3D Printing and Blue Sustainability: Taking Advantage of Process-Induced Defects for the Metallic Ion Removal from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AM of FOMM
2.3. Characterization of FOMM
2.3.1. Chemical Analysis
2.3.2. Morphological Analysis
2.3.3. Porosity Characterization
2.3.4. Thermal Characterization
2.3.5. Swelling
2.3.6. Ion Sorption
2.3.7. Statistical Analysis
3. Results and Discussion
3.1. Exploratory/Preliminary Studies
3.2. Chemical Characterization
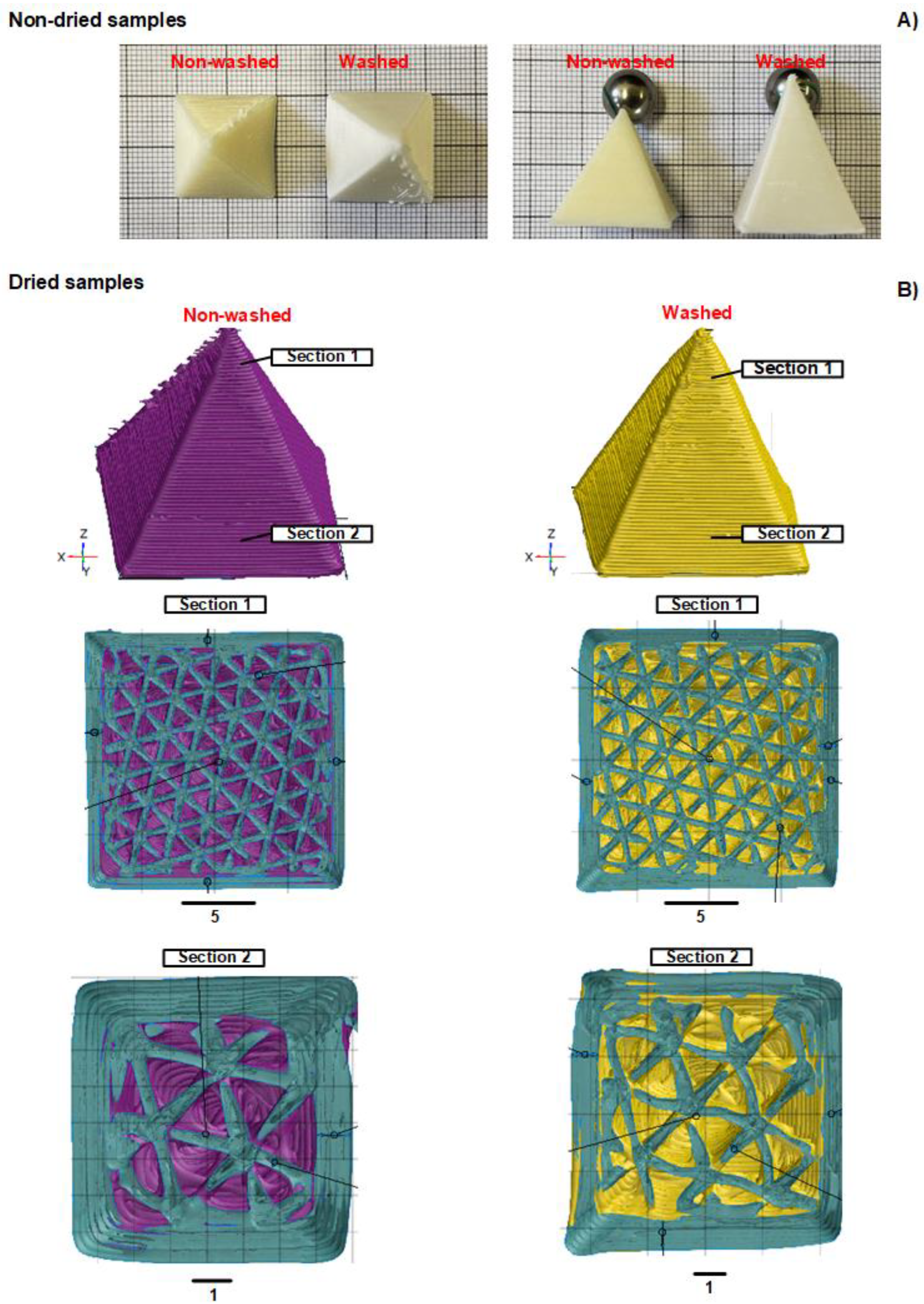
3.3. Morphological Characterization
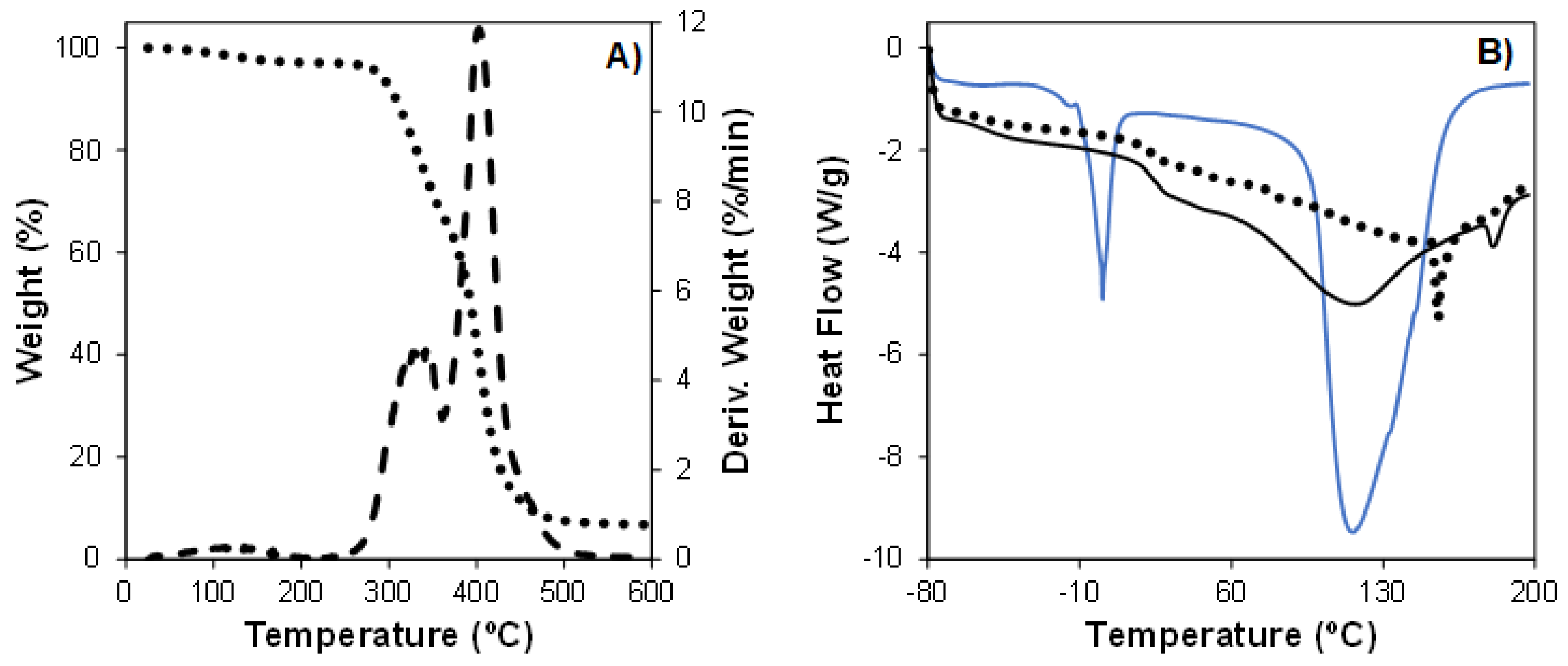
3.4. Thermal Characterization
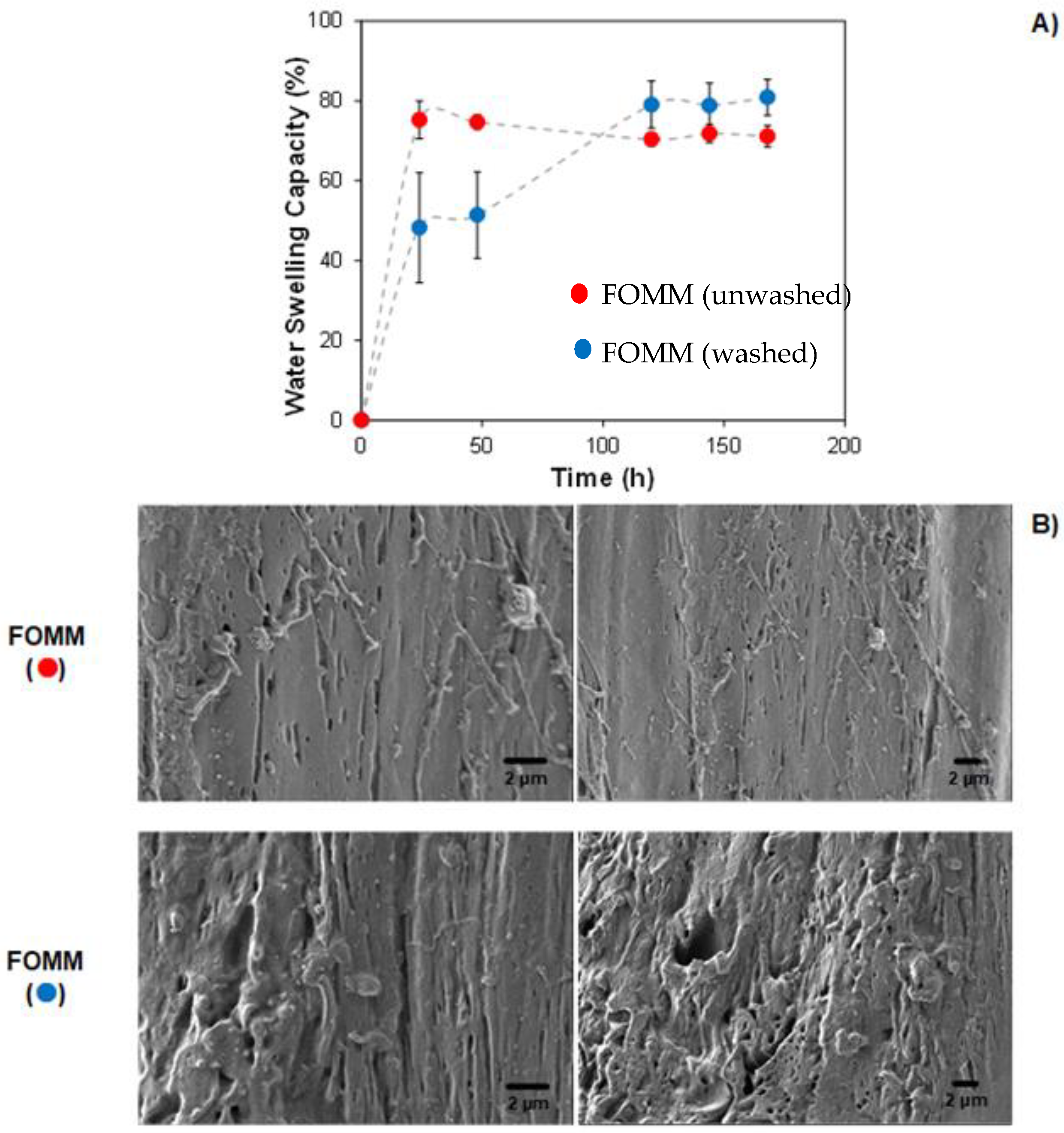
3.5. Water Swelling Capacity (WSC)
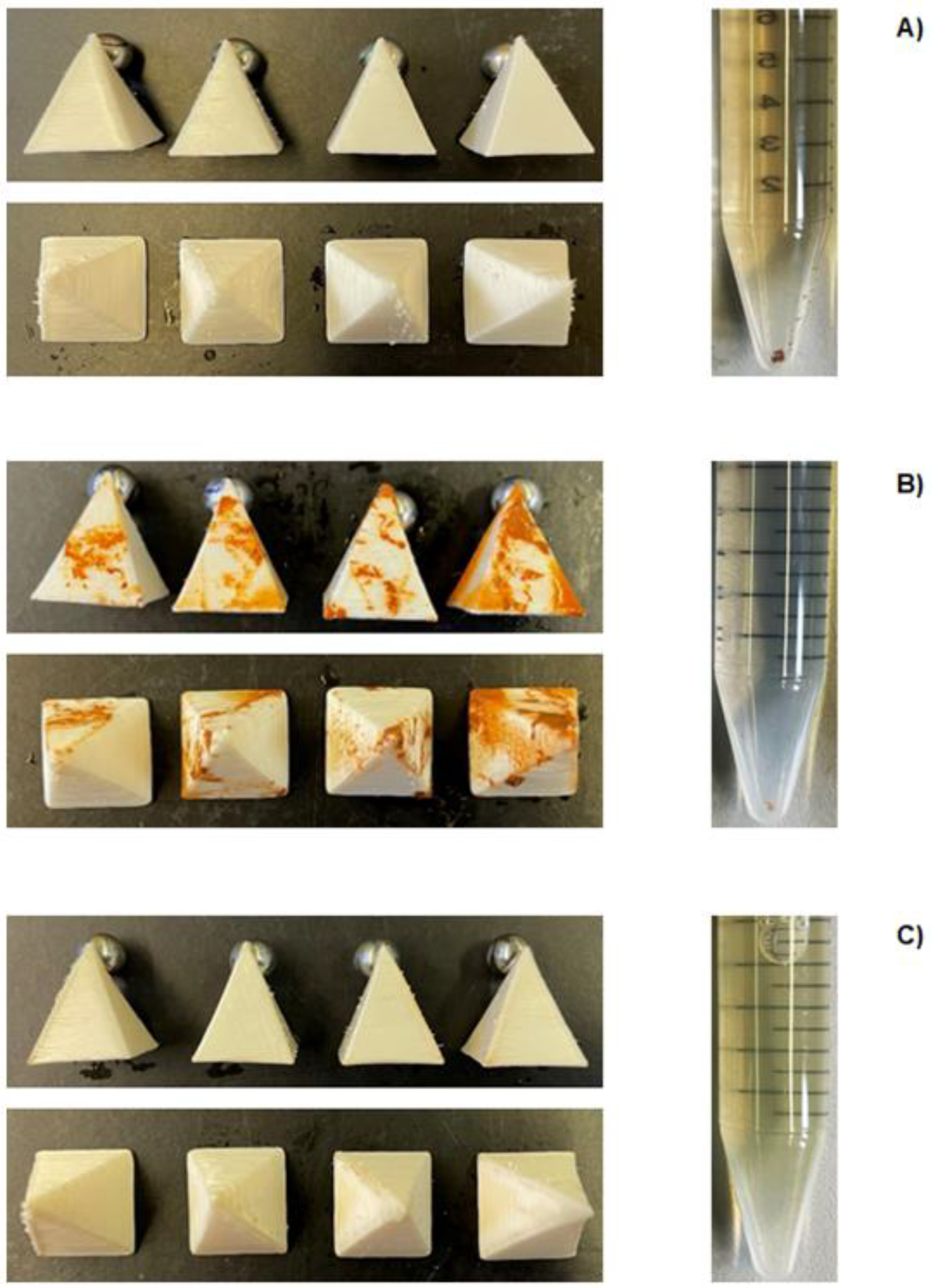
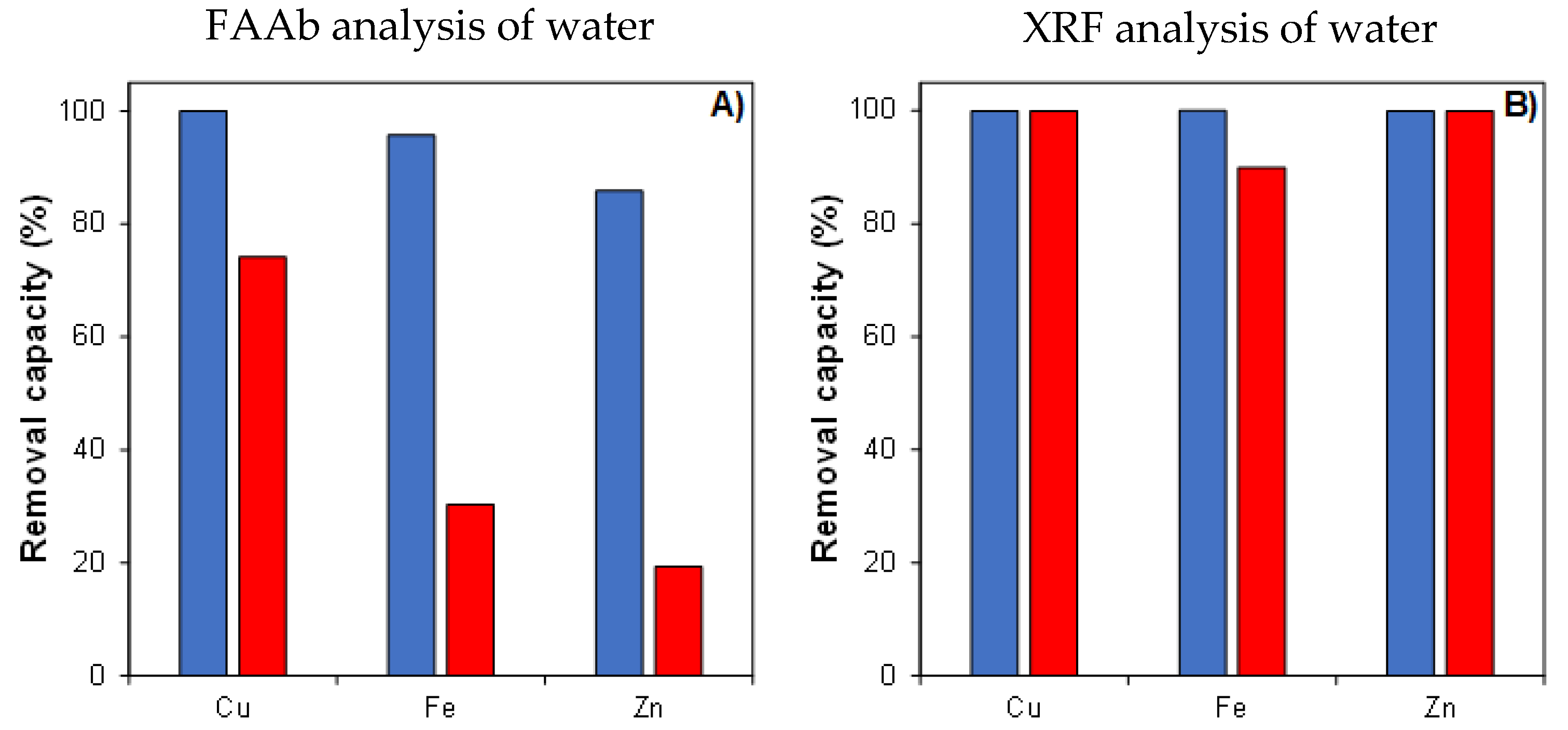
3.6. Metallic Ion Removal Capacity of 3D-Printed FOMM Sorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Tridimensional |
| ABS | Poly(acrylonitrile-butadiene-styrene) |
| AM | Additive manufacturing |
| AI | Artificial intelligence |
| CAD | Computer-aided design |
| CT | Computer tomography |
| DSC | Differential scanning calorimetry |
| DTG | Differential thermogram |
| FAAb | Flame atomic absorption spectrometry |
| FFF | Fused filament fabrication |
| FTIR | Fourier transform infrared spectroscopy |
| PDMS | Poly(dimethyl siloxane) |
| PEG | Poly(ethylene glycol) |
| PLA | Poly(lactic acid) |
| PP | Polypropylene |
| PU | Polyurethane |
| PVA | Poly(vinyl alcohol) |
| SEM | Scanning electron microscopy |
| TGA | Thermogravimetric analysis |
| WSC | Water sorption capacity |
| XRF | X-ray fluorescence |
References
- Maja, M.M.; Ayano, S.F. The Impact of Population Growth on Natural Resources and Farmers’ Capacity to Adapt to Climate Change in Low-Income Countries. Earth Syst. Environ. 2021, 5, 271–283. [Google Scholar] [CrossRef]
- UUNN. World Population Prospects 2019. Available online: https://www.ined.fr/fichier/s_rubrique/29368/wpp2019_10.key.findings_embargoed.version.en.pdf (accessed on 18 March 2024).
- Molotoks, A.; Stehfest, E.; Doelman, J.; Albanito, F.; Fitton, N.; Dawson, T.P.; Smith, P. Global projections of future cropland expansion to 2050 and direct impacts on biodiversity and carbon storage. Glob. Chang. Biol. 2018, 24, 5895–5908. [Google Scholar] [CrossRef] [PubMed]
- The Global Risks Report 2022. Available online: https://www.marshmclennan.com/insights/publications/2022/january/global-risks-report.html (accessed on 18 March 2024).
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Gu, H.; Liu, X.; Wang, S.; Chen, Z.; Yang, H.; Hu, B.; Shen, C.; Wang, X. COF-Based Composites: Extraordinary Removal Performance for Heavy Metals and Radionuclides from Aqueous Solutions. Rev. Environ. Contam. Toxicol. 2022, 260, 23. [Google Scholar] [CrossRef]
- Hao, M.; Liu, Y.; Wu, W.; Wang, S.; Yang, X.; Chen, Z.; Tang, Z.; Huang, Q.; Wang, S.; Yang, H.; et al. Advanced porous adsorbents for radionuclides elimination. Energy Chem. 2023, 5, 100101. [Google Scholar] [CrossRef]
- Brett, A.M.C.F.O.; Gil, M.H.; Piedade, A.P. An electrochemical bienzyme membrane sensor for free cholesterol. Bioeletrochem. Bioenerg. 1992, 28, 105–115. [Google Scholar] [CrossRef]
- Alves da Silva, M.; Gil, M.H.; Piedade, A.P.; Redinha, J.S.; Brett, A.M.; Costa, J.M. Immobilization of catalase on membranes of poly(ethylene)-g-co-acrylic acid and poly(tetrafluoroethylene)-g-co-acrylic acid and their application in hydrogen peroxide electrochemical sensors. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 269–274. [Google Scholar] [CrossRef]
- Piedade, A.P.; Gil, M.H.; Cavaco, M.C.; Andrade, M.E. Behaviour of catalase immobilised on poly(acrylonitrile)-g.co-hydroxyethyl methacrylate when used in a continuous system. Polym. Int. 1995, 38, 269–275. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, K.; Laghari, I.A.; Samykano, M.; Kothari, R.; Abusorrah, A.M.; Sharma, K.; Tyagi, V.V. Utilization of solar energy for wastewater treatment: Challenges and progressive research trends. J. Environ. Manag. 2021, 297, 113300. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Carbon-based sustainable nanomaterials for water treatment: State-of-art and future perspectives. Chemosphere 2021, 263, 128005. [Google Scholar] [CrossRef]
- Nishu, S.K. Smart and innovative nanotechnology applications for water purification. Hybrid Adv. 2023, 3, 100044. [Google Scholar] [CrossRef]
- Thomas, R.R.D.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Binod, P.; Pugazhendhi, A.; Sirohi, R.; Tarafdar, A.; Pandey, A. Potential of nanocellulose for wastewater treatment. Chemosphere 2021, 281, 130738. [Google Scholar]
- Kamali, M.; Appels, L.; Yu, X.; Aminabhavi, T.M.; Dewil, R. Artificial intelligence as a sustainable tool in wastewater treatment using membrane bioreactors. Chem. Eng. J. 2021, 417, 128070. [Google Scholar] [CrossRef]
- Ghosal, P.; Gupta, B.; Ambekar, R.S.; Rahman, M.M.; Ajayan, P.M.; Aich, N.; Gupta, A.K.; Tiwary, C.S. 3D Printed Materials in Water Treatment Applications. Adv. Sustainable Syst. 2022, 6, 2100282. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Review of manufacturing three-dimensional-printed membranes for water treatment. Environ. Sci. Pollut. Res. 2020, 27, 36091–36108. [Google Scholar] [CrossRef] [PubMed]
- Tijing, L.D.; Dizon, J.R.C.; Ibrahim, I.; Nisay, A.R.N.; Shon, H.K.; Advincula, R.C. 3D printing for membrane separation, desalination and water treatment. Appl. Mater. Today 2020, 18, 100486. [Google Scholar] [CrossRef]
- Yanar, N.; Kallem, P.; Son, M.; Park, H.; Kang, S.; Choi, H. A New era of water treatment technologies: 3D printing for membranes. J. Ind. Eng. Chem. 2020, 91, 1–14. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Ginn, M.; Rastogi, V. A review of 3D printing techniques for environmental applications. Curr. Opin. Chem. Eng. 2020, 28, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Ahmed, F.E.; Hilal, N. The emerging role of 3D printing in water desalination. Sci. Total Environ. 2021, 790, 148238. [Google Scholar] [CrossRef]
- Shuaib, M.; Haleem, A.; Kumar, S.; Javaid, M. Impact of 3D Printing on the environment: A literature-based study. Sustain. Oper. Comput. 2021, 2, 57–63. [Google Scholar] [CrossRef]
- Pinho, A.C.; Piedade, A.P. Influence of build orientation, geometry and artificial saliva aging on the mechanical properties of 3d printed poly(ε-caprolactone). Materials 2021, 14, 3335. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.; Amaro, A.M.; Piedade, A.P. Understanding atherosclerosis pathophysiology: Can additive manufacturing be helpful? Polymers 2023, 15, 480. [Google Scholar] [CrossRef]
- Shahbazi, M.; Jäger, H.; Ahmadi, S.J.; Lacroix, M. Electron beam crosslinking of alginate/nanoclay ink to improve functional properties of 3D printed hydrogel for removing heavy metal ions. Carbohydr. Polym. 2020, 240, 116211. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Fan, L.; Zhao, F.; Xiao, J.; Yang, Y.; Lai, A.; Zhou, S.F.; Zhan, G. 3D-Printed metal-organic frameworks within biocompatible polymers as excellent adsorbents for organic dyes removal. J. Hazard. Mater. 2020, 384, 121418. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.L.; Mondini, A.; Del Dottore, E.; Filippeschi, C.; Pignatelli, F.; Mazzolai, B. 3D printed composites from heat extruded polycaprolactone/sodium alginate filaments and their heavy metal adsorption properties. Mater. Chem. Front. 2020, 4, 2472–2483. [Google Scholar] [CrossRef]
- Appuhamillage, G.A.; Berry, D.R.; Benjamin, C.E.; Luzuriaga, M.A.; Reagan, J.C.; Gassensmith, J.J.; Smaldone, R.A. A biopolymer-based 3D printable hydrogel for toxic metal adsorption from water. Polym. Int. 2019, 68, 964–971. [Google Scholar] [CrossRef]
- Zhang, D.; Xiao, J.; Guo, Q.; Yang, J. 3D-printed highly porous and reusable chitosan monoliths for Cu(II) removal. J. Mater. Sci. 2019, 54, 6728–6741. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Jiang, Y.; Sui, G. Self-Thickening and Self-Strengthening 3D Printing Magnetic Cellulose-Based Aerogel for Adsorption and Recovery of Methylene Blue. Adv. Sustain. Syst. 2022, 6, 2100329. [Google Scholar] [CrossRef]
- Miao, Y.; Peng, W.; Wang, W.; Cao, Y.; Li, H.; Chang, L.; Huang, Y.; Fan, G.; Yi, H.; Zhao, Y.; et al. 3D-printed montmorillonite nanosheets based hydrogel with biocompatible polymers as excellent adsorbent for Pb(II) removal. Sep. Purif. Technol. 2022, 283, 120176. [Google Scholar] [CrossRef]
- Franchin, G.; Pesonen, J.; Luukkonen, T.; Bai, C.; Scanferla, P.; Botti, R.; Carturan, S.; Innocentini, M.; Colombo, P. Removal of ammonium from wastewater with geopolymer sorbents fabricated via additive manufacturing. Mater. Des. 2020, 195, 109006. [Google Scholar] [CrossRef]
- Shin, J.H.; Heo, J.H.; Jeon, S.; Park, J.H.; Kim, S.; Kang, H.W. Bio-inspired hollow PDMS sponge for enhanced oil–water separation. J. Hazard. Mater. 2019, 365, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Lagalante, L.A.; Lagalante, A.J.; Lagalante, A.F. 3D printed solid-phase extraction sorbents for removal of volatile organic compounds from water. J. Water Proc. Eng. 2020, 35, 101194. [Google Scholar] [CrossRef]
- Fijoł, N.; Abdelhamid, H.N.; Pillai, B.; Hall, S.A.; Thomas, N.; Mathew, A.P. 3D-printed monolithic biofilters based on a polylactic acid (PLA)-hydroxyapatite (HAp) composite for heavy metal removal from an aqueous medium. RSC Adv. 2021, 11, 32408–32418. [Google Scholar] [CrossRef]
- Lahtinen, E.; Hänninen, M.M.; Kinnunen, K.; Tuononen, H.M.; Väisänen, A.; Rissanen, K.; Haukka, M. Porous 3D Printed Scavenger Filters for Selective Recovery of Precious Metals from Electronic Waste. Adv. Sustain. Syst. 2018, 2, 1800048. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, X.; Li, W.; Xiao, L.; Sun, X.; Luo, F.; Chen, Q.; Qian, Q. In situ growth of ca2+-based metal–organic framework on casio3/abs/tpu 3d skeleton for methylene blue removal. Materials 2020, 13, 4403. [Google Scholar] [CrossRef]
- Oberoi, G.; Nitsch, S.; Janjić, K.; Shokoohi-Tabrizi, H.; Moritz, A.; Moscato, F.; Unger, E.; Agis, H. The impact of 3D-printed LAY-FOMM 40 and LAY-FOMM 60 on L929 cells and human oral fibroblasts. Clin. Oral Investig. 2021, 25, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Konieczna, L.; Belka, M.; Okońska, M.; Pyszka, M.; Bączek, T. New 3D-printed sorbent for extraction of steroids from human plasma preceding LC–MS analysis. J. Chromatogr. A 2018, 1545, 1–11. [Google Scholar] [CrossRef]
- Khan, Z.; He, H.; Chen, X.; Sydänheimo, L.; Ukkonen, L.; Virkki, J. Testing the effects of fabrication parameters on the post-fabrication shape change of a three-dimensional printed textile platform. Text. Res. J. 2021, 91, 2157–2166. [Google Scholar] [CrossRef]
- Delgado, G.F.; Pinho, A.C.; Piedade, A.P. 3D Printing for Cartilage Replacement: A Preliminary Study to Explore New Polymers. Polymers 2022, 14, 1044. [Google Scholar] [CrossRef]
- Belka, M.; Ulenberg, S.; Ba̧czek, T. Fused Deposition Modeling Enables the Low-Cost Fabrication of Porous, Customized-Shape Sorbents for Small-Molecule Extraction. Anal. Chem. 2017, 89, 4373–4376. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, X.; Cao, C.; Li, W.; Zeng, L.; Xiao, L.; Yan, P.; Huang, B.; Liu, X.; Qian, Q.; et al. Efficient Removal of Organic Contaminants from Aqueous Solution by Highly Compressible Reusable Three-Dimensional Printing Sponges. 3D Print. Addit. Manuf. 2021, 8, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.; Pinho, A.C.; Piedade, A.P. Mechanical properties of 3D printed mouthguards: Influence of layer height and device thickness. Mater. Des. 2021, 203, 109624. [Google Scholar] [CrossRef]
- Kaur, G.; Marmur, A.; Magdassi, S. Fabrication of superhydrophobic 3D objects by Digital Light Processing. Addit. Manuf. 2020, 36, 101669. [Google Scholar] [CrossRef]
- Yan, C.; Jiang, P.; Jia, X.; Wang, X. 3D printing of bioinspired textured surfaces with superamphiphobicity. Nanoscale 2020, 12, 2924–2938. [Google Scholar] [CrossRef] [PubMed]
- Dalawai, S.P.; Saad Aly, M.A.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.S.; Sadasivuni, K.K.; Liu, S. Recent Advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Pitaru, A.A.; Lacombe, J.G.; Cooke, M.E.; Beckman, L.; Steffen, T.; Weber, M.H.; Martineau, P.A.; Rosenzweig, D.H. Investigating commercial filaments for 3D printing of stiff and elastic constructs with ligament-like mechanics. Micromachines 2020, 11, 846. [Google Scholar] [CrossRef]
- Ahangar, P.; Akoury, E.; Luna, A.S.R.G.; Nour, A.; Weber, M.W.; Rosenzweig, D.H. Nanoporous 3D-printed scaffolds for local doxorubicin delivery in bone metastases secondary to prostate cancer. Materials 2018, 11, 1485. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Vazquez, N.A.; Sánchez-López, C.; Rodríguez Felix, F. Spectroscopy analyses of polyurethane/polyaniline IPN using computational simulation (Amber, MM+ and PM3 method). Polimeros 2014, 24, 453–463. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Li, M.; Lei, M.; Wang, Y.; Wei, Q. 3D printing thermo-responsive shape memory polymer composite based on PCL/TPU blends. J. Polym. Res. 2022, 29, 243. [Google Scholar] [CrossRef]
- Maciulyte, S.; Mamaviciute, I.; Straksys, A.; Kochane, T.; Budriene, S. New poly(urethane-urea) microcapsules from PVA modified with APTES: Preparation, characterization and enzyme encapsulation. Polym. Bull. 2021, 78, 1867–1886. [Google Scholar] [CrossRef]
- Cong, K.; He, J.; Yang, R. Using twin screw extrusion reaction (TSER) to produce thermoplastic polyurethane (TPU): Tunable, stoichiometric and eco-friendly. Polym. Adv. Technol. 2021, 32, 3495–3504. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Cuo, T.; Chen, Y. Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Crișan, A.G.; Porfire, A.; Ambrus, R.; Katona, G.; Rus, L.M.; Porav, A.S.; Ilyés, K.; Tomuță, I. Polyvinyl alcohol-based 3d printed tablets: Novel insight into the influence of polymer particle size on filament preparation and drug release performance. Pharmaceuticals 2021, 14, 418. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.; Kim, H.; Lee, S. Physical property of 3D-printed sinusoidal pattern using shape memory TPU filament. Text. Res. J. 2020, 90, 2399–2410. [Google Scholar] [CrossRef]
- Ghodbane, S.A.; Murthy, N.S.; Dunn, M.G.; Kohn, J. Achieving molecular orientation in thermally extruded 3D printed objects. Biofabrication 2019, 11, 045004. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, S.; Masania, K.; Woigk, W.; Sesseg, J.P.W.; Tervoort, T.A.; Studart, A.R. Three-dimensional printing of hierarchical liquid-crystal-polymer structures. Nature 2018, 561, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Cui, H.; Zhang, H.; Wang, F.; Zhan, X.; Mayer, F.; Nestler, B.; Wegener, M.; Levkin, P.A. 3D printing of inherently nanoporous polymers via polymerization-induced phase separation. Nat. Commun. 2021, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Radzi, A.M.; Sapuan, S.M.; Jawaid, M.; Mansor, M.R. Water absorption, thickness swelling and thermal properties of roselle/sugar palm fibre reinforced thermoplastic polyurethane hybrid composites. J. Mater. Res. Technol. 2019, 8, 3988–3994. [Google Scholar] [CrossRef]
- Atiqah, A.; Jawaid, M.; Ishak, M.R.; Sapuan, S.M. Moisture Absorption and Thickness Swelling Behaviour of Sugar Palm Fibre Reinforced Thermoplastic Polyurethane. Procedia Eng. 2017, 184, 581–586. [Google Scholar] [CrossRef]
- Kalaivani, S.S.; Muthukrishnaraj, A.; Sivanesan, S.; Ravikumar, L. Novel hyperbranched polyurethane resins for the removal of heavy metal ions from aqueous solution. Process Saf. Environ. Prot. 2016, 104, 11–23. [Google Scholar] [CrossRef]

| FOMM Unwashed | FOMM washed | |
|---|---|---|
| Total pore area (m2/g) | 8.7 | 11.7 |
| Median pore diameter (volume) (μm) | 30.5 | 49.7 |
| Median pore diameter (area) (nm) | 8.6 | 9.3 |
| Bulk density (g/mL) | 1.42 | 1.00 |
| Apparent density (g/mL) | 1.60 | 1.23 |
| Porosity (%) | 11.0 | 18.2 |
| Metallic Ions in the FOMM (%) | |||||||
|---|---|---|---|---|---|---|---|
| Specimens | Fe | Zn | Cl | S | Cu | Si | Total |
| Before experiment | 2.18 | 0.83 | 20.83 | 4.08 | 1.63 | 70.51 | 100 |
| After continuous | 28.67 | 8.33 | 8.85 | 1.83 | 1.21 | 51.12 | |
| After ultrasound | 6.53 | 4.11 | 15.72 | 2.90 | 1.03 | 69.70 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanaan, A.F.; Piedade, A.P. 3D Printing and Blue Sustainability: Taking Advantage of Process-Induced Defects for the Metallic Ion Removal from Water. Polymers 2024, 16, 1992. https://doi.org/10.3390/polym16141992
Kanaan AF, Piedade AP. 3D Printing and Blue Sustainability: Taking Advantage of Process-Induced Defects for the Metallic Ion Removal from Water. Polymers. 2024; 16(14):1992. https://doi.org/10.3390/polym16141992
Chicago/Turabian StyleKanaan, Akel F., and Ana P. Piedade. 2024. "3D Printing and Blue Sustainability: Taking Advantage of Process-Induced Defects for the Metallic Ion Removal from Water" Polymers 16, no. 14: 1992. https://doi.org/10.3390/polym16141992
APA StyleKanaan, A. F., & Piedade, A. P. (2024). 3D Printing and Blue Sustainability: Taking Advantage of Process-Induced Defects for the Metallic Ion Removal from Water. Polymers, 16(14), 1992. https://doi.org/10.3390/polym16141992







