C.; Sivasankarapillai, G.; Gleuwitz, F.R.; Rolauffs, B.; Laborie, M.-P.; Hart, M.L. Characterization and In Vitro Cytotoxicity Safety Screening of Fractionated Organosolv Lignin on Diverse Primary Human Cell Types Commonly Used in Tissue Engineering. Biology 2022, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hatfield, R.D.; Ralph, S.A.; Christensen, J.H.; et al. Lignins: Natural Polymers from Oxidative Coupling of 4-Hydroxyphenyl-Propanoids. Phytochem. Rev. 2004, 3, 29–60. [Google Scholar] [CrossRef]
- Akinosho, H.O.; Yoo, C.G.; Dumitrache, A.; Natzke, J.; Muchero, W.; Brown, S.D.; Ragauskas, A.J. Elucidating the Structural Changes to Populus Lignin during Consolidated Bioprocessing with Clostridium thermocellum. ACS Sustain. Chem. Eng. 2017, 5, 7486–7491. [Google Scholar] [CrossRef]
- Marton, J.; Ingemar Falkehag, S. (Eds.) Chromophores in Kraft Lignin. In Lignin Structure and Reactions; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1966; Volume 59, ISBN 978-0-8412-0060-9. [Google Scholar]
- Lanzalunga, O.; Bietti, M. Photo- and Radiation Chemical Induced Degradation of Lignin Model Compounds. J. Photochem. hotobiol. B Biol. 2000, 56, 85–108. [Google Scholar] [CrossRef] [PubMed]
- Barsberg, S.; Elder, T.; Felby, C. Lignin−Quinone Interactions: Implications for Optical Properties of Lignin. Chem. Mater. 2003, 15, 649–655. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker—A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Abu-Omar, M.M. Chemicals From Lignin. In Encyclopedia of Sustainable Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 573–585. ISBN 978-0-12-804792-7. [Google Scholar]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaün, F.; Malucelli, G.; Guan, J.-P. An Overview on the Use of Lignin and Its Derivatives in Fire Retardant Polymer Systems. In Lignin—Trends and Applications; Poletto, M., Ed.; InTech: London, UK, 2018; ISBN 978-953-51-3901-0. [Google Scholar]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery Review: Wide-Reaching Products through Kraft Lignin. BioResources 2019, 14, 7543–7581. [Google Scholar] [CrossRef]
- Strassberger, Z.; Tanase, S.; Rothenberg, G. The Pros and Cons of Lignin Valorisation in an Integrated Biorefinery. RSC Adv. 2014, 4, 25310–25318. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in Industrial Applications of Technical Lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Eraghi Kazzaz, A.; Fatehi, P. Technical Lignin and Its Potential Modification Routes: A Mini-Review. Ind. Crops Prod. 2020, 154, 112732. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of Lignocellulosic Wastes for Biofuel Production: A Critical Review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Ab Rasid, N.S.; Shamjuddin, A.; Abdul Rahman, A.Z.; Amin, N.A.S. Recent Advances in Green Pre-Treatment Methods of Lignocellulosic Biomass for Enhanced Biofuel Production. J. Clean. Prod. 2021, 321, 129038. [Google Scholar] [CrossRef]
- Matsakas, L.; Karnaouri, A.; Cwirzen, A.; Rova, U.; Christakopoulos, P. Formation of Lignin Nanoparticles by Combining Organosolv Pretreatment of Birch Biomass and Homogenization Processes. Molecules 2018, 23, 1822. [Google Scholar] [CrossRef]
- Adamcyk, J.; Beisl, S.; Amini, S.; Jung, T.; Zikeli, F.; Labidi, J.; Friedl, A. Production and Properties of Lignin Nanoparticles from Ethanol Organosolv Liquors—Influence of Origin and Pretreatment Conditions. Polymers 2021, 13, 384. [Google Scholar] [CrossRef]
- Raj, S.; Jose, S.; Sumod, U.; Sabitha, M. Nanotechnology in Cosmetics: Opportunities and Challenges. J. Pharm. Bioall. Sci. 2012, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Larese Filon, F.; Mauro, M.; Adami, G.; Bovenzi, M.; Crosera, M. Nanoparticles Skin Absorption: New Aspects for a Safety Profile Evaluation. Regul. Toxicol. Pharmacol. 2015, 72, 310–322. [Google Scholar] [CrossRef]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Synthesis and Characterization of Biodegradable Lignin Nanoparticles with Tunable Surface Properties. Langmuir 2016, 32, 6468–6477. [Google Scholar] [CrossRef] [PubMed]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A Simple Process for Lignin Nanoparticle Preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Ju, T.; Zhang, Z.; Li, Y.; Miao, X.; Ji, J. Continuous Production of Lignin Nanoparticles Using a Microchannel Reactor and Its Application in UV-Shielding Films. RSC Adv. 2019, 9, 24915–24921. [Google Scholar] [CrossRef] [PubMed]
- Conner, C.G.; Veleva, A.N.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Scalable Formation of Concentrated Monodisperse Lignin Nanoparticles by Recirculation-Enhanced Flash Nanoprecipitation. Part. Part. Syst. Charact. 2020, 37, 2000122. [Google Scholar] [CrossRef]
- Ma, M.; Dai, L.; Xu, J.; Liu, Z.; Ni, Y. A Simple and Effective Approach to Fabricate Lignin Nanoparticles with Tunable Sizes Based on Lignin Fractionation. Green Chem. 2020, 22, 211–217. [Google Scholar] [CrossRef]
- Morsali, M.; Moreno, A.; Loukovitou, A.; Pylypchuk, I.; Sipponen, M.H. Stabilized Lignin Nanoparticles for Versatile Hybrid and Functional Nanomaterials. Biomacromolecules 2022, 23, 4597–4606. [Google Scholar] [CrossRef]
- Gilca, I.A.; Popa, V.I.; Crestini, C. Obtaining Lignin Nanoparticles by Sonication. Ultrason. Sonochem. 2015, 23, 369–375. [Google Scholar] [CrossRef]
- Nair, S.S.; Sharma, S.; Pu, Y.; Sun, Q.; Pan, S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High Shear Homogenization of Lignin to Nanolignin and Thermal Stability of Nanolignin-Polyvinyl Alcohol Blends. ChemSusChem 2014, 7, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Juikar, S.J.; Vigneshwaran, N. Extraction of Nanolignin from Coconut Fibers by Controlled Microbial Hydrolysis. Ind. Crops Prod. 2017, 109, 420–425. [Google Scholar] [CrossRef]
- Garcia Gonzalez, M.N.; Levi, M.; Turri, S.; Griffini, G. Lignin Nanoparticles by Ultrasonication and Their Incorporation in Waterborne Polymer Nanocomposites: ARTICLE. J. Appl. Polym. Sci. 2017, 134, 45318. [Google Scholar] [CrossRef]
- Mili, M.; Hashmi, S.A.R.; Tilwari, A.; Rathore, S.K.S.; Naik, A.; Srivastava, A.K.; Verma, S. Preparation of Nanolignin Rich Fraction from Bamboo Stem via Green Technology: Assessment of Its Antioxidant, Antibacterial and UV Blocking Properties. Environ. Technol. 2023, 44, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Abbati de Assis, C.; Greca, L.G.; Ago, M.; Balakshin, M.Y.; Jameel, H.; Gonzalez, R.; Rojas, O.J. Techno-Economic Assessment, Scalability, and Applications of Aerosol Lignin Micro- and Nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 11853–11868. [Google Scholar] [CrossRef]
- Hussin, M.H.; Appaturi, J.N.; Poh, N.E.; Latif, N.H.A.; Brosse, N.; Ziegler-Devin, I.; Vahabi, H.; Syamani, F.A.; Fatriasari, W.; Solihat, N.N.; et al. A Recent Advancement on Preparation, Characterization and Application of Nanolignin. Int. J. Biol. Macromol. 2022, 200, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Liu, D.; Qian, Y.; Wang, J.; Huang, J.; Yi, C.; Qiu, X.; Qin, Y. Towards Better UV-Blocking and Antioxidant Performance of Varnish via Additives Based on Lignin and Its Colloids. Holzforschung 2019, 73, 485–491. [Google Scholar] [CrossRef]
- Girard, V.; Chapuis, H.; Brosse, N.; Canilho, N.; Marchal-Heussler, L.; Ziegler-Devin, I. Lignin Nanoparticles: Contribution of Biomass Types and Fractionation for an Eco-Friendly Production. ACS Sustain. Chem. Eng. 2024, 12, 7055–7068. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of Milled Wood Lignin and Ethanol Organosolv Lignin from Miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Steinmetz, V.; Villain-Gambier, M.; Klem, A.; Gambier, F.; Dumarcay, S.; Trebouet, D. Unveiling TMP Process Water Potential As an Industrial Sourcing of Valuable Lignin–Carbohydrate Complexes toward Zero-Waste Biorefineries. ACS Sustain. Chem. Eng. 2019, 7, 6390–6400. [Google Scholar] [CrossRef]
- He, Q.; Ziegler-Devin, I.; Chrusciel, L.; Obame, S.N.; Hong, L.; Lu, X.; Brosse, N. Lignin-First Integrated Steam Explosion Process for Green Wood Adhesive Application. ACS Sustain. Chem. Eng. 2020, 8, 5380–5392. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Z.; Ji, X.-X.; Zhang, F. Light-Colored Lignin Extraction by Ultrafiltration Membrane Fractionation for Lignin Nanoparticles Preparation as UV-Blocking Sunscreen. Int. J. Biol. Macromol. 2023, 231, 123244. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A Comparison of in Vivo and in Vitro Testing of Sunscreening Formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Brosse, N.; El Hage, R.; Chaouch, M.; Pétrissans, M.; Dumarçay, S.; Gérardin, P. Investigation of the Chemical Modifications of Beech Wood Lignin during Heat Treatment. Polym. Degrad. Stab. 2010, 95, 1721–1726. [Google Scholar] [CrossRef]
- Siika-aho, M.; Varhimo, A.; Sirviö, J.; Kruus, K. Sugars from Biomass—High Cellulose Hydrolysability of Oxygen Alkali Treated Spruce, Beech and Wheat Straw. In Proceedings of the 6th NordicWood Biorefinery Conference 2015 (NWBC), Helsinki, Finland, 20–22 October 2015; ISBN 978-951-38-8352-2. [Google Scholar]
- Schneider, W.D.H.; Dillon, A.J.P.; Camassola, M. Lignin Nanoparticles Enter the Scene: A Promising Versatile Green Tool for Multiple Applications. Biotechnol. Adv. 2021, 47, 107685. [Google Scholar] [CrossRef] [PubMed]
- Pylypchuk, I.V.; Riazanova, A.; Lindström, M.E.; Sevastyanova, O. Structural and Molecular-Weight-Dependency in the Formation of Lignin Nanoparticles from Fractionated Soft- and Hardwood Lignins. Green Chem. 2021, 23, 3061–3072. [Google Scholar] [CrossRef]
- Zwilling, J.D.; Jiang, X.; Zambrano, F.; Venditti, R.A.; Jameel, H.; Velev, O.D.; Rojas, O.J.; Gonzalez, R. Understanding Lignin Micro- and Nanoparticle Nucleation and Growth in Aqueous Suspensions by Solvent Fractionation. Green Chem. 2021, 23, 1001–1012. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Valorizing Recalcitrant Cellulolytic Enzyme Lignin via Lignin Nanoparticles Fabrication in an Integrated Biorefinery. ACS Sustain. Chem. Eng. 2017, 5, 2702–2710. [Google Scholar] [CrossRef]
- Ma, Y.; Liao, Y.; Jiang, Z.; Sun, Q.; Guo, X.; Zhang, W.; Hu, C.; Luque, R.; Shi, B.; Sels, B.F. Solvent Effect on the Production of Spherical Lignin Nanoparticles. Green Chem. 2023, 25, 993–1003. [Google Scholar] [CrossRef]
- Zhang, Y.; Naebe, M. Lignin: A Review on Structure, Properties, and Applications as a Light-Colored UV Absorber. ACS Sustain. Chem. Eng. 2021, 9, 1427–1442. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, D.; Shen, F.; Yang, G.; Long, L.; He, J.; Song, C.; Zhang, J.; Zhu, Y.; Huang, C.; et al. Transparent Cellulose/Technical Lignin Composite Films for Advanced Packaging. Polymers 2019, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Chen, Z.; Xia, Y.; Lin, M.; Li, J.; Lan, W.; Zhang, L.; Yue, F. Influence of the Lignin Extraction Methods on the Content of Tricin in Grass Lignins. Front. Energy Res. 2021, 9, 756285. [Google Scholar] [CrossRef]
- Lim, J.; Sana, B.; Krishnan, R.; Seayad, J.; Ghadessy, F.J.; Jana, S.; Ramalingam, B. Laccase-Catalyzed Synthesis of Low-Molecular-Weight Lignin-Like Oligomers and Their Application as UV-Blocking Materials. Chemistry 2018, 13, 284–291. [Google Scholar] [CrossRef]
- Yearla, S.R.; Padmasree, K. Preparation and Characterisation of Lignin Nanoparticles: Evaluation of Their Potential as Antioxidants and UV Protectants. J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the Structural and Chemical Changes of Plant Biomass Following Steam Explosion Pretreatment. Biotechnol Biofuels 2017, 10, 36. [Google Scholar] [CrossRef] [PubMed]




| LMPs Characterizations | Organosolv 200 °C 60 min 60/40 EtOH/H2O (v/v) | Kraft | ||
|---|---|---|---|---|
| Beech | Spruce | Wheat Straw | ||
| Extraction yields (wt%) | 69.7 ± 0.8 | 35.9 ± 1.2 | 44.8 ± 0.4 | / |
| Purity (%) | 93.0 ± 0.4 | 96.1 ± 0.1 | 96.3 ± 0.7 | 91.5 ± 0.3 |
| Sulfur content (%) | ˂0.05 | ˂0.05 | ˂0.05 | 1.8 |
| Mw (kDa) | 20.8 | 15.7 | 17.2 | 29.4 |
| 31P Nuclear Magnetic Resonance (NMR)—Hydroxyl Groups (mmol.g−1) | ||||
|---|---|---|---|---|
| LMPs | Beech | Spruce | Wheat Straw | Kraft |
| Total -OH | 4.19 | 3.71 | 2.94 | 4.62 |
| Aliphatic -OH | 2.26 | 1.71 | 1.27 | 1.68 |
| Phenolic -OH | 1.93 | 1.86 | 1.44 | 2.53 |
| Syringyl | 1.18 | 0.14 | 0.59 | 0.36 |
| Guaiacyl | 0.74 | 1.59 | 0.61 | 2.17 |
| p-Hydroxypenyl | 0.01 | 0.13 | 0.24 | 0 |
| COOH | 0 | 0.14 | 0.23 | 0.41 |
| Heteronuclear single quantum coherence (HSQC) NMR spectroscopy—linkages (%) | ||||
| LMPs | Beech | Spruce | Wheat straw | Kraft |
| β-O-4 (%) | 25.28 | 17.89 | 16.09 | 9.65 |
| β-5 (%) | 3.94 | 13.71 | 3.94 | 2.44 |
| β-β (%) | 6.76 | 3.06 | 1.40 | 3.72 |
| S/G | 1.87 | 0 | 1.03 | 0 |
| LMPs (wt%) | 0 a | Beech | Spruce | Wheat Straw | Kraft | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 10 | 1 | 5 | 10 | 1 | 5 | 10 | 1 | 5 | 10 | ||||||
| Pure cream | 1.08 ± 0.02 | 1.48 ± 0.03 | 6.77 ± 1.09 | 11.24 ± 0.24 | 1.45 ± 0.05 | 2.76 ± 0.13 | 9.65 ± 1.23 | 1.66 ± 0.02 | 7.33 ± 1.37 | 25.30 ± 4.31 | 1.27 ± 0.02 | 2.51 ± 0.65 | 8.85 ± 1.21 | ||||
| LMPs (wt%) | 0 b | 1 | 5 | 1 | 5 | 1 | 5 | 1 | 5 | ||||||||
| * SPF 10 | 9.39 ± 0.53 | 27.43 | 50+ | 13.70 | 26.17 | 18.29 | 50+ | 11.88 | 22.65 | ||||||||
| LMPs (wt%) | 0 c | 1 | 1 | 1 | 1 | ||||||||||||
| * SPF 30 | 30.93 ± 4.97 | 50+ | 50+ | 50+ | 50+ | ||||||||||||
| LNPs (wt%) | 0 a | Beech | Spruce | Wheat straw | |||||||||||||
| 1 | 5 | 1 | 5 | 1 | 5 | ||||||||||||
| Pure cream | 1.08 ± 0.02 | 4.93 ± 0.53 | 20.17 ± 2.20 | 3.41 ± 0.96 | 11.39 ± 1.32 | 7.88 ± 0.84 | 41.97 ± 7.38 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard, V.; Fragnières, L.; Chapuis, H.; Brosse, N.; Marchal-Heussler, L.; Canilho, N.; Parant, S.; Ziegler-Devin, I. The Impact of Lignin Biopolymer Sources, Isolation, and Size Reduction from the Macro- to Nanoscale on the Performances of Next-Generation Sunscreen. Polymers 2024, 16, 1901. https://doi.org/10.3390/polym16131901
Girard V, Fragnières L, Chapuis H, Brosse N, Marchal-Heussler L, Canilho N, Parant S, Ziegler-Devin I. The Impact of Lignin Biopolymer Sources, Isolation, and Size Reduction from the Macro- to Nanoscale on the Performances of Next-Generation Sunscreen. Polymers. 2024; 16(13):1901. https://doi.org/10.3390/polym16131901
Chicago/Turabian StyleGirard, Victor, Léane Fragnières, Hubert Chapuis, Nicolas Brosse, Laurent Marchal-Heussler, Nadia Canilho, Stéphane Parant, and Isabelle Ziegler-Devin. 2024. "The Impact of Lignin Biopolymer Sources, Isolation, and Size Reduction from the Macro- to Nanoscale on the Performances of Next-Generation Sunscreen" Polymers 16, no. 13: 1901. https://doi.org/10.3390/polym16131901
APA StyleGirard, V., Fragnières, L., Chapuis, H., Brosse, N., Marchal-Heussler, L., Canilho, N., Parant, S., & Ziegler-Devin, I. (2024). The Impact of Lignin Biopolymer Sources, Isolation, and Size Reduction from the Macro- to Nanoscale on the Performances of Next-Generation Sunscreen. Polymers, 16(13), 1901. https://doi.org/10.3390/polym16131901








