Cactus Pear Mucilage (Opuntia spp.) as a Novel Functional Biopolymer: Mucilage Extraction, Rheology and Biofilm Development
Abstract
1. Introduction
2. Mucilage Chemical Structure
2.1. Mucilage Fractions
2.2. Mineral Composition of Mucilage
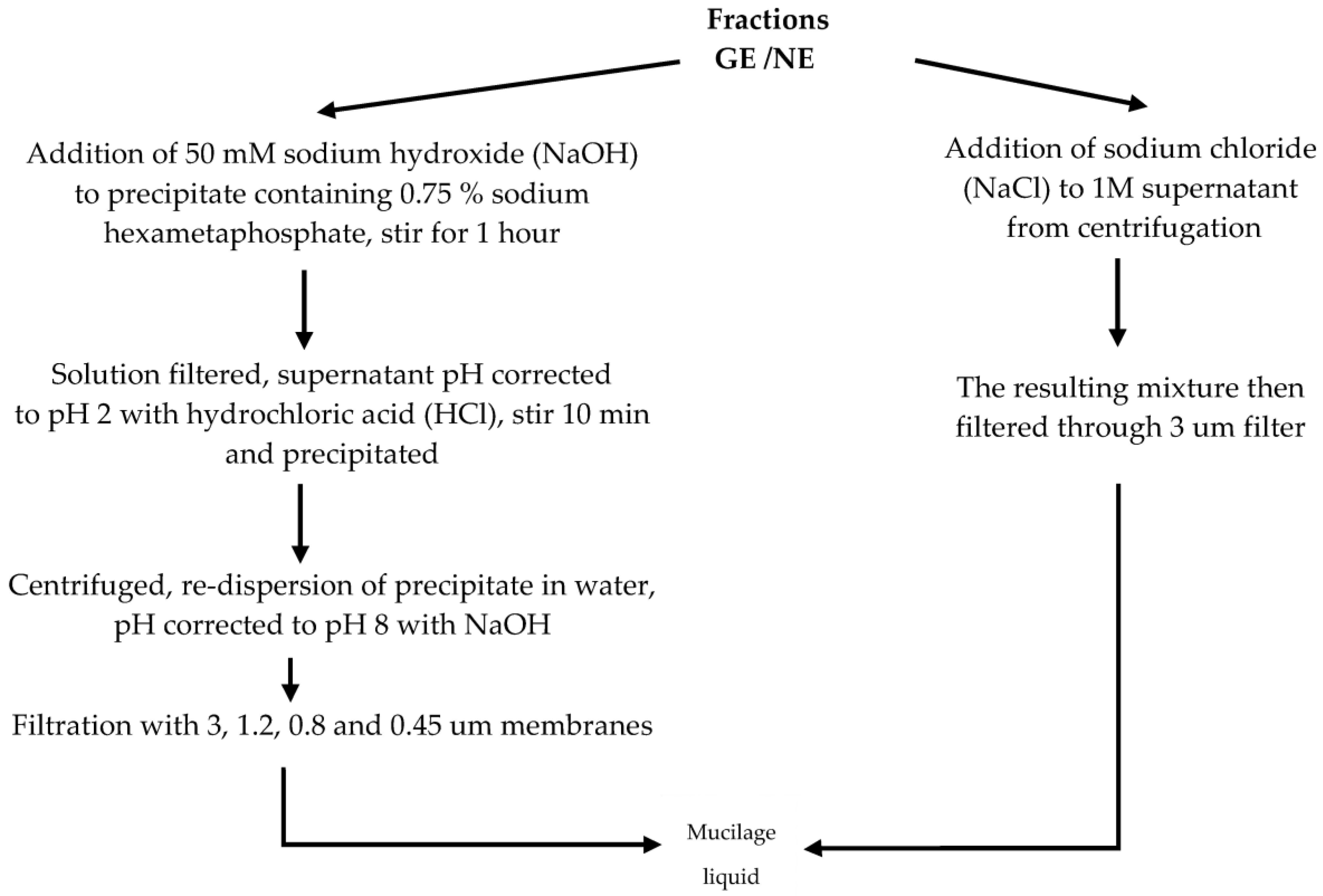
3. Extraction Methods
4. Functional Properties Associated with Mucilage
4.1. Rheology-Altering Properties of Mucilage
Factors Influencing the Rheology of a Mucilage Solution
4.2. Mucilage in the Development of Biofilms
4.2.1. Homopolymeric Mucilage Biofilms
4.2.2. Composite/Blended Mucilage Biofilms
4.3. Limitation and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nath, P.C.; Sharma, R.; Debnath, S.; Sharma, M.; Inbaraj, B.S.; Dikkala, P.K.; Nayak, P.K.; Sridhar, K. Recent Trends in Polysaccharide-Based Biodegradable Polymers for Smart Food Packaging Industry. Int. J. Biol. Macromol. 2023, 253, 127524. [Google Scholar] [CrossRef] [PubMed]
- Zena, Y.; Periyasamy, S.; Tesfaye, M.; Tumssa, Z.; Mohamed, B.A.; Karthik, V.; Asaithambi, P.; Getachew, D.; Aminabhavi, T.M. Trends on Barrier Characteristics Improvement of Emerging Biopolymeric Composite Films Using Nanoparticles—A Review. J. Taiwan Inst. Chem. Eng. 2024, 105488. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J.; Hugo, A. Effect of Native Mucilage on the Mechanical Properties of Pectin-Based and Alginate-Based Polymeric Films. Coatings 2023, 13, 1611. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Tkaczewska, J. Peptides and Protein Hydrolysates as Food Preservatives and Bioactive Components of Edible Films and Coatings—A Review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J. Cactus Pear (Opuntia spp.) Crop Applications and Emerging Biopolymer Innovations. Acta Hortic. 2023, 1380, 129–134. [Google Scholar] [CrossRef]
- Gheribi, R.; Khwaldia, K. Cactus Mucilage for Food Packaging Applications. Coatings 2019, 9, 655. [Google Scholar] [CrossRef]
- Sandoval, D.C.G.; Sosa, B.L.; Martínez-Ávila, G.C.G.; Fuentes, H.R.; Abarca, V.H.A.; Rojas, R. Formulation and Characterization of Edible Films Based on Organic Mucilage from Mexican Opuntia ficus-indica. Coatings 2019, 9, 506. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J.; Hugo, A. Microstructural and Mechanical Properties of Calcium-Treated Cactus Pear Mucilage (Opuntia spp.), Pectin and Alginate Single-Biopolymer Films. Polymers 2023, 15, 4295. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J.; Hugo, A. Effect of pH on the Mechanical Properties of Single-Biopolymer Mucilage (Opuntia ficus-indica), Pectin and Alginate Films: Development and Mechanical Characterisation. Polymers 2023, 15, 4640. [Google Scholar] [CrossRef]
- Majdoub, H.; Roudesli, S.; Picton, L.; Le Cerf, D.; Muller, G.; Grisel, M. Prickly Pear Nopals Pectin from Opuntia ficus-indica Physico-Chemical Study in Dilute and Semi-Dilute Solutions. Carbohydr. Polym. 2001, 46, 69–79. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Lillo, L.E.; Sáenz, C.; Urzúa, C.C.; Zárate, O. Chemical Characterization of the Mucilage from Fruits of Opuntia ficus indica. Carbohydr. Polym. 2006, 63, 263–267. [Google Scholar] [CrossRef]
- Monrroy, M.; García, E.; Ríos, K.; García, J.R. Extraction and Physicochemical Characterization of Mucilage from Opuntia cochenillifera (L.) Miller. J. Chem. 2017, 2017, 4301901. [Google Scholar] [CrossRef]
- Rodríguez-González, S.; Martínez-Flores, H.E.; Chávez-Moreno, C.K.; Macías-Rodríguez, L.I.; Zavala-Mendoza, E.; Garnica-Romo, M.G.; Chacõn-García, L. Extraction and Characterization of Mucilage from Wild Species of Opuntia. J. Food Process Eng. 2014, 37, 285–292. [Google Scholar] [CrossRef]
- Goycoolea, F.M.; Cárdenas, A. Pectins from Opuntia Spp.: A Short Review. J. Prof. Assoc. Cactus Dev. 2003, 5, 17–29. [Google Scholar]
- Cárdenas, A.; Goycoolea, F.M.; Rinaudo, M. On the Gelling Behaviour of “nopal” (Opuntia ficus indica) Low Methoxyl Pectin. Carbohydr. Polym. 2008, 73, 212–222. [Google Scholar] [CrossRef]
- Du Toit, A.; De Wit, M. Patent PA153178P A Process for Extracting Mucilage from Opuntia ficus-indica, Aloe Barbadensis and Agave Americana. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2021. [Google Scholar] [CrossRef]
- Feugang, J.M. Nutritional and Medicinal Use of Cactus Pear (Opuntia spp.) Cladodes and Fruits. Front. Biosci. 2006, 11, 2574. [Google Scholar] [CrossRef] [PubMed]
- Van Rooyen, B.; De Wit, M.; Osthoff, G. Gelling Potential of Native Cactus Pear Mucilage. Acta Hortic. 2022, 1343, 489–496. [Google Scholar] [CrossRef]
- Van Rooyen, B.; De Wit, M.; Osthoff, G. Functionality of Native Mucilage from Cactus Pears as a Potential Functional Food Ingredient at Industrial Scale. Acta Hortic. 2022, 1343, 481–488. [Google Scholar] [CrossRef]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia Spp. Mucilage’s: A Functional Component with Industrial Perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and Characterization of Mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Brito-De La Fuente, E.; Torrestiana-Sanchez, B.; Katthain, R. Rheological Properties of the Mucilage Gum (Opuntia ficus indica). Food Hydrocoll. 2000, 14, 417–424. [Google Scholar] [CrossRef]
- Felkai-Haddache, L.; Dahmoune, F.; Remini, H.; Lefsih, K.; Mouni, L.; Madani, K. Microwave Optimization of Mucilage Extraction from Opuntia ficus indica Cladodes. Int. J. Biol. Macromol. 2016, 84, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S.; Harper, B.A. Dried Ca-Alginate Films: Effects of Glycerol, Relative Humidity, Soy Fibers, and Carrageenan. LWT 2019, 103, 260–265. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K.; Da Silva, M.A.; Kieckbusch, T.G. Natamycin Release from Alginate/Pectin Films for Food Packaging Applications. J. Food Eng. 2012, 110, 18–25. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and Pectin Composite Films Crosslinked with Ca2+ Ions: Effect of the Plasticizer Concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Galicia-García, T.; Martínez-Bustos, F.; Ferreira Grosso, R.; González-Núñez, R. Effects of Nopal Mucilage (Opuntia ficus-indica) as Plasticizer in the Fabrication of Laminated and Tubular Films of Extruded Acetylated Starches. Int. J. Polym. Sci. 2021, 2021, 6638756. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Zawiślak, G.; Maj, G.; Chawla, P. Starch–Mucilage Composite Films: An Inclusive on Physicochemical and Biological Perspective. Polymers 2021, 13, 2588. [Google Scholar] [CrossRef]
- Garfias Silva, V.; Cordova Aguilar, M.S.; Ascanio, G.; Aguayo, J.P.; Pérez-Salas, K.Y.; Susunaga Notario, A.D.C. Acid Hydrolysis of Pectin and Mucilage from Cactus (Opuntia ficus) for Identification and Quantification of Monosaccharides. Molecules 2022, 27, 5830. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; Ornelas-Paz, J.D.J.; Martínez-Téllez, M.A.; Santillán, C.; Barbosa-Cánovas, G.V.; Zamudio-Flores, P.B.; Olivas, G.I. Development and Characterization of Edible Films Based on Mucilage of Opuntia ficus-indica (L.). J. Food Sci. 2010, 75, 347–352. [Google Scholar] [CrossRef]
- Du Toit, A.; De Wit, M.; Fouché, H.J.; Taljaard, M.; Venter, S.L.; Hugo, A. Mucilage Powder from Cactus Pears as Functional Ingredient: Influence of Cultivar and Harvest Month on the Physicochemical and Technological Properties. J. Food Sci. Technol. 2019, 56, 2404–2416. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, A.; De Wit, M.; Seroto, K.D.; Fouché, H.J.; Hugo, A.; Venter, S.L. Rheological Characterization of Cactus Pear Mucilage for Application in Nutraceutical Food Products. Acta Hortic. 2019, 1247, 63–72. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Vargas-Rodríguez, L.; Núñez-Colín, C.A.; González-García, G.; Peña-Caballero, V.; Núñez-Gastélum, J.A.; Gallegos-Vázquez, C.; Rodríguez-Núñez, J.R. Mucilage from Cladodes of Opuntia Spinulifera Salm-Dyck: Chemical, Morphological, Structural and Thermal Characterization. CYTA—J. Food 2018, 16, 650–657. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Miya, S.; De Wit, M.; Van Biljon, A.; Venter, S.L.; Amonsou, E. Opuntia ficus-indica Mill. and O. robusta Cladode Mucilage: Carbohydrates. Acta Hortic. 2022, 1343, 511–518. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Rodríguez-García, M.E.; Gutiérrez-Cortez, E.; Valderrama-Bravo, M.d.C.; Rojas-Molina, J.I.; Rivera-Muñoz, E.M. Physicochemical and Rheological Characterization of Opuntia Ficus Mucilage at Three Different Maturity Stages of Cladode. Eur. Polym. J. 2016, 78, 226–234. [Google Scholar] [CrossRef]
- De Wit, M.; Du Toit, A.; Fouché, H.J.; Hugo, A.; Venter, S.L. Screening of Cladodes from 42 South African Spineless Cactus Pear Cultivars for Morphology, Mucilage Yield and Mucilage Viscosity. Acta Hortic. 2019, 1247, 47–55. [Google Scholar] [CrossRef]
- Soukoulis, C.; Gaiani, C.; Hoffmann, L. Plant Seed Mucilage as Emerging Biopolymer in Food Industry Applications. Curr. Opin. Food Sci. 2018, 22, 28–42. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and Characterization of Composite Edible Films Based on Sodium Alginate and Pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of Plasticized Edible Films from Opuntia ficus-indica Mucilage: A Comparative Study of Various Polyol Plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Influence of Calcium on Tensile, Optical and Water Vapour Permeability Properties of Sodium Caseinate Edible Films. J. Food Eng. 2010, 96, 356–364. [Google Scholar] [CrossRef]
- Lira-Vargas, A.A.; Lira-Vargas, A.A.; Corrales-Garcia, J.J.E.; Valle-Guadarrama, S.; Peña-Valdivia, C.B.; Trejo-Marquez, M.A. Biopolymeric Films Based on Cactus (Opuntia ficus-indica) Mucilage Incorporated with Gelatin and Beeswax. J. Prof. Assoc. Cactus Dev. 2014, 16, 51–70. [Google Scholar]
- Allegra, A.; Inglese, P.; Sortino, G.; Settanni, L.; Todaro, A.; Liguori, G. The Influence of Opuntia ficus-indica Mucilage Edible Coating on the Quality of “Hayward” Kiwifruit Slices. Postharvest Biol. Technol. 2016, 120, 45–51. [Google Scholar] [CrossRef]
- Damas, M.S.P.; Junior, V.A.P.; Nishihora, R.K.; Quadri, M.G.N. Edible Films from Mucilage of Cereus Hildmannianus Fruits: Development and Characterization. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Zibaei, R.; Hasanvand, S.; Hashami, Z.; Roshandel, Z.; Rouhi, M.; Guimarães, J.d.T.; Mortazavian, A.M.; Sarlak, Z.; Mohammadi, R. Applications of Emerging Botanical Hydrocolloids for Edible Films: A Review. Carbohydr. Polym. 2021, 256, 117554. [Google Scholar] [CrossRef] [PubMed]
- Livney, Y.D.; Schwan, A.L.; Dalgleish, D.G. A Study of β-Casein Tertiary Structure by Intramolecular Crosslinking and Mass Spectrometry. J. Dairy Sci. 2004, 87, 3638–3647. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Biliaderis, C.G.; Kontogiorgos, V. Molecular Weight Effects on Solution Rheology of Pullulan and Mechanical Properties of Its Films. Carbohydr. Polym. 2003, 52, 151–166. [Google Scholar] [CrossRef]
- Gheribi, R.; Gharbi, M.A.; Ouni, M.E.; Khwaldia, K. Enhancement of the Physical, Mechanical and Thermal Properties of Cactus Mucilage Films by Blending with Polyvinyl Alcohol. Food Packag. Shelf Life 2019, 22, 100386. [Google Scholar] [CrossRef]
- Luna-Sosa, B.; Martínez-Ávila, G.C.G.; Rodríguez-Fuentes, H.; Azevedo, A.G.; Pastrana, L.M.; Rojas, R.; Cerqueira, M.A. Pectin-Based Films Loaded with Hydroponic Nopal Mucilages: Development and Physicochemical Characterization. Coatings 2020, 10, 467. [Google Scholar] [CrossRef]
- Scognamiglio, F.S.; Gattia, D.M.; Roselli, G.; Persia, F.; De Angelis, U.; Santulli, C. Thermoplastic Starch (TPS) Films Added with Mucilage from Opuntia ficus indica: Mechanical, Microstructural and Thermal Characterization. Materials 2020, 13, 1000. [Google Scholar] [CrossRef]

| Sugars Investigated ** | Pectin Fraction (A) | Pectin Fraction (B) | Mucilage Fraction (A) | Mucilage Fraction (C) | Mucilage Fraction (D) |
|---|---|---|---|---|---|
| Uronic acid * | 56.30 | 85.40 | 11.00 | 19.4 | 13.91 |
| Arabinose | 5.60 | 6.00 | 17.93 | 33.10 | 35.36 |
| Galactose | 6.50 | 7.00 | 20.99 | 20.30 | 27.26 |
| Rhamnose | 0.50 | 0.60 | 1.75 | 6.90 | 1.93 |
| Xylose | 0.90 | 1.00 | 3.06 | 18.7 | 16.32 |
| Research performed by: | |||||
| (A) Goycoolea and Cárdenas [15]; (B) Cárdenas et al. [16]; (C) Majdoub et al. [11]; (D) Rodriguez-González et al. [14]. | |||||
| Publication | Heating/Microwave Treatment | Maceration/Blending/Milling | Filtration | Centrifugation | Ethanol Extraction | Moisture Removal | Mucilage Yield |
|---|---|---|---|---|---|---|---|
| Monrroy et al. [13] | √ | √ | √ | – | √ | Oven-dried | ~24–31% |
| Felkai-Haddache et al. [24] | √ | √ | √ Cheesecloth | √ | √ | Freeze-drying | ~6.82–25.56% |
| Du Toit and De Wit PA153178P [17] | √ | √ | – | √ | – | Freeze-drying | ~39–62% Native Mucilage *** |
| Majdoub et al. [11] | – | √ | Filtration + Ultrafiltration | √ | – | Freeze-drying | Separate Fractions ** |
| A review: Goycoolea and Cárdenas [15] | √ | √ | Complex Filtration; pH Adjustment | √ | √ Multi-step | * DNS | Separate Fractions ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Rooyen, B.; De Wit, M.; Osthoff, G.; Van Niekerk, J. Cactus Pear Mucilage (Opuntia spp.) as a Novel Functional Biopolymer: Mucilage Extraction, Rheology and Biofilm Development. Polymers 2024, 16, 1993. https://doi.org/10.3390/polym16141993
Van Rooyen B, De Wit M, Osthoff G, Van Niekerk J. Cactus Pear Mucilage (Opuntia spp.) as a Novel Functional Biopolymer: Mucilage Extraction, Rheology and Biofilm Development. Polymers. 2024; 16(14):1993. https://doi.org/10.3390/polym16141993
Chicago/Turabian StyleVan Rooyen, Brandon, Maryna De Wit, Gernot Osthoff, and Johan Van Niekerk. 2024. "Cactus Pear Mucilage (Opuntia spp.) as a Novel Functional Biopolymer: Mucilage Extraction, Rheology and Biofilm Development" Polymers 16, no. 14: 1993. https://doi.org/10.3390/polym16141993
APA StyleVan Rooyen, B., De Wit, M., Osthoff, G., & Van Niekerk, J. (2024). Cactus Pear Mucilage (Opuntia spp.) as a Novel Functional Biopolymer: Mucilage Extraction, Rheology and Biofilm Development. Polymers, 16(14), 1993. https://doi.org/10.3390/polym16141993








