Dispersion and Lubrication of Zinc Stearate in Polypropylene/Sodium 4-[(4-chlorobenzoyl) amino] Benzoate Nucleating Agent Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the SCAB-Znst Composite Nucleating Agent
2.3. Characterization of SCAB-Znst Composite Nucleating Agent
2.4. Preparation of the PP/SCAB Composite
2.5. Characterization of the PP/SCAB Composite
3. Results and Discussion
3.1. FTIR Spectra of the SCAB-Znst Composite Nucleating Agent
3.2. SEM Photograph of the SCAB-Znst Composite Nucleating Agent
3.3. TG Analyses of the SCAB-Znst Composite Nucleating Agent
3.4. Non-Isothermal Crystallization Process of the PP/SCAB Composite
3.5. Non-Isothermal Crystallization Kinetics Analysis of the PP/SCAB Composite
3.6. POM of the PP/SCAB Composite
3.7. Lubrication Mechanism of the PP/SCAB Composite
3.8. Mechanical Properties of the PP/SCAB Composite
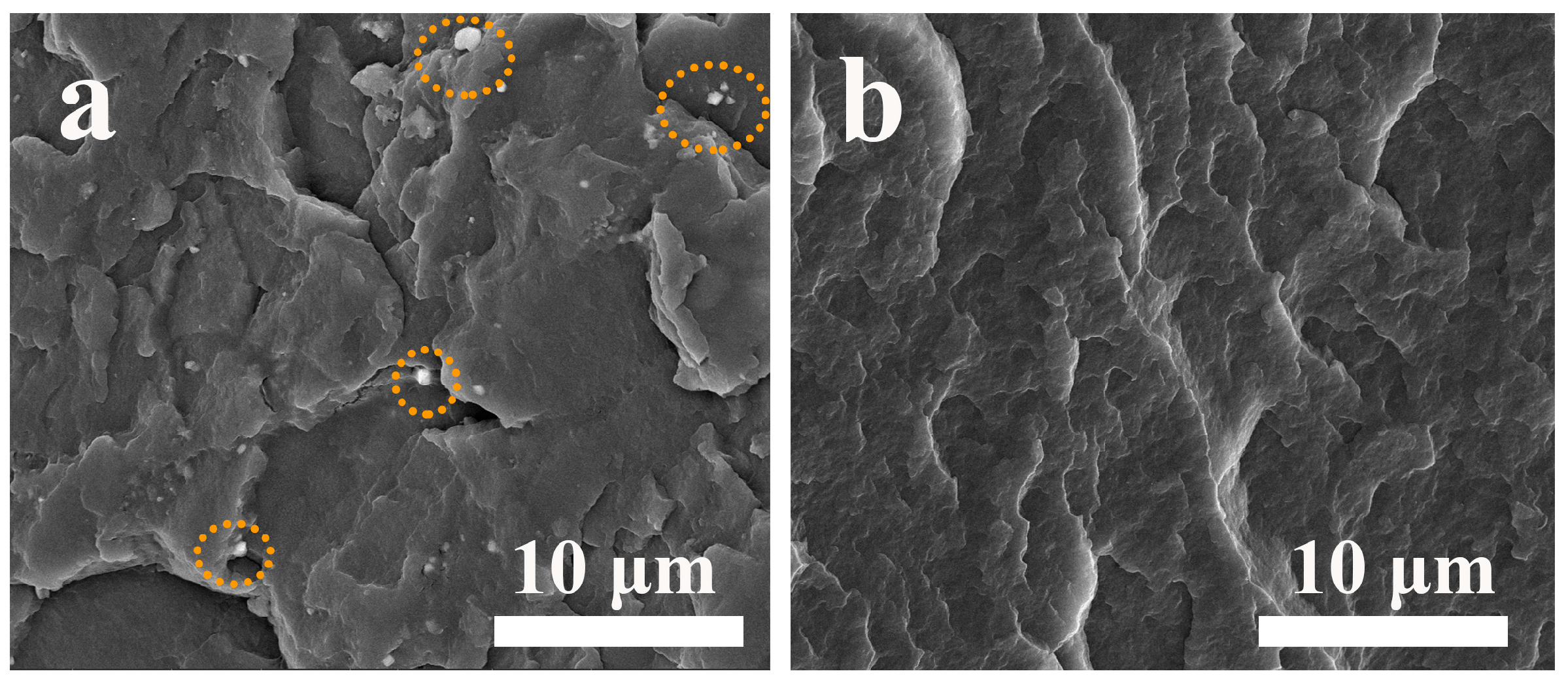
3.9. Dispersion Property of SCAB in the PP Matrix
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tadele, D.; Roy, P.; Defersha, F.; Misra, M.; Mohanty, A.K. A Comparative Life-Cycle Assessment of Talc- and Biochar-Reinforced Composites for Lightweight Automotive Parts. Clean Technol. Environ. Policy 2020, 22, 639–649. [Google Scholar] [CrossRef]
- Pal, S.; Bhattacharjee, P. Polypropylene-Based Packaging Materials for Shelf-Life Enhancement of Yellow Corn (Zea mays) Kernels: Effects on Lutein, Aflatoxin Content, Sensory, and Nutritional Profiles. J. Food Process. Preserv. 2018, 42, e13618. [Google Scholar] [CrossRef]
- Ghanbari, A.; Seyedin, S.; Haddadi, S.A.; Nofar, M.; Ameli, A. Reinforcing Potential of Recycled Carbon Fibers in Compatibilized Polypropylene Composites. J. Polym. Res. 2021, 28, 145. [Google Scholar] [CrossRef]
- Wang, K.; Chen, L.; Gao, Y.; Jiang, D.; Quan, Y.; Yan, S. Effect of Morphology Development on the Low-Temperature Tensile Properties of PP/POE Blends. J. Appl. Polym. Sci. 2022, 139, e52192. [Google Scholar] [CrossRef]
- Ong, M.Y.; Chow, W.S. Kinetics of Crystallization for Polypropylene/Polyethylene/Halloysite Nanotube Nanocomposites. J. Thermoplast. Compos. Mater. 2020, 33, 451–463. [Google Scholar] [CrossRef]
- Huang, C.-W.; Yang, T.-C.; Hung, K.-C.; Xu, J.-W.; Wu, J.-H. The Effect of Maleated Polypropylene on the Non-Isothermal Crystallization Kinetics of Wood Fiber-Reinforced Polypropylene Composites. Polymers 2018, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zheng, X.; Xu, W.; Ren, Y.; Leng, H.; Liang, L.; Zheng, D.; Chen, J.; Jiang, H. β-Nucleated Polypropylene: Preparation, Nucleating Efficiency, Composite, and Future Prospects. Polymers 2023, 15, 3107. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Meng, X.; Li, C.; Yao, Z.; Gong, W. Effects of Halloysite Nanotubes Modified by Organic Phosphate on the Performance Improvement for Polypropylene. J. Appl. Polym. Sci. 2023, 140, e53703. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Hu, S.; Zhou, J.; Li, L.; Huo, H. Optimizing Nanoscale Morphology and Improving Carrier Transport of PCDTBT-PCBM Bulk Heterojunction by Cyclic Carboxylate Nucleating Agents. Org. Electron. 2019, 65, 222–231. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; He, B.; Hou, H.-H.; Guo, L.-H. Isothermal Crystallization of Isotactic Polypropylene Nucleated with a Novel Aromatic Heterocyclic Phosphate Nucleating Agent. J. Macromol. Sci. Part B-Phys. 2017, 56, 811–820. [Google Scholar] [CrossRef]
- Shan, H.; He, J.; Zhu, B.; Zhou, J.; Huo, H. The Role of the Commercial Nucleating Agent HPN-68L in the Stretchable and Electrical Properties of Solvent Vapor Annealed P3HT. J. Mater. Chem. C 2022, 10, 17583–17593. [Google Scholar] [CrossRef]
- Zenzingerova, S.; Kudlacek, M.; Navratilova, J.; Gajzlerova, L.; Jaska, D.; Benicek, L.; Cermak, R. The Competition between Self-Seeding and Specific Nucleation in Crystallization of Long-Chain Branched Polypropylene. Express Polym. Lett. 2023, 17, 1110–1120. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, W.; Liu, G.; Mueller, A.J.; Zhao, Y.; Dong, X.; Wang, K.; Wang, D. Probing into the Epitaxial Crystallization of β Form Isotactic Polypropylene: From Experimental Observations to Molecular Mechanics Computation. J. Polym. Sci. PART B-Polym. Phys. 2017, 55, 418–424. [Google Scholar] [CrossRef]
- Li, X.; JinRong, Z.; Yan, L.; YueFei, Z. In Situ Synthesis of Calcium Pimelate as a Highly Dispersed Β-nucleating Agent for Improving the Crystallization Behavior and Mechanical Properties of Isotactic Polypropylene. Polym. Adv. Technol. 2022, 34, 377–385. [Google Scholar]
- Fuhua, L.; Mi, Z.; Shuangdan, M.; Jianjun, Z.; Kezhi, W.; Jun, L.; Xinde, C.; Bo, W.; Yinghui, W. The Influence of Metal Lithium and Alkyl Chain in the Nucleating Agent Lauroyloxy-Substituted Aryl Aluminum Phosphate on the Crystallization and Optical Properties for iPP. Polymers 2022, 14, 3637. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Nie, M.; Wang, Q. Root-like Glass Fiber with Branched Fiber Prepared via Molecular Self-Assembly. RSC Adv. 2016, 6, 45492–45494. [Google Scholar] [CrossRef]
- Zhao, T.-J.; Lin, F.-H.; Mao, S.; Dong, Y.-P.; Zhao, J.-L.; Cui, W.-J.; Wang, S.-H.; Ning, D.-Y.; Lu, J.-Q.; Wang, B. Functionalization Modification of the Fischer-Tropsch Wax to Improve the Mechanicaland Crystallization Properties of the Recycled Polypropylene/Attapulgite Composites. Polymer 2024, 48, 253–264. [Google Scholar]
- Mao, S.-D.; Zhang, M.; Lin, F.-H.; Li, X.-Y.; Zhao, Y.-Y.; Zhang, Y.-L.; Gao, Y.-F.; Luo, J.; Chen, X.-D.; Wang, B. Attapulgite Structure Reset to Accelerate the Crystal Transformation of Isotactic Polybutene. Polymers 2022, 14, 3820. [Google Scholar] [CrossRef] [PubMed]
- Gang, W.U.; Zhen-bin, C.; Yang-dong, L.I.U.; Si-yuan, L.U. Effect of Stearate on Performance of Transparent Modified Polypropylene with Sorbitol-Based Nucleating Agent. Plast. Sci. Technol. Suliao Ke-Ji 2023, 51, 54. [Google Scholar]
- Balkaev, D.; Neklyudov, V.; Starshinova, V.; Stolov, M.; Amirova, L.M.; Ziyatdinova, A.; Amirov, R.R. Novel Nucleating Agents for Polypropylene and Modifier of Its Physical-Mechanical Properties. Mater. Today Commun. 2021, 26, 101783. [Google Scholar] [CrossRef]
- Dotson, D.L. Nucleating Agents for Polyethylene. In Handbook of Industrial Polyethylene and Technology; Spalding, M.A., Chatterjee, A.M., Eds.; Wiley: New York, NY, USA, 2017; pp. 935–965. [Google Scholar]
- Turgut, G.; Isiksel, E.; Kahraman, G.; Eren, T.; Özkoç, G. Synthesis of Phosphorus- and Phenyl-Based ROMP Polymers and Investigation of Their Effects on the Thermomechanical and Flammability Properties of a Polypropylene-IFR System. J. Appl. Polym. Sci. 2018, 135, 45998. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.-R.; Huang, C.; Xiong, L.; Luo, J.; Chen, X. Study on Non-Isothermal Crystallization Behavior of Isotactic Polypropylene/Bacterial Cellulose Composites. RSC Adv. 2017, 7, 42113–42122. [Google Scholar] [CrossRef]
- Seven, K.; Cogen, J.; Gilchrist, J. Nucleating Agents for High-Density Polyethylene-A Review. Polym. Eng. Sci. 2016, 56, 541–554. [Google Scholar] [CrossRef]
- Gholami, F.; Pircheraghi, G.; Rashedi, R.; Sepahi, A. Correlation between Isothermal Crystallization Properties and Slow Crack Growth Resistance of Polyethylene Pipe Materials. Polym. Test. 2019, 80, 106128. [Google Scholar] [CrossRef]
- Kaizuka, M.; Sato, H.; Ozaki, Y.; Sato, H. Visualization of Recrystallization Induced by Ultraviolet Degradation of a Polypropylene Film Using Raman Imaging. Appl. Spectrosc. 2024, 78, 517–522. [Google Scholar] [CrossRef]
- Tian, M.; Yang, Y.; He, W.; Li, J.; Qin, S.; Yu, J. Preparation, Characterization and Application of Silica Nanoparticle Micro-Aggregates with Circular Structures. Chem. J. Chin. Univ.-Chin. 2016, 37, 180–188. [Google Scholar]
- Xu, R.R.; Du, B.X.; Xiao, M.; Li, J.; Liu, H.L.; Ran, Z.Y.; Xing, J.W. Dielectric Properties Dependent on Crystalline Morphology of PP Film for HVDC Capacitors Application. Polymer 2021, 213, 123204. [Google Scholar] [CrossRef]
- Lei, X.; Liang, M.; Zou, H.; Zhou, S. A Holistic Evaluation of the Influence of Shear Rates and Matrix Viscosity on the Properties of Polypropylene/Multi-Walled Carbon Nanotubes Composites. Polym. Adv. Technol. 2023, 34, 317–331. [Google Scholar] [CrossRef]
- Dai, L.; Wang, X.; Zhang, J.; Wang, F.; Ou, R.; Song, Y. Effects of Lubricants on the Rheological and Mechanical Properties of Wood Flour/Polypropylene Composites. J. Appl. Polym. Sci. 2019, 136, 47667. [Google Scholar] [CrossRef]
- Martin-Alfonso, J.E.; Valencia, C.; Sanchez, M.C.; Franco, J.M.; Gallegos, C. The Effect of Recycled Polymer Addition on the Thermorheological Behavior of Modified Lubricating Greases. Polym. Eng. Sci. 2013, 53, 818–826. [Google Scholar] [CrossRef]
- Lugt, P.M. On the Use of the Arrhenius Equation to Describe the Impact of Temperature on Grease Life. Tribol. Int. 2023, 179, 108142. [Google Scholar] [CrossRef]
- Li, F.-J.; Tan, L.-C.; Zhang, S.-D.; Zhu, B. Compatibility, Steady and Dynamic Rheological Behaviors of Polylactide/Poly(Ethylene Glycol) Blends. J. Appl. Polym. Sci. 2016, 133, 42919. [Google Scholar] [CrossRef]
- Gong, L.; Yin, B.; Li, L.; Yang, M. Morphology and Properties of PP/EPDM Binary Blends and PP/EPDM/Nano-CaCO3 Ternary Blends. J. Appl. Polym. Sci. 2012, 123, 510–519. [Google Scholar] [CrossRef]
- Liu, M.; Chen, K.; Yu, S.; Zhang, R.; Jia, M.; Pan, K.; Xue, P. Light-Weight and High-Strength PP/CaCO3 Composites by Die Drawing: Effect of Drawing Ratios. Polym. Eng. Sci. 2022, 62, 3809–3819. [Google Scholar] [CrossRef]
- Han, H.; Hu, S.; Feng, J.; Gao, H. Effect of Stearic Acid, Zinc Stearate Coating on the Properties of Synthetic Hydromagnesite. Appl. Surf. Sci. 2011, 257, 2677–2682. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Song, B.; Fan, X.; Zhou, Z.; Li, Y. Nucleating Agent Induced Impact Fracture Behavior Change in PP/POE Blend. Polym. Bull. 2009, 62, 405–419. [Google Scholar] [CrossRef]
- Burbano-Garcia, C.; Araya-Letelier, G.; Astroza, R.; Silva, Y.F. Adobe Mixtures Reinforced with Fibrillated Polypropylene Fibers: Physical/Mechanical/Fracture/Durability Performance and Its Limits Due to Fiber Clustering. Constr. Build. Mater. 2022, 343, 128102. [Google Scholar] [CrossRef]
- Yousefi, A.A.; Rezaei, M.; Naderpour, N. Hybrid Multiwalled-Carbon Nanotube/Nanosilica/Polypropylene Nanocomposites: Morphology, Rheology, and Mechanical Properties. Polym. Compos. 2023, 44, 5464–5479. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, K.; Fang, Y.; Guo, Z.; Yang, X.; Sheng, K. Improvements in Compatibility and Properties of Biocomposites Modified through Nanosilica Attachment. Iran. Polym. J. 2022, 31, 1387–1398. [Google Scholar] [CrossRef]












| Sample Name | PP (g) | SCAB (g) | Znst (g) |
|---|---|---|---|
| PP | 200 | - | - |
| PP/SCAB | 200 | 0.6 | - |
| PP/ZnSt | 200 | - | 0.6 |
| PP/SCAB-ZnSt | 200 | 0.6 | 0.6 |
| Sample | Tc (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) |
|---|---|---|---|---|
| PP | 117.20 | 169.05 | 95.36 | 53.88 |
| PP/SCAB | 130.68 | 167.17 | 99.87 | 56.42 |
| PP/Znst | 117.76 | 169.91 | 95.43 | 53.92 |
| PP/SCAB-Znst | 132.16 | 167.06 | 99.91 | 56.44 |
| Sample | φ | Tc (°C) | ΔHc (J/g) | n | log kc | t1/2 (min) |
|---|---|---|---|---|---|---|
| PP | 5 | 120.14 | 109.8 | 2.5 | −2.44108 | 1.87 |
| 10 | 117.20 | 109.7 | 2.3 | −0.01653 | 1.13 | |
| 20 | 113.80 | 104.7 | 2.5 | −1.08751 | 0.71 | |
| PP/SCAB | 5 | 134.33 | 114.2 | 3.2 | −0.65311 | 1.74 |
| 10 | 130.68 | 111.3 | 3.8 | −0.01444 | 1.01 | |
| 20 | 126.85 | 111.5 | 3.4 | −0.46192 | 0.52 | |
| PP/ZnSt | 5 | 120.27 | 109.8 | 2.4 | −6.26352 | 1.82 |
| 10 | 117.76 | 110.2 | 2.6 | −3.23878 | 1.09 | |
| 20 | 115.00 | 104.9 | 2.2 | −4.00041 | 0.68 | |
| PP/SCAB-ZnSt | 5 | 135.49 | 115.8 | 3.4 | −0.02088 | 1.71 |
| 10 | 132.16 | 116.1 | 4.1 | 0.10153 | 0.89 | |
| 20 | 128.50 | 113.5 | 3.4 | 0.87485 | 0.45 |
| Samples | lnA | ΔE | R2 |
|---|---|---|---|
| PP | 3.84 | 34.47 | 0.99825 |
| PP/SCAB | 3.89 | 44.68 | 0.99888 |
| PP/Znst | 2.88 | 24.62 | 0.99985 |
| PP/SCAB-Znst | 3.02 | 30.36 | 0.99996 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Lin, F.; Zhao, T.; Wang, M.; Ning, D.; Hao, X.; Zhang, Y.; Zhou, D.; Zhao, Y.; Chen, X.; et al. Dispersion and Lubrication of Zinc Stearate in Polypropylene/Sodium 4-[(4-chlorobenzoyl) amino] Benzoate Nucleating Agent Composite. Polymers 2024, 16, 1942. https://doi.org/10.3390/polym16131942
Dong Y, Lin F, Zhao T, Wang M, Ning D, Hao X, Zhang Y, Zhou D, Zhao Y, Chen X, et al. Dispersion and Lubrication of Zinc Stearate in Polypropylene/Sodium 4-[(4-chlorobenzoyl) amino] Benzoate Nucleating Agent Composite. Polymers. 2024; 16(13):1942. https://doi.org/10.3390/polym16131942
Chicago/Turabian StyleDong, Yapeng, Fuhua Lin, Tianjiao Zhao, Meizhen Wang, Dingyi Ning, Xinyu Hao, Yanli Zhang, Dan Zhou, Yuying Zhao, Xinde Chen, and et al. 2024. "Dispersion and Lubrication of Zinc Stearate in Polypropylene/Sodium 4-[(4-chlorobenzoyl) amino] Benzoate Nucleating Agent Composite" Polymers 16, no. 13: 1942. https://doi.org/10.3390/polym16131942
APA StyleDong, Y., Lin, F., Zhao, T., Wang, M., Ning, D., Hao, X., Zhang, Y., Zhou, D., Zhao, Y., Chen, X., & Wang, B. (2024). Dispersion and Lubrication of Zinc Stearate in Polypropylene/Sodium 4-[(4-chlorobenzoyl) amino] Benzoate Nucleating Agent Composite. Polymers, 16(13), 1942. https://doi.org/10.3390/polym16131942







