Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies
Abstract
:1. Introduction
2. Heavy Metals: Sources and Toxicity
| Heavy Metal | Major Source(s) | Common Effects | Reference |
|---|---|---|---|
| Arsenic (As) | Insecticides and pesticides, treated wooden pulps, and smelting metals. | Cancer, skin color changes, Blackfoot disease, diabetes, and others. | [31,32] |
| Zinc (Zn) | Plating, galvanizing, other metal finishing processes, paper and pulp industries, battery industry. | Kidney failure, lung fibrosis and cancer, anemia, vomiting, and others. | [33] |
| Chromium (Cr) | Electroplating, leather tanning, and textile industries. | Dermatitis, kidney and gastric damage, lung cancer, respiratory tract and eyes irritation, and others. | [34,35] |
| Mercury (Hg) | Oil refinery processes, pesticides, and burning coal for power generation. | Brain, central nervous system, heart, alimentary tract, kidney, and liver significant damages. | [36] |
| Nickel (Ni) | Electroplating, printing and dyeing, and pharmaceutical and metallurgical industries. | Skin irritation, asthma, conjunctivitis, and cancer. | [37,38] |
| Bismuth (Bi) | Chemical and pharmaceutical industries. | Hypotension, insanity, and renal failure. | [39] |
3. Elimination of Heavy Metals from Wastewater: Conventional Methods
3.1. Coagulation and Flocculation
3.2. Precipitation
3.3. Ion Exchange
3.4. Flotation
3.5. Electrochemical Separation
4. Elimination of Heavy Metals from Wastewater: Advanced Methods
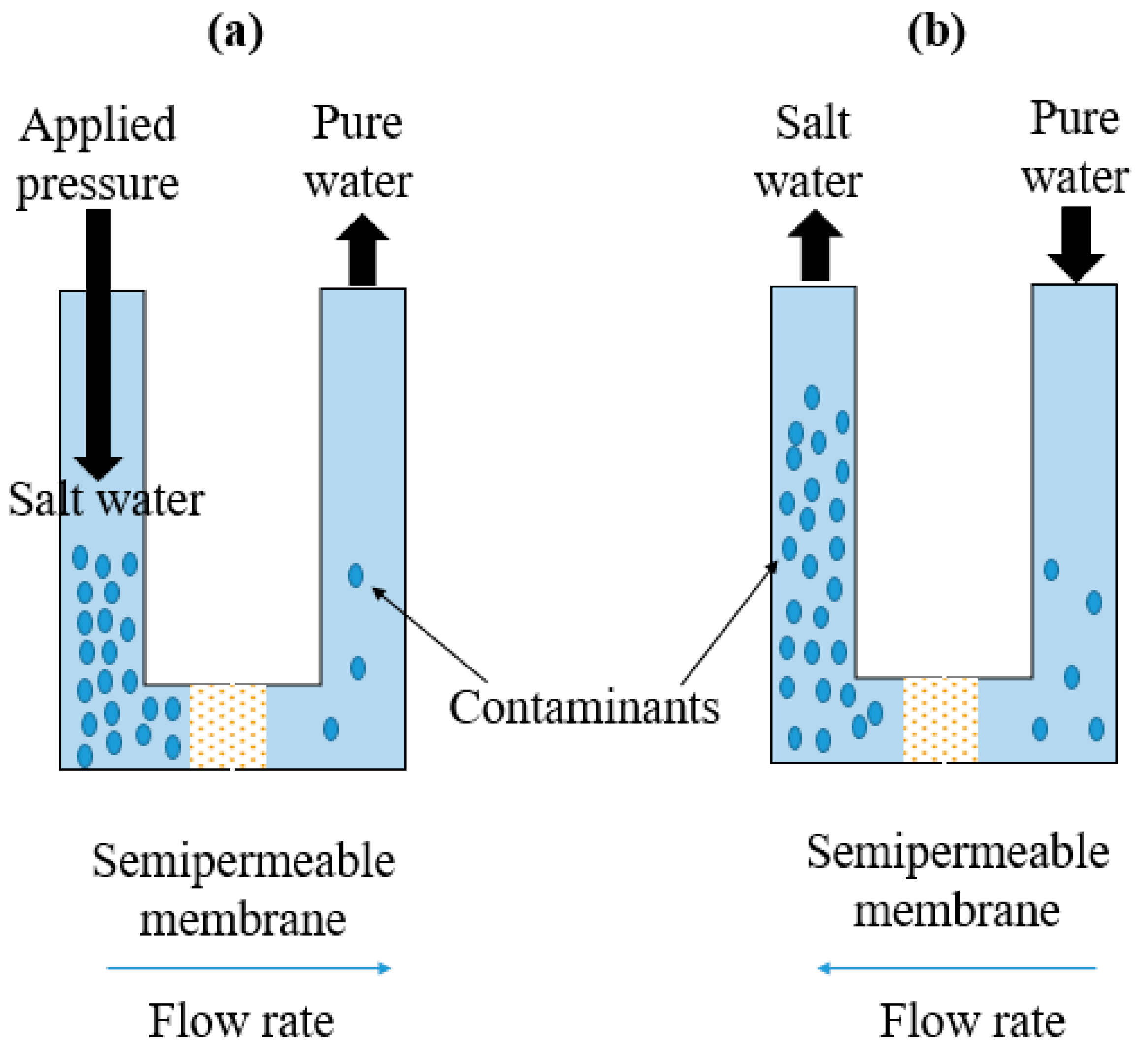
4.1. Membrane Filtration
4.2. Adsorption
4.2.1. Filters: Classification and Applications
Synthetic Filters
Natural Filters
Hybrid Filters
4.2.2. Adsorbents Regeneration/Reuse
- -
- Magnetic separation leverages the magnetic properties of adsorbents to separate them from the wastewater after they have adsorbed heavy metals. By applying an external magnetic field, the magnetic adsorbents can be easily retrieved from the solution, facilitating their regeneration and reuse. The primary advantages are efficiency and simplicity, allowing for quick recovery of adsorbents, reducing the need for complex filtration methods, and lowering operational costs while minimizing secondary pollution [152,153]. However, the stability of the magnetic properties over multiple regeneration cycles needs to be ensured.
- -
- Thermal desorption involves heating the adsorbent to volatilize the adsorbed metals, which are then captured and treated separately. Regeneration temperatures typically range from 200 to 800 °C [147]. Thermal desorption can achieve high regeneration efficiencies, often exceeding 90%, making it suitable for large-scale industrial applications [152]. It is effective for various adsorbents, including activated carbons, zeolites, and metal-organic frameworks (MOFs), but has drawbacks like high energy requirements and potential degradation of adsorbents over multiple cycles.
- -
- Solvent regeneration involves using various solvents to desorb metals from the adsorbent surface, effectively restoring its adsorption capacity. Common solvents include acids (e.g., hydrochloric acid, nitric acid), alkalis (e.g., sodium hydroxide), and organic solvents (e.g., ethanol, methanol) [148,152,154]. This method is particularly effective for adsorbents with a strong affinity for certain heavy metals and is relatively cost-effective. However, it poses environmental risks due to the disposal of spent solvents and can lead to partial degradation of the adsorbent material over time. Recent advancements focus on developing eco-friendly solvents and integrating solvent recovery systems to enhance sustainability [152,154].
- -
- Microwave irradiation heats the adsorbent using microwave energy, volatilizing the adsorbed contaminants and allowing for efficient desorption and regeneration. Studies have shown that microwave-assisted regeneration can recover up to 90% of the adsorptive capacity of activated carbon used in wastewater treatment [152,155]. This method is highly effective at targeting specific adsorbed species and is energy-efficient and environmentally friendly, offering rapid and selective heating that reduces operational costs and environmental impact [149].
- -
- Supercritical fluid regeneration uses supercritical fluids, typically supercritical CO2, which operate under conditions above their critical temperature and pressure. This method achieves high efficiency in desorbing heavy metals from adsorbents and can achieve metal recovery rates exceeding 90%, making it highly effective for industrial applications. Supercritical CO2 is non-toxic, non-flammable, and relatively low-cost. The process minimizes waste and environmental impact and preserves the structural integrity of the adsorbent material over multiple regeneration cycles. However, it requires high initial setup costs and precise control of operational parameters to optimize regeneration efficiency [156].
- -
- Advanced oxidation processes (AOPs) generate highly reactive species, such as hydroxyl radicals, which degrade contaminants adsorbed onto the adsorbents, restoring their adsorption capacity [157]. AOPs can regenerate adsorbents without significant material degradation, allowing for multiple reuse cycles and reducing operational costs. These processes target a wide range of heavy metals, ensuring comprehensive removal and recovery. However, they require high initial setup costs and precise control of operational parameters [157].
5. Comparison between Filters and Other Methods
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient Techniques for the Removal of Toxic Heavy Metals from Aquatic Environment: A Review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Bica, J.; Chatterley, C.; Dönmez, A.; Johnston, R.; Mitis, F.; Slaymaker, T. Progress on Household Drinking Water, Sanitation and Hygiene 2000–2022: Special Focus on Gender; World Health Organization: New York, NY, USA, 2023. [Google Scholar]
- Carstea, E.M.; Bridgeman, J.; Baker, A.; Reynolds, D.M. Fluorescence Spectroscopy for Wastewater Monitoring: A Review. Water Res. 2016, 95, 205–219. [Google Scholar] [CrossRef] [PubMed]
- John, M.J.; Anandjiwala, R.D. Recent Developments in Chemical Modification and Characterization of Natural Fiber-Reinforced Composites. Polym. Compos. 2008, 29, 187–207. [Google Scholar] [CrossRef]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. Adsorption of Acid Dyes on Chitosan—Equilibrium Isotherm Analyses. Process Biochem. 2004, 39, 695–704. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of Iron Oxide Nanomaterials in Wastewater Treatment: A Review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yenkie, K.M. Integrating the Three E’s in Wastewater Treatment: Efficient Design, Economic Viability, and Environmental Sustainability. Curr. Opin. Chem. Eng. 2019, 26, 131–138. [Google Scholar] [CrossRef]
- António Guterres Water and Sanitation—United Nations Sustainable Development. Available online: https://www.un.org/sustainabledevelopment/water-and-sanitation/ (accessed on 7 July 2023).
- Gallego-Schmid, A.; Tarpani, R.R.Z. Life Cycle Assessment of Wastewater Treatment in Developing Countries: A Review. Water Res. 2019, 153, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Adsorptive Removal of Nickel(II) Ions from Aqueous Environment: A Review. J. Environ. Manag. 2016, 179, 1–20. [Google Scholar] [CrossRef]
- Lee, M.; Tebo, B.M. Surface Charge Properties of and Cu(II) Adsorption by Spores of the Marine Bacillus Sp. Strain SG-1. Appl. Environ. Microbiol. 1998, 64, 1123–1129. [Google Scholar] [CrossRef]
- Meena, A.K.; Mishra, G.K.; Rai, P.K.; Rajagopal, C.; Nagar, P.N. Removal of Heavy Metal Ions from Aqueous Solutions Using Carbon Aerogel as an Adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef]
- Zinicovscaia, I. Conventional Methods of Wastewater Treatment. In Cyanobacteria for Bioremediation of Wastewaters; Zinicovscaia, I., Cepoi, L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 17–25. ISBN 978-3-319-26749-4. [Google Scholar]
- Alikaj, M.; Brahushi, F. Heavy Metals Assessment in the Macrophytes of Viroi Lake. Albanian J. Agric. Sci. 2017, special edition, 245–252. [Google Scholar]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Elkady, M.; Shokry, H.; Hamad, H. New Activated Carbon from Mine Coal for Adsorption of Dye in Simulated Water or Multiple Heavy Metals in Real Wastewater. Materials 2020, 13, 2498. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.S.U.; Kim, I.; Han, J.-I. Adsorption of Methylene Blue Dye from Aqueous Solution by Sugar Extracted Spent Rice Biomass. Carbohydr. Polym. 2012, 90, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- El malti, W.; Hijazi, A.; Khalil, Z.A.; Yaghi, Z.; Medlej, M.K.; Reda, M. Comparative Study of the Elimination of Copper, Cadmium, and Methylene Blue from Water by Adsorption on the Citrus Sinensis Peel and Its Activated Carbon. RSC Adv. 2022, 12, 10186–10197. [Google Scholar] [CrossRef]
- Wassim El Malti, W.E.; Hamieh, M.; Noaman, A.; El-Dine, R.N.; Hijazi, A.; Al-Khatib, W. Polyurethane Loaded with Vegetable Activated Carbon for Heavy Metals Removal from Water. J. Ecol. Eng. 2021, 22, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Ho, S.-H.; Wang, X.; Li, Y.; Wang, C. Application of Biodegradable Cellulose-Based Biomass Materials in Wastewater Treatment. Environ. Pollut. 2021, 290, 118087. [Google Scholar] [CrossRef]
- Herawati, N.; Suzuki, S.; Hayashi, I.; Rivai, I.F.; Koyama, H. Cadmium, Copper, and Zinc Levels in Rice and Soil of Japan, Indonesia, and China by Soil Type. Bull. Environ. Contam. Toxicol. 2000, 64, 33–39. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Soil–Plant Transfer of Trace Elements—An Environmental Issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Cr(VI) Removal from Synthetic Wastewater Using Coconut Shell Charcoal and Commercial Activated Carbon Modified with Oxidizing Agents and/or Chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef]
- Ab Hamid, N.H.; bin Mohd Tahir, M.I.H.; Chowdhury, A.; Nordin, A.H.; Alshaikh, A.A.; Suid, M.A.; Nazaruddin, N.I.; Nozaizeli, N.D.; Sharma, S.; Rushdan, A.I. The Current State-Of-Art of Copper Removal from Wastewater: A Review. Water 2022, 14, 3086. [Google Scholar] [CrossRef]
- Fewtrell, L.; Kay, D.; MacGill, S. A Review of the Science behind Drinking Water Standards for Copper. Int. J. Environ. Health Res. 2001, 11, 161–167. [Google Scholar] [CrossRef]
- Schweitzer, L.; Noblet, J. Water Contamination and Pollution. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 261–290. ISBN 978-0-12-809270-5. [Google Scholar]
- Al-Saydeh, S.A.; El-Naas, M.H.; Zaidi, S.J. Copper Removal from Industrial Wastewater: A Comprehensive Review. J. Ind. Eng. Chem. 2017, 56, 35–44. [Google Scholar] [CrossRef]
- Badr, E.A.E.; Agrama, A.A.E.; Badr, S.A.E. Heavy Metals in Drinking Water and Human Health, Egypt. Nutr. Food Sci. 2011, 41, 210–217. [Google Scholar] [CrossRef]
- Sun, L.; Lu, M.; Li, Q.; Jiang, H.; Yin, S. Research Progress of Arsenic Removal from Wastewater. IOP Conf. Ser. Earth Environ. Sci. 2019, 218, 012142. [Google Scholar] [CrossRef]
- Lu, Z.; Qi, X.; Zhu, X.; Li, X.; Li, K.; Wang, H. Highly Effective Remediation of High-Arsenic Wastewater Using Red Mud through Formation of AlAsO4@silicate Precipitate. Environ. Pollut. 2021, 287, 117484. [Google Scholar] [CrossRef]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J. Removal of Lead and Zinc from Battery Industry Wastewater Using Electrocoagulation Process: Influence of Direct and Alternating Current by Using Iron and Stainless Steel Rod Electrodes. Sep. Purif. Technol. 2014, 135, 165–175. [Google Scholar] [CrossRef]
- Mitra, S.; Sarkar, A.; Sen, S. Removal of Chromium from Industrial Effluents Using Nanotechnology: A Review. Nanotechnol. Environ. Eng. 2017, 2, 11. [Google Scholar] [CrossRef]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.S.; Rezania, S.; Radwan, N.; Alam, J. Chromium Contamination and Effect on Environmental Health and Its Remediation: A Sustainable Approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Peng, D.; Deng, S.; Chen, J.; Duan, C. Efficient Treatment of Mercury(Ⅱ)-Containing Wastewater in Aerated Constructed Wetland Microcosms Packed with Biochar. Chemosphere 2022, 290, 133302. [Google Scholar] [CrossRef] [PubMed]
- Dindin, D.; Rani, W.; Hariyadi, H.R.; Wulan, D.R.; Cahyaningsih, S. Removal of Nickel Ion from Electroplating Wastewater Using Double Chamber Electrodeposition Cell (DCEC) Reactor Partitioned with Water Hyacinth (Eichhornia Crassipes) Leaves. IOP Conf. Ser. Earth Environ. Sci. 2017, 60, 012020. [Google Scholar] [CrossRef]
- Li, N.; Yuan, M.; Lu, S.; Xiong, X.; Xie, Z.; Liu, Y.; Guan, W. Highly Effective Removal of Nickel Ions from Wastewater by Calcium-Iron Layered Double Hydroxide. Front. Chem. 2023, 10, 1089690. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Chen, L.; Liao, Y.-L.; Wu, Z.-Z.; Yu, Y.-Q.; Yang, J.-Y. Removal of Bismuth Ion from Aqueous Solution by Pulverized Eggshells. Des. Water Treat. 2021, 213, 395–405. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for Heavy Metals Removal and Their Future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Rodríguez, D.; Lizardi-Mendoza, J.; López-Maldonado, E.A.; Oropeza-Guzmán, M.T. Capacity of ‘Nopal’ Pectin as a Dual Coagulant-Flocculant Agent for Heavy Metals Removal. Chem. Eng. J. 2017, 323, 19–28. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, M.; Wang, J. Removal of Cu2+ and Turbidity from Wastewater by Mercaptoacetyl Chitosan. J. Hazard. Mater. 2009, 169, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, D.; Rakhila, Y.; Elmchaouri, A.; Abouri, M.; Souabi, S.; Jada, A. Optimization of Coagulation Flocculation Process for the Removal of Heavy Metals from Real Textile Wastewater. In Advanced Intelligent Systems for Sustainable Development (AI2SD’2018); Ezziyyani, M., Ed.; Advances in Intelligent Systems and Computing; Springer International Publishing: Cham, Switzerland, 2019; Volume 913, pp. 257–266. ISBN 978-3-030-11880-8. [Google Scholar]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. Performance Evaluation of Reverse Osmosis Process in the Post-Treatment of Mining Wastewaters: Case Study of Costerfield Mining Operations, Victoria, Australia. J. Water Process Eng. 2020, 34, 101116. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Yadav, M.; Gupta, R.; Sharma, R.K. Green and Sustainable Pathways for Wastewater Purification. In Advances in Water Purification Techniques; Elsevier: Amsterdam, Netherlands, 2019; pp. 355–383. ISBN 978-0-12-814790-0. [Google Scholar]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean. Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Kumar, M.; Nandi, M.; Pakshirajan, K. Recent Advances in Heavy Metal Recovery from Wastewater by Biogenic Sulfide Precipitation. J. Environ. Manag. 2021, 278, 111555. [Google Scholar] [CrossRef] [PubMed]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective Removal of the Heavy Metal Ions from Waters and Industrial Wastewaters by Ion-Exchange Method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Tenório, J.A.; Espinosa, D.C. Treatment of Chromium Plating Process Effluents with Ion Exchange Resins. Waste Manag. 2001, 21, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-Y.; Lee, J.-U.; Moon, S.-H.; Kim, K.-W. Competitive Adsorption Characteristics of Co2+, Ni2+, and Cr3+ by IRN-77 Cation Exchange Resin in Synthesized Wastewater. Chemosphere 2004, 56, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, S.A.; Fernandes, S.; Quina, M.M.; Ferreira, L.M. Removal of Chromium from Electroplating Industry Effluents by Ion Exchange Resins. J. Hazard. Mater. 2007, 144, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–Organic Frameworks for Heavy Metal Removal from Water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Fang, Q.-R.; Yuan, D.-Q.; Sculley, J.; Li, J.-R.; Han, Z.-B.; Zhou, H.-C. Functional Mesoporous Metal−Organic Frameworks for the Capture of Heavy Metal Ions and Size-Selective Catalysis. Inorg. Chem. 2010, 49, 11637–11642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, Z.; Wang, Z.; Feng, X.; Wang, Y.; Wu, A. Unveiling the Adsorption Mechanism of Zeolitic Imidazolate Framework-8 with High Efficiency for Removal of Copper Ions from Aqueous Solutions. Dalton Trans. 2016, 45, 12653–12660. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Ai, L.; Jiang, J. Mechanistic Insight into the Interaction and Adsorption of Cr(VI) with Zeolitic Imidazolate Framework-67 Microcrystals from Aqueous Solution. Chem. Eng. J. 2015, 274, 238–246. [Google Scholar] [CrossRef]
- Shah, R. Ligational, Potentiometric and Floatation Studies on Cu(II) Complexes of Hydrazones Derived from p and o-Vanillin Condensed with Diketo Hydrazide. J. Mol. Liq. 2016, 220, 939–953. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Karimi, B.; Rajaei, M.S. The Effect of Aeration on Advanced Coagulation, Flotation and Advanced Oxidation Processes for Color Removal from Wastewater. J. Mol. Liq. 2016, 223, 75–80. [Google Scholar] [CrossRef]
- Elehinafe, F.B.; Agboola, O.; Vershima, A.D.; Bamigboye, G.O. Insights on the Advanced Separation Processes in Water Pollution Analyses and Wastewater Treatment—A Review. S. Afr. J. Chem. Eng. 2022, 42, 188–200. [Google Scholar] [CrossRef]
- Wang, G.; Nguyen, A.V.; Mitra, S.; Joshi, J.B.; Jameson, G.J.; Evans, G.M. A Review of the Mechanisms and Models of Bubble-Particle Detachment in Froth Flotation. Sep. Purif. Technol. 2016, 170, 155–172. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Kyzas, G.Z.; Matis, K.A. Various Flotation Techniques for Metal Ions Removal. J. Mol. Liq. 2017, 225, 260–264. [Google Scholar] [CrossRef]
- Jia, K.; Yi, Y.; Ma, W.; Cao, Y.; Li, G.; Liu, S.; Wang, T.; An, N. Ion Flotation of Heavy Metal Ions by Using Biodegradable Biosurfactant as Collector: Application and Removal Mechanism. Miner. Eng. 2022, 176, 107338. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous Removal of Organics and Heavy Metals from Industrial Wastewater: A Review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Fu, Y.; Hu, M.; Wang, S.; Liu, Z. Highly Efficient SnS-Decorated Bi2O3 Nanosheets for Simultaneous Electrochemical Detection and Removal of Cd(II) and Pb(II). J. Electroanal. Chem. 2020, 856, 113744. [Google Scholar] [CrossRef]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct Membrane Filtration for Wastewater Treatment and Resource Recovery: A Review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-Based Seawater Desalination: Present and Future Prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and Its Control in Membrane Distillation—A Review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Ozaki, H.; Sharma, K.; Saktaywin, W. Performance of an Ultra-Low-Pressure Reverse Osmosis Membrane (ULPROM) for Separating Heavy Metal: Effects of Interference Parameters. Desalination 2002, 144, 287–294. [Google Scholar] [CrossRef]
- Kajitvichyanukul, P.; Hung, Y.-T.; Wang, L.K. Membrane Technologies for Oil–Water Separation. In Membrane and Desalination Technologies; Wang, L.K., Chen, J.P., Hung, Y.-T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 639–668. ISBN 978-1-58829-940-6. [Google Scholar]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent Trends of Heavy Metal Removal from Water/Wastewater by Membrane Technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- He, M.; Wang, L.; Lv, Y.; Wang, X.; Zhu, J.; Zhang, Y.; Liu, T. Novel Polydopamine/Metal Organic Framework Thin Film Nanocomposite Forward Osmosis Membrane for Salt Rejection and Heavy Metal Removal. Chem. Eng. J. 2020, 389, 124452. [Google Scholar] [CrossRef]
- Cui, Y.; Ge, Q.; Liu, X.-Y.; Chung, T.-S. Novel Forward Osmosis Process to Effectively Remove Heavy Metal Ions. J. Membr. Sci. 2014, 467, 188–194. [Google Scholar] [CrossRef]
- Abdullah, N.; Tajuddin, M.H.; Yusof, N. Forward Osmosis (FO) for Removal of Heavy Metals. In Nanotechnology in Water and Wastewater Treatment; Elsevier: Amsterdam, Netherlands, 2019; pp. 177–204. ISBN 978-0-12-813902-8. [Google Scholar]
- Cui, Y.; Wang, H.; Wang, H.; Chung, T.-S. Micro-Morphology and Formation of Layer-by-Layer Membranes and Their Performance in Osmotically Driven Processes. Chem. Eng. Sci. 2013, 101, 13–26. [Google Scholar] [CrossRef]
- Ge, Q.; Ling, M.; Chung, T.-S. Draw Solutions for Forward Osmosis Processes: Developments, Challenges, and Prospects for the Future. J. Membr. Sci. 2013, 442, 225–237. [Google Scholar] [CrossRef]
- Chung, T.-S.; Zhang, S.; Wang, K.Y.; Su, J.; Ling, M.M. Forward Osmosis Processes: Yesterday, Today and Tomorrow. Desalination 2012, 287, 78–81. [Google Scholar] [CrossRef]
- Groves, G.R.; Buckley, C.A.; Cox, J.M.; Kirk, A.; Macmillan, C.D.; Simpson, M.J. Dynamic Membrane Ultrafiltration and Hyperfiltration for the Treatment of Industrial Effluents for Water Reuse. Desalination 1983, 47, 305–312. [Google Scholar] [CrossRef]
- Zheng, G.; Jiang, J.; Wang, X.; Li, W.; Liu, J.; Fu, G.; Lin, L. Nanofiber Membranes by Multi-Jet Electrospinning Arranged as Arc-Array with Sheath Gas for Electrodialysis Applications. Mater. Des. 2020, 189, 108504. [Google Scholar] [CrossRef]
- Swanckaert, B.; Geltmeyer, J.; Rabaey, K.; De Buysser, K.; Bonin, L.; De Clerck, K. A Review on Ion-Exchange Nanofiber Membranes: Properties, Structure and Application in Electrochemical (Waste)Water Treatment. Sep. Purif. Technol. 2022, 287, 120529. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal Communication: When I’m Calling You, Will You Answer Too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Kim, J.H.; Park, K.Y. Characteristics of Heavy Metal Separation and Determination of Limiting Current Density in a Pilot-Scale Electrodialysis Process for Plating Wastewater Treatment. Sci. Total Environ. 2021, 757, 143762. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, K.; Maan, A.; Zia, R.; Giorno, L.; Schroën, K. Membrane Separation Technology for the Recovery of Nutraceuticals from Food Industrial Streams. Trends Food Sci. Technol. 2019, 86, 426–438. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface Functional Groups of Carbon-Based Adsorbents and Their Roles in the Removal of Heavy Metals from Aqueous Solutions: A Critical Review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Houari, B.; Louhibi, S.; Tizaoui, K.; Boukli-hacene, L.; Benguella, B.; Roisnel, T.; Dorcet, V. New Synthetic Material Removing Heavy Metals from Aqueous Solutions and Wastewater. Arab. J. Chem. 2019, 12, 5040–5048. [Google Scholar] [CrossRef]
- Di Natale, F.; Gargiulo, V.; Alfè, M. Adsorption of Heavy Metals on Silica-Supported Hydrophilic Carbonaceous Nanoparticles (SHNPs). J. Hazard. Mater. 2020, 393, 122374. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Demiral, İ.; Samdan, C.; Demiral, H. Enrichment of the surface functional groups of activated carbon by modification method. Surf. Interfaces 2021, 22, 100873. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of Heavy Metals on Conventional and Nanostructured Materials for Wastewater Treatment Purposes: A Review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Hayder, G.; Latiff, A.A.A.; Aziz, N.A.A.; Umaru, I.; Ghaleb, A.A.S.; Abubakar, S.; Lawal, I.M.; Nasara, M.A. Sustainable Use of Natural and Chemical Coagulants for Contaminants Removal from Palm Oil Mill Effluent: A Comparative Analysis. Ain Shams Eng. J. 2020, 11, 951–960. [Google Scholar] [CrossRef]
- Badawi, A.K.; Zaher, K. Hybrid Treatment System for Real Textile Wastewater Remediation Based on Coagulation/Flocculation, Adsorption and Filtration Processes: Performance and Economic Evaluation. J. Water Process Eng. 2021, 40, 101963. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial Behavior of Intraparticle Diffusion Model Used in the Description of Adsorption Kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. Utilization of Agro-Industrial and Municipal Waste Materials as Potential Adsorbents for Water Treatment—A Review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Singh, K.K.; Rastogi, R.; Hasan, S.H. Removal of Cr(VI) from Wastewater Using Rice Bran. J. Colloid. Interface Sci. 2005, 290, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Karthikeyan, J. Removal of Lignin and Tannin Colour from Aqueous Solution by Adsorption onto Activated Charcoal. Environ. Pollut. 1997, 97, 183–187. [Google Scholar] [CrossRef]
- Gupta, V.K.; Ali, I.; Saleh, T.A.; Nayak, A.; Agarwal, S. Chemical Treatment Technologies for Waste-Water Recycling—An Overview. RSC Adv. 2012, 2, 6380–6388. [Google Scholar] [CrossRef]
- Bessbousse, H.; Rhlalou, T.; Verchère, J.-F.; Lebrun, L. Removal of Heavy Metal Ions from Aqueous Solutions by Filtration with a Novel Complexing Membrane Containing Poly(Ethyleneimine) in a Poly(Vinyl Alcohol) Matrix. J. Membr. Sci. 2008, 307, 249–259. [Google Scholar] [CrossRef]
- Li, P.; Jiang, H.; Barr, A.; Ren, Z.; Gao, R.; Wang, H.; Fan, W.; Zhu, M.; Xu, G.; Li, J. Reusable Polyacrylonitrile-Sulfur Extractor of Heavy Metal Ions from Wastewater. Adv. Funct. Mater. 2021, 31, 2105845. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef] [PubMed]
- Pereao, O.; Bode-Aluko, C.; Laatikainen, K.; Nechaev, A.; Petrik, L. Morphology, Modification and Characterisation of Electrospun Polymer Nanofiber Adsorbent Material Used in Metal Ion Removal. J. Polym. Environ. 2019, 27, 1843–1860. [Google Scholar] [CrossRef]
- Santos, S.; Botelho, C.; Pintor, A. (Eds.) Adsorbents for Water and Wastewater Treatment and Resource Recovery; MDPI: Basel, Switzerland, 2022; ISBN 978-3-0365-4721-3. [Google Scholar]
- Zhou, W.; Wu, P.; Zhang, L.; Zhu, D.; Zhao, X.; Cai, Y. Heavy Metal Ions and Particulate Pollutants Can Be Effectively Removed by a Gravity-Driven Ceramic Foam Filter Optimized by Carbon Nanotube Implantation. J. Hazard. Mater. 2022, 421, 126721. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Zhao, R.; Lu, X.; Xu, X.; Jia, X.; Wang, C.; Li, L. Efficient Adsorption of Gold Ions from Aqueous Systems with Thioamide-Group Chelating Nanofiber Membranes. Chem. Eng. J. 2013, 229, 420–428. [Google Scholar] [CrossRef]
- Meng, J.; Cao, J.; Xu, R.; Wang, Z.; Sun, R. Hyperbranched Grafting Enabling Simultaneous Enhancement of the Boric Acid Uptake and the Adsorption Rate of a Complexing Membrane. J. Mater. Chem. A 2016, 4, 11656–11665. [Google Scholar] [CrossRef]
- Horzum, N.; Shahwan, T.; Parlak, O.; Demir, M.M. Synthesis of Amidoximated Polyacrylonitrile Fibers and Its Application for Sorption of Aqueous Uranyl Ions under Continuous Flow. Chem. Eng. J. 2012, 213, 41–49. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Isawi, H. Using Zeolite/Polyvinyl Alcohol/Sodium Alginate Nanocomposite Beads for Removal of Some Heavy Metals from Wastewater. Arab. J. Chem. 2020, 13, 5691–5716. [Google Scholar] [CrossRef]
- Zhao, Y. Review of the Natural, Modified, and Synthetic Zeolites for Heavy Metals Removal from Wastewater. Environ. Eng. Sci. 2016, 33, 443–454. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and Plant Derived Biomass for Removal of Heavy Metals from Wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Shreya; Verma, A.K.; Dash, A.K.; Bhunia, P.; Dash, R.R. Removal of Surfactants in Greywater Using Low-Cost Natural Adsorbents: A Review. Surf. Interfaces 2021, 27, 101532. [Google Scholar] [CrossRef]
- Ahmad, A.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Exploring the Extraction Methods for Plant-Based Coagulants and Their Future Approaches. Sci. Total Environ. 2022, 818, 151668. [Google Scholar] [CrossRef] [PubMed]
- Ueda Yamaguchi, N.; Cusioli, L.F.; Quesada, H.B.; Camargo Ferreira, M.E.; Fagundes-Klen, M.R.; Salcedo Vieira, A.M.; Gomes, R.G.; Vieira, M.F.; Bergamasco, R. A Review of Moringa Oleifera Seeds in Water Treatment: Trends and Future Challenges. Process Saf. Environ. Prot. 2021, 147, 405–420. [Google Scholar] [CrossRef]
- Raghuwanshi, P.K.; Mandloi, M.; Sharma, A.J.; Malviya, H.S.; Chaudhari, S. Improving Filtrate Quality Using Agrobased Materials as Coagulant Aid. Water Qual. Res. J. 2002, 37, 745–756. [Google Scholar] [CrossRef]
- Nand, V.; Maata, M.; Koshy, K.; Sotheeswaran, S. Water Purification Using Moringa Oleifera and Other Locally Available Seeds in Fiji for Heavy Metal Removal. Int. J. Appl. Sci. Technol. 2012, 2, 125–129. [Google Scholar]
- Etorki, A.M.; El-Rais, M.; Mahabbis, M.T.; Moussa, N.M. Removal of Some Heavy Metals from Wastewater by Using of Fava Beans. Am. J. Anal. Chem. 2014, 05, 225–234. [Google Scholar] [CrossRef]
- Zvinowanda, C.M.; Okonkwo, J.O.; Shabalala, P.N.; Agyei, N.M. A Novel Adsorbent for Heavy Metal Remediation in Aqueous Environments. Int. J. Environ. Sci. Technol. 2009, 6, 425–434. [Google Scholar] [CrossRef]
- Muyibi, S.A.; Mohd. Noor, M.J.M.; Leong, T.K.; Loon, L.H. Effects of Oil Extraction from Moringa Oleifera Seeds On Coagulation Of Turbid Water. Int. J. Environ. Stud. 2002, 59, 243–254. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of Cadmium (II) Ions from Aqueous Solution by a New Low-Cost Adsorbent—Bamboo Charcoal. J. Hazard. Mater. 2010, 177, 300–306. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, D.; Chen, L.; Yu, X.J. Investigation of Cu(II) Adsorption from Aqueous Solutions by NKF-6 Zeolite. Water Sci. Technol. 2011, 63, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Sen Gupta, B.; Curran, M.; Hasan, S.; Ghosh, T.K. Adsorption Characteristics of Cu and Ni on Irish Peat Moss. J. Environ. Manag. 2009, 90, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Meez, E.; Rahdar, A.; Kyzas, G.Z. Sawdust for the Removal of Heavy Metals from Water: A Review. Molecules 2021, 26, 4318. [Google Scholar] [CrossRef] [PubMed]
- Caliceti, M.; Argese, E.; Sfriso, A.; Pavoni, B. Heavy Metal Contamination in the Seaweeds of the Venice Lagoon. Chemosphere 2002, 47, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Marine Drugs 2018, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Alpat, S.K.; Özbayrak, Ö.; Alpat, Ş.; Akçay, H. The Adsorption Kinetics and Removal of Cationic Dye, Toluidine Blue O, from Aqueous Solution with Turkish Zeolite. J. Hazard. Mater. 2008, 151, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kallo, D. Applications of Natural Zeolites in Water and Wastewater Treatment. Rev. Mineral. Geochem. 2001, 45, 519–550. [Google Scholar] [CrossRef]
- Bahrodin, M.B.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.D.; Syafiuddin, A. Recent Advances on Coagulation-Based Treatment of Wastewater: Transition from Chemical to Natural Coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Yimer, A.; Dame, B. Papaya Seed Extract as Coagulant for Potable Water Treatment in the Case of Tulte River for the Community of Yekuset District, Ethiopia. Environ. Chall. 2021, 4, 100198. [Google Scholar] [CrossRef]
- Merenstein, J.L.; Bennett, I.J. Bridging Patterns of Neurocognitive Aging across the Older Adult Lifespan. Neurosci. Biobehav. Rev. 2022, 135, 104594. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, F.; Ni, Y.; Kokot, S. Effects of Aluminum on Amyloid-Beta Aggregation in the Context of Alzheimer’s Disease. Arab. J. Chem. 2019, 12, 2897–2904. [Google Scholar] [CrossRef]
- Rahman, N.S.A.; Yhaya, M.F.; Azahari, B.; Ismail, W.R. Utilisation of Natural Cellulose Fibres in Wastewater Treatment. Cellulose 2018, 25, 4887–4903. [Google Scholar] [CrossRef]
- Rima, J.; Rahme, K.; Assaker, K. The Use of Modified Beetroot Fibers by Sodium Dodecyl Sulfate (SDS) Cleaning Water Contaminated by Organic and Inorganic Compounds. J. Food Res. 2014, 3, 19. [Google Scholar] [CrossRef]
- Rima, J.; Assaker, K. B-Cyclodextrin Polyurethanes Copolymerised with Beetroot Fibers (Bio-Polymer), for the Removal of Organic and Inorganic Contaminants from Water. J. Food Res. 2013, 2, 150. [Google Scholar] [CrossRef]
- Rima, J.; Ghauch, A.; Ghaouch, M.; Martin-Bouyer, M. Cleaning of Water Contaminated by Heavy Metals Using Beetroot Fibers as Biofilter. Toxicol. Environ. Chem. 2000, 75, 89–97. [Google Scholar] [CrossRef]
- Abdulla, G.; Kareem, M.M.; Hashim, K.S.; Muradov, M.; Kot, P.; Mubarak, H.A.; Abdellatif, M.; Abdulhadi, B. Removal of Iron from Wastewater Using a Hybrid Filter. IOP Conf. Ser. Mater. Sci. Eng. 2020, 888, 012035. [Google Scholar] [CrossRef]
- Hussain, S.; Aziz, H.A.; Isa, M.H.; Ahmad, A.; Van Leeuwen, J.; Zou, L.; Beecham, S.; Umar, M. Orthophosphate Removal from Domestic Wastewater Using Limestone and Granular Activated Carbon. Desalination 2011, 271, 265–272. [Google Scholar] [CrossRef]
- Bakar, A.A.A.; Razak, N.F.; Akbar, N.A.; Daud, N.M.; Ali, K.A.M. Removal of Cr (III) from Industrial Wastewater Using Coconut Shell Carbon and Limestone as Adsorbent. IOP Conf. Ser. Earth Environ. Sci. 2021, 646, 012063. [Google Scholar] [CrossRef]
- Li, Z.; Pan, Z.; Wang, Y. Preparation of Ternary Amino-Functionalized Magnetic Nano-Sized Illite-Smectite Clay for Adsorption of Pb(II) Ions in Aqueous Solution. Environ. Sci. Pollut. Res. 2020, 27, 11683–11696. [Google Scholar] [CrossRef]
- He, H.; Meng, X.; Yue, Q.; Yin, W.; Gao, Y.; Fang, P.; Shen, L. Thiol-Ene Click Chemistry Synthesis of a Novel Magnetic Mesoporous Silica/Chitosan Composite for Selective Hg(II) Capture and High Catalytic Activity of Spent Hg(II) Adsorbent. Chem. Eng. J. 2021, 405, 126743. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Hafez, O.F.; Alrefaay, A.; Osman, M.M. Performance Evaluation of Hybrid Inorganic/Organic Adsorbents in Removal and Preconcentration of Heavy Metals from Drinking and Industrial Waste Water. Desalination 2010, 253, 9–15. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, Z.; Hanigan, D.; Westerhoff, P.; Zhao, H.; Ni, J. Coagulation Behaviors of New Covalently Bound Hybrid Coagulants (CBHyC) in Surface Water Treatment. Sep. Purif. Technol. 2018, 192, 322–328. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wen, H.; Luo, Y.; Huang, R.; Rong, L.; Xie, J. Recent Advance in Delivery System and Tissue Engineering Applications of Chondroitin Sulfate. Carbohydr. Polym. 2020, 230, 115650. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Ahmad, R.; Mirza, A.; Mittal, J. Sequestration of Toxic Congo Red Dye from Aqueous Solution Using Ecofriendly Guar Gum/ Activated Carbon Nanocomposite. Int. J. Biol. Macromol. 2020, 158, 1310–1318. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Vijayaraghavan, K.; Kim, J.A.; Yun, Y.-S. Valorisation of Post-Sorption Materials: Opportunities, Strategies, and Challenges. Adv. Colloid. Interface Sci. 2017, 242, 35–58. [Google Scholar] [CrossRef]
- Tamjidi, S.; Esmaeili, H.; Moghadas, B.K. Application of Magnetic Adsorbents for Removal of Heavy Metals from Wastewater: A Review Study. Mater. Res. Express 2019, 6, 102004. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lee, G.B.; Kim, J.H.; Hong, B.U.; Park, J.E. Pre-Treatment Methods for Regeneration of Spent Activated Carbon. Molecules 2020, 25, 4561. [Google Scholar] [CrossRef]
- Jiang, D.; Chu, B.; Amano, Y.; Machida, M. Removal and Recovery of Phosphate from Water by Mg-Laden Biochar: Batch and Column Studies. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 429–437. [Google Scholar] [CrossRef]
- Zhang, X.N.; Mao, G.Y.; Jiao, Y.B.; Shang, Y.; Han, R.P. Adsorption of Anionic Dye on Magnesium Hydroxide-Coated Pyrolytic Bio-Char and Reuse by Microwave Irradiation. Int. J. Environ. Sci. Technol. 2014, 11, 1439–1448. [Google Scholar] [CrossRef]
- Momina, M.; Shahadat, M.; Isamil, S. Regeneration Performance of Clay-Based Adsorbents for the Removal of Industrial Dyes: A Review. RSC Adv. 2018, 8, 24571–24587. [Google Scholar] [CrossRef]
- Acevedo-García, V.; Rosales, E.; Puga, A.; Pazos, M.; Sanromán, M.A. Synthesis and Use of Efficient Adsorbents under the Principles of Circular Economy: Waste Valorisation and Electroadvanced Oxidation Process Regeneration. Sep. Purif. Technol. 2020, 242, 116796. [Google Scholar] [CrossRef]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of Adsorbents and Recovery of Heavy Metals: A Review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef]
- Ruthiraan, M.; Mubarak, N.M.; Abdullah, E.C.; Khalid, M.; Nizamuddin, S.; Walvekar, R.; Karri, R.R. An Overview of Magnetic Material: Preparation and Adsorption Removal of Heavy Metals from Wastewater. In Magnetic Nanostructures: Environmental and Agricultural Applications; Abd-Elsalam, K.A., Mohamed, M.A., Prasad, R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 131–159. ISBN 978-3-030-16439-3. [Google Scholar]
- Younas, F.; Mustafa, A.; Farooqi, Z.U.R.; Wang, X.; Younas, S.; Mohy-Ud-Din, W.; Ashir Hameed, M.; Mohsin Abrar, M.; Maitlo, A.A.; Noreen, S.; et al. Current and Emerging Adsorbent Technologies for Wastewater Treatment: Trends, Limitations, and Environmental Implications. Water 2021, 13, 215. [Google Scholar] [CrossRef]
- El Messaoudi, N.; El Khomri, M.; El Mouden, A.; Bouich, A.; Jada, A.; Lacherai, A.; Iqbal, H.M.N.; Mulla, S.I.; Kumar, V.; Américo-Pinheiro, J.H.P. Regeneration and Reusability of Non-Conventional Low-Cost Adsorbents to Remove Dyes from Wastewaters in Multiple Consecutive Adsorption–Desorption Cycles: A Review. Biomass Convers. Biorefinery 2022, 14, 11739–11756. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pal, S.L.; Srivastava, N.; Shah, M.; Ahmad, I.; Alshahrani, M.Y.; Pal, D.B. Bioadsorbent and Adsorbent-Based Heavy Metal Removal Technologies from Wastewater: New Insight. Biomass Convers. Biorefinery 2023, 13, 13335–13356. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A. Synergy of Adsorption and Advanced Oxidation Processes in Recalcitrant Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 1125–1142. [Google Scholar] [CrossRef]
- Ahalya, N.; Kanamadi, R.D.; Ramachandra, T.V. Biosorption of Chromium (VI) from Aqueous Solutions by the Husk of Bengal Gram (Cicer Arientinum). Electron. J. Biotechnol. 2005, 8, 258–264. [Google Scholar] [CrossRef]
- Wang, W.; Wang, R.; Zhang, C.; Lu, S.; Liu, T. Synthesis, Characterization and Self-Assembly Behavior in Water as Fluorescent Sensors of Cationic Water-Soluble Conjugated Polyfluorene-b-Poly(N-Isopropylacrylamide) Diblock Copolymers. Polymer 2009, 50, 1236–1245. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.-H.; Babel, S. Physico–Chemical Treatment Techniques for Wastewater Laden with Heavy Metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Barakat, M.; Chen, Y.; Huang, C. Removal of Toxic Cyanide and Cu(II) Ions from Water by Illuminated TiO2 Catalyst. Appl. Catal. B Environ. 2004, 53, 13–20. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of Heavy Metal Ions by Various Low-Cost Adsorbents: A Review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. (Eds.) Adsorption Processes for Water Treatment and Purification; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-58135-4. [Google Scholar]
- Abdullah, R.; Abustan, I.; Ibrahim, A.N.M. Wastewater Treatment Using Bentonite, the Combinations of Bentonite—Zeolite, Bentonite-Alum, and Bentonite-Limestone as Adsorbent and Coagulant. Int. J. Environ. Sci. 2013, 4, 379–391. [Google Scholar]
- Zheng, R.; Chen, Y.; Wang, J.; Song, J.; Li, X.-M.; He, T. Preparation of Omniphobic PVDF Membrane with Hierarchical Structure for Treating Saline Oily Wastewater Using Direct Contact Membrane Distillation. J. Membr. Sci. 2018, 555, 197–205. [Google Scholar] [CrossRef]
- Boulaadjoul, S.; Zemmouri, H.; Bendjama, Z.; Drouiche, N. A Novel Use of Moringa Oleifera Seed Powder in Enhancing the Primary Treatment of Paper Mill Effluent. Chemosphere 2018, 206, 142–149. [Google Scholar] [CrossRef]








| Synthetic Filter | Targeted Metals | Metal Ions Removal (%) | Reference |
|---|---|---|---|
| PEI | Pb2+ | 80 | [99] |
| Cu2+ | 94 | ||
| Cd2+ | 99 | ||
| PAN | Cu2+ | 96 | [100] |
| Synthetic Zeolites | Pb2+ | 95 | [101] |
| Cd2+ | 90 | ||
| Ni2+ | 85 | ||
| Dithiocarbamate resins | Pb2+ | 45 | [102] |
| Cu2+ | 78 | ||
| Hg2+ | 80 | ||
| As3+ | 55 | ||
| Cr3+ | 76 | ||
| Metal Oxides (e.g., MnO₂, Fe₃O₄) | As3+ | 80 | [103] |
| Pb2+ | 85 | ||
| Cd2+ | 65 | ||
| Cr3+ | 55 | ||
| MWCNTs | Cd2+ | 96 | [104] |
| Natural Adsorbents | Removal Capacity (%) | Reference | ||||
|---|---|---|---|---|---|---|
| Cu2+ | Pb2+ | Cd2+ | Cr3+ | Zn2+ | ||
| Urad | 30 | 40 | 10 | 20 | 15 | [115] |
| Peanut | 4 | 50 | 8 | 40 | 22 | [116] |
| Bean | 3 | 29 | 10 | 40 | 30 | [117] |
| Corn | 10 | 13 | 40 | 10 | 20 | [118] |
| Moringa | 90 | 90 | 60 | 50 | 55 | [119] |
| Clay Minerals (e.g., Bentonite, Kaolinite) | - | 80 | 70 | 50 | - | [120] |
| Zeolites | 90 | 75 | - | - | 60 | [121] |
| Peat Moss | 52 | 50 | 70 | - | - | [122] |
| Sawdust | 60 | 20 | 30 | 80 | - | [123] |
| Algae (Biomass) | 50 | 40 | - | 60 | 45 | [124] |
| Hybrid Filter | Targeted Metals | Metal Ions Removal (%) | Reference |
|---|---|---|---|
| Beetroot fibers and SDS | Pb2+ | 100 | [132,133,134,135] |
| Zn2+ | 99 | ||
| Ni2+ | 99 | ||
| Cu2+ | 99 | ||
| Limestone and activated carbon | Fe2+ | 100 | [136,137,138] |
| Clay-EDTA | Pb2+ | 95 | [139] |
| Chitosan Modified with Thiol Groups | Hg2+ | 90 | [140] |
| Treatment Method | Advantages | Disadvantages | Reference | |
|---|---|---|---|---|
| Coagulation and flocculation or precipitation | Low cost; simple operation | Sludge generation; the extra operational cost of sludge disposal | [160] | |
| Ion exchange | Removal of metals and organic pollutants simultaneously; less harmful byproduct | Long duration time; limited application | [161] | |
| Membrane filtration, forward and reverses osmosis | Small space requirement; low pressure; high separation selectivity | High operational cost due to membrane fouling | [160] | |
| Adsorption | Availability; high efficiency; less expensive than other techniques; works in a wide pH range | Hard separation of the adsorbent from metals | [162,163] | |
| Adsorption by Filters | Synthetic filters | High efficiency | High energy consumption | [164] |
| Natural filters | Low-cost; eco-friendly | Moderate efficiency | [165] | |
| Hybrid filters | High separation selectivity; economical; 50% eco friendly | 50% harmful effect on the environment | [166] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayach, J.; El Malti, W.; Duma, L.; Lalevée, J.; Al Ajami, M.; Hamad, H.; Hijazi, A. Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies. Polymers 2024, 16, 1959. https://doi.org/10.3390/polym16141959
Ayach J, El Malti W, Duma L, Lalevée J, Al Ajami M, Hamad H, Hijazi A. Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies. Polymers. 2024; 16(14):1959. https://doi.org/10.3390/polym16141959
Chicago/Turabian StyleAyach, Jana, Wassim El Malti, Luminita Duma, Jacques Lalevée, Mohamad Al Ajami, Hussein Hamad, and Akram Hijazi. 2024. "Comparing Conventional and Advanced Approaches for Heavy Metal Removal in Wastewater Treatment: An In-Depth Review Emphasizing Filter-Based Strategies" Polymers 16, no. 14: 1959. https://doi.org/10.3390/polym16141959






