Valorization of Sugarcane Bagasse for Co-Production of Poly(3-hydroxybutyrate) and Bacteriocin Using Bacillus cereus Strain S356
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria
2.2. Sugarcane Bagasse Hydrolysate Preparation
2.3. Screening of P(3HB)-Producing Bacteria
2.4. Quantitative Analysis of P(3HB) Production
2.5. Identification of the Most Potent P(3HB)-Producing Isolate
2.6. Optimization of Culture Medium for P(3HB) Production
2.7. Transmission Electron Microscopy (TEM)
2.8. P(3HB) Film Preparation
2.9. Characterization of the Extracted P(3HB) Polymer
2.9.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.9.2. Fourier Transform Infrared (FTIR) Spectroscopy
2.10. Antibacterial Activity Assay
2.11. Effects of Temperature and Proteolytic Enzymes on Bacteriocin-like Substances
2.12. Statistical Analysis
3. Results
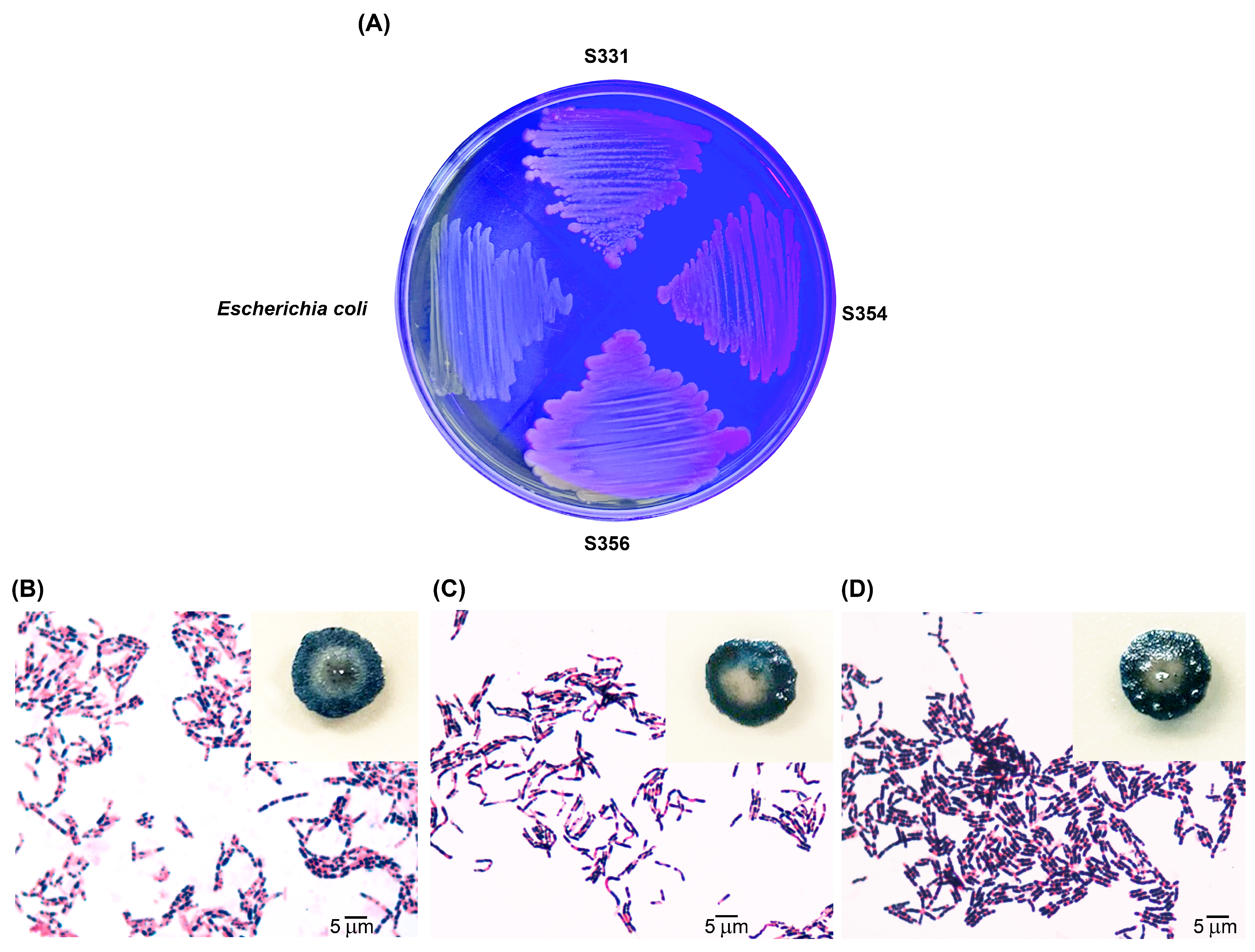
3.1. Screening of P(3HB)-Producing Bacteria
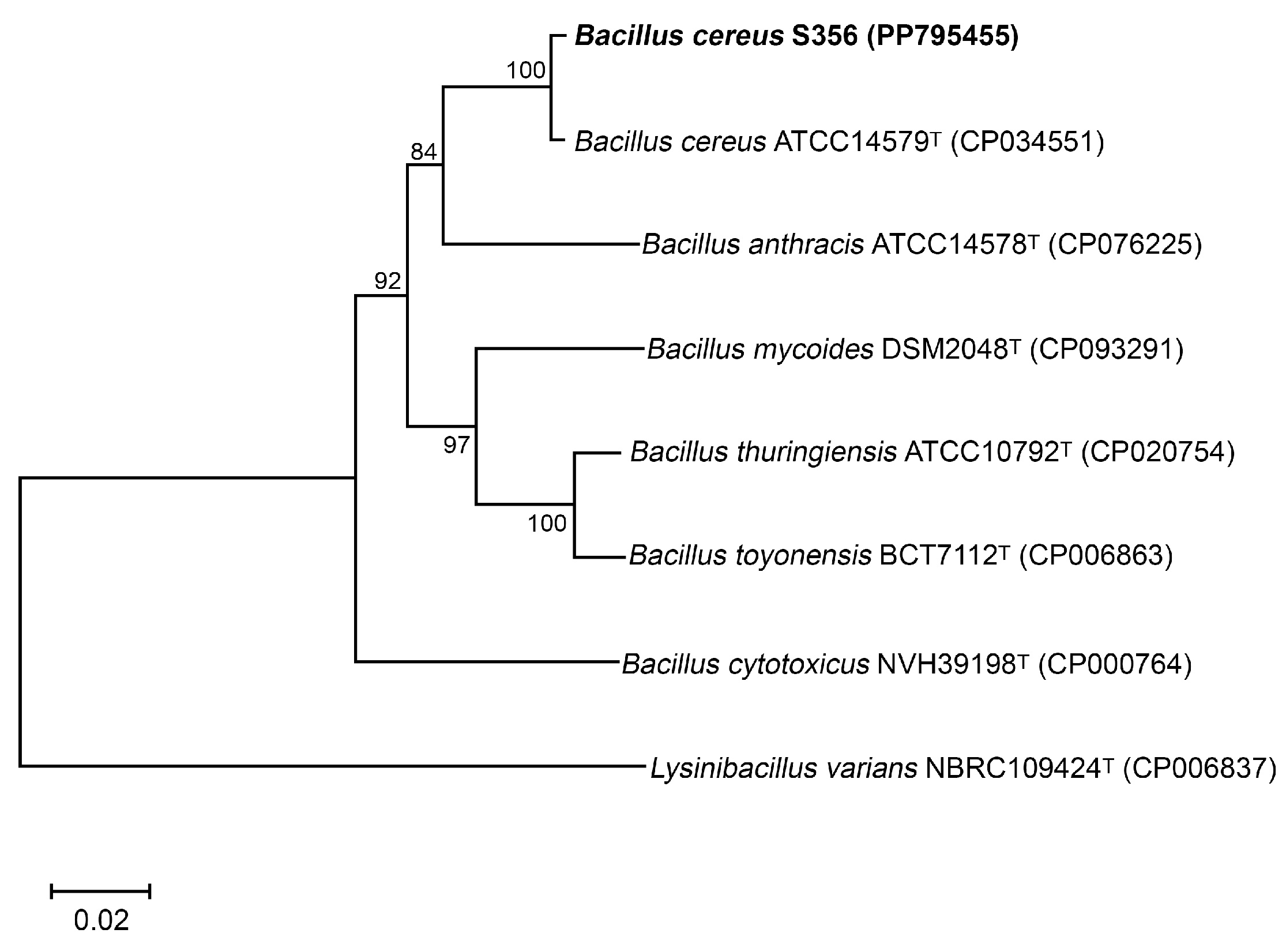
3.2. Identification of The Most Potent P(3HB)-Producing Isolate
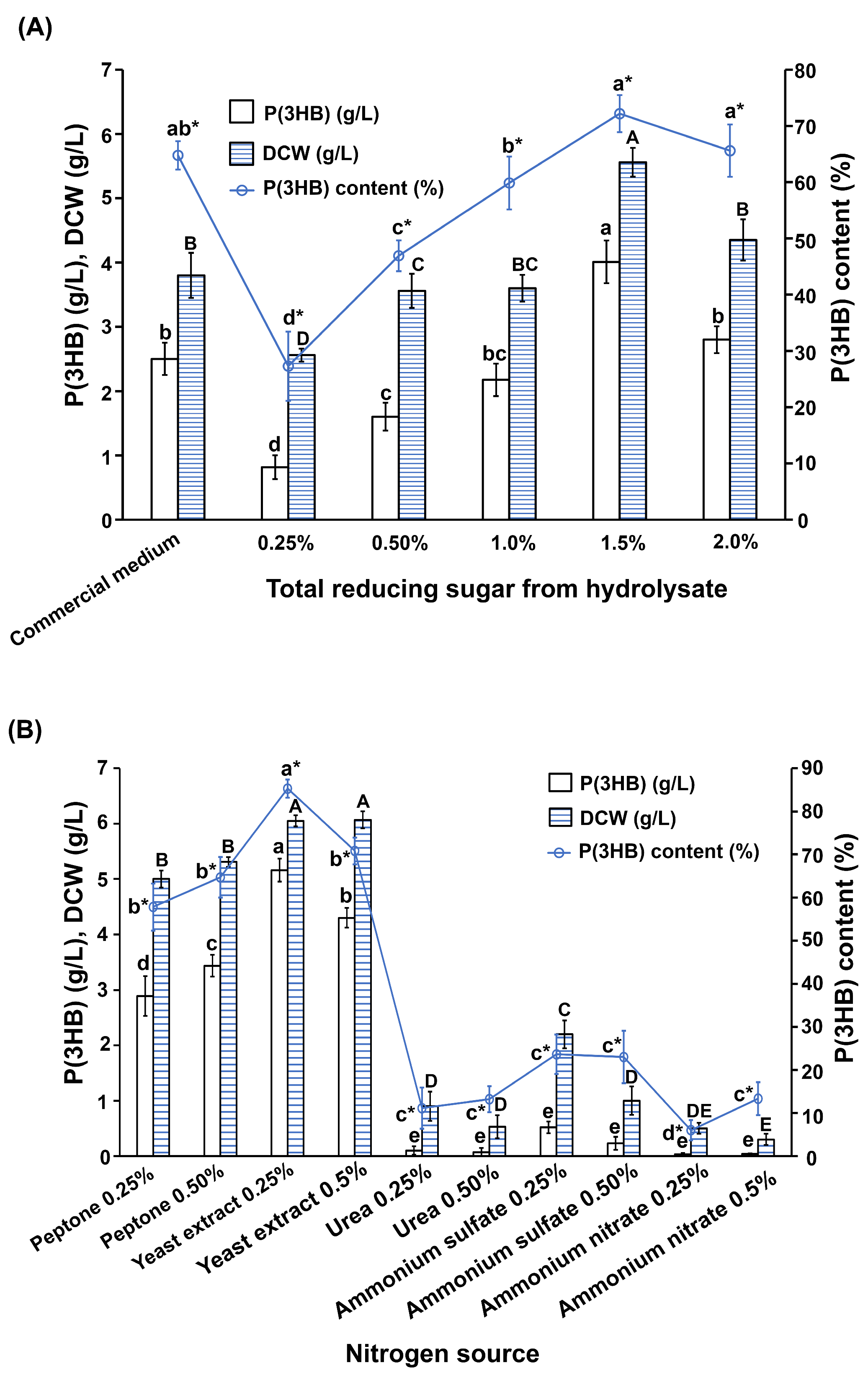
3.3. Optimization of Culture Medium for P(3HB) Production by the Most Potent B. cereus S356
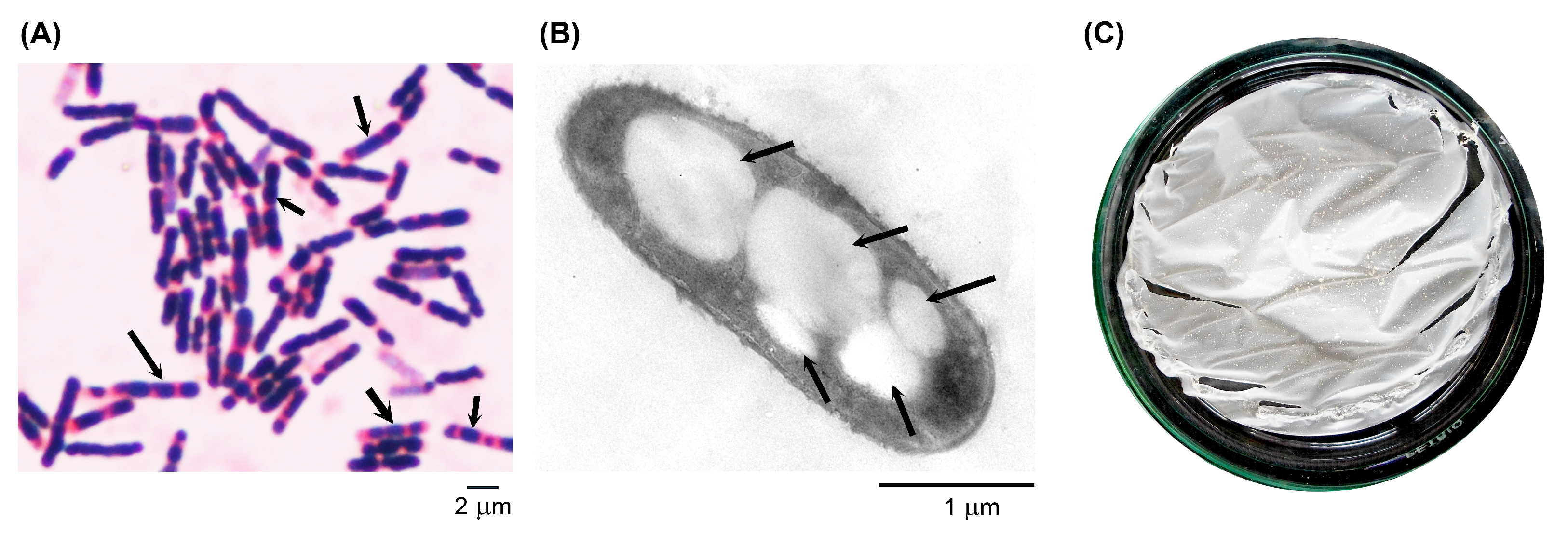
3.4. Characterization of The Most Potent Isolate and Its Extracted Polymer
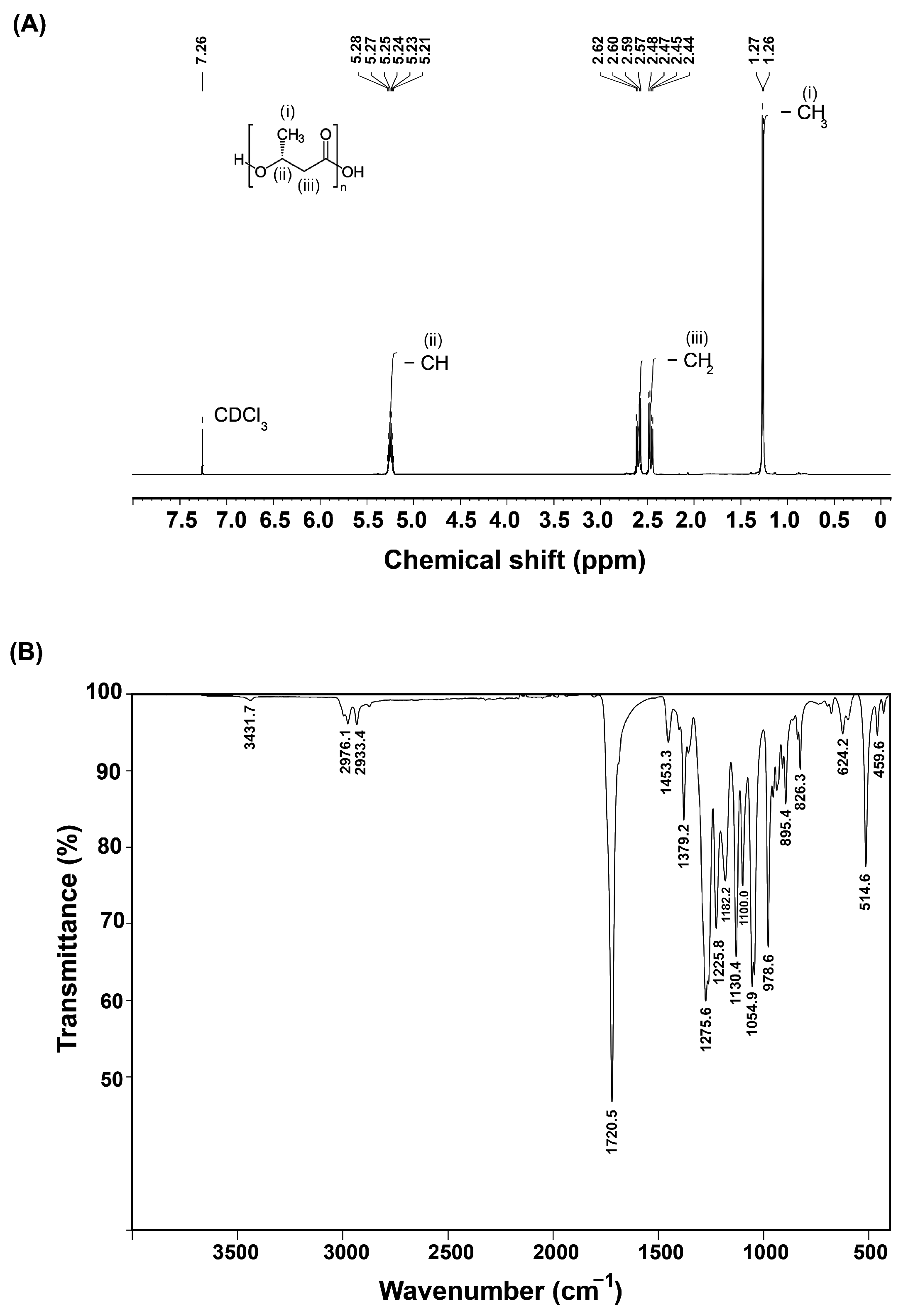
3.4.1. 1H NMR Analysis
3.4.2. FTIR Analysis
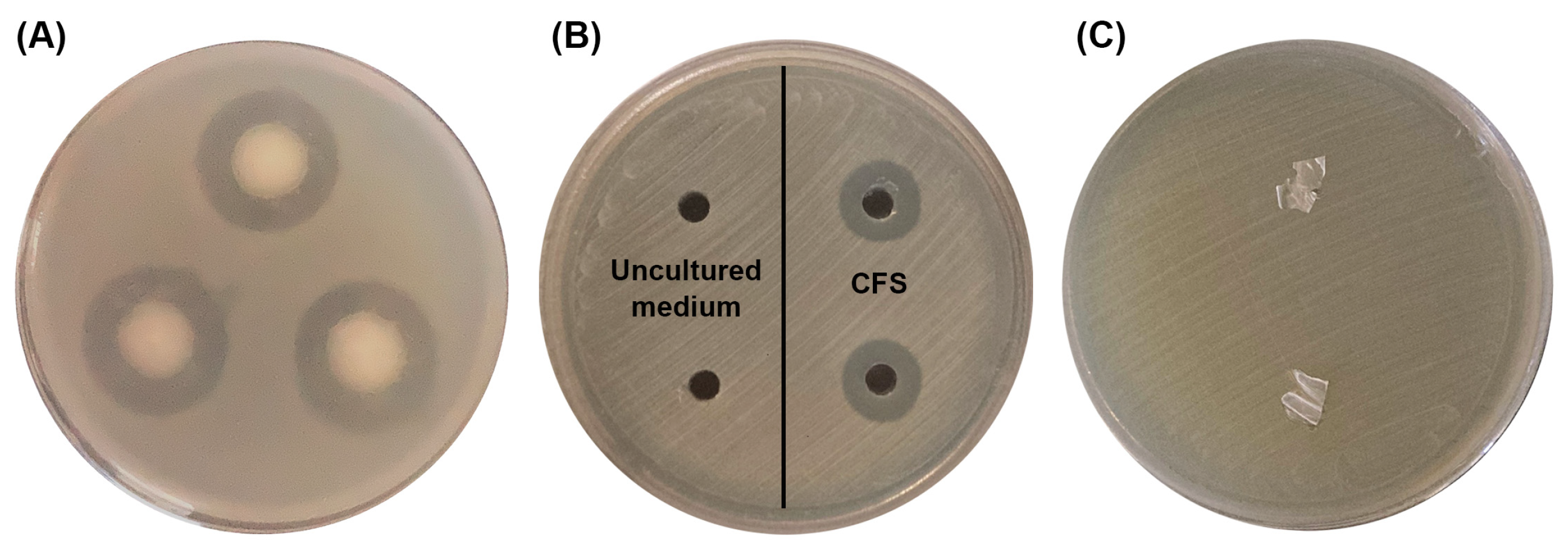
3.5. Antibacterial Activity Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe. Plastics—The Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 10 April 2024).
- Attapong, M.; Chatgasem, C.; Siripornadulsil, W.; Siripornadulsil, S. Ability of converting sugarcane bagasse hydrolysate into polyhydroxybutyrate (PHB) by bacteria isolated from stressed environmental soils. Biocatal. Agric. Biotechnol. 2023, 50, 102676. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Khatami, K.; Perez-Zabaleta, M.; Owusu-Agyeman, I.; Cetecioglu, Z. Waste to bioplastics: How close are we to sustainable polyhydroxyalkanoates production? Waste Manag. 2021, 119, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, D.; Yashchuk, O.; Noseda, D.G.; Araoz, B.; Hermida, É.B. Improved fermentation strategies in a bioreactor for enhancing poly(3-hydroxybutyrate) (PHB) production by wild type Cupriavidus necator from fructose. Heliyon 2021, 7, e05979. [Google Scholar] [CrossRef] [PubMed]
- Müller-Santos, M.; Koskimäki, J.J.; Alves, L.P.S.; de Souza, E.M.; Jendrossek, D.; Pirttilä, A.M. The protective role of PHB and its degradation products against stress situations in bacteria. FEMS Microbiol. Rev. 2021, 45, fuaa058. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Prakash Pandey, J.; Kumar Gaur, V.; Gnansounou, E.; Sindhu, R. Critical overview of biomass feedstocks as sustainable substrates for the production of polyhydroxybutyrate (PHB). Bioresour. Technol. 2020, 311, 123536. [Google Scholar] [CrossRef] [PubMed]
- Rathna, R.P.; Kulandhaivel, M. Sustainable synthesis of biopolymer polyhydroxybutyrate (PHB) from agro-residue by Brevibacterium casei with emphasis on degradation analysis. J. Pure Appl. Microbiol. 2024, 18, 347–366. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Liu, S. The production, recovery, and valorization of polyhydroxybutyrate (PHB) based on circular bioeconomy. Biotechnol. Adv. 2024, 72, 108340. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Ghodake, G.S.; Bharagava, R.N.; Kim, D.S.; Nair, S.; Shin, H.S. Efficient bioconversion of sugarcane bagasse into polyhydroxybutyrate (PHB) by Lysinibacillus sp. and its characterization. Bioresour. Technol. 2021, 324, 124673. [Google Scholar] [CrossRef]
- Hassan, S.; Ngo, T.; Ball, A.S. Valorisation of sugarcane bagasse for the sustainable production of polyhydroxyalkanoates. Sustainability 2024, 16, 2200. [Google Scholar] [CrossRef]
- FAO. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/?#data/QCL (accessed on 14 April 2024).
- Meghana, M.; Shastri, Y. Sustainable valorization of sugar industry waste: Status, opportunities, and challenges. Bioresour. Technol. 2020, 303, 122929. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Wang, R.; Yan, X.; Wang, J.; Wang, X.; Chen, S.; Bai, F.; He, Q.; Yang, S. Metabolic engineering of Zymomonas mobilis for continuous co-production of bioethanol and poly-3-hydroxybutyrate (PHB). Green Chem. 2022, 24, 2588–2601. [Google Scholar] [CrossRef]
- Cui, P.; Shao, Y.; Wang, Y.; Zhao, R.; Zhan, H.; Zhong, W. Co-production of polyhydroxybutyrate (PHB) and coenzyme Q10 (CoQ10) via no-sugar fermentation—A case by Methylobacterium sp. XJLW. Ann. Microbiol. 2021, 71, 20. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, Y.; Huang, W.; Zhang, L.; Wu, F.; Ye, J.; Chen, G.Q. Rational flux-tuning of Halomonas bluephagenesis for co-production of bioplastic PHB and ectoine. Nat. Commun. 2020, 11, 3313. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vidaurri, J.A.; Hernández-Rosas, F.; Ríos-Corripio, M.A.; Loeza-Corte, J.M.; Rojas-López, M.; Hernández-Martínez, R. Coproduction of polyhydroxyalkanoates and exopolysaccharide by submerged fermentation using autochthonous bacterial strains. Chem. Pap. 2022, 76, 2419–2429. [Google Scholar] [CrossRef]
- Song, H.-S.; Jeon, J.-M.; Bhatia, S.K.; Choi, T.-R.; Lee, S.M.; Park, S.L.; Lee, H.S.; Yoon, J.-J.; Ahn, J.; Lee, H.; et al. Enhanced isobutanol production by co-production of polyhydroxybutyrate and cofactor engineering. J. Biotechnol. 2020, 320, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Hacıosmanoğlu, G.G.; Eroğlu, M.S.; Kasavi, C.; Genç, S.; Can, Z.S.; Toksoy Oner, E. Halomonas smyrnensis as a cell factory for co-production of PHB and levan. Int. J. Biol. Macromol. 2018, 118, 1238–1246. [Google Scholar] [CrossRef]
- Kumar, V.; Darnal, S.; Kumar, S.; Kumar, S.; Singh, D. Bioprocess for co-production of polyhydroxybutyrate and violacein using Himalayan bacterium Iodobacter sp. PCH194. Bioresour. Technol. 2021, 319, 124235. [Google Scholar] [CrossRef]
- Sathya, P.B.; Soundrya, S.L.; Stalin, T.; Charles, J.B.; Lo, H.-M. Enhanced co-production of biopolymer polyhydroxybutyrate (PHB) and biopigment (carotenoid) through wild Bacillus chungangensis by utilizing agro-waste substrates. Int. J. Appl. Sci. Eng. 2024, 21, 1–13. [Google Scholar] [CrossRef]
- Toksinnya, B. Toxins of foodborne pathogen Bacillus cereus and the regulatory factors controlling the biosynthesis of its toxins. Sains Malays. 2021, 50, 1651–1662. [Google Scholar] [CrossRef]
- Martínez-Herrera, R.E.; Alemán-Huerta, M.E.; Rutiaga-Quiñones, O.M.; de Luna-Santillana, E.d.J.; Elufisan, T.O. A comprehensive view of Bacillus cereus as a polyhydroxyalkanoate (PHA) producer: A promising alternative to petroplastics. Process Biochem. 2023, 129, 281–292. [Google Scholar] [CrossRef]
- Morandini, L.; Caulier, S.; Bragard, C.; Mahillon, J. Bacillus cereus sensu lato antimicrobial arsenal: An overview. Microbiol. Res. 2024, 283, 127697. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Zheng, J.; Xu, Z.; Song, X.; Ruan, L.; Peng, D.; Sun, M. The Bacillus cereus group is an excellent reservoir of novel lanthipeptides. Appl. Environ. Microbiol. 2015, 81, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Thammasittirong, A.; Saechow, S.; Thammasittirong, S.N.R. Efficient polyhydroxybutyrate production from Bacillus thuringiensis using sugarcane juice substrate. Turk. J. Biol. 2017, 41, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Supmeeprom, S.; Thammasittirong, A.; Jeennor, S.; Sopalun, K.; Thammasittirong, S.N.-R. Valorisation of sawdust-based spent mushroom substrate for sustainable xylooligosaccharides production using low-cost crude xylanases from Aspergillus flavus KUB2. Mycology 2024. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Thammasittirong, S.N.-R.; Chatwachirawong, P.; Khemdee, K.; Thammasittirong, A. Evaluating the potential of newly developed energy cane clones for first- and second-generation ethanol production. Fermentation 2023, 9, 267. [Google Scholar] [CrossRef]
- Muneer, F.; Rasul, I.; Qasim, M.; Sajid, A.; Nadeem, H. Optimization, production and characterization of polyhydroxyalkanoate (PHA) from indigenously isolated novel bacteria. J. Environ. Polym. Degrad. 2022, 30, 3523–3533. [Google Scholar] [CrossRef]
- Kadriye İnan, B.; Can, K.; Belduz, A.O. Isolation and screening of polyhydroxybutyrate (PHB) producing bacteria from soils. Biol. Bull. 2023, 50, 319–328. [Google Scholar] [CrossRef]
- Yamamoto, S.; Harayama, S. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl. Environ. Microbiol. 1995, 61, 1104–1109. [Google Scholar] [CrossRef]
- Ketsakhon, P.; Thammasittirong, A.; Thammasittirong, S.N.-R. Adding value to rice straw waste for high-level xylanase production using a new isolate of Bacillus altitudinis RS3025. Folia Microbiol. 2023, 68, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Bonhi, K.L.R.; Imran, S. A comparative profile of bactericidal action of a partially purified bacteriocin from lactic acid bacteria with antibiotics. Malays. J. Microbiol. 2021, 17, 143–154. [Google Scholar] [CrossRef]
- Madhumathi, R.; Muthukumar, K.; Velan, M. Optimization of polyhydroxybutyrate production by Bacillus safensis EBT1. Clean 2016, 44, 1066–1074. [Google Scholar] [CrossRef]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes 2016, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Gowda, V.; Shivakumar, S. Agrowaste-based polyhydroxyalkanoate (PHA) production using hydrolytic potential of Bacillus thuringiensis IAM 12077. Braz. Arch. Biol. Technol. 2014, 57, 55–61. [Google Scholar] [CrossRef]
- Trakunjae, C.; Boondaeng, A.; Apiwatanapiwat, W.; Kosugi, A.; Arai, T.; Sudesh, K.; Vaithanomsat, P. Enhanced polyhydroxybutyrate (PHB) production by newly isolated rare actinomycetes Rhodococcus sp. strain BSRT1-1 using response surface methodology. Sci. Rep. 2021, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Alokika; Anu; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges and future perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, H.; Fan, F.; Zhang, J.; Liu, H.; Cao, J. Survival of community-acquired Bacillus cereus sepsis with venous sinus thrombosis in an immunocompetent adult man—A case report and literature review. BMC Infect. Dis. 2023, 23, 213. [Google Scholar] [CrossRef]
- Martínez-Herrera, R.E.; Alemán-Huerta, M.E.; Flores-Rodríguez, P.; Almaguer-Cantú, V.; Valencia-Vázquez, R.; Rosas-Flores, W.; Medrano-Roldán, H.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M. Utilization of Agave durangensis leaves by Bacillus cereus 4N for polyhydroxybutyrate (PHB) biosynthesis. Int. J. Biol. Macromol. 2021, 175, 199–208. [Google Scholar] [CrossRef]
- Yustinah; Hidayat, N.; Alamsyah, R.; Roslan, A.M.; Hermansyah, H.; Gozan, M. Production of polyhydroxybutyrate from oil palm empty fruit bunch (OPEFB) hydrolysates by Bacillus cereus suaeda B-001. Biocatal. Agric. Biotechnol. 2019, 18, 101019. [Google Scholar] [CrossRef]
- Andler, R.; Pino, V.; Moya, F.; Soto, E.; Valdés, C.; Andreeßen, C. Synthesis of poly-3-hydroxybutyrate (PHB) by Bacillus cereus using grape residues as sole carbon source. Int. J. Biobased Plast. 2021, 3, 98–111. [Google Scholar] [CrossRef]
- Martínez-Herrera, R.E.; Alemán-Huerta, M.E.; Almaguer-Cantú, V.; Rosas-Flores, W.; Martínez-Gómez, V.J.; Quintero-Zapata, I.; Rivera, G.; Rutiaga-Quiñones, O.M. Efficient recovery of thermostable polyhydroxybutyrate (PHB) by a rapid and solvent-free extraction protocol assisted by ultrasound. Int. J. Biol. Macromol. 2020, 164, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Drusilla Wendy, Y.B.; Nor Fauziah, M.Z.; Siti Baidurah, Y.; Tong, W.Y.; Lee, C.K. Production and characterization of polyhydroxybutyrate (PHB) by Burkholderia cepacia BPT1213 using waste glycerol as carbon source. Biocatal. Agric. Biotechnol. 2022, 41, 102310. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Chaabouni, I.; Ouertani, A.; Koubaa, N.; Sassi, I.; Barkallah, I.; Saidi, M.; Cherif, A. Ecological role of widespread bacteriocin production among Bacillus cereus contaminating dairy products and characterization of three cereins. Microbiology 2023, 92, 398–407. [Google Scholar] [CrossRef]
| Bacterial Strain | Pretreatment/Hydrolysis of SCB | Cultivation Conditions | SCB Hydrolysate in Medium | P(3HB) (g/L) | Dry Cell Weight (g/L) | P(3HB) Accumulation (% of DCW) | Reference |
|---|---|---|---|---|---|---|---|
| Bacillus cereus | 2% NaOH, 80 °C for 3 h/enzymatic hydrolysis | 37 °C, 48 h | 1.5% (TRS) | 5.16 | 6.05 | 85.3 | This study |
| Bacillus safensis EBT1 | NR | 45 °C, 48 h | 1% (w/v) | 5.92 | 6.56 | 90.2 | [36] |
| Bacillus sp. | Zinc chloride/ acid hydrolysis, 70–180 °C | 37 °C, 48 h | 4% (v/v) | 5.00 | 9.00 | 55.5 | [37] |
| Bacillus thuringiensis IAM 12077 | 0.5–5.0% H2SO4, autoclave 121 °C, 30 min | room temperature for 48 h | 10% (v/v) | 4.20 | 10.60 | 39.6 | [38] |
| Burkholderia cepacia ASL22 | 1% H2SO4, autoclave at 121 °C, 15 min | 30 °C, 72 h | 2.5% (TRS) | 1.03 | 10 | 10.3 | [2] |
| Lysinibacillus sp. | 2% peracetic acid solution, autoclave 121 °C, 15 min/enzymatic hydrolysis | 37 °C 48 h | 2% (TRS) | 3.05 | 6.05 | 50.5 | [10] |
| Lysinibacillus sp. | Ultrasound, 2% NaOH, autoclave 121 °C, 15 min/enzymatic hydrolysis | 37 °C 48 h | 2% (TRS) | 4.32 | 7.81 | 55.4 | [10] |
| Lysinibacillus sp. | 2%NaOH, autoclave 121 °C, 15 min/enzymatic hydrolysis | 37 °C 48 h | 2% (TRS) | 3.54 | 6.88 | 51.5 | [10] |
| Priestia megaterium KKR5 | 1% H2SO4, autoclave at 121 °C, 15 min | 30 °C, 72 h | 2.5% (TRS) | 1.36 | 11.62 | 11.7 | [2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamberk, S.; Thammasittirong, S.N.-R.; Thammasittirong, A. Valorization of Sugarcane Bagasse for Co-Production of Poly(3-hydroxybutyrate) and Bacteriocin Using Bacillus cereus Strain S356. Polymers 2024, 16, 2015. https://doi.org/10.3390/polym16142015
Khamberk S, Thammasittirong SN-R, Thammasittirong A. Valorization of Sugarcane Bagasse for Co-Production of Poly(3-hydroxybutyrate) and Bacteriocin Using Bacillus cereus Strain S356. Polymers. 2024; 16(14):2015. https://doi.org/10.3390/polym16142015
Chicago/Turabian StyleKhamberk, Sunisa, Sutticha Na-Ranong Thammasittirong, and Anon Thammasittirong. 2024. "Valorization of Sugarcane Bagasse for Co-Production of Poly(3-hydroxybutyrate) and Bacteriocin Using Bacillus cereus Strain S356" Polymers 16, no. 14: 2015. https://doi.org/10.3390/polym16142015







