Sustainable All-Cellulose Biocomposites from Renewable Biomass Resources Fabricated in a Water-Based Processing System by the Vacuum-Filtration-Assisted Impregnation Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
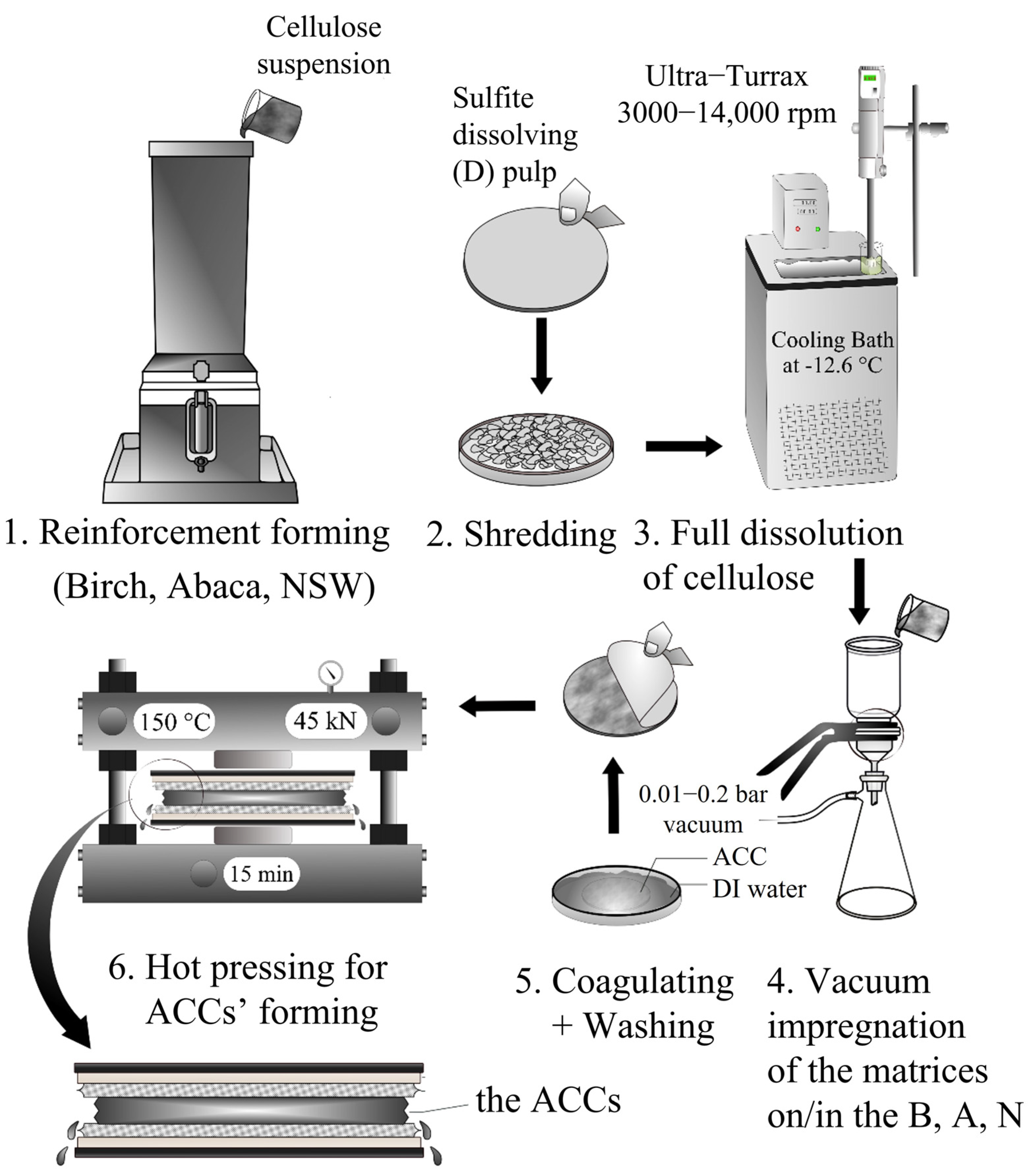
2.2. Cellulose Reinforcement Preparation
2.3. Cellulose Matrix Preparation
2.4. Preparation of the ACCs
2.5. Regenerated Cellulose Film Preparation
3. Characterizations
3.1. Degree of Polymerization (DP)
3.2. Thickness and Density
3.3. Field Emission Scanning Electron Microscopy (FE-SEM)
3.4. X-ray Diffraction (XRD)
3.5. Mechanical Testing
4. Results and Discussion
4.1. Degree of Polymerization (DP)
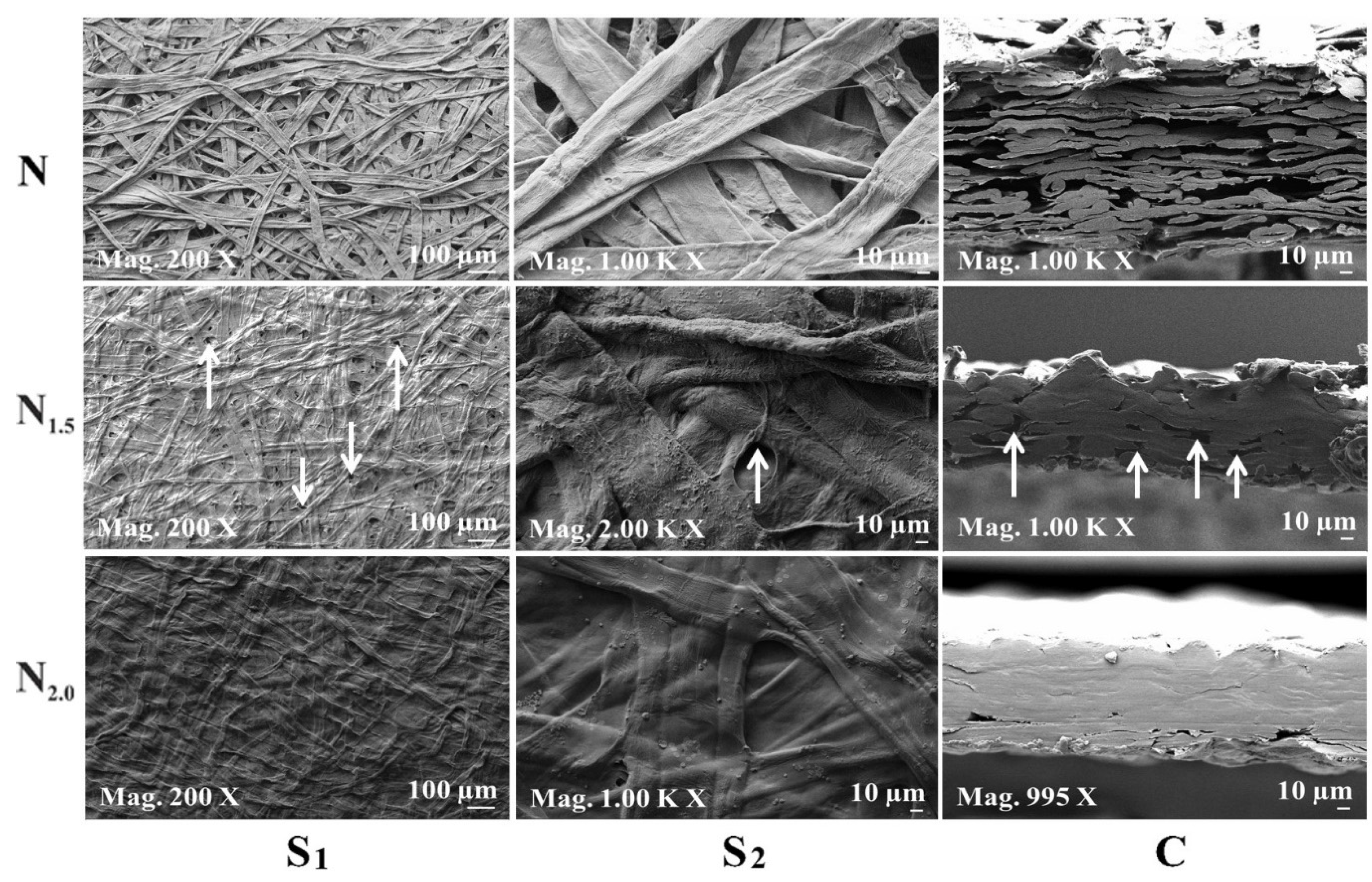
4.2. FE-SEM
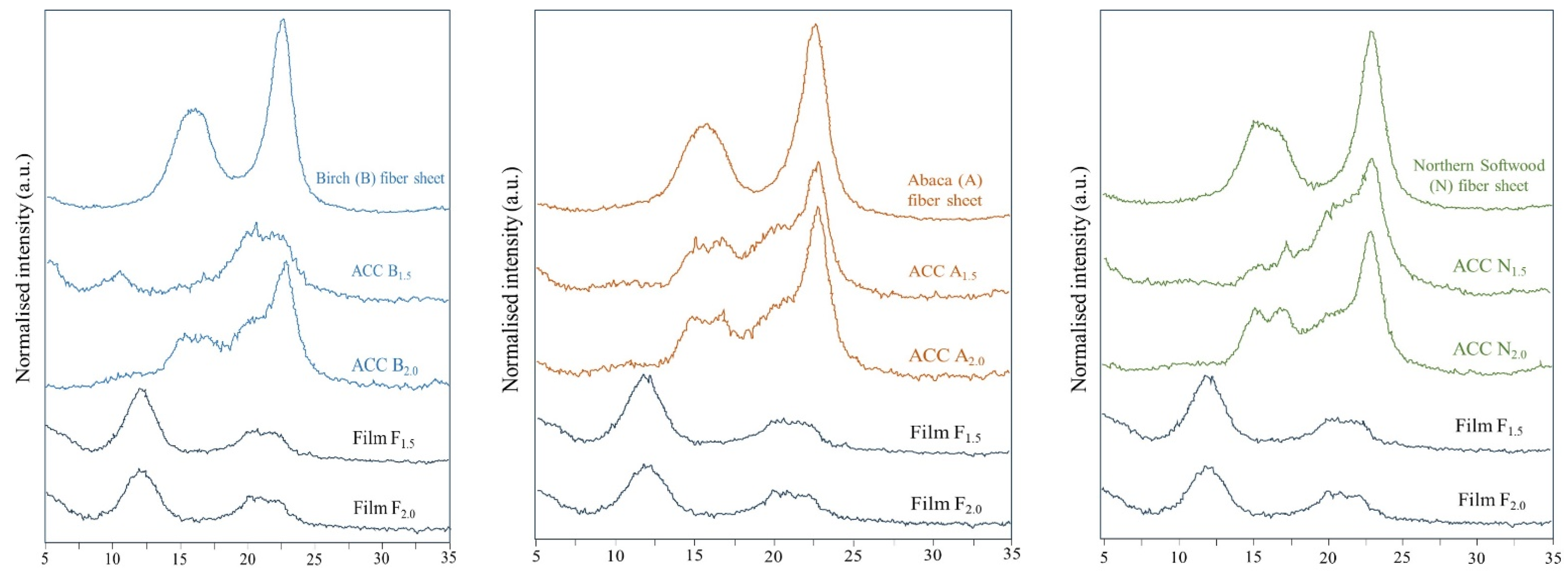
4.3. XRD
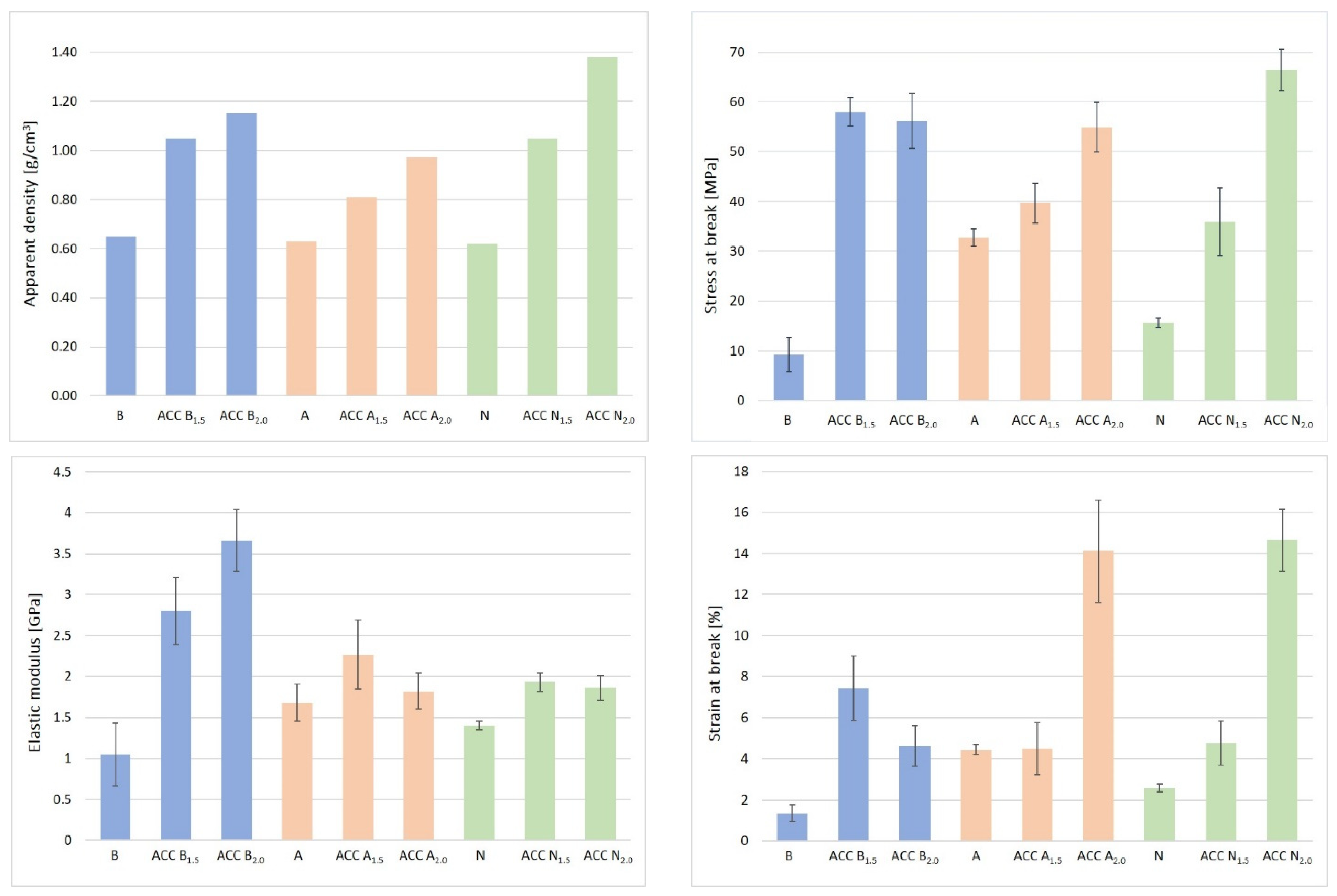
4.4. Mechanical Properties
5. Conclusions
- ○
- A 532% increase in stress at break for sample ACC B1.5 (birch fiber sheet reinforced with a 1.5 wt.% dissolved cellulose matrix) from the initial 9.24 MPa to 58.4 MPa;
- ○
- A 248% increase in elastic modulus for sample ACC B2.0 (birch fiber sheet reinforced with a 2.0 wt.% dissolved cellulose matrix) from the initial 1.05 GPa to 3.66 GPa;
- ○
- A 466% increase in strain at break for sample ACC N2.0 (northern softwood
- ○
- fiber sheet reinforced with a 2.0 dissolved cellulose matrix) from the initial 2.59% to 14.65%.
Future Perspectives of this Study
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishino, T.; Arimoto, N. All-Cellulose Composite Prepared by Selective Dissolving of Fiber Surface. Biomacromolecules 2007, 8, 2712–2716. [Google Scholar] [CrossRef] [PubMed]
- Green Composites from Natural Resources; Thakur, V.K. (Ed.) CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4665-7070-2. [Google Scholar]
- Acharya, S.; Liyanage, S.; Parajuli, P.; Rumi, S.S.; Shamshina, J.L.; Abidi, N. Utilization of Cellulose to Its Full Potential: A Review on Cellulose Dissolution, Regeneration, and Applications. Polymers 2021, 13, 4344. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, S.; Fernandes, C.; Pedrosa, J.F.S.; Alves, L.; Medronho, B.; Ferreira, P.J.T.; Rasteiro, M.D.G. Eco-Friendly Methods for Extraction and Modification of Cellulose: An Overview. Polymers 2023, 15, 3138. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Shokri, S.; Hedjazi, S.; Lê, H.Q.; Abdulkhani, A.; Sixta, H. High-purity cellulose production from birch wood by γ-valerolactone/water fractionation and IONCELL-P process. Carbohydr. Polym. 2022, 288, 119364. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Jolly, R.; Fatima, T.; Shakir, M. Extraction processes for deriving cellulose: A comprehensive review on green approaches. Polym. Adv. Technol. 2022, 33, 2069–2090. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Chanthathamrongsiri, N.; Petchsomrit, A.; Leelakanok, N.; Siranonthana, N.; Sirirak, T. The comparison of the properties of nanocellulose isolated from colonial and solitary marine tunicates. Heliyon 2021, 7, e07819. [Google Scholar] [CrossRef]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal cellulose, production and potential use in plastics: Challenges and opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, J.A.; Ismael, M.I.; Anjo, C.M.S.; Duarte, A.P. Cellulose and Derivatives from Wood and Fibers as Renewable Sources of Raw-Materials. In Carbohydrates in Sustainable Development I; Rauter, A.P., Vogel, P., Queneau, Y., Eds.; Topics in Current Chemistry; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2010; Volume 294, pp. 117–128. ISBN 978-3-642-14836-1. [Google Scholar]
- Piltonen, P.; Hildebrandt, N.C.; Westerlind, B.; Valkama, J.-P.; Tervahartiala, T.; Illikainen, M. Green and efficient method for preparing all-cellulose composites with NaOH/urea solvent. Compos. Sci. Technol. 2016, 135, 153–158. [Google Scholar] [CrossRef]
- Yousefi, H.; Faezipour, M.; Nishino, T.; Shakeri, A.; Ebrahimi, G. All-cellulose composite and nanocomposite made from partially dissolved micro-and nanofibers of canola straw. Polym. J. 2011, 43, 559–564. [Google Scholar] [CrossRef]
- Gindl-Altmutter, W.; Keckes, J.; Plackner, J.; Liebner, F.; Englund, K.; Laborie, M.-P. All-cellulose composites prepared from flax and lyocell fibres compared to epoxy–matrix composites. Compos. Sci. Technol. 2012, 72, 1304–1309. [Google Scholar] [CrossRef]
- Gindl, W.; Keckes, J. All-cellulose nanocomposite. Polymer 2005, 46, 10221–10225. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Arimoto, N.; Nishino, T.; Peijs, T. All-cellulose composites by surface selective dissolution of aligned ligno-cellulosic fibres. Compos. Sci. Technol. 2008, 68, 2201–2207. [Google Scholar] [CrossRef]
- Shakeri, A.; Mathew, A.P.; Oksman, K. Self-reinforced nanocomposite by partial dissolution of cellulose microfibrils in ionic liquid. J. Compos. Mater. 2012, 46, 1305–1311. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, N.; Zhang, X.; Xu, L.; Wu, J.; Yu, J.; He, J.; Zhang, J. All-Cellulose Nanocomposites Reinforced with in Situ Retained Cellulose Nanocrystals during Selective Dissolution of Cellulose in an Ionic Liquid. ACS Sustain. Chem. Eng. 2016, 4, 4417–4423. [Google Scholar] [CrossRef]
- Kalka, S.; Huber, T.; Steinberg, J.; Baronian, K.; Müssig, J.; Staiger, M.P. Biodegradability of all-cellulose composite laminates. Compos. Part Appl. Sci. Manuf. 2014, 59, 37–44. [Google Scholar] [CrossRef]
- Uusi-Tarkka, E.-K.; Skrifvars, M.; Haapala, A. Fabricating Sustainable All-Cellulose Composites. Appl. Sci. 2021, 11, 10069. [Google Scholar] [CrossRef]
- Adak, B.; Mukhopadhyay, S. A comparative study on lyocell-fabric based all-cellulose composite laminates produced by different processes. Cellulose 2017, 24, 835–849. [Google Scholar] [CrossRef]
- Pullawan, T. Interfacial Micromechanics of All-Cellulose Nanocomposites Using Raman Spectroscopy. Doctoral Dissertation, Department of Materials, The University of Manchester, Manchester, UK, 2013. [Google Scholar]
- Duchemin, B. Structure, Property and Processing Relationships of All-Cellulose Composites; University of Canterbury: Christchurch, New Zealand, 2008. [Google Scholar]
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836. [Google Scholar] [CrossRef] [PubMed]
- Ouajai, S.; Shanks, R.A. Preparation, structure and mechanical properties of all-hemp cellulose biocomposites. Compos. Sci. Technol. 2009, 69, 2119–2126. [Google Scholar] [CrossRef]
- Han, D.; Yan, L. Preparation of all-cellulose composite by selective dissolving of cellulose surface in PEG/NaOH aqueous solution. Carbohydr. Polym. 2010, 79, 614–619. [Google Scholar] [CrossRef]
- Huber, T.; Bickerton, S.; Müssig, J.; Pang, S.; Staiger, M.P. Solvent infusion processing of all-cellulose composite materials. Carbohydr. Polym. 2012, 90, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Duchemin, B.; Le Corre, D.; Leray, N.; Dufresne, A.; Staiger, M.P. All-cellulose composites based on microfibrillated cellulose and filter paper via a NaOH-urea solvent system. Cellulose 2016, 23, 593–609. [Google Scholar] [CrossRef]
- Hu, F.; Wang, M.; Wang, N.; Hu, Y.; Gan, M.; Liu, D.; Xie, Y.; Feng, Q. All-cellulose composites prepared by partially dissolving cellulose using NaOH /thiourea aqueous solution. J. Appl. Polym. Sci. 2021, 138, 51298. [Google Scholar] [CrossRef]
- Delgado, J.F.; Salvay, A.G.; Arroyo, S.; Bernal, C.R.; Foresti, M.L. Effect of Dissolution Time on the Development of All-Cellulose Composites Using the NaOH/Urea Solvent System. Polysaccharides 2023, 4, 65–77. [Google Scholar] [CrossRef]
- Uusi-Tarkka, E.-K.; Skrifvars, M.; Khalili, P.; Heräjärvi, H.; Kadi, N.; Haapala, A. Mechanical and Thermal Properties of Wood-Fiber-Based All-Cellulose Composites and Cellulose-Polypropylene Biocomposites. Polymers 2023, 15, 475. [Google Scholar] [CrossRef]
- Suthatho, A.; Rattanawongkun, P.; Tawichai, N.; Tanpichai, S.; Boonmahitthisud, A.; Soykeabkaew, N. Low-Density All-Cellulose Composites Made from Cotton Textile Waste with Promising Thermal Insulation and Acoustic Absorption Properties. ACS Appl. Polym. Mater. 2024, 6, 390–397. [Google Scholar] [CrossRef]
- Nishino, T.; Matsuda, I.; Hirao, K. All-Cellulose Composite. Macromolecules 2004, 37, 7683–7687. [Google Scholar] [CrossRef]
- Zhao, Q.; Yam, R.C.M.; Zhang, B.; Yang, Y.; Cheng, X.; Li, R.K.Y. Novel all-cellulose ecocomposites prepared in ionic liquids. Cellulose 2009, 16, 217–226. [Google Scholar] [CrossRef]
- Qin, C.; Soykeabkaew, N.; Xiuyuan, N.; Peijs, T. The effect of fibre volume fraction and mercerization on the properties of all-cellulose composites. Carbohydr. Polym. 2008, 71, 458–467. [Google Scholar] [CrossRef]
- Pullawan, T.; Wilkinson, A.N.; Zhang, L.N.; Eichhorn, S.J. Deformation micromechanics of all-cellulose nanocomposites: Comparing matrix and reinforcing components. Carbohydr. Polym. 2014, 100, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Saito, T.; Berglund, L.A.; Isogai, A. Cellulose nanofibrils improve the properties of all-cellulose composites by the nano-reinforcement mechanism and nanofibril-induced crystallization. Nanoscale 2015, 7, 17957–17963. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, N.; Singh, H.; Chatterjee, A. All-cellulose composite from cotton fabric and cellulose solution. Cellul. Chem. Technol. 2020, 54, 757–764. [Google Scholar] [CrossRef]
- Chen, F.; Sawada, D.; Hummel, M.; Sixta, H.; Budtova, T. Unidirectional All-Cellulose Composites from Flax via Controlled Impregnation with Ionic Liquid. Polymers 2020, 12, 1010. [Google Scholar] [CrossRef]
- Jaafar, M.Z.; Mohd Ridzuan, F.F.; Mohamad Kassim, M.H.; Abu, F. The Role of Dissolution Time on the Properties of All-Cellulose Composites Obtained from Oil Palm Empty Fruit Bunch. Polymers 2023, 15, 691. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Hildebrandt, N.C. Rapid preparation of all-cellulose composites by solvent welding based on the use of aqueous solvent. Eur. Polym. J. 2017, 97, 292–298. [Google Scholar] [CrossRef]
- Gan, M.; Tian, L.; Chen, Y.; Xin, J.; Si, H.; Xie, Y.; Feng, Q. All-cellulose composites fabricated by in-situ welding. BioResources 2023, 18, 3044–3055. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Hao, S.; Huang, J. Preparation of All-Cellulose Composites Based on Controlled Dissolution Procedure. Starch-Stärke 2021, 73, 2000280. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, F.; Gan, M.; Xie, Y.; Feng, Q. Facile one-step fabrication of all cellulose composites with unique optical performance from wood and bamboo pulp. Carbohydr. Polym. 2021, 274, 118630. [Google Scholar] [CrossRef] [PubMed]
- Abou-Yousef, H.; Kamel, S. Physico-mechanical properties of all-cellulose composites prepared by different approaches from micro-fibrillated bagasse pulp fibers. Mater. Today Commun. 2023, 35, 105672. [Google Scholar] [CrossRef]
- Wang, C.; Wu, S.; Zhang, N.; Jiang, Z.; Hou, X.; Huang, L.; Deng, T. Efficient oil-water separation by novel biodegradable all cellulose composite filter paper. Green Energy Environ. 2023, 8, 1673–1682. [Google Scholar] [CrossRef]
- Baghaei, B.; Johansson, B.; Skrifvars, M.; Kadi, N. All-Cellulose Composites Properties from Pre- and Post-Consumer Denim Wastes: Comparative Study. J. Compos. Sci. 2022, 6, 130. [Google Scholar] [CrossRef]
- He, X.; Xiao, Q.; Lu, C.; Wang, Y.; Zhang, X.; Zhao, J.; Zhang, W.; Zhang, X.; Deng, Y. Uniaxially Aligned Electrospun All-Cellulose Nanocomposite Nanofibers Reinforced with Cellulose Nanocrystals: Scaffold for Tissue Engineering. Biomacromolecules 2014, 15, 618–627. [Google Scholar] [CrossRef]
- Hai, L.V.; Kim, H.C.; Kafy, A.; Zhai, L.; Kim, J.W.; Kim, J. Green all-cellulose nanocomposites made with cellulose nanofibers reinforced in dissolved cellulose matrix without heat treatment. Cellulose 2017, 24, 3301–3311. [Google Scholar] [CrossRef]
- Labidi, K.; Korhonen, O.; Zrida, M.; Hamzaoui, A.H.; Budtova, T. All-cellulose composites from alfa and wood fibers. Ind. Crops Prod. 2019, 127, 135–141. [Google Scholar] [CrossRef]
- Qi, H.; Cai, J.; Zhang, L.; Kuga, S. Properties of Films Composed of Cellulose Nanowhiskers and a Cellulose Matrix Regenerated from Alkali/Urea Solution. Biomacromolecules 2009, 10, 1597–1602. [Google Scholar] [CrossRef]
- Yang, Q.; Lue, A.; Zhang, L. Reinforcement of ramie fibers on regenerated cellulose films. Compos. Sci. Technol. 2010, 70, 2319–2324. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Impacts of nanowhisker on formation kinetics and properties of all-cellulose composite gels. Carbohydr. Polym. 2011, 83, 1937–1946. [Google Scholar] [CrossRef]
- Venu Nadhan, A.; Varada Rajulu, A.; Li, R.; Jie, C.; Zhang, L. Properties of Regenerated Cellulose Short Fibers/Cellulose Green Composite Films. J. Polym. Environ. 2012, 20, 454–458. [Google Scholar] [CrossRef]
- Senthil Muthu Kumar, T.; Rajini, N.; Obi Reddy, K.; Varada Rajulu, A.; Siengchin, S.; Ayrilmis, N. All-cellulose composite films with cellulose matrix and Napier grass cellulose fibril fillers. Int. J. Biol. Macromol. 2018, 112, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, W.L.E.; Cao, X.; Ramires, M.A.; Lucia, L.A. Novel all-cellulose composite displaying aligned cellulose nanofibers reinforced with cellulose nanocrystals. Tappi J. 2011, 10, 19–25. [Google Scholar] [CrossRef]
- Baghaei, B.; Compiet, S.; Skrifvars, M. Mechanical properties of all-cellulose composites from end-of-life textiles. J. Polym. Res. 2020, 27, 260. [Google Scholar] [CrossRef]
- Lourdin, D.; Peixinho, J.; Bréard, J.; Cathala, B.; Leroy, E.; Duchemin, B. Concentration driven cocrystallisation and percolation in all-cellulose nanocomposites. Cellulose 2016, 23, 529–543. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Jiang, Z.; Gao, X.; Sun, C.; Zhang, L. Green Preparation and Functional Properties of Reinforced All-Cellulose Membranes Made from Corn Straw. Membranes 2024, 14, 16. [Google Scholar] [CrossRef]
- Jiang, Z.; Fang, Y.; Xiang, J.; Ma, Y.; Lu, A.; Kang, H.; Huang, Y.; Guo, H.; Liu, R.; Zhang, L. Intermolecular Interactions and 3D Structure in Cellulose–NaOH–Urea Aqueous System. J. Phys. Chem. B 2014, 118, 10250–10257. [Google Scholar] [CrossRef]
- Battista, O.A. Molecular Weight of Cellulose Measurement of Average Degree of Polymerization. Ind. Eng. Chem. Anal. Ed. 1944, 16, 351–354. [Google Scholar] [CrossRef]
- Kargupta, W.; Seifert, R.; Martinez, M.; Olson, J.; Tanner, J.; Batchelor, W. Preparation and benchmarking of novel cellulose nanopaper. Cellulose 2022, 29, 4393–4411. [Google Scholar] [CrossRef]
- Al Tamimi, Z.; Chen, L.; Ji, X.; Vanderlaan, G.; Gacura, M.D.; Piovesan, D. Preparation of Nanopaper for Colorimetric Food Spoilage Indication. Polymers 2023, 15, 3098. [Google Scholar] [CrossRef] [PubMed]
- ASTM D1795-13(2021); D01 Committee Test Method for Intrinsic Viscosity of Cellulose. ASTM: West Conshohocken, PA, USA, 2021. [CrossRef]
- Kihlman, M.; Medronho, B.F.; Romano, A.L.; Germgård, U.; Lindman, B. Cellulose Dissolution in an Alkali Based Solvent: Influence of Additives and Pretreatments. J. Braz. Chem. Soc. 2013, 24, 295–303. [Google Scholar] [CrossRef]
- ASTM D645/D645M-97; D06 Committee Test Method for Thickness of Paper and Paperboard. ASTM: West Conshohocken, PA, USA, 2021. [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- ASTM D882-18; D20 Committee Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM E104-02(2012); D22 Committee Practice for Maintaining Constant Relative Humidity by Means of Aqueous Solutions. ASTM: West Conshohocken, PA, USA, 2020. [CrossRef]
- Chen, X.; Burger, C.; Wan, F.; Zhang, J.; Rong, L.; Hsiao, B.S.; Chu, B.; Cai, J.; Zhang, L. Structure Study of Cellulose Fibers Wet-Spun from Environmentally Friendly NaOH/Urea Aqueous Solutions. Biomacromolecules 2007, 8, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, S.; Lu, A.; Zhang, L. Dissolution of cellulose from different sources in an NaOH/urea aqueous system at low temperature. Cellulose 2015, 22, 339–349. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Xu, M. Novel regenerated cellulose films prepared by coagulating with water: Structure and properties. Carbohydr. Polym. 2012, 87, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Maltais, A.; Liu, J.; Wang, Y. Wood cellulose films regenerated from NaOH/urea aqueous solution and treated by hot pressing for food packaging application. Food Biosci. 2022, 50, 102177. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Nugraha, F.W.; Laroybafih, M.B.; Ismail, R.; Bayuseno, A.P.; Muhamadin, R.C.; Ramadan, M.B.; Qudus, A.R.; Siregar, J.P. The effect of hydroxyapatite concentration on the mechanical properties and degradation rate of biocomposite for biomedical applications. IOP Conf. Ser. Earth Environ. Sci. 2022, 969, 012045. [Google Scholar] [CrossRef]
- Uusi-Tarkka, E.-K.; Levanič, J.; Heräjärvi, H.; Kadi, N.; Skrifvars, M.; Haapala, A. All-Cellulose Composite Laminates Made from Wood-Based Textiles: Effects of Process Conditions and the Addition of TEMPO-Oxidized Nanocellulose. Polymers 2022, 14, 3959. [Google Scholar] [CrossRef]


| Matrices of the ACCs | Reinforcements of the ACCs | Immobilization Method (and Anti-Solvent) | Stress at Break (MPa) (±%) | Elastic Modulus (Gpa) (±%) | Strain at Break (%) (±%) | Ref. |
|---|---|---|---|---|---|---|
| Cotton linter pulp (DP: 500) | Regenerated cellulose (RC) film (reference without reinforcement) | Casting method (5 wt.% H2SO4) | 87.0 | 3.92 | 9.5 | [53] |
| Cellulose nanowhiskers (CNWs: 10 wt.%) reinforced ACC films | Casting method (5 wt.% H2SO4) | 124.0 +47% | 5.10 +30% | 6.0 −37% | ||
| Cellulose nanowhiskers (CNWs: 20 wt.%) reinforced ACC films | Casting method (5 wt.% H2SO4) | 117.0 +34% | 5.87 +50% | 4.0 −58% | ||
| Cotton linter pulps (DP: unspecified) | RC film (reference without reinforcement) | Casting method (5 wt.% H2SO4) | 81.4 | 2.5 | 7.8 | [38] |
| Tunicate nanowhiskers (T-NWs: 15 v/v%) reinforced ACC films | Casting method (5 wt.% H2SO4) | 137.1 +68% | 9.8 +392% | 4.1 −47% | ||
| Cotton nanowhiskers (C-NWs: 15 v/v%) reinforced ACC films | Casting method (5 wt.% H2SO4) | 127.4 +56% | 7.2 +188% | 4.3 −45% | ||
| Cotton linter pulps (α-cellulose ≥ 95%) (DP: 617) * | RC film (reference without reinforcement) | Casting method (5 wt.% H2SO4) | 98.4 | 3.93 | 8.9 | [54] |
| Ramie (RA) fibers (delignified, cleaned, cut into short fibers in 5 nm lengths) (RA: 15 wt.%) reinforced ACC films | Casting method (5 wt.% H2SO4) | 124.3 +26% | 5.25 +33% | 4.9 +56% | ||
| Ramie (RA) fibers (delignified, cleaned, cut into short fibers in 5 nm lengths) (RA: 25 wt.%) reinforced ACC films | Casting method (5 wt.% H2SO4) | 83.0 −15% | 5.94 +51% | 2.5 −72% | ||
| Cotton linter pulps (DP: 620) | RC film (reference without reinforcement) | Casting method (5 wt.% H2SO4) | 48.1 | 4.21 | 2.2 | [56] |
| Wet spinning of RC fibers (RCF) in a NaOH–urea solution and cut into ~1 mm lengths: (5 wt.% RCF) reinforced ACC films | Casting method (5 wt.% H2SO4) | 76.0 +58% | 6.9 +64% | 2.9 +32% | ||
| Cotton linter pulps (DP: 617) * | RC gels (reference without reinforcement) | Injection method (running H2O) | 0.4 | (unspecified) | 56 | [55] |
| Cellulose nanowhiskers (CNW powder: 50 wt. %) reinforced ACC gels | Injection method (running H2O) | 0.7 +75% | (unspecified) (N/A) | 55 −1.8% | ||
| Cotton fabrics (spun from 20 s Ne Rotor yarn) (plain weave type) (reference without full dissolved cellulose matrix) | 216.28 kPa | 7.32 | 18.04 | [40] | ||
| Viscose fibers (DP: unspecified) | Cotton fabrics (spun from 20 s Ne Rotor yarn) (twill weave type) reinforced ACCs dip-padded with 1.5% of fully dissolved cellulose matrix | Dip-padding method (H2O) | 219.12 kPa +1% | 4.22 −42% | 26.98 +50% | |
| Cotton fabrics (spun from 20 s Ne Rotor yarn) (twill weave type) (reference without fully dissolved cellulose matrix) | 208.40 | 8.63 | 16.13 | |||
| Viscose fibers (DP: unspecified) | Cotton fabrics (spun from 20 s Ne Rotor yarn) (twill weave type) reinforced ACCs dip-padded with 1.5% of fully dissolved cellulose matrix | Dip-padding method (H2O) | 210.68 kPa +0.5% | 4.67 −46% | 25.55 +58% | |
| Cotton fabrics (spun from 20 s Ne Rotor yarn) (satin weave type) (reference without fully dissolved cellulose matrix) | 203.88 kPa | 11.71 | 13.86 | |||
| Viscose fibers (DP: unspecified) | Cotton fabrics (spun from 20 s Ne Rotor yarn) (satin weave type) reinforced ACCs dip-padded with 1.5% of fully dissolved cellulose matrix | Dip-padding method (H2O) | 208.91 kPa +3% | 5.40 −54% | 17.41 +26% | |
| Cotton linter pulp (DP: unspecified) | RC film from 4 wt.% alkali/urea/cellulose (AUC) matrix solution of cotton linter pulp (reference without reinforcement) | Casting method (5 wt.% H2SO4) | 111.0 | 3.2 | 12.0 | [39] |
| TEMPO-oxidized cellulose nanofibrils (TOCN) from never-dried softwood bleached kraft pulp (SBKP) (~90% α-cellulose and ~10% hemicelluloses): (TOCN: 1 wt. %) reinforced ACCs with 4 wt.% AUC matrix solution | Casting method (5 wt.% H2SO4) | 167.0 +50% | 6.2 +94% | 10.0 −17% | ||
| Birch (B) fiber sheets (prepared from birch wood pulps by sheet forming) (reference without the impregnation of dissolved cellulose matrices of the D pulps) | 9.24 | 1.05 | 1.36 | This study | ||
| Sulfite dissolving (D) pulp (DP: 580.73) | Birch (B) fiber sheets (prepared from birch wood pulps by sheet forming) reinforced ACCs with the impregnation of 1.5 wt.% dissolved cellulose matrix | Vacuum-filtration-assisted impregnation method (deionized H2O) | 58.4 +532% | 2.80 +167% | 7.43 +446% | |
| Birch (B) fiber sheets (prepared from birch wood pulps by sheet forming) reinforced ACCs with the impregnation of a 2.0 wt.% dissolved cellulose matrices of the D pulps) | Vacuum-filtration-assisted impregnation method (deionized H2O) | 56.21 +508% | 3.66 +248% | 4.62 +240% | ||
| Abaca (A) fiber sheets (prepared from abaca leaf-based pulps by sheet forming) (reference without the impregnation of dissolved cellulose matrices of the D pulps) | 32.76 | 1.68 | 4.44 | |||
| Sulfite dissolving (D) pulp (DP: 580.73) | Abaca fiber sheets (A) (prepared from abaca leaf-based pulps beforehand) reinforced ACCs with the impregnation of a 1.5 wt.% dissolved cellulose matrices of the D pulps) | Vacuum-filtration-assisted impregnation method (deionized H2O) | 39.68 +21% | 2.27 +35% | 4.5 +1% | |
| Abaca fiber sheets (A) (prepared from abaca leaf-based pulps beforehand) reinforced ACCs with the impregnation of a 2.0 wt.% dissolved cellulose matrices of the D pulps) | Vacuum-filtration-assisted impregnation method (deionized H2O) | 54.93 +68% | 1.82 +8% | 14.11 +218% | ||
| Northern softwood (N) fiber sheets (prepared from the N wood pulps by sheet forming) (reference without the impregnation of the dissolved cellulose matrices of the D pulps) | 15.64 | 1.4 | 2.59 | |||
| Sulfite dissolving (D) pulp (DP: 580.73) | Northern softwood (N) fiber sheets (prepared from the N wood pulps by sheet forming) reinforced ACCs with the impregnation of a 1.5 wt.% dissolved cellulose matrices of the D pulps) | Vacuum-filtration-assisted impregnation method (deionized H2O) | 35.84 +129% | 1.93 +38% | 4.77 +84% | |
| Northern softwood (N) fiber sheets (prepared from the N wood pulps by sheet forming) reinforced ACCs with the impregnation of a 2.0 wt.% dissolved cellulose matrices of the D pulps) | Vacuum-filtration-assisted impregnation method (deionized H2O) | 66.43 +325% | 1.86 +33% | 14.65 +466% | ||
| (*) | The DP values were calculated only for Table 1, based on their reported number-average molecular weight (Mη = 1 × 105) by calculation from the Mη of natural cellulose, which is 162.1406 g/mol. | [63] | ||||
| Sample | Crystalline Index Crl (%) |
|---|---|
| Birch fiber sheets (B) | 73.1 |
| All-cellulose composite (ACC) (B1.5) | 44.4 |
| All-cellulose composite (ACC) (B2.0) | 58.3 |
| Abaca fiber sheets (A) | 76.8 |
| All-cellulose composite (ACC) (A1.5) | 60.6 |
| All-cellulose composite (ACC) (A2.0) | 61.5 |
| Northern softwood fiber sheets (N) | 78.3 |
| All-cellulose composite (ACC) (N1.5) | 59.2 |
| All-cellulose composite (ACC) (N2.0) | 66.2 |
| Regenerated cellulose film (F1.5) | 43.7 |
| Regenerated cellulose film (F2.0) | 43.9 |
| Samples | Thickness (µm) | Apparent Density (g/cm3) | Stress at Break (MPa) | Elastic Modulus (GPa) | Strain at Break (%) |
|---|---|---|---|---|---|
| B (birch fiber sheet) | 138 ± 3 | 0.65 | 9.24 ± 3.4 | 1.05 ± 0.38 | 1.36 ± 0.43 |
| ACC, B1.5 | 102 ± 1 | 1.05 | 58.04 + 2.93 | 2.80 ± 0.41 | 7.43 ± 1.57 |
| ACC, B2.0 | 145 ± 5 | 1.15 | 56.21 ± 5.5 | 3.66 ± 0.38 | 4.62 ± 0.98 |
| A (abaca fiber sheet) | 136 ± 4 | 0.63 | 32.76 ± 1.74 | 1.68 ± 0.23 | 4.44 ± 0.24 |
| ACC, A1.5 | 129 ± 3 | 0.81 | 39.68 ± 4.02 | 2.27 ± 0.42 | 4.5 ± 1.26 |
| ACC, A2.0 | 142 ± 3 | 0.97 | 54.93 ± 5.01 | 1.82 ± 0.22 | 14.11 ± 2.5 |
| N (NSW fiber sheet) | 131 ± 1 | 0.62 | 15.64 ± 0.94 | 1.4 ± 0.05 | 2.59 ± 0.18 |
| ACC, N1.5 | 139 ± 7 | 1.05 | 35.84 ± 6.76 | 1.93 ± 0.11 | 4.77 ± 1.08 |
| ACC, N2.0 | 117 ± 3 | 1.38 | 66.43 ± 4.2 | 1.86 ± 0.15 | 14.65 ± 1.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yapar, Ö.; Piltonen, P.; Hadela, A.; Lobnik, A. Sustainable All-Cellulose Biocomposites from Renewable Biomass Resources Fabricated in a Water-Based Processing System by the Vacuum-Filtration-Assisted Impregnation Method. Polymers 2024, 16, 1921. https://doi.org/10.3390/polym16131921
Yapar Ö, Piltonen P, Hadela A, Lobnik A. Sustainable All-Cellulose Biocomposites from Renewable Biomass Resources Fabricated in a Water-Based Processing System by the Vacuum-Filtration-Assisted Impregnation Method. Polymers. 2024; 16(13):1921. https://doi.org/10.3390/polym16131921
Chicago/Turabian StyleYapar, Özkan, Petteri Piltonen, Ajra Hadela, and Aleksandra Lobnik. 2024. "Sustainable All-Cellulose Biocomposites from Renewable Biomass Resources Fabricated in a Water-Based Processing System by the Vacuum-Filtration-Assisted Impregnation Method" Polymers 16, no. 13: 1921. https://doi.org/10.3390/polym16131921






