Delivery of Lutein by Using Modified Burdock Polysaccharide Aggregates: Preparation, Characterization, and In Vitro Release Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Lutein-Loaded SA-Modified BP Aggregates
2.2.1. Preparation of BP
2.2.2. Synthesis of SA-Modified BP
2.2.3. Preparation of Lutein-Loaded SA-BP Aggregates
2.3. Lutein Encapsulation Efficiency and Loading Capacity
2.4. Structural Characterization
2.5. Storage Stability
2.6. In Vitro Simulated Digestion Test
2.6.1. In Vitro Simulation of Gastrointestinal Digestion
2.6.2. Determination of Lutein Bioavailability
2.6.3. Determination of Cumulative Release Rate of Lutein
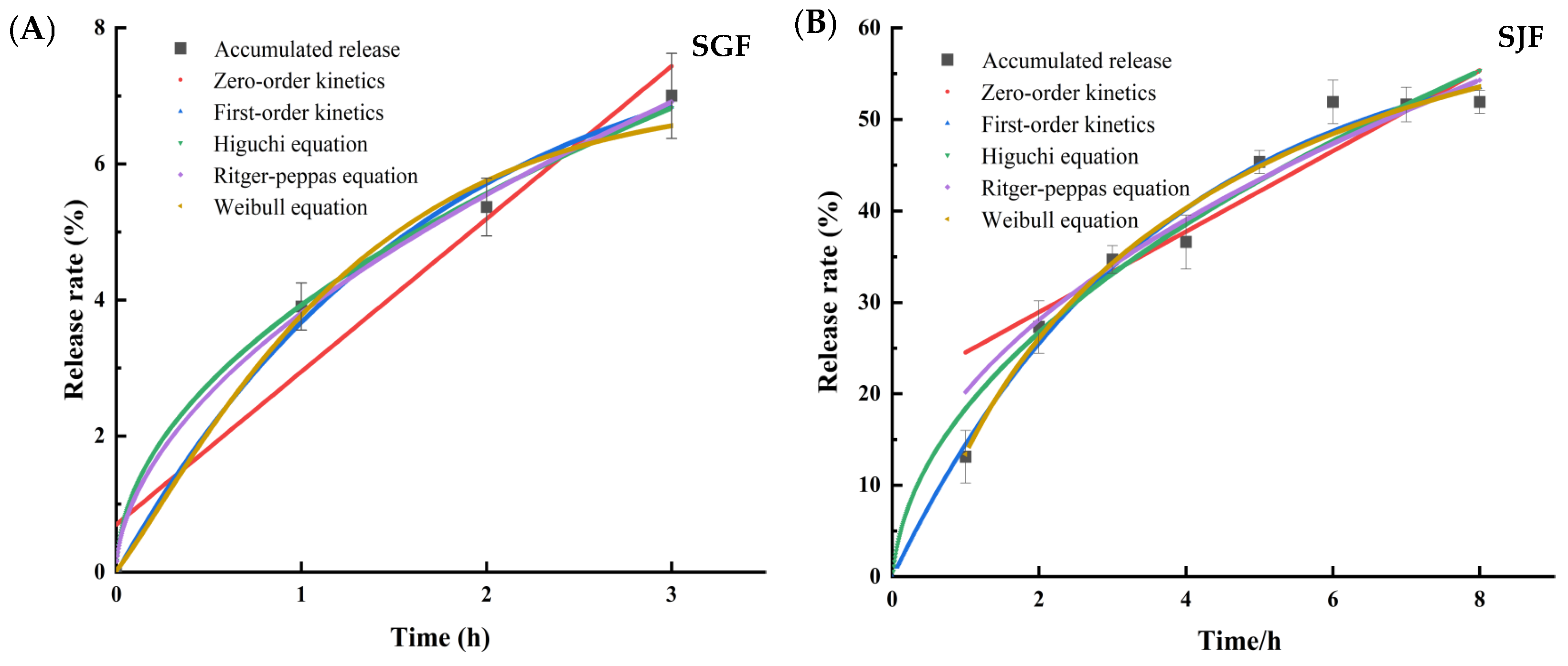
2.6.4. Establishment of Sustained Release Kinetics Model
2.7. Cellular Absorption and Transport Characteristics
2.7.1. Establishment of Caco-2 Cell Monolayer Model
2.7.2. Cytotoxicity Test
2.7.3. Determination of Apparent Permeability Coefficient (Papp)
2.7.4. Cell Uptake Inhibition Test
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of SA-BP Carrier on the EE and LC of Lutein
3.2. Structural Characterization
3.2.1. Microstructure, Particle Size, and Zeta Potential
3.2.2. XRD and DSC Analysis
3.2.3. FTIR and FS Analysis
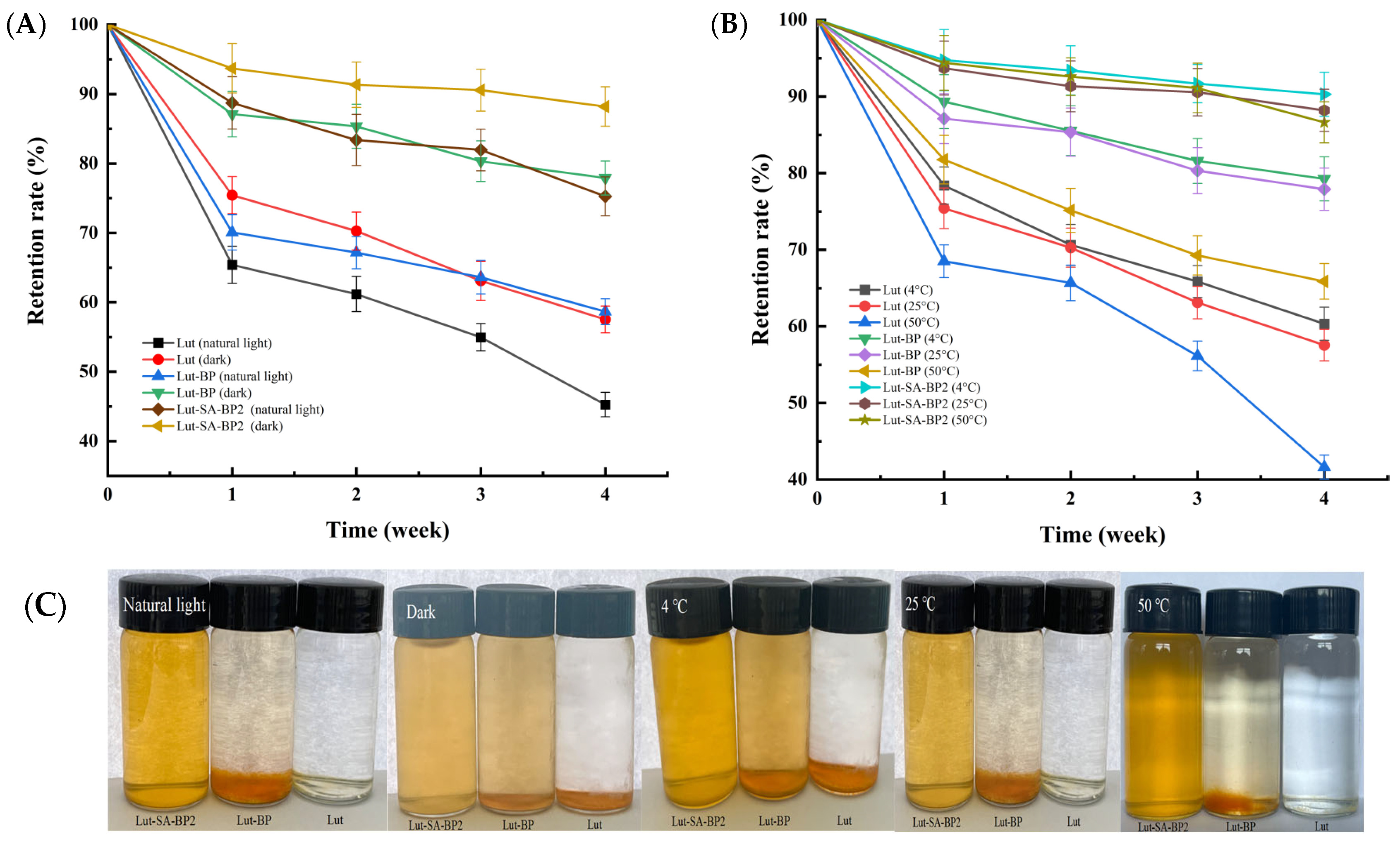
3.3. Storage Stability
3.4. In Vitro Release Properties
3.4.1. In Vitro Bioavailability
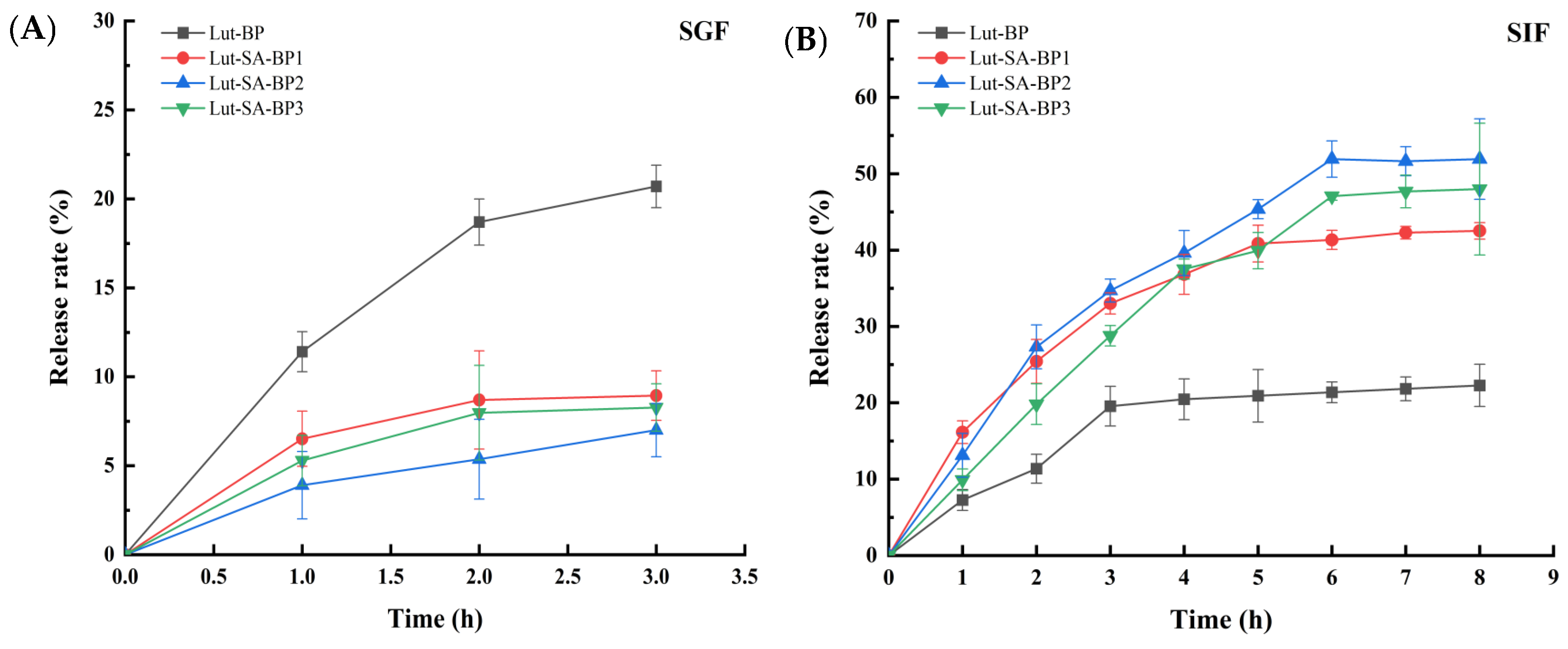
3.4.2. Release Characteristics of Lutein in Simulated Gastrointestinal Fluid
3.5. Cellular Osmotic Transport Characteristics
3.5.1. The Cototoxicity
3.5.2. Papp and Inhibition of Cellular Uptake
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.J.; Chang, J.S. Recent advances in lutein production from microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Yi, J.; Fan, Y.; Yokoyama, W.; Zhang, Y.; Zhao, L. Characterization of milk proteins–lutein complexes and the impact on lutein chemical stability. Food Chem. 2016, 200, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, S.; Kong, L.; Tan, L. Developing biopolymer-stabilized emulsions for improved stability and bioaccessibility of lutein. Int. J. Biol. Macromol. 2024, 259, 129202. [Google Scholar] [CrossRef]
- Algan, A.H.; Gungor-Ak, A.; Karatas, A. Nanoscale delivery systems of lutein: An updated review from a pharmaceutical perspective. Pharmaceutics 2022, 14, 1852. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, J. Polymeric materials reinforced by noncovalent aggregates of polymer chains. Aggregate 2021, 2, e109. [Google Scholar] [CrossRef]
- Chen, S.; Wu, J.; Tang, Q.; Xu, C.; Huang, Y.; Huang, D.; Luo, F.; Wu, Y.; Yan, F.; Wen, Z.; et al. Nano-micelles based on hydroxyethyl starch-curcumin conjugates for improved stability, antioxidant and anticancer activity of curcumin. Carbohyd. Polym. 2020, 228, 115398. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Feng, J.; Zhang, Q.; Wu, W.; Mo, H.; Huang, L.; Zhang, W. l-Carnitine conjugated chitosan-stearic acid polymeric micelles for improving the oral bioavailability of paclitaxel. Drug Deliv. 2020, 27, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, H.; Zhao, X.; Ye, F.; Zhao, G. Hydrophobically modified polysaccharides and their self-assembled systems: A review on structures and food applications. Carbohyd. Polym. 2022, 284, 119182. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Zhang, G.; Sun, D.; Wang, Y.; Liu, K.; Wang, M.; Sun, C.; Zhang, Z.; Li, B.; Lv, J. In vitro and in vivo evaluation of docetaxel-loaded stearic acid-modified Bletilla striata polysaccharide copolymer micelles. PLoS ONE 2017, 12, e0173172. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Chen, Q.; Zhang, C.; Li, L.; Zheng, Z. Structure analysis and anti-inflammatory activity evaluation of neutral polysaccharides from Arctium lappa L. Sci. Technol. Food Ind. 2023, 12, 45–54. [Google Scholar]
- Zeng, F.; Li, Y.; Zhang, X.; Shen, L.; Zhao, X.; Beta, T.; Li, B.; Chen, R.; Huang, W. Immune regulation and inflammation inhibition of Arctium lappa L. polysaccharides by TLR4/NF-κB signaling pathway in cells. Int. J. Biol. Macromol. 2024, 254, 127700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, H.; Zhang, Y.; Song, J.; Li, Y. Preparation and characterization of selfaggregating micelles from stearic acid-modified burdock root polysaccharide. Food Prod. Process. Nutr. 2024, 6, 11. [Google Scholar] [CrossRef]
- Varavinit, S.; Chaokasem, N.; Shobsngob, S. Studies of flavor encapsulation by agents produced from modified sago and tapioca starches. Starch-Stärke 2001, 53, 281–287. [Google Scholar] [CrossRef]
- Muhoza, B.; Zhang, Y.; Xia, S.; Cai, J.; Zhang, X.; Su, J. Improved stability and controlled release of lutein-loaded micelles based on glycosylated casein via Maillard reaction. J. Funct. Foods 2018, 45, 1–9. [Google Scholar] [CrossRef]
- Shi, Z.P. Stability Quality and Bioavailability of Bovine-Serum-Albumin-Dextran-Lutein Nanoparticles. Master’s Thesis, Tianjin University of Commerce, Tianjin, China, 2019. [Google Scholar]
- Mutsokoti, L.; Panozzo, A.; Pallares, A.P.; Jaiswal, S.; Van Loey, A.; Grauwet, T.; Hendrickx, M. Carotenoid bioaccessibility and the relation to lipid digestion: A kinetic study. Food Chem. 2017, 232, 124–134. [Google Scholar] [CrossRef]
- Luo, S.; Song, J.; Xu, P.; Dai, Z.; Wu, C.; Li, Y. Release characteristics and bioaccessibility of lutein from calcium alginate hydrogels during simulated digestion in vitro. Int. J. Food Sci. Technol. 2022, 57, 6122–6129. [Google Scholar] [CrossRef]
- Liang, R.; Shoemaker, C.F.; Yang, X.; Zhong, F.; Huang, Q. Stability and bioaccessibility of β-carotene in nanoemulsions stabilized by modified starches. J. Agric. Food Chem. 2013, 61, 1249–1257. [Google Scholar] [CrossRef]
- Ganje, M.; Jafari, S.M.; Tamadon, A.M.; Niakosari, M.; Maghsoudlou, Y. Mathematical and fuzzy modeling of limonene release from amylose nanostructures and evaluation of its release kinetics. Food Hydrocoll. 2019, 95, 186–194. [Google Scholar] [CrossRef]
- Peng, F.; Lin, G. Study on absorption and transportation of flavonoids and their monomers in extract of Zhishi (Aurantii Fructus Immaturus) in Caco-2 cells model. Chin. Arch. Tradit. Chin. Med. 2021, 39, 107–110. [Google Scholar]
- Bittermann, K.; Goss, K.U. Predicting apparent passive permeability of Caco-2 and MDCK cell-monolayers: A mechanistic model. PLoS ONE 2017, 12, e0190319. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, X.Y.; Gong, W.; Li, X.; Huang, H.B.; Zhu, X.M. Nanoparticles based on carboxymethylcellulose-modified rice protein for efficient delivery of lutein. Food Funct. 2020, 11, 2380–2394. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, R.; Prashanth KV, H.; Baskaran, V. Promising interaction between nanoencapsulated lutein with low molecular weight chitosan: Characterization and bioavailability of lutein in vitro and in vivo. Food Chem. 2013, 141, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Negahban, Z.; Shojaosadati, S.A.; Hamedi, S. A novel self-assembled micelles based on stearic acid modified schizophyllan for efficient delivery of paclitaxel. Colloid Surf. B 2021, 199, 111524. [Google Scholar] [CrossRef] [PubMed]
- Duval-Terrié, C.; Huguet, J.; Muller, G. Self-assembly and hydrophobic clusters of amphiphilic polysaccharides. Colloid Surf. A 2003, 220, 105–115. [Google Scholar] [CrossRef]
- Srivastava, A.; Yañez, O.; Cantero-López, P. Mixed micellization of bile salts and transglycosylated stevia and enhanced binding and solubility of non-steroidal anti-inflammatory drugs using mixed micelle. J. Mol. Liq. 2020, 311, 113341. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Wang, D.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Toragall, V.; Jayapala, N.; Vallikannan, B. Chitosan-oleic acid-sodium alginate a hybrid nanocarrier as an efficient delivery system for enhancement of lutein stability and bioavailability. Int. J. Biol. Macromol. 2020, 150, 578–594. [Google Scholar] [CrossRef]
- Xu, P.; Song, J.; Dai, Z.; Xu, Y.; Li, D.; Wu, C. Effect of Ca2+ cross-linking on the properties and structure of lutein-loaded sodium alginate hydrogels. Int. J. Biol. Macromol. 2021, 193, 53–63. [Google Scholar] [CrossRef]
- Chang, Y.; Jiao, Y.; Li, D.J.; Liu, X.L.; Han, H. Glycosylated zein as a novel nanodelivery vehicle for lutein. Food Chem. 2022, 376, 131927. [Google Scholar] [CrossRef]
- Liu, P.; Wang, K.; Liang, S.; Li, P.; Du, B. Advances in understanding the structure and biological activity of Poria cocos polysaccharides. Food Sci. 2023, 44, 380–391. [Google Scholar]
- Chang, Y.; Jiao, Y.; Liu, Q.; Liu, Q.; Sun, X.; Han, H. Structural characterization and release property of lutein loaded corn peptide nanoparticles. Food Sci. Technol. 2021, 46, 238–243. [Google Scholar]
- Shen, F.; Zhong, H.; Ge, W.; Ren, J.; Wang, X. Quercetin/chitosan-graft-alpha lipoic acid micelles: A versatile antioxidant water dispersion with high stability. Carbohyd. Polym. 2020, 34, 115927. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, Y.; Bai, L.; Liu, F.; Deng, Y.; McClements, D.J. Fabrication of β-carotene nanoemulsion-based delivery systems using dual-channel microfluidization: Physical and chemical stability. J. Colloid Interface Sci. 2017, 490, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Li, D.; Chen, M.; Wang, X.; Liu, S.; Sun, R. Characterization and antioxidant activity of β-carotene loaded chitosan-graft-poly (lactide) nanomicelles. Carbohyd. Polym. 2015, 117, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Yang, J.; Ge, S.; Zhao, M.; Liang, C.; Xiong, L.; Sun, Q. Synthesis and self-assembly of octenyl succinic anhydride modified short glucan chains based amphiphilic biopolymer: Micelles, ultrasmall micelles, vesicles, and lutein encapsulation/release. Food Hydrocoll. 2017, 67, 14–26. [Google Scholar] [CrossRef]
- Qi, X.; Liu, P.; Li, S.; Fan, Z. Preparation and drug release behavior of paclitaxel loaded polylactide-poly (ethylene glycol) triblock copolymer micelles. J. Fudan Univ. Nat. Sci. 2013, 52, 1–8. [Google Scholar]
- Yi, X.; Hou, H.; Meng, P.; Gui, J.; Liu, H.; Xiao, X. Release model of W/O/W multiple emulsion of coix-seed oil in vitro. J. Chin. Inst. Food Sci. Technol. 2018, 18, 53–59. [Google Scholar]
- Yang, C.; Liu, Y.; He, Y.; Du, Y.; Wang, W.; Shi, X.; Gao, F. The use of HA oligosaccharide-loaded nanoparticles to breach the endogenous hyaluronan glycocalyx for breast cancer therapy. Biomaterials 2013, 34, 6829–6838. [Google Scholar] [CrossRef]
- Xiong, S.; Li, J.; Mu, Y.; Zhang, Z. The absorption of oral morroniside in rats: In vivo, in situ and in vitro studies. Acta Pharm. 2019, 69, 287–296. [Google Scholar] [CrossRef]



| Sample | Lut-BP | Lut-SA-BP1 | Lut-SA-BP2 | Lut-SA-BP3 |
|---|---|---|---|---|
| Bioavailability (%) | 12.53 ± 3.24 c | 36.01 ± 4.32 b | 54.72 ± 4.54 a | 42.72 ± 3.88 b |
| Model | Fitted Equation | a | b | R2 | |
|---|---|---|---|---|---|
| Simulated gastric juice | Zero-order kinetics | Q(t) = 2.24t + 0.7 | 2.24 | 0.70 | 0.9089 |
| First-order kinetics | Q(t) = 8.24 (1 − e−0.59t) | 8.24 | 0.59 | 0.9889 | |
| Higuchi equation | Q(t) = 3.96t0.5 + 0.04 | 3.96 | 0.04 | 0.9959 | |
| Ritger-Peppas equation | Q(t) = 3.81 (t0.54) | 3.81 | 0.54 | 0.9972 | |
| Weibull equation | Q(t) = 7(1 −) | 0.796 | 1.167 | 0.9865 | |
| Simulated intestinal fluids | Zero-order kinetics | Q(t) = 4.4t + 20.13 | 4.40 | 20.13 | 0.8378 |
| First-order kinetics | Q(t) = 60.02 (1 − e−0.18t) | 60.02 | 0.18 | 0.9764 | |
| Higuchi equation | Q(t) = 20.25t0.5 − 1.97 | 20.25 | 1.97 | 0.9701 | |
| Ritger-Peppas | Q(t) = 18.31 (t0.55) | 18.31 | 0.55 | 0.9504 | |
| Weibull equation | Q(t) = 65.48 (1 − ) | 0.267 | 0.762 | 0.9775 |
| Sample | Papp/×10−5 (cm/s) | ||
|---|---|---|---|
| AP→BL | BL→AP | ||
| Lutein | 1.79 ± 0.47 c | 1.34 ± 0.32 c | 0.75 ± 0.03 B |
| Lut-BP | 2.20 ± 0.51 b | 2.65 ± 0.24 b | 1.20 ± 0.03 A |
| Lut-SA-BP2 | 5.31 ± 0.40 a | 2.38 ± 0.54 b | 0.45 ± 0.02 C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhang, Y.; Song, J.; Wang, H.; Wu, C.; Li, Y. Delivery of Lutein by Using Modified Burdock Polysaccharide Aggregates: Preparation, Characterization, and In Vitro Release Properties. Polymers 2024, 16, 1982. https://doi.org/10.3390/polym16141982
Zhang C, Zhang Y, Song J, Wang H, Wu C, Li Y. Delivery of Lutein by Using Modified Burdock Polysaccharide Aggregates: Preparation, Characterization, and In Vitro Release Properties. Polymers. 2024; 16(14):1982. https://doi.org/10.3390/polym16141982
Chicago/Turabian StyleZhang, Chenchen, Yan Zhang, Jiangfeng Song, Hongjuan Wang, Caie Wu, and Ying Li. 2024. "Delivery of Lutein by Using Modified Burdock Polysaccharide Aggregates: Preparation, Characterization, and In Vitro Release Properties" Polymers 16, no. 14: 1982. https://doi.org/10.3390/polym16141982
APA StyleZhang, C., Zhang, Y., Song, J., Wang, H., Wu, C., & Li, Y. (2024). Delivery of Lutein by Using Modified Burdock Polysaccharide Aggregates: Preparation, Characterization, and In Vitro Release Properties. Polymers, 16(14), 1982. https://doi.org/10.3390/polym16141982








