Recent Advances in Poly(vinyl alcohol)-Based Hydrogels
Abstract
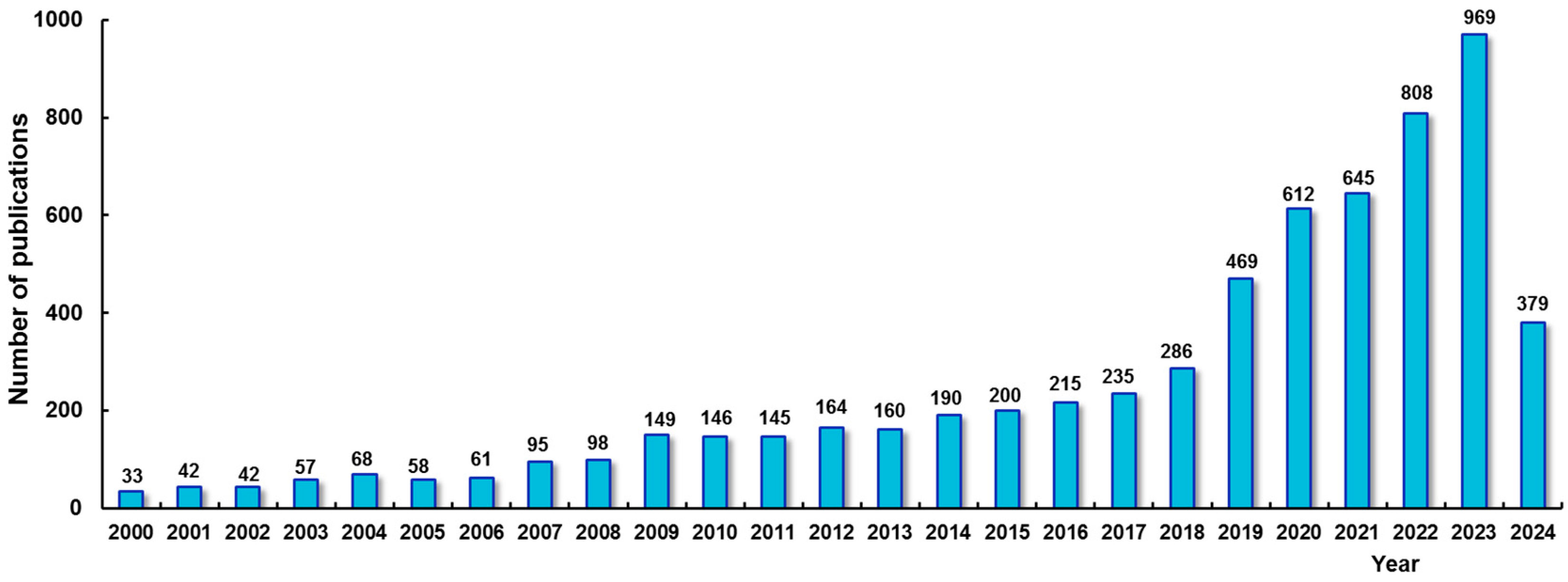
:1. Introduction
2. Structure and Properties of PVA
3. Preparation Methods of PVA Hydrogels
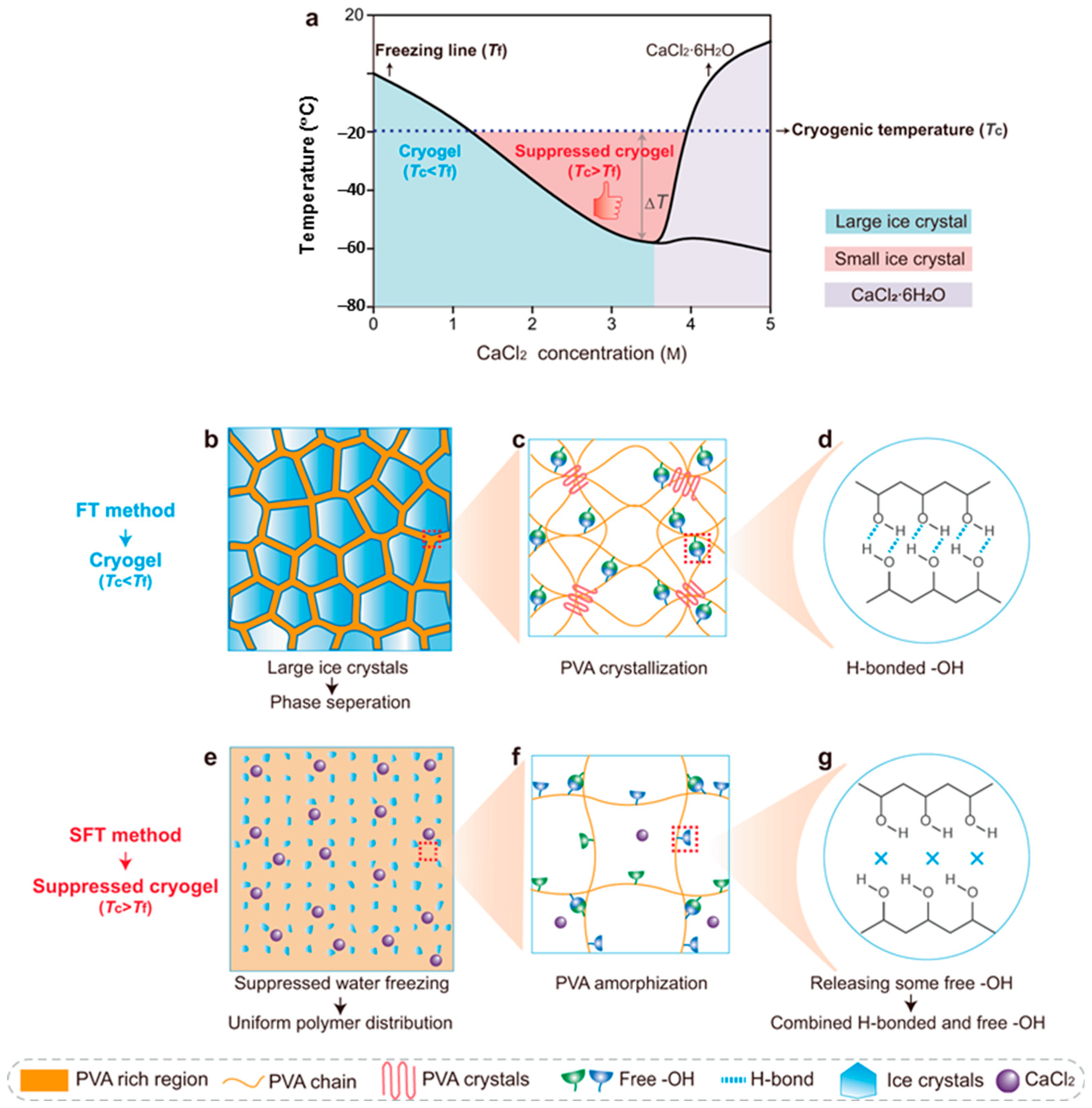
3.1. PVA Hydrogels Obtained by Repeated Freezing/Thawing Cycles Applied to Aqueous Solutions
3.2. Non-Cryogenic Physical Gelation
3.3. Chemical Crosslinking of PVA
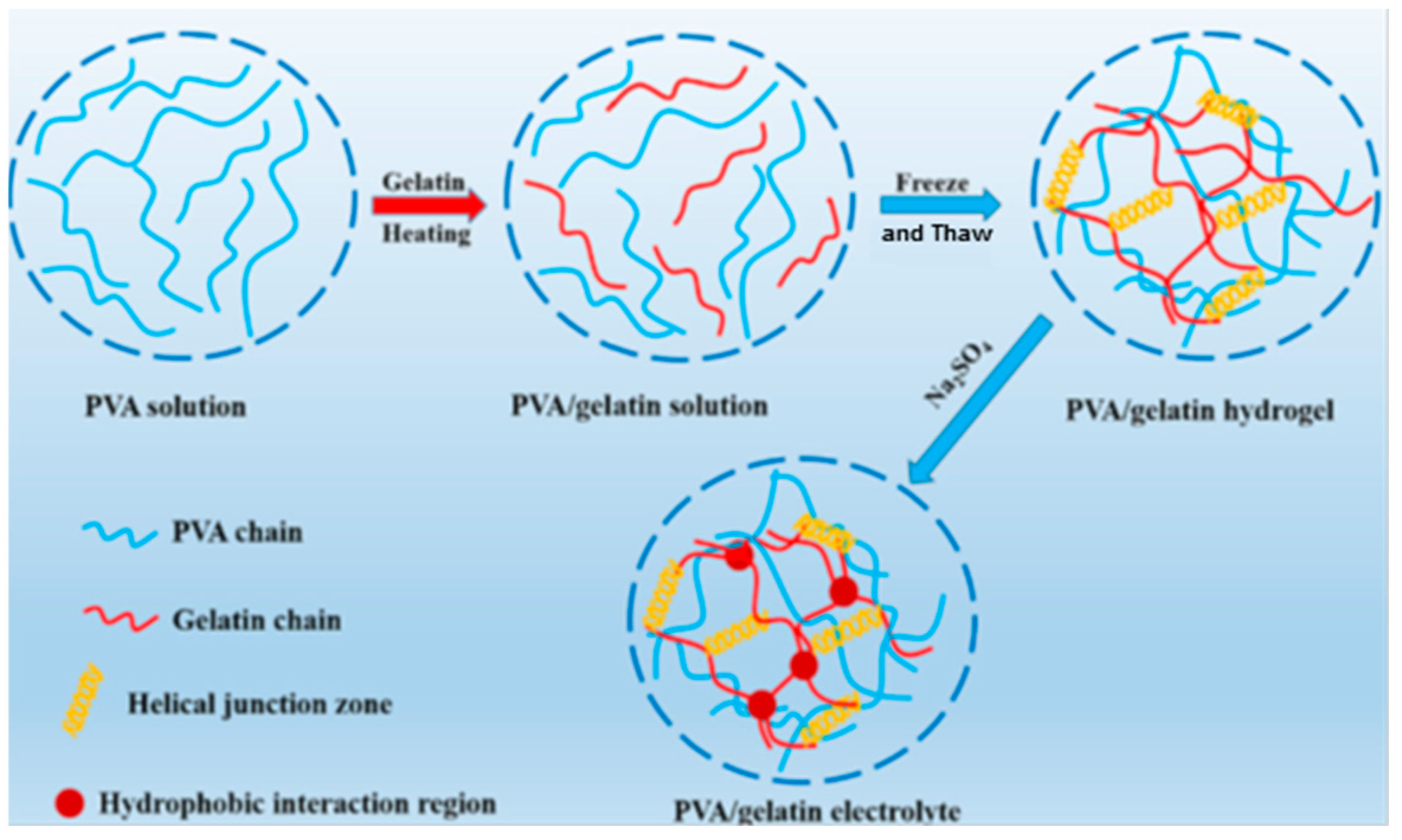
3.4. PVA Hydrogels Obtained by Combined Methods
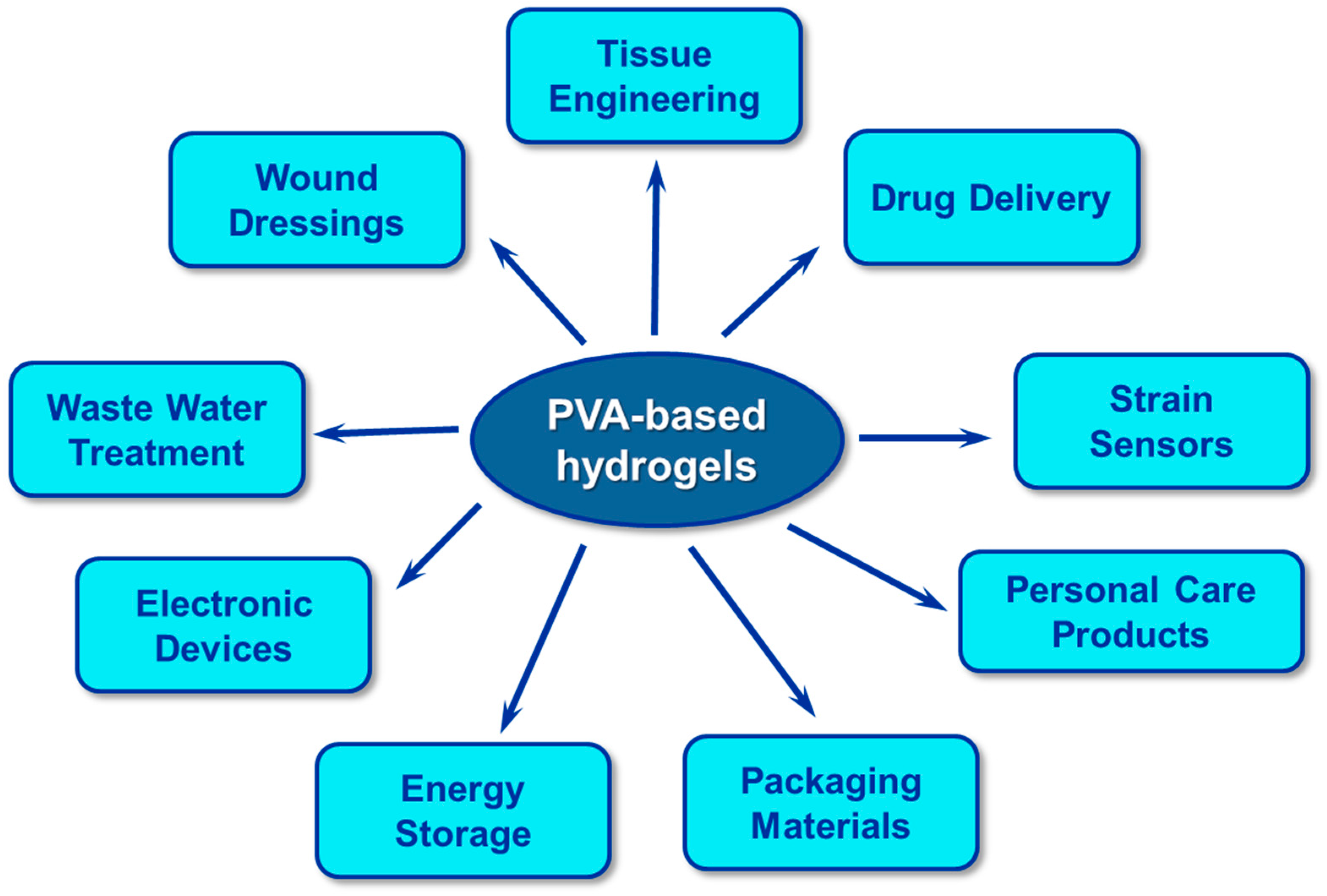
4. Applications of PVA-Based Hydrogels
4.1. Wound Dressings
4.2. High-Performance PVA-Based Hydrogels for Tissue Engineering
4.3. Sensors
4.4. Other New and Promising Applications of PVA-Based Hydrogels
Funding
Conflicts of Interest
Abbreviations
| CNF | cellulose nanofibril |
| DH | degree of hydrolysis |
| DN | double network |
| ESR | equilibrium swelling ratio |
| FT | freezing/thawing |
| GA | glutaraldehyde |
| GF | gauge factor |
| GG | gellan gum |
| HGG | high-acyl gellan gum |
| LGG | low-acyl gellan gum |
| GO | graphene oxide |
| GMA | glycidyl methacrylate |
| H-bond | hydrogen bonding |
| MC | microcrystalline cellulose |
| n | polymerization degree |
| PA | phytic acid |
| PVA | poly(vinyl alcohol) |
| PAm | polyacrylamide |
| PANI | polyaniline |
| PDA | polydopamine |
| SA | sodium alginate |
| SFT | suppressed freezing/thawing |
| TA | tannic acid |
| TEOS | tetraethyl orthosilicate |
| ZIF-8 | zeolitic imidazolate framework |
References
- Web of Science. Available online: http://webofknowledge.com (accessed on 15 May 2024).
- Zhang, Y.; Wang, Y.; Bao, Y.; Lin, B.; Cheng, G.; Yuan, N.; Ding, J. Multifunctional PVA/gelatin DN hydrogels with strong mechanical properties enhanced by Hofmeister effect. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133833. [Google Scholar] [CrossRef]
- Kolosova, O.Y.; Vasil’ev, V.G.; Novikov, I.A.; Sorokina, E.V.; Lozinsky, V.I. Cryostructuring of polymeric systems: 67. Properties and microstructure of poly(vinyl alcohol) cryogels formed in the presence of phenol or bis-phenols introduced into the aqueous polymeric solutions prior to their freeze–thaw processing. Polymers 2024, 16, 675. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, O.Y.; Shaikhaliev, A.I.; Krasnov, M.S.; Bondar, I.M.; Sidorskii, E.V.; Sorokina, E.V.; Lozinsky, V.I. Cryostructuring of polymeric systems: 64. Preparation and properties of poly(vinyl alcohol)-based cryogels loaded with antimicrobial drugs and assessment of the potential of such gel materials to perform as gel implants for the treatment of infected wounds. Gels 2023, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Sun, F.; Li, Y.; Li, L.; Liu, M.; Wang, S.; Wang, Y.; Li, T.; Liu, L.; Feng, S.; et al. Biological tissue-inspired ultrasoft, ultrathin, and mechanically enhanced microfiber composite hydrogel for flexible bioelectronics. Nano-Micro Lett. 2023, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, H.; Xu, C.; Dong, X.; Wang, Y.; Fu, A.; Li, H.; Lee, J.Y.; Zhang, S.; Ni, J.; et al. Skin-like cryogel electronics from suppressed-freezing tuned polymer amorphization. Nat. Commun. 2023, 14, 5010. [Google Scholar] [CrossRef] [PubMed]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgan, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Feldman, D. Poly(vinyl alcohol) nanocomposites: Recent contributions to engineering and medicine. AIMS Mater. Sci. 2021, 8, 119–129. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of poly(vinyl alcohol) and natural polymers. Polym. Rev. 2018, 58, 247–287. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Aruldass, S.; Mathivanan, V.; Mohamed, A.R.; Tye, C.T. Factors affecting hydrolysis of polyvinyl acetate to polyvinyl alcohol. J. Environ. Chem. Eng. 2019, 7, 103238. [Google Scholar] [CrossRef]
- Suleiman, G.S.A.; Zeng, X.; Chakma, R.; Wakai, I.Y.; Feng, Y. Recent advances and challenges in thermal stability of PVA-based film: A review. Polym. Adv. Technol. 2024, 35, e6327. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Y.; Xu, Z.; Xiao, X.; Li, P.; Wang, X.; Guo, H. Solubilization of fully hydrolyzed poly(vinyl alcohol) at room temperature for fabricating recyclable hydrogels. ACS Macro Lett. 2023, 12, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Han, Q. Molecular dynamics study of the influence of solvents on the structure and mechanical properties of poly(vinyl alcohol) gels. J. Mol. Model. 2018, 24, 325. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.D.; Huang, H.T. Effect of co-solvent complex on preferential adsorption phenomenon in polyvinyl alcohol ternary solutions. Polymer 2000, 41, 6195–6204. [Google Scholar] [CrossRef]
- Chen, S.; Yang, H.; Huang, K.; Ge, X.; Yao, H.; Tang, J.; Ren, J.; Ren, S.; Ma, Y. Quantitative study on solubility parameters and related thermodynamic parameters of PVA with different alcoholysis degrees. Polymers 2021, 13, 3778. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M.; Morariu, S.; Rusu, D. In-situ gelation of aqueous solutions of entangled poly(vinyl alcohol). Soft Matter 2013, 9, 1244–1253. [Google Scholar] [CrossRef]
- Bercea, M.; Ioan, C.; Ioan, S.; Simionescu, B.C.; Simionescu, C.I. Ultrahigh molecular weight polymers in dilute solutions. Progr. Polym. Sci. 1999, 24, 379–424. [Google Scholar] [CrossRef]
- Ricciardi, R.; Mangiapia, G.; Lo Celso, F.; Paduano, L.; Triolo, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. Structural organization of poly(vinyl alcohol) hydrogels obtained by freezing and thawing techniques: A SANS study. Chem. Mater. 2005, 17, 1183. [Google Scholar] [CrossRef]
- Chiellini, E.; Cinelli, P.; Ilieva, V.I.; Martera, M. Biodegradable thermoplastic composites based on polyvinyl alcohol and algae. Biomacromolecules 2008, 9, 1007–1013. [Google Scholar] [CrossRef]
- Bercea, M.; Gradinaru, L.M.; Barbalata-Mandru, M.; Vlad, S.; Nita, L.E.; Plugariu, I.A.; Albulescu, R. Shear flow of associative polymers in aqueous solutions. J. Mol. Struct. 2021, 1238, 130441. [Google Scholar] [CrossRef]
- Stephans, L.E.; Foster, N. Magnetization-transfer NMR analysis of aqueous poly(vinyl alcohol) gels: Effect of hydrolysis and storage temperature on network formation. Macromolecules 1998, 31, 1644–1651. [Google Scholar] [CrossRef]
- Sau, S.; Pandit, S.; Kundu, S. Crosslinked poly (vinyl alcohol): Structural, optical and mechanical properties. Surf. Interfaces 2021, 25, 101198. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Liu, L.; Song, P.; Cao, Q.; Xu, Z.; Fang, Z.; Wang, H. Water governs the mechanical properties of poly(vinyl alcohol). Polymer 2021, 213, 123330. [Google Scholar] [CrossRef]
- Jain, N.; Singh, V.K.; Chauhan, S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mater. 2018, 26, 213–222. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Abdeen, Z. Preparation and characterization of hydrogel based on poly(vinyl alcohol) crosslinked by different crosslinkers used to dry organic solvents. J. Polym. Environ. 2010, 18, 576–583. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Mamtsis, A.M.; Titova, E.F.; Belavtseva, E.M.; Rogozhin, S.V. Study of cryostructurization of polymer systems. VII. Structure formation under freezing of poly(vinyl alcohol) aqueous solutions. Colloid Polym. Sci. 1986, 264, 19–24. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Kolosova, O.Y.; Michurov, D.A.; Dubovik, A.S.; Vasil’ev, V.G.; Grinberg, V. Cryostructuring of polymeric systems. 49. Unexpected “kosmotropic-like” impact of organic chaotropes on freeze–thaw-induced gelation of PVA in DMSO. Gels 2018, 4, 81. [Google Scholar] [CrossRef]
- Bercea, M.; Gradinaru, L.M.; Mandru, M.; Tigau, D.L.; Ciobanu, C. Intermolecular interactions and self-assembling of polyurethane with poly(vinyl alcohol) in aqueous solutions. J. Mol. Liq. 2019, 274, 562–567. [Google Scholar] [CrossRef]
- Plugariu, I.A.; Bercea, M. The viscosity of globular proteins in the presence of an “inert” macromolecular cosolute. J. Mol. Liq. 2021, 337, 116382. [Google Scholar] [CrossRef]
- Plugariu, I.A.; Bercea, M.; Gradinaru, L.M.; Rusu, D.; Lupu, A. Poly(vinyl alcohol)/pullulan composite hydrogels for wound dressing applications. Gels 2023, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.F.; Song, Y.F.; Chen, J.W.; Yang, S.; Gong, L.H.; Shi, D.J.; Dong, W.F.; Zhang, H.J. Strong and tough conductive PVA hydrogels based on the synergistic effect of acetic acid induction and salting-out for flexible solid-state supercapacitors. J. Mater. Chem. C 2023, 11, 15934–15944. [Google Scholar] [CrossRef]
- Karyappa, R.; Nagaraju, N.; Yamagishi, K.; Koh, X.Q.; Zhu, Q.; Hashimoto, M. 3D printing of polyvinyl alcohol hydrogels enabled by aqueous two-phase system. Mater. Horiz. 2024, 11, 2701–2717. [Google Scholar] [CrossRef]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; David, G.; Serbezeanu, D.; Rîmbu, C.M.; Daraba, O.; Enache, A.A.; Bercea, M. Phosphorylated curdlan/poly(vinyl alcohol) electrospun nanofibers loaded with clove oil with antibacterial activity. Gels 2022, 8, 439. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, E.; Suzuki, A. A simple method to obtain a swollen PVA gel crosslinked by hydrogen bonds. J. Appl. Polym. Sci. 2009, 114, 10–16. [Google Scholar] [CrossRef]
- Chen, W.; Li, N.; Ma, Y.; Minus, M.L.; Benson, K.; Lu, X.; Wang, X.; Ling, X.; Zhu, H. Superstrong and tough hydrogel through physical cross-linking and molecular alignment. Biomacromolecules 2019, 20, 4476–4484. [Google Scholar] [CrossRef]
- Hojo, N.; Shirai, H.; Hayashi, S. Complex formation between poly(vinyl alcohol) and metallic ions in aqueous solution. J. Polym. Sci. Polym. Symp. 2007, 47, 299–307. [Google Scholar] [CrossRef]
- Ohgi, H.; Sato, T.; Watanabe, H.; Horii, F. Highly isotactic poly(vinyl alcohol) derived from tert-butyl vinyl ether V. Viscoelastic behavior of highly isotactic poly(vinyl alcohol) films. Polym. J. 2006, 38, 1055–1060. [Google Scholar] [CrossRef]
- Moritani, T.; Kuruma, I.; Shibatani, K.; Fujiwara, Y. Tacticity of poly(vinyl alcohol) studied by Nuclear Magnetic Resonance of hydroxyl protons. Macromolecules 1972, 5, 577–580. [Google Scholar] [CrossRef]
- Choi, J.H.; Ko, S.-W.; Kim, B.C.; Blackwell, J.; Lyoo, W.S. Phase behavior and physical gelation of high molecular weight syndiotactic poly(vinyl alcohol) solution. Macromolecules 2001, 34, 2964–2972. [Google Scholar] [CrossRef]
- Choi, J.H.; Lyoo, W.S.; Ghim, H.D.; Ko, S.-W. High molecular weight syndiotacticity-rich poly(vinyl alcohol) gel in aging process. Colloid Polym. Sci. 2000, 278, 1198–1204. [Google Scholar] [CrossRef]
- Fukae, R.; Yoshimura, M.; Yamamoto, T.; Nishinari, K. Effect of stereoregularity and molecular weight on the mechanical properties of poly(vinyl alcohol) hydrogel. J. Appl. Polym. Sci. 2011, 120, 573–578. [Google Scholar] [CrossRef]
- Peppas, N.A. Turbidimetric studies of aqueous poly (vinyl alcohol) solutions. Makromol. Chem. (Macromol. Chem. Phys.) 1975, 176, 3433–3440. [Google Scholar] [CrossRef]
- Trieu, H.; Qutubuddin, S. Poly(vinyl alcohol) hydrogels. 2. Effects of processing parameters on structure and properties. Polymer 1995, 36, 2531–2539. [Google Scholar] [CrossRef]
- Viana, J.L.; França, T.; Cena, C. Solution blow spinning poly(vinyl alcohol) sub-microfibers produced from different solvents. Orbital Electron. J. Chem. 2020, 12, 1–6. [Google Scholar] [CrossRef]
- Lee, H.W.; Karim, M.R.; Park, J.H.; Bae, D.G.; Oh, W.; Cheong, I.W.; Yeum, J.H. Electrospinning and characterisation of poly(vinyl alcohol) blend submicron fibres in aqueous solutions. Polym. Polym. Compos. 2009, 17, 47–54. [Google Scholar] [CrossRef]
- Sapalidis, A.A. Porous polyvinyl alcohol membranes: Preparation methods and applications. Symmetry 2020, 12, 960. [Google Scholar] [CrossRef]
- Corti, A.; Solaro, R.; Chiellini, E. Biodegradation of poly(vinyl alcohol) in selected mixed microbial culture and relevant culture filtrate. Polym. Degrad. Stab. 2002, 75, 447–458. [Google Scholar] [CrossRef]
- Halima, N.B. Poly(vinyl alcohol): Review of its promising applications and insights into biodegradation. RSC Adv. 2016, 6, 39823–39832. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- von Haugwitz, G.; Donnelly, K.; Di Filippo, M.; Breite, D.; Phippard, M.; Schulze, A.; Wei, R.; Baumann, M.; Bornscheuer, U.T. Synthesis of modified poly(vinyl alcohol)s and their degradation using an enzymatic cascade. Angew. Chem. Int. Ed. 2023, 62, e202216962. [Google Scholar] [CrossRef] [PubMed]
- Abbondandolo, A.G.; Brewer, E.C. Assessing the degradation profiles of a thermoresponsive polyvinyl alcohol (PVA)-based hydrogel for biomedical applications. Polym. Adv. Technol. 2024, 35, e6305. [Google Scholar] [CrossRef]
- Bercea, M.; Plugariu, I.-A.; Dinu, M.V.; Pelin, I.M.; Lupu, A.; Bele, A.; Gradinaru, V.R. Poly(vinyl alcohol)/bovine serum albumin hybrid hydrogels with tunable mechanical properties. Polymers 2023, 15, 4611. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M.; Plugariu, I.A.; Gradinaru, L.M.; Avadanei, M.; Doroftei, F.; Gradinaru, V.R. Hybrid hydrogels for neomycin delivery: Synergistic effects of natural/synthetic polymers and proteins. Polymers 2023, 15, 630. [Google Scholar] [CrossRef] [PubMed]
- Asadpour, S.; Raeisi vanani, A.; Kooravand, M.; Asfaram, A. A review on zinc oxide/poly(vinyl alcohol) nanocomposites: Synthesis, characterization and applications. J. Clean. Prod. 2022, 362, 132297. [Google Scholar] [CrossRef]
- Bercea, M. Self-Healing behavior of polymer/protein hybrid hydrogels. Polymers 2022, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Morariu, S.; Bercea, M.; Gradinaru, L.M.; Roşca, I.; Avadanei, M. Versatile poly(vinyl alcohol)/clay physical hydrogels with tailorable structure as potential candidates for wound healing applications. Mater. Sci. Eng. C 2020, 109, 110395. [Google Scholar] [CrossRef]
- Abdollahi, M.; Andablib, S.; Ghorbani, R.; Afshar, D.; Gholinejad, M.; Abdollahi, H.; Akbari, A.; Nikfarjam, N. Polydopamine contained hydrogel nanocomposites with combined antimicrobial and antioxidant properties for accelerated wound healing. Int. J. Biol. Macromol. 2024, 40, 131700. [Google Scholar] [CrossRef]
- Mandru, M.; Bercea, M.; Gradinaru, L.M.; Ciobanu, C.; Drobota, M.; Vlad, S.; Albulescu, R. Preparation and characterization of polyurethane/poly(vinyl alcohol) hydrogels for drug delivery. Eur. Polym. J. 2019, 118, 137–145. [Google Scholar] [CrossRef]
- Cao, J.; Wu, B.; Yuan, P.; Liu, Y.; Hu, C. Progress of research on conductive hydrogels in flexible wearable sensors. Gels 2024, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.F.; Smith, A.A.A.; Zelikin, A.N. Microstructured, Functional PVA hydrogels through bioconjugation with oligopeptides under physiological conditions. Small 2013, 9, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.-H.; Jensen, B.E.B.; Smith, A.A.A.; Zelikin, A.N. Poly(vinyl alcohol) physical hydrogels: New vista on a long serving biomaterial. Macromol. Biosci. 2011, 11, 1293–1313. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Sonego, J.M.; Flórez-Castillo, J.M.; Jobbágy, M. Highly structured polyvinyl alcohol porous carriers: Tuning inherent stability and release kinetics in water. ACS Omega 2018, 3, 2390–2395. [Google Scholar] [CrossRef] [PubMed]
- Elgharbawy, A.S.; El Demerdash, A.-G.M.; Sadik, W.A.; Kasaby, M.A.; Lotfy, A.H.; Osman, A.I. Synthetic degradable polyvinyl alcohol polymer and its blends with starch and cellulose—A comprehensive overview. Polymers 2024, 16, 1356. [Google Scholar] [CrossRef] [PubMed]
- Razmgar, K.; Nasiraee, M. Polyvinyl alcohol -based membranes for filtration of aqueous solutions: A comprehensive review. Polym. Eng. Sci. 2022, 62, 25–43. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Triolo, R. Slow crystallization kinetics of poly(vinyl alcohol) in confined environment during cryotropic gelation of aqueous solutions. Macromolecules 2006, 39, 9429. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Romero-Montero, A.; Hernández-Parra, H.; Peña-Corona, S.I.; Del Prado-Audelo, M.L.; Alcalá-Alcalá, S.; Cortés, H.; Kiyekbayeva, L.; Sharifi-Rad, J.; Leyva-Gómez, G. Enhancing chemical and physical stability of pharmaceuticals using freeze-thaw method: Challenges and opportunities for process optimization through quality by design approach. J. Biol. Eng. 2023, 17, 35. [Google Scholar] [CrossRef]
- Morariu, S.; Bercea, M.; Brunchi, C.E. Effect of cryogenic treatment on the rheological properties of chitosan/poly(vinyl alcohol) hydrogels. Ind. Eng. Chem. Res. 2015, 54, 11475–11482. [Google Scholar] [CrossRef]
- Teodorescu, M.; Morariu, S.; Bercea, M.; Sacarescu, L. Viscoelastic and structural properties of poly(vinyl alcohol)/poly(vinyl pyrrolidone) hydrogels. RSC Adv. 2016, 6, 39718–39727. [Google Scholar] [CrossRef]
- Morariu, S.; Bercea, M.; Teodorescu, M.; Avadanei, M. Tailoring the properties of PVA/PVP hydrogels for biomedical application. Eur. Polym. J. 2016, 84, 313–325. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Teodorescu, M. Rheological investigation of poly(vinyl alcohol)/poly(N-vinyl pyrrolidone) mixtures in aqueous solution and hydrogel state. J. Polym. Res. 2016, 23, 142. [Google Scholar] [CrossRef]
- Suzuki, A.; Sasaki, S. Swelling and mechanical properties of physically crosslinked poly(vinyl alcohol) hydrogels. J. Eng. Med. 2015, 229, 828–844. [Google Scholar] [CrossRef] [PubMed]
- Podorozhko, E.A.; Vasil’ev, V.G.; Buzin, M.I.; Golubev, E.K.; Shcherbina, M.A. Change in the morphology and properties of poly(vinyl alcohol) cryogels depending on the annealing temperature. Polym. Sci. Ser. A 2023, 65, 734–743. [Google Scholar] [CrossRef]
- Bercea, M.; Gradinaru, L.-M.; Morariu, S.; Plugariu, I.-A.; Gradinaru, R.V. Tailoring the properties of PVA/HPC/BSA hydrogels for wound dressing applications. React. Funct. Polym. 2022, 170, 105094. [Google Scholar] [CrossRef]
- Cretu, B.-E.-B.; Dodi, G.; Gardikiotis, I.; Balan, V.; Nacu, I.; Stoica, I.; Stoleru, E.; Rusu, A.G.; Ghilan, A.; Nita, L.E.; et al. Bioactive composite cryogels based on poly(vinyl alcohol) and a polymacro-lactone as tissue engineering scaffolds: In vitro and in vivo studies. Pharmaceutics 2023, 15, 2730. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Ding, Z.; Tao, X.; Nellist, P.D.; Assender, H.E. Atomic-scale imaging of polyvinyl alcohol crystallinity using electron ptychography. Polymer 2023, 284, 126305. [Google Scholar] [CrossRef]
- Yokoyama, F.; Masada, I.; Shimamura, K.; Ikawa, T.; Monobe, K. Morphology and structure of highly elastic poly (vinyl alcohol) hydrogel prepared by repeated freezing-and-melting. Colloid Polym. Sci. 1986, 264, 595–601. [Google Scholar] [CrossRef]
- Wu, S.W.; Hua, M.; Alsaid, Y.; Du, Y.; Ma, Y.; Zhao, Y.; Lo, C.-Y.; Wang, C.; Wu, D.; Yao, B.; et al. Poly(vinyl alcohol) hydrogels with broad-range tunable mechanical properties via the Hofmeister effect. Adv. Mater. 2021, 33, 2007829. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Kawase, M.; Sugiura, Y.; Takematsu, S.; Hara, C. Phase separation behavior of poly(vinyl alcohol) solutions in relation to the drawability of films prepared from the solutions. Macromolecules 1993, 26, 4461–4471. [Google Scholar] [CrossRef]
- Bao, Q.B. Dehydration of Hydrogels. U.S. Patent US5705780A, 6 January 1998. [Google Scholar]
- Yao, L.; Haas, T.W.; Guiseppi-Elie, A.; Bowlin, G.L.; Simpson, D.G.; Wnek, G.E. Electrospinning and stabilization of fully hydrolyzed poly(vinyl alcohol) fibers. Chem. Mater. 2003, 15, 1860–1864. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Wang, Y.; Ashammakhi, N.; Alem, H.; Erdem, A.; Chang, Q.; Xu, K.; Liu, Y.; Luo, G.; et al. An alkaline based method for generating crystalline, strong, and shape memory polyvinyl alcohol biomaterials. Adv. Mater. 2020, 7, 1902740. [Google Scholar] [CrossRef]
- Türkoğlu, G.C.; Khomarloo, N.; Mohsenzadeh, E.; Gospodinova, D.N.; Neznakomova, M.; Salaün, F. PVA-based electrospun materials—A promising route to designing nanofiber mats with desired morphological shape—A review. Int. J. Mol. Sci. 2024, 25, 1668. [Google Scholar] [CrossRef]
- Supaphol, P.; Chuangchote, S. On the electrospinning of poly(vinyl alcohol) nanofiber mats: A revisit. J. Appl. Polym. Sci. 2008, 108, 969–978. [Google Scholar] [CrossRef]
- Lim, H.J.; Lee, S.J.; Bae, H.J.; Noh, S.K.; Lee, Y.R.; Han, S.S.; Jeon, H.Y.; Park, W.H.; Lyoo, W.S. Effects of the tacticities of poly(vinyl alcohol) on the structure and morphology of poly(vinyl alcohol) nanowebs prepared by electrospinning. J. Appl. Polym. Sci. 2007, 106, 3282–3289. [Google Scholar] [CrossRef]
- Guerrini, L.M.; de Oliveira, M.P.; Branciforti, M.C.; Custódio, T.A.; Bretas, R.E.S. Thermal and structural characterization of nanofibers of poly(vinyl alcohol) produced by electrospinning. J. Appl. Polym. Sci. 2009, 112, 1680–1687. [Google Scholar] [CrossRef]
- Gautam, L.; Warkar, S.G.; Ahmad, S.I.; Kant, R.; Jain, M. A review on carboxylic acid cross-linked polyvinyl alcohol: Properties and applications. Polym. Eng. Sci. 2022, 62, 225–246. [Google Scholar] [CrossRef]
- Moulay, S. Review: Poly(vinyl alcohol) functionalizations and applications. Polym. Plast. Technol. Eng. 2015, 54, 1289–1319. [Google Scholar] [CrossRef]
- Shui, T.; Pan, M.; Li, A.; Fan, H.; Wu, J.; Liu, Q.; Zeng, H. Poly(vinyl alcohol) (PVA)-based hydrogel scaffold with isotropic ultratoughness enabled by dynamic amine–catechol interactions. Chem. Mater. 2022, 34, 8613–8628. [Google Scholar] [CrossRef]
- Zhong, Y.; Lin, Q.; Yu, H.; Shao, L.; Cui, X.; Pang, Q.; Zhu, Y.; Hou, R. Construction methods and biomedical applications of PVA-based hydrogels. Front. Chem. 2024, 12, 1376799. [Google Scholar] [CrossRef] [PubMed]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) hydrogels: The old and new functional materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Chen, Y.; Song, J.; Wang, S.; Liu, W. PVA-based hydrogels: Promising candidates for articular cartilage repair. Macromol. Biosci. 2021, 21, e2100147. [Google Scholar] [CrossRef]
- Park, J.S.; Park, J.W.; Ruckenstein, E. On the viscoelastic properties of poly(vinyl alcohol) and chemically crosslinked poly(vinyl alcohol). J. Appl. Polym. Sci. 2001, 82, 1816–1823. [Google Scholar] [CrossRef]
- Pisanova, E.; Schmidt, R.; Tseitlin, A. Poly(vinyl alcohol)-Based Formaldehyde-Free Curable Aqueous Composition. European Patent EP1885785A1, 13 February 2008. [Google Scholar]
- Zhang, Y.; Snover, D.A. Methods for Preparing Stable Urea Formaldehyde Polyvinyl Alcohol Colloids. U.S. Patent 9,217,046 B1, 22 December 2015. [Google Scholar]
- Gao, X.-P.; Liu, I.J.; Zheng, X.-J.; Tang, K.; Zhang, Y.-Q. Influence of crosslinking of formaldehyde on structure and properties of gelatin/PVA blend films. J. Funct. Biomater. 2014, 45, 5049–5052. [Google Scholar] [CrossRef]
- Teramoto, N.; Saitoh, M.; Kuroiwa, J.; Shibata, M.; Yosomiya, R. Morphology and mechanical properties of pullulan/poly(vinyl alcohol) blends crosslinked with glyoxal. J. Appl. Polym. Sci. 2001, 82, 2273–2280. [Google Scholar] [CrossRef]
- Krishna, D.V.; Sankar, M.R. Functionally graded gelatine/PVA-based composite hydrogel for repairing the soft tissues of diarthrodial joints. Mater. Today Commun. 2024, 38, 108070. [Google Scholar] [CrossRef]
- Locarno, S.; Arosio, P.; Curtoni, F.; Piazzoni, M.; Pignoli, E.; Gallo, S. Microscopic and macroscopic characterization of hydrogels based on poly(vinyl-alcohol)–glutaraldehyde mixtures for fricke gel dosimetry. Gels 2024, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Morandim-Giannetti, A.d.A.; Rubio, S.R.; Nogueira, R.F.; Ortega, F.d.S.; Magalhães, O., Jr.; Schor, P.; Bersanetti, P.A. Characterization of PVA/glutaraldehyde hydrogels obtained using central composite rotatable design (CCRD). J. Biomed. Mater. Res.—B Appl. Biomater. 2018, 106, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, T.; Zaman, M.N.; Prasad, B.B.; Shukla, A.; Aggarwal, S.; Singh, R. Rapid immobilization of viable Bacillus pseudomycoides in polyvinyl alcohol/glutaraldehyde hydrogel for biological treatment of municipal wastewater. Environ. Sci. Pollut. Res. 2020, 27, 9167–9180. [Google Scholar] [CrossRef] [PubMed]
- Aflatounian, A.; Sharzehee, M.; Mashroteh, H.A. Preparation of stable polyvinyl alcohol/polyurea hydrogel using oligomeric compounds containing urea-bonded. Results Mater. 2024, 22, 100564. [Google Scholar] [CrossRef]
- Gautam, L.; Warkar, S.G.; Jain, M. Physicochemical evaluation of polyvinyl alcohol films crosslinked with saturated and unsaturated dicarboxylic acids: A comparative study. Polym. Eng. Sci. 2024. [Google Scholar] [CrossRef]
- Lu, B.; Lin, F.; Jiang, X.; Cheng, J.; Lu, Q.; Song, J.; Chen, C.; Huang, B. One-pot assembly of microfibrillated cellulose reinforced PVA–borax hydrogels with self-healing and pH-responsive properties. ACS Sustain. Chem. Eng. 2017, 5, 948–956. [Google Scholar] [CrossRef]
- Karaman, B.; Bozkurt, A. Enhanced performance of supercapacitor based on boric acid doped PVA-H2SO4 gel polymer electrolyte system. Int. J. Hydrogen Energy 2018, 43, 6229–6237. [Google Scholar] [CrossRef]
- Ailincai, D.; Gavril, G.; Marin, L. Polyvinyl alcohol boric acid—A promising tool for the development of sustained release drug delivery systems. Mater. Sci. Eng. C 2020, 107, 110316. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Yang, S.; Li, Z.; Wang, J.; Han, M.; Hou, K. In-situ gelation based on rapid crosslinking: A versatile bionic water-based lubrication strategy. Chem. Eng. J. 2023, 477, 146863. [Google Scholar] [CrossRef]
- El-Naggar, A.W.M.; Senna, M.M.; Mostafa, T.A.; Helal, R.H. Radiation synthesis and drug delivery properties of interpenetrating networks (IPNs) based on poly (vinyl alcohol)/methylcellulose blend hydrogels. Int. J. Biol. Macromol. 2017, 102, 1045–1051. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Kamoun, E.A.; El-Gendi, H. Gamma radiation-induced crosslinked composite membranes based on polyvinyl alcohol/chitosan/AgNO3/vitamin E for biomedical applications. Int. J. Biol. Macromol. 2019, 37, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly(vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Yusuf, N.M.; Ooi, B.S. Preparation and modification of poly(vinyl alcohol) membrane: Effect of crosslinking time towards its morphology. Desalination 2012, 287, 35–40. [Google Scholar] [CrossRef]
- Figueiredo, K.C.S.; Alves, T.L.M.; Borges, C.P. Poly(vinyl alcohol) films crosslinked by glutaraldehyde under mild conditions. J. Appl. Polym. Sci. 2009, 111, 3074–3080. [Google Scholar] [CrossRef]
- Gopakumar, A.N.; Ccanccapa-Cartagena, A.; Bell, K.; Salehi, M. Development of crosslinked polyvinyl alcohol nanofibrous membrane for microplastic removal from water. J. Appl. Polym. Sci. 2024, 141, e55428. [Google Scholar] [CrossRef]
- Yeom, C.-K.; Lee, K.-H. Pervaporation separation of water-acetic acid mixtures through poly(vinyl alcohol) membranes crosslinked with glutaraldehyde. J. Membr. Sci. 1996, 109, 257–265. [Google Scholar] [CrossRef]
- Zhang, Z.D.; Liu, Y.; Lin, S.D.; Wang, Q. Preparation and properties of glutaraldehyde crosslinked poly(vinyl alcohol) membrane with gradient structure. J. Polym. Res. 2020, 27, 228. [Google Scholar] [CrossRef]
- Dai, S.; Barbari, T.A. Hydrogel membranes with mesh size asymmetry based on the gradient crosslinking of poly(vinyl alcohol). J. Membr. Sci. 1999, 156, 67–79. [Google Scholar] [CrossRef]
- Vatanpour, V.; Teber, O.O.; Mehrabi, M.; Koyuncu, I. Polyvinyl alcohol-based separation membranes: A comprehensive review on fabrication techniques, applications and future prospective. Mater. Today Chem. 2023, 28, 101381. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, M.S.; Choi, Y.; Lee, K.B.; Kim, J.H. Synthesis of PVA-g-POEM graft copolymers and their use in highly permeable thin film composite membranes. Chem. Eng. J. 2018, 346, 739–747. [Google Scholar] [CrossRef]
- Zhong, W.F.; Song, Y.F.; Yang, S.; Gong, L.H.; Shi, D.J.; Dong, W.F.; Zhang, H.J. A monocarboxylic acid induction strategy to prepare tough and thermo-reversible poly(vinyl alcohol) physical gels with high transparency. Soft Matter 2023, 19, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, F.; Jiang, M.; Ye, G.; Xu, J. A novel method to prepare poly(vinyl alcohol) water-soluble fiber with narrowly dissolving temperature range. J. Appl. Polym. Sci. 2012, 125, 2956–2962. [Google Scholar] [CrossRef]
- Lin, M.; Wang, Y.; Xing, S.; Lv, C.; Fan, Z.; Li, G.; Liao, X. A new strategy to fabricate polyvinyl alcohol (PVA) foam with low thermal conductivity using PVA-citric acid solution in supercritical carbon dioxide. Eur. Polym. J. 2024, 208, 112869. [Google Scholar] [CrossRef]
- Nataraj, D.; Reddy, R.; Reddy, N. Crosslinking electrospun poly (vinyl) alcohol fibers with citric acid to impart aqueous stability for medical applications. Eur. Polym. J. 2020, 124, 109484. [Google Scholar] [CrossRef]
- De Castro, D.P.; Santana, R.M.C. Influence of chemical nature of citric and tartaric acids on reaction time of the crosslinking of polyvinyl alcohol hydrogels. An. Acad. Bras. Cienc. 2024, 96, e20230092. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ye, H.; Wang, L.; Liu, M. Experimental investigation of the effects of crosslinking processes on the swelling and hygroscopic performances of a poly(vinyl alcohol) membrane. J. Appl. Polym. Sci. 2017, 134, 7. [Google Scholar] [CrossRef]
- Bandelli, D.; Casini, A.; Guaragnone, T.; Baglioni, M.; Mastrangelo, R.; Buemi, L.P.; Chelazzi, D.; Baglioni, P. Tailoring the properties of poly(vinyl alcohol) “twin-chain” gels via sebacic acid decoration. J. Colloid Interface Sci. 2024, 657, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Yu, T.L. Dynamic light scattering of poly(vinyl alcohol)-borax aqueous solution near overlap concentration. Polymer 1997, 38, 2019–2025. [Google Scholar] [CrossRef]
- Ossipov, D.A.; Piskounova, S.; Hilborn, J. Poly(vinyl alcohol) cross-linkers for in vivo injectable hydrogels. Macromolecules 2008, 41, 3971–3982. [Google Scholar] [CrossRef]
- Lambertini, L.; Coccarelli, G.; Toto, E.; Santonicola, M.G.; Laurenzi, S. Poly(vinyl alcohol) gels cross-linked by boric acid for radiation protection of astronauts. Acta Astronaut. 2024, 221, 142–154. [Google Scholar] [CrossRef]
- Ramamoorthy, T.; Soloman, A.M.; Annamalai, D.; Thiruppathi, V.; Srinivasan, P.; Masilamani, D.; Gopinath, A.; Madhan, B. Facile crosslinking of PVA scaffolds using quercetin for biomaterial applications. Mater. Lett. 2024, 364, 136307. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Eldin, M.S.M.; Kenawy, E.-R.-S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Yan, M.; Wang, Q. High strength and toughness poly(vinyl alcohol)/gelatin double network hydrogel fabricated via Hofmeister effect for polymer electrolyte. Eur. Polym. J. 2023, 185, 111826. [Google Scholar] [CrossRef]
- Yin, C.; Huang, Z.; Zhang, Y.; Ren, K.; Liu, S.; Luo, H.; Zhang, Q.; Wan, Y. Strong, tough, and elastic poly(vinyl alcohol)/polyacrylamide DN hydrogels based on the Hofmeister effect for articular cartilage replacement. J. Mater. Chem. B 2024, 12, 3079–3091. [Google Scholar] [CrossRef] [PubMed]
- Rufato, K.B.; Veregue, F.R.; Medeiro, R.D.; Francisco, C.B.; Souza, P.R.; Popat, K.C.; Kipper, M.J.; Martins, A.F. Electrospinning of poly(vinyl alcohol) and poly(vinyl alcohol)/tannin solutions: A critical viewpoint about crosslinking. Mater. Today Commun. 2023, 35, 106271. [Google Scholar] [CrossRef]
- Li, Y.B.; Yao, S. High stability under extreme condition of the poly(vinyl alcohol) nanofibers crosslinked by glutaraldehyde in organic medium. Polym. Degrad. Stab. 2017, 137, 229–237. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Kang, L.; Gao, X.; Zhao, K. Antibacterial, efficient and sustainable CS/PVA/GA electrospun nanofiber membrane for air filtration. Mater. Res. Express 2022, 9, 125002. [Google Scholar] [CrossRef]
- Qin, X.H.; Wang, S.Y. Electrospun nanofibers from crosslinked poly(vinyl alcohol) and its filtration efficiency. J. Appl. Polym. Sci. 2008, 109, 951–956. [Google Scholar] [CrossRef]
- Yuan, J.; Mo, H.; Wang, M.; Li, L.; Zhang, J.; Shen, J. Reactive electrospinning of poly(vinyl alcohol) nanofibers. J. Appl. Polym. Sci. 2012, 124, 1067–1073. [Google Scholar] [CrossRef]
- Wan, X.L.; Zhang, M.Y.; Wang, Y.; Chen, B.; Gui, Z.P.; Xu, Y.H.; Zhang, Y.; Chen, D.H.; Du, Z.; Jah, T.N. Molecule structure design by synergistic crosslinking in the PVA matrix and its physical properties regulation. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 133104. [Google Scholar] [CrossRef]
- Sonker, A.K.; Roy, S.; Wagner, H.D.; Zak, A.; Sui, X.M.; Bajpai, R. Synergistic effect of crosslinking and dual reinforcement on the thermal and mechanical properties of polyvinyl alcohol. Polym. Compos. 2021, 42, 1214–1223. [Google Scholar] [CrossRef]
- Sonker, A.K.; Verma, V. Influence of crosslinking methods toward poly(vinyl alcohol) properties: Microwave irradiation and conventional heating. J. Appl. Polym. Sci. 2018, 135, 46125. [Google Scholar] [CrossRef]
- Bates, N.M.; Puy, C.; Jurney, P.L.; McCarty, O.J.T.; Hinds, M.T. Evaluation of the effect of crosslinking method of poly(vinyl alcohol) hydrogels on thrombogenicity. Cardiovasc. Eng. Technol. 2020, 11, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Faase, R.A.; Keeling, N.M.; Plaut, J.S.; Leycam, C.; Munares, G.A.; Hinds, M.T.; Baio, J.E.; Jurney, P.L. Temporal changes in the surface chemistry and topography of reactive ion plasma-treated poly(vinyl alcohol) alter endothelialization potential. ACS Appl. Mater. Interfaces 2024, 16, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Brazel, C.S. Modifying the release of proxyphylline from PVA hydrogels using surface crosslinking. Int. J. Pharm. 2008, 349, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Ramya, K.A.; Srinivasan, R.; Deshpande, A.P. Nonlinear measures and modeling to examine the role of physical and chemical crosslinking in poly(vinyl alcohol)-based crosslinked systems. Rheol. Acta 2018, 57, 181–195. [Google Scholar] [CrossRef]
- Sparks, H.D.; Mandla, S.; Vizely, K.; Rosin, N.; Radisic, M.; Biernaskie, J. Application of an instructive hydrogel accelerates re-epithelialization of xenografted human skin wounds. Sci. Rep. 2022, 12, 14233. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.R.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.G. Production and application of biomaterials based on polyvinyl alcohol (PVA) as wound dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- Meng, L.; Li, J.; Fan, X.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Improved mechanical and antibacterial properties of polyvinyl alcohol composite films using quaternized cellulose nanocrystals as nanofillers. Compos. Sci. Technol. 2023, 232, 109885. [Google Scholar] [CrossRef]
- Popescu, M.C. Structure and sorption properties of CNC reinforced PVA films. Int. J. Biol. Macromol. 2017, 101, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Paduraru, O.M.; Ciolacu, D.; Darie, R.N.; Vasile, C. Synthesis and characterization of polyvinyl alcohol/cellulose cryogels and their testing as carriers for a bioactive component. Mat. Sci. Eng. C 2012, 32, 2508–2515. [Google Scholar] [CrossRef]
- Baron, R.I.; Bercea, M.; Avadanei, M.; Lisa, G.; Biliuta, G.; Coseri, S. Green route for the fabrication of self-healable hydrogels based on tricarboxy cellulose and poly(vinyl alcohol). Int. J. Biol. Macromol. 2019, 123, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Sankarganesh, P.; Parthasarathy, V.; Ganesh Kumar, A.; Ragu, S.; Saraniya, M.; Udayakumari, N.; Anbarasan, R. Preparation of cellulose-PVA blended hydrogels for wound healing applications with controlled release of the antibacterial drug: An in vitro anticancer activity. Biomass Convers. Bioref. 2024, 14, 3385–3395. [Google Scholar] [CrossRef]
- Offia-Kalu, N.E.; Nwanonenyi, S.C.; Abdulhakeem, B.; Dzade, N.Y.; Onwalu, P.A. Theoretical investigation of electronic, energetic, and mechanical properties of polyvinyl alcohol/cellulose composite hydrogel electrolyte. J. Mol. Graph. Model. 2024, 127, 108667. [Google Scholar] [CrossRef] [PubMed]
- Caselli, L.; Malmsten, M. Skin and wound delivery systems for antimicrobial peptides. Curr. Opin. Colloid Interface Sci. 2023, 65, 101701. [Google Scholar] [CrossRef]
- Kanaujia, K.A.; Mishra, N.; Rajinikanth, P.S.; Saraf, A.A. Antimicrobial peptides as antimicrobials for wound care management: A comprehensive review. J. Drug Deliv. Sci. Technol. 2024, 95, 105570. [Google Scholar] [CrossRef]
- Pelin, I.M.; Silion, M.; Popescu, I.; Rîmbu, C.M.; Fundueanu, G.; Constantin, M. Pullulan/poly(vinyl alcohol) hydrogels loaded with Calendula officinalis extract: Design and in vitro evaluation for wound healing applications. Pharmaceutics 2023, 15, 1674. [Google Scholar] [CrossRef] [PubMed]
- Nita, L.E.; Cretu, B.E.B.; Serban, A.M.; Rusu, A.G.; Rosca, I.; Pamfil, D.; Chiriac, A.P. New cryogels based on poly (vinyl alcohol) and a copolymacrolactone system. II. Antibacterial properties of the network embedded with thymol bioactive agent. React. Funct. Polym. 2023, 182, 105461. [Google Scholar] [CrossRef]
- Popescu, I.; Constantin, M.; Solcan, G.; Ichim, D.L.; Rata, D.M.; Horodincu, L.; Solcan, C. Composite hydrogels with embedded silver nanoparticles and ibuprofen as wound dressing. Gels 2023, 9, 654. [Google Scholar] [CrossRef]
- Iswarya, S.; Bharathi, M.; Hariram, N.; Theivasanthi, T.; Gopinath, S.C.B. Solid polymer electrolyte and antimicrobial performance of polyvinyl alcohol/silver nanoparticles composite film. Results Chem. 2024, 7, 101431. [Google Scholar] [CrossRef]
- Pan, T.; Zhang, Y.; Qu, X.; Liang, X.; Zhao, Y. AgNPs/g-C3N4/PVA antibacterial hydrogel with excellent photocatalytic antibacterial activity for wound dressing. New J. Chem. 2024, 48, 7785–7798. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A. Therapeutic potential of green synthesized silver nanoparticles loaded PVA hydrogel patches for wound healing. J. Drug Deliv. Sci. Technol. 2019, 54, 101308. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Nath, V.K.Y.; Thomas, J.; Augustine, A.; Kalarikkal, N.; Al Moustafa, A.E.; Thomas, S. Electrospun polyvinyl alcohol membranes incorporated with green synthesized silver nanoparticles for wound dressing applications. J. Mater. Sci. Mater. Med. 2018, 29, 163. [Google Scholar] [CrossRef] [PubMed]
- Sabrin, S.; Hong, S.-H.; Kumar, S.K.C.; Oh, J.-S.; Derrick-Roberts, A.L.K.; Karmokar, D.K.; Habibullah, H.; Short, R.D.; Ghimire, B.; Fitridge, R.; et al. Electrochemically enhanced antimicrobial action of plasma-activated poly(vinyl alcohol) hydrogel dressings. Adv. Mater. 2024, 34, 2314345. [Google Scholar] [CrossRef]
- He, G.; Zhou, Y.; Chen, X.; Ma, T.; Yin, Y.; Chu, Y.; Fan, L.; Cai, W. Preparation of poly (vinyl alcohol)/polydopamine/tannic acid composite hydrogels with dual adhesive, antioxidant and antibacterial properties. Eur. Polym. J. 2024, 205, 112708. [Google Scholar] [CrossRef]
- Hou, Z.; Zeng, S.; Shen, K.; Healey, P.R.; Schipper, H.J.; Zhang, L.; Zhang, M.; Jones, M.D.; Sun, L. Interactive deformable electroluminescent devices enabled by an adaptable hydrogel system with optical/photothermal/mechanical tunability. Mater. Horiz. 2023, 10, 5931–5941. [Google Scholar] [CrossRef] [PubMed]
- Amir, F.; Niazi, M.B.K.; Malik, U.S.; Jahan, Z.; Andleeb, A.; Ahmad, T.; Mustansar, Z. A multifunctional vanillin-infused chitosan-PVA hydrogel reinforced by nanocellulose and CuO-Ag nanoparticles as antibacterial wound dressing. Int. J. Biol. Macromol. 2024, 258, 128831. [Google Scholar] [CrossRef]
- Lan, X.; Yang, H.; Xiong, Y.; Zeng, G.; Dong, F. Polyvinyl alcohol/chitosan quaternary ammonium salt composite hydrogel with directional macroporous structure for photothermal synergistic antibacterial and wound healing promotion. Int. J. Biol. Macromol. 2024, 267, 131549. [Google Scholar] [CrossRef]
- Stoica, A.E.; Albulet, D.; Birca, A.C.; Iordache, F.; Ficai, A.; Grumezescu, A.M.; Vasile, B.S.; Andronescu, E.; Marinescu, F.; Holban, A.M. Electrospun nanofibrous mesh based on PVA, chitosan, and usnic acid for applications in wound healing. Int. J. Mol. Sci. 2023, 24, 11037. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinia, A.; Morowvat, M.H.; Niknezhad, S.V.; Baghbadorani, M.A.; Michalek, M.; Chen, S.; Nemati, M.M.; Negahdaripour, M.; Heidari, R.; Azadi, A.; et al. A photocrosslinkable and hemostatic bilayer wound dressing based on gelatin methacrylate hydrogel and polyvinyl alcohol foam for skin regeneration. Int. J. Biol. Macromol. 2024, 266, 131231. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic acid with antiviral and antibacterial activity as a promising component of biomaterials—A minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Fan, D.; Cao, W.; Zhu, C.; Duan, Z.; Fu, R.; Li, X.; Ma, X. Preparation and characterization of breathable hemostatic hydrogel dressings and determination of their effects on full-thickness defects. Polymers 2017, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Temel-Soylu, T.M.; Keçeciler-Emir, C.; Rababah, T.; Özel, C.; Yücel, S.; Basaran-Elalmis, Y.; Altan, D.; Kirgiz, Ö.; Seçinti, I.E.; Kaya, U.; et al. Green electrospun poly(vinyl alcohol)/gelatin-based nanofibrous membrane by incorporating 45S5 bioglass nanoparticles and urea for wound dressing applications: Characterization and in vitro and in vivo evaluations. ACS Omega 2024, 9, 21187–21203. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yang, W.; Wei, W.; Liu, Z.; Tremblay, P.L.; Zhang, T. An electroconductive and antibacterial adhesive nanocomposite hydrogel for high-performance skin wound healing. Adv. Healthc. Mater. 2024, 13, 2303138. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, F.; Guo, M.; Lei, K.; Jiao, Z.; Li, Z.; Li, H.; Liu, D.; He, M.; Wang, Z.; et al. Shikonin delivery strategy through alkali-crosslinked polyvinyl alcohol hydrogel promotes effective wound healing. New J. Chem. 2024, 48, 3492–3500. [Google Scholar] [CrossRef]
- Paik, J.J.; Jang, B.; Nam, S.; Guo, L.J. A Transparent poly(vinyl alcohol) ion-conducting organohydrogel for skin-based strain-sensing applications. Adv. Healthc. Mater. 2023, 12, 2300076. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Zhang, H.; Wang, G.; Wei, H.; Zhang, X.; Ma, N. Bioinspired conductive hydrogel with ultrahigh toughness and stable antiswelling properties for articular cartilage replacement. ACS Mater. Lett. 2021, 3, 807–814. [Google Scholar] [CrossRef]
- Yan, M.; Cai, J.; Fang, Z.; Wang, H.; Qiu, X.; Liu, W. Anisotropic muscle-like conductive composite hydrogel reinforced by lignin and cellulose nanofibrils. ACS Sustain. Chem. Eng. 2022, 10, 12993–13003. [Google Scholar] [CrossRef]
- Osolnik, U.; Vek, V.; Korošec, R.C.; Oven, P.; Poljanšek, I. Integration of wood-based components—Cellulose nanofibrils and tannic acid-into a poly(vinyl alcohol) matrix to improve functional properties. Int. J. Biol. Macromol. 2024, 256, 128495. [Google Scholar] [CrossRef]
- Khan, R.; Khan, M.U.A.; Stojanović, G.M.; Javed, A.; Haider, S.; Razak, S.I.A. Fabrication of bilayer nanofibrous-hydrogel scaffold from bacterial cellulose, PVA, and gelatin as advanced dressing for wound healing and soft tissue engineering. ACS Omega 2024, 9, 6527–6536. [Google Scholar] [CrossRef]
- Lei, L.; Cong, R.; Ni, Y.; Cui, X.; Wang, X.; Ren, H.; Wang, Z.; Liu, M.; Tu, J.; Jiang, L. Dual-functional injectable hydrogel for osteoarthritis treatments. Adv. Healthc. Mater. 2024, 13, 2302551. [Google Scholar] [CrossRef] [PubMed]
- Jeencham, R.; Sinna, J.; Ruksakulpiwat, C.; Tawonsawatruk, T.; Numpaisal, P.-o.; Ruksakulpiwat, Y. Development of biphasic injectable hydrogels for meniscus scaffold from photocrosslinked glycidyl methacrylate-modified poly(vinyl alcohol)/glycidyl methacrylate-modified silk fibroin. Polymers 2024, 16, 1093. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, H.; Song, F.; Yang, W.; Yang, B.; Ding, D.; Liu, Z.; Hui, L.; Zhang, F. A highly sensitive and anti-freezing conductive strain sensor based on polypyrrole/cellulose nanofiber crosslinked polyvinyl alcohol hydrogel for human motion detection. Int. J. Biol. Macromol. 2024, 257, 128800. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, B.; Yu, J.; Yan, Z.; Liu, R.; Yu, C.; Zhao, Z.; Zhang, H.; Yao, F.; Li, J. A polypyrrole-dopamine/poly(vinyl alcohol) anisotropic hydrogel for strain sensor and bioelectrodes. Chem. Eng. J. 2024, 486, 150182. [Google Scholar] [CrossRef]
- Cao, X.; He, T.; Sui, J.; Yan, Y.; Liu, X.; Liu, L.; Lv, S. PVA/KGM dual network hydrogels doped with carbon nanotube-collagen corona as flexible sensors for human motion monitoring. J. Mater. Chem. C 2024, 12, 3333–3344. [Google Scholar] [CrossRef]
- Shi, X.; Lee, A.; Yang, B.; Gao, L.; Ning, H.; Huang, K.; Luo, X.; Zhang, L.; Zhang, J.; Yang, C.; et al. A 3D cross-linked hierarchical hydrogel E-skin with sensing of touch position and pressure. Carbon 2024, 216, 118514. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, L.; Sun, T.; Qin, C.; Wang, J.; Sun, J.; Dai, L. A transparent, self-adhesive and fully recyclable conductive PVA based hydrogel for wearable strain sensor. Polymer 2023, 283, 126281. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Xiao, Y.Y.; Kang, Y.; Pan, M.; Li, B.J.; Zhang, S. Shape memory polymers based on supramolecular interactions. ACS Appl. Mater. Interfaces 2017, 9, 20276–20293. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, J.; Li, S.; Wu, Z.; Mondal, A.K. Anti-swellable, stretchable, self-healable, shape-memory and supramolecular conductive TA-based hydrogels for amphibious motion sensors. Eur. Polym. J. 2024, 211, 113034. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Peng, L.; Liu, T.; Wang, Y.; Shi, S.; Wang, H. Poly(vinyl alcohol)-tannic acid hydrogels with excellent mechanical properties and shape memory behaviors. ACS Appl. Mater. Interfaces 2016, 8, 27199–27206. [Google Scholar] [CrossRef]
- Chen, Y.N.; Jiao, C.; Zhao, Y.; Zhang, J.; Wang, H. Self-assembled polyvinyl alcohol–tannic acid hydrogels with diverse microstructures and good mechanical properties. ACS Omega 2018, 3, 11788–11795. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, J.; Jin, Z. Tough, swelling-resistant, self-healing, and adhesive dual-cross-linked hydrogels based on polymer–tannic acid multiple hydrogen bonds. Macromolecules 2018, 51, 1696–1705. [Google Scholar] [CrossRef]
- Niu, W.; Zhu, Y.; Wang, R.; Lu, Z.; Liu, X.; Sun, J. Remalleable, healable, and highly sustainable supramolecular polymeric materials combining superhigh strength and ultrahigh toughness. ACS Appl. Mater. Interfaces 2020, 12, 30805–30814. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Sun, T.; Liu, R.; Li, L.; Gong, Y.; Xiao, Z. Robust hydrogel sensor with good mechanical properties, conductivity, anti-swelling ability, water tolerance and biocompatibility. Green Chem. 2024, 26, 3926–3939. [Google Scholar] [CrossRef]
- Wan, Z.; Qu, R.; Sun, Y.; Gao, Y.; Gao, G.; Chen, K.; Liu, T. Physically crosslinked polyvinyl alcohol, phytic acid and glycerol hydrogels for wearable sensors with biocompatibility, antimicrobial stability and anti-freezing. Eur. Polym. J. 2024, 211, 112974. [Google Scholar] [CrossRef]
- Wei, C.; Wang, Y.; Liang, Y.; Wu, J.; Li, F.; Luo, Q.; Lu, Y.; Liu, C.; Zhang, R.; Lu, Z.; et al. Low-hysteresis and highly linear sensors based on environmentally stable, adhesive, and antibacterial hydrogels. J. Mater. Chem. A 2024, 12, 10392–10402. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, Y.; Ji, Q.; Xing, Y.; Ma, X. Elastic and conductive gellan gum/polyvinyl alcohol physical hydrogels with low swelling potential for sustainable flexible electronics. J. Clean. Prod. 2024, 435, 140503. [Google Scholar] [CrossRef]
- Liu, P.; Yao, D.; Lu, C.; Gao, X.; Dong, P. Highly sensitive strain sensors based on PVA hydrogels with a conductive surface layer of graphene. J. Mater. Sci. Mater. Electron. 2024, 35, 97. [Google Scholar] [CrossRef]
- Pierchala, M.K.; Kadumudi, F.B.; Mehrali, M.; Zsurzsan, T.G.; Kempen, P.J.; Serdeczny, M.P.; Spangenberg, J.; Andresen, T.L.; Dolatshahi-Pirouz, A. Soft electronic materials with combinatorial properties generated via mussel-inspired chemistry and halloysite nanotube reinforcement. ACS Nano 2021, 15, 9531–9549. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Zhou, S.; Zhang, J.; Zong, L. Biomimetic multiscale oriented PVA/NRL hydrogel enabled multistimulus responsive and smart shape memory actuator. Small 2024, 20, 2311240. [Google Scholar] [CrossRef]
- Jin, T.; Zou, J.; Jing, X. Designing a small waist structure to enhance the sensitivity of PVA hydrogels. Mater. Lett. 2024, 355, 135568. [Google Scholar] [CrossRef]
- Qin, Y.; Wei, E.; Cui, C.; Xie, J. High tensile, antibacterial, and conductive hydrogel sensor with multiple cross-linked networks based on PVA/sodium alginate/zinc oxide. ACS Omega 2024, 9, 16851–16859. [Google Scholar] [CrossRef] [PubMed]
- Uto, K.; Liu, Y.; Mu, M.; Yamamoto, R.; Azechi, C.; Tenjimbayashi, M.; Kaeser, A.; Marliac, M.-A.; Alam, M.M.; Sasai, J.; et al. Humidity-responsive polyvinyl alcohol/microcrystalline cellulose composites with shape memory features for hair-styling applications. Adv. Mater. Interfaces 2024, 11, 2300274. [Google Scholar] [CrossRef]
- Jonjaroen, V.; Thinkohkaew, K.; Nakseno, B.; Payongsri, P.; Niamsiri, N.; Charoenrat, T.; Chittapun, S. Algal cellulose reinforced polyvinyl alcohol composite hydrogel with controlled niacinamide release for cosmeceutical applications. Materialia 2024, 33, 102012. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, J.T.; Chathuranga, K.; Lee, J.S.; Park, S.W.; Park, W.H. Tannic-acid-enriched poly(vinyl alcohol) nanofibrous membrane as a UV-shielding and antibacterial face mask filter material. ACS Appl. Mater. Interfaces 2023, 15, 20435–20443. [Google Scholar] [CrossRef]
- Devi, L.S.; Palathinkal, R.P.; Dasmahapatra, A.K. Preparation of cross-linked PANI/PVA conductive hydrogels for electrochemical energy storage and sensing applications. Polymer 2024, 293, 126673. [Google Scholar] [CrossRef]
- Stejskal, J.; Bober, P.; Trchová, M.; Kovalcik, A.; Hodan, J.; Hromádková, J.; Prokeš, J. Polyaniline cryogels supported with poly(vinyl alcohol): Soft and conducting. Macromolecules 2017, 50, 972–978. [Google Scholar] [CrossRef]
- Honciuc, A.; Solonaru, A.-M.; Teodorescu, M. Flexible composites with variable conductivity and memory of deformation obtained by polymerization of polyaniline in PVA hydrogel. Polymers 2022, 14, 4638. [Google Scholar] [CrossRef] [PubMed]
- D’Altri, G.; Yeasmin, L.; Di Matteo, V.; Scurti, S.; Giovagnoli, A.; Di Filippo, M.F.; Gualandi, I.; Cassani, M.C.; Caretti, D.; Panzavolta, S.; et al. Preparation and characterization of self-healing PVA–H2SO4 hydrogel for flexible energy storage. ACS Omega 2024, 9, 6391–6402. [Google Scholar] [CrossRef]
- Taoka, Y.; Saari, R.A.; Kida, T.; Yamaguchi, M.; Matsumura, K. Enhancing the mechanical properties of poly(vinyl alcohol) fibers by lithium iodide addition. ACS Omega 2023, 8, 32623–32634. [Google Scholar] [CrossRef]
- Chikkatti, B.S.; Sajjan, A.M.; Kalahal, P.B.; Banapurmath, N.R.; Angadi, A.R. Insight into the performance of VRLA battery using PVA-TEOS hybrid gel electrolytes with titania nanoparticles. J. Energy Storage 2023, 72, 108572. [Google Scholar] [CrossRef]
- M’barki, O.; Hanafia, A.; Bouyer, D.; Faur, C.; Sescousse, R.; Delabre, U.; Blot, C.; Guenoun, P.; Deratani, A.; Quemener, D.; et al. Greener method to prepare porous polymer membranes by combining thermally induced phase separation and crosslinking of poly(vinyl alcohol) in water. J. Membr. Sci. 2014, 458, 225–235. [Google Scholar] [CrossRef]
- Bercea, M.; Wolf, B.A. Vitrification of polymer solutions as a function of solvent quality, analyzed via vapor pressures. J. Chem. Phys. 2006, 124, 174902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, T.; Xu, L.; Miao, G.; Li, F.; Miao, X.; Lu, J.; Hou, Z.; Ren, G.; Zhu, X. Mechanical robust GO/PVA hydrogel for strong and recyclable adhesion in air, underwater, and underoil environments. Langmuir 2024, 40, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hwang, H.; Kim, S.J.; Park, J.; Youn, D.; Kim, D.; Hahn, J.; Seo, M.; Lee, H. VATA: A poly(vinyl alcohol)- and tannic acid-based nontoxic underwater adhesive. ACS Appl. Mater. Interfaces 2020, 12, 20933–20941. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, J.; Zeng, G.; Xu, H.; Lv, Y.; Liang, X.; Jin, L.; Jiang, X. Chitosan-crosslinked polyvinyl alcohol anti-swelling hydrogel designed to prevent abdominal wall adhesion. Mater. Today Bio 2024, 24, 100931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Chen, H.; Gao, Y.; Zhou, L.; Wan, J.; Li, Y.; Tang, M.; Peng, Y.; Wang, B.; et al. Efficient phosphate removal from water by multi-engineered PVA/SA matrix double network hydrogels: Influencing factors and removal mechanism. Sep. Purif. Technol. 2024, 336, 126261. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, D.; Huang, L.; Fang, Z.; Jiang, H.; Mao, Q.; Wang, H.; Tang, P.; Li, J.; Wang, G.; et al. Ultra-high strength sodium alginate/PVA/PHMB double-network hydrogels for marine antifouling. Prog. Org. Coat. 2024, 187, 108175. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Wang, H.; Cavaco-Paulo, A.; Su, J. Co-immobilizing laccase-mediator system by in-situ synthesis of MOF in PVA hydrogels for enhanced laccase stability and dye decolorization efficiency. J. Environ. Manag. 2024, 353, 120114. [Google Scholar] [CrossRef]
- Jung, S.; Yun, H.; Kim, J.; Kim, J.; Yeo, H.; Choi, I.G.; Kwak, H.W. Lignin/PVA hydrogel with enhanced structural stability for cationic dye removal. Int. J. Biol. Macromol. 2024, 257, 128810. [Google Scholar] [CrossRef]
- Filimon, A.; Albu, R.M.; Stoica, I.; Avram, E. Blends based on ionic polysulfones with improved conformational and microstructural characteristics: Perspectives for biomedical applications. Compos. Part B Eng. 2016, 93, 1–11. [Google Scholar] [CrossRef]
- Alkhouzaam, A.; Khraisheh, M. Development of eco-friendly coating for the fabrication of high performing loose nanofiltration membranes for dye-contaminated wastewater treatment. Desalination 2024, 581, 117609. [Google Scholar] [CrossRef]
- Gong, W.; He, W.; Hou, Y.; Li, Y.; Hu, Y.; Zhu, B.; Hu, J. Polyvinyl alcohol-based multifunctional hydrogel film: A novel strategy for food preservation packaging. Food Biosci. 2024, 59, 104125. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, S.; Xia, X.; Tan, M.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Du, J.; Wang, H. High-performance carboxymethyl cellulose-based hydrogel film for food packaging and preservation system. Int. J. Biol. Macromol. 2022, 223, 1126–1137. [Google Scholar] [CrossRef]
- Raschip, I.E.; Darie-Nita, R.N.; Fifere, N.; Hitruc, G.-E.; Dinu, M.V. Correlation between mechanical and morphological properties of polyphenol-laden xanthan gum/poly(vinyl alcohol) composite cryogels. Gels 2023, 9, 281. [Google Scholar] [CrossRef]
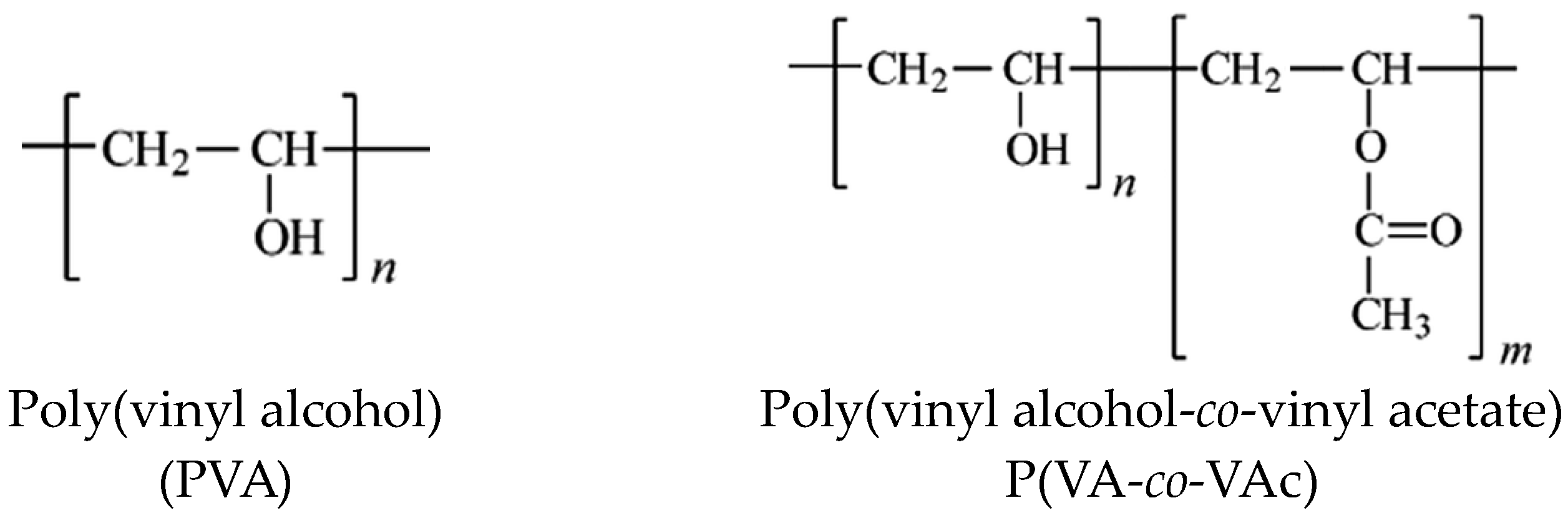



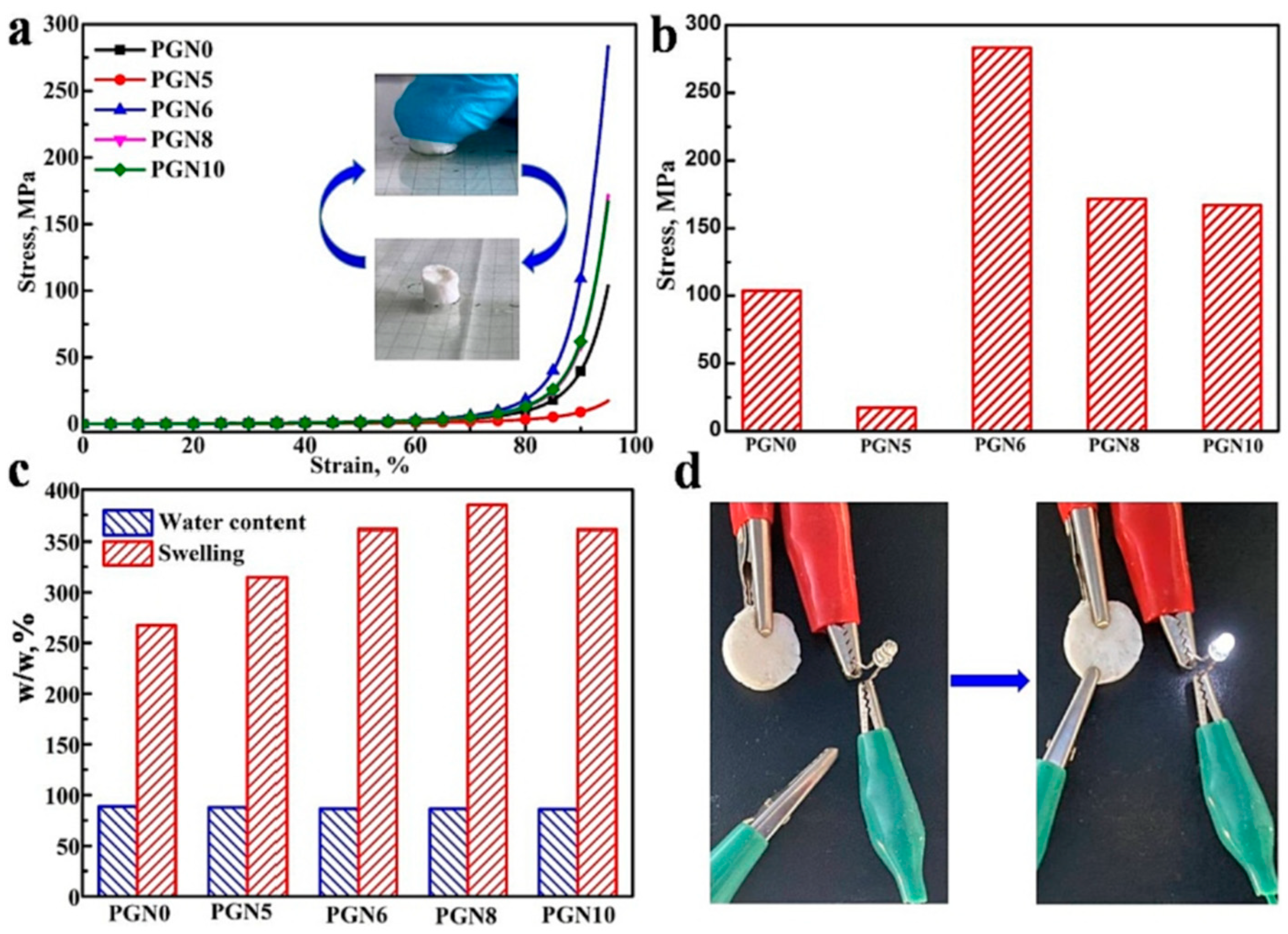

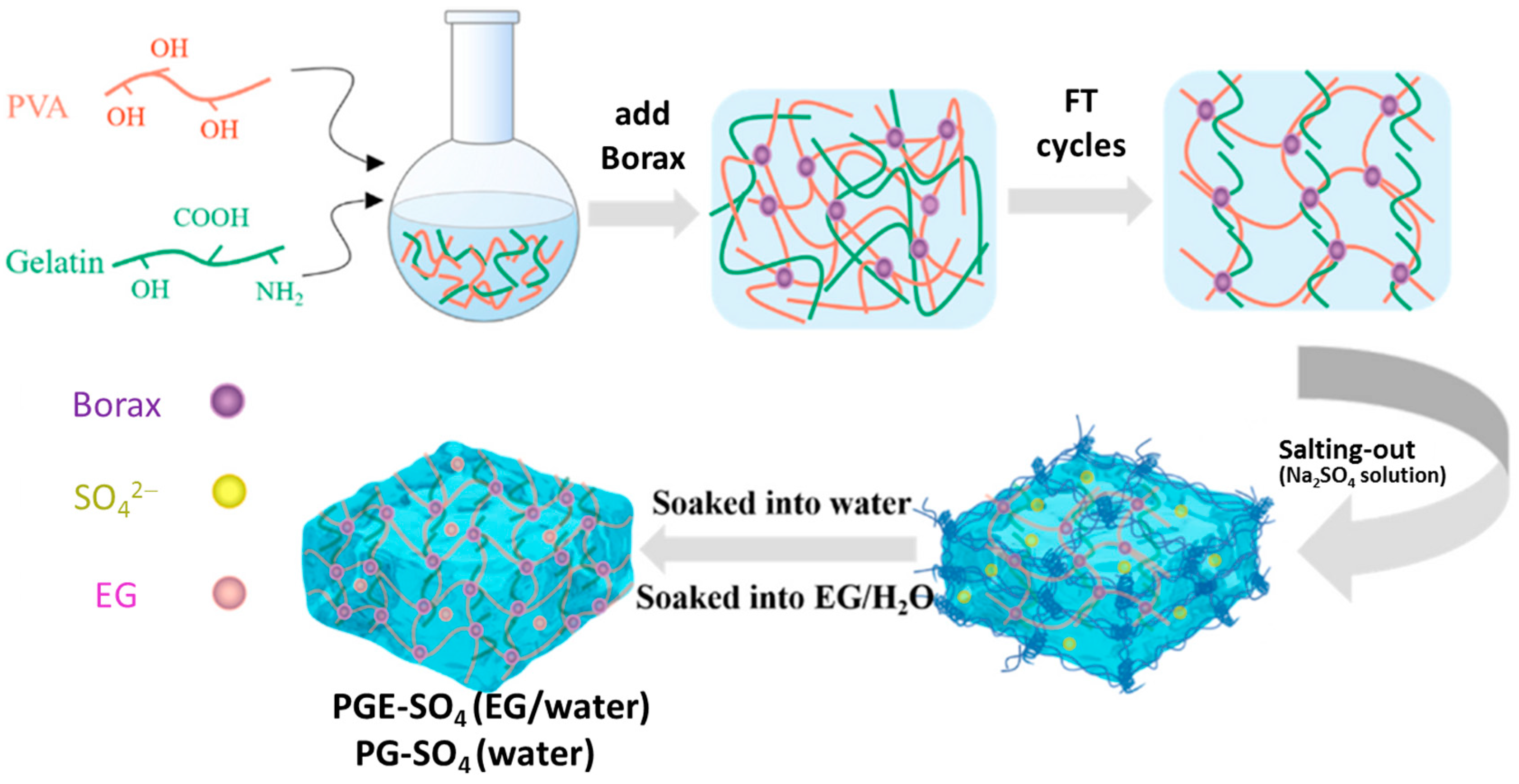

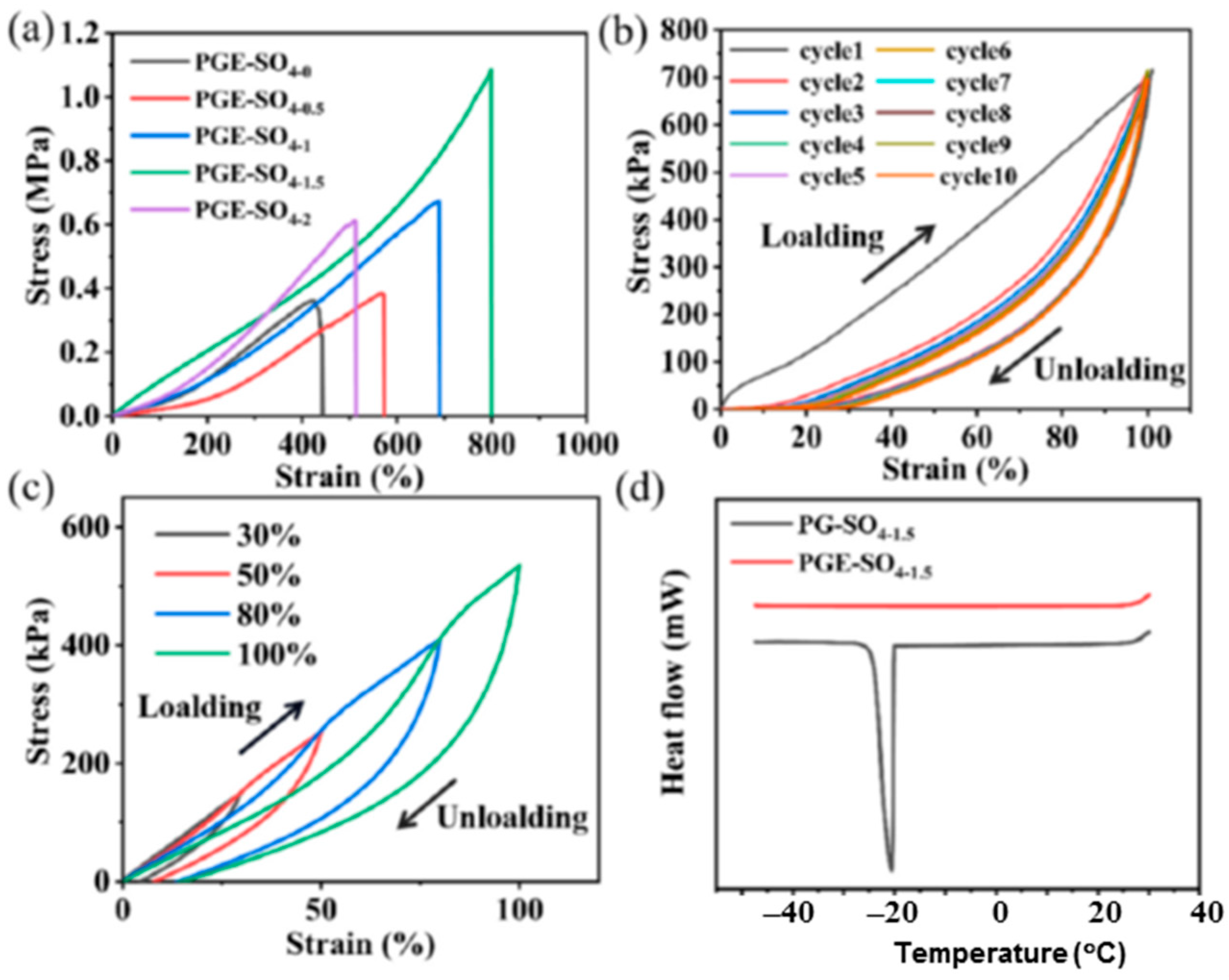
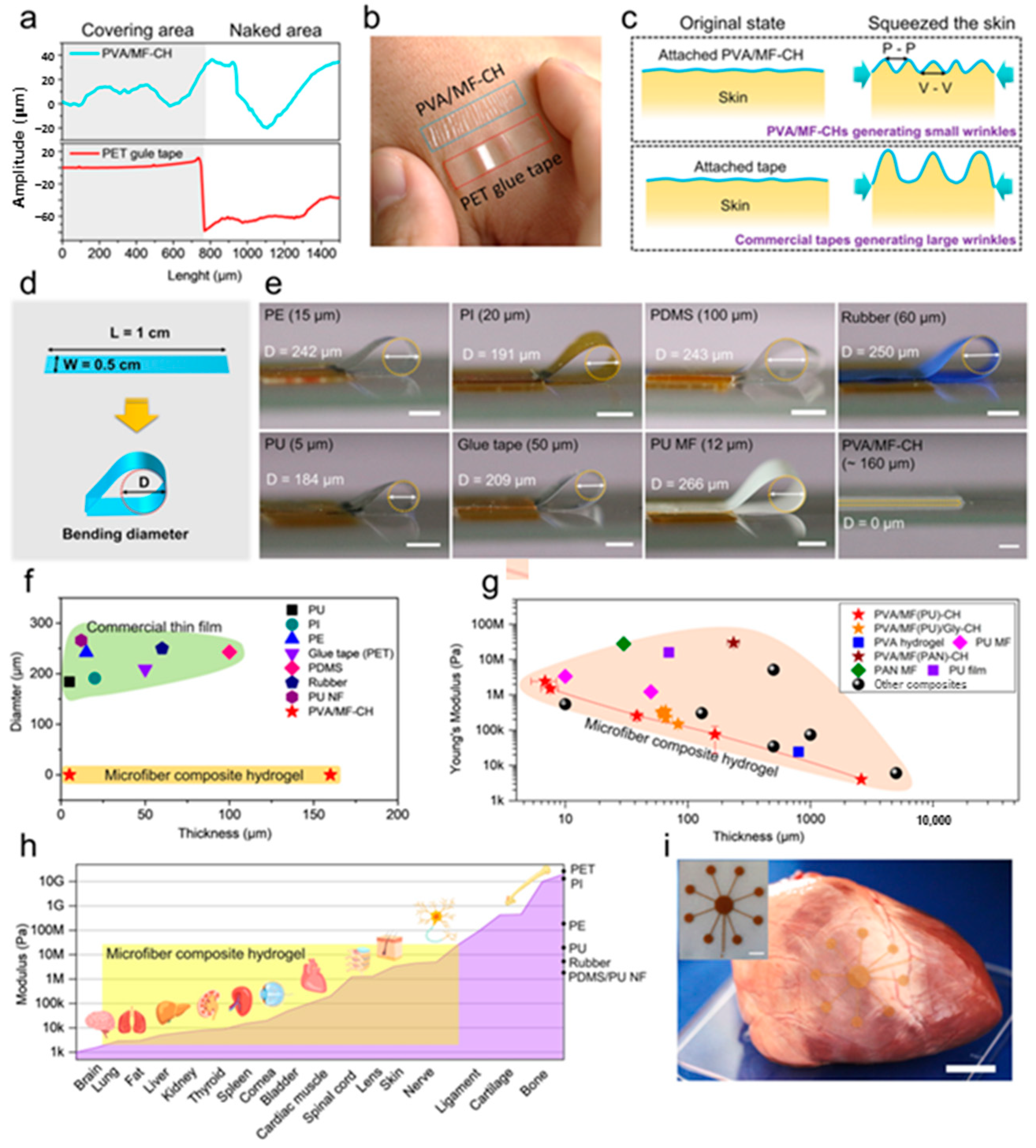

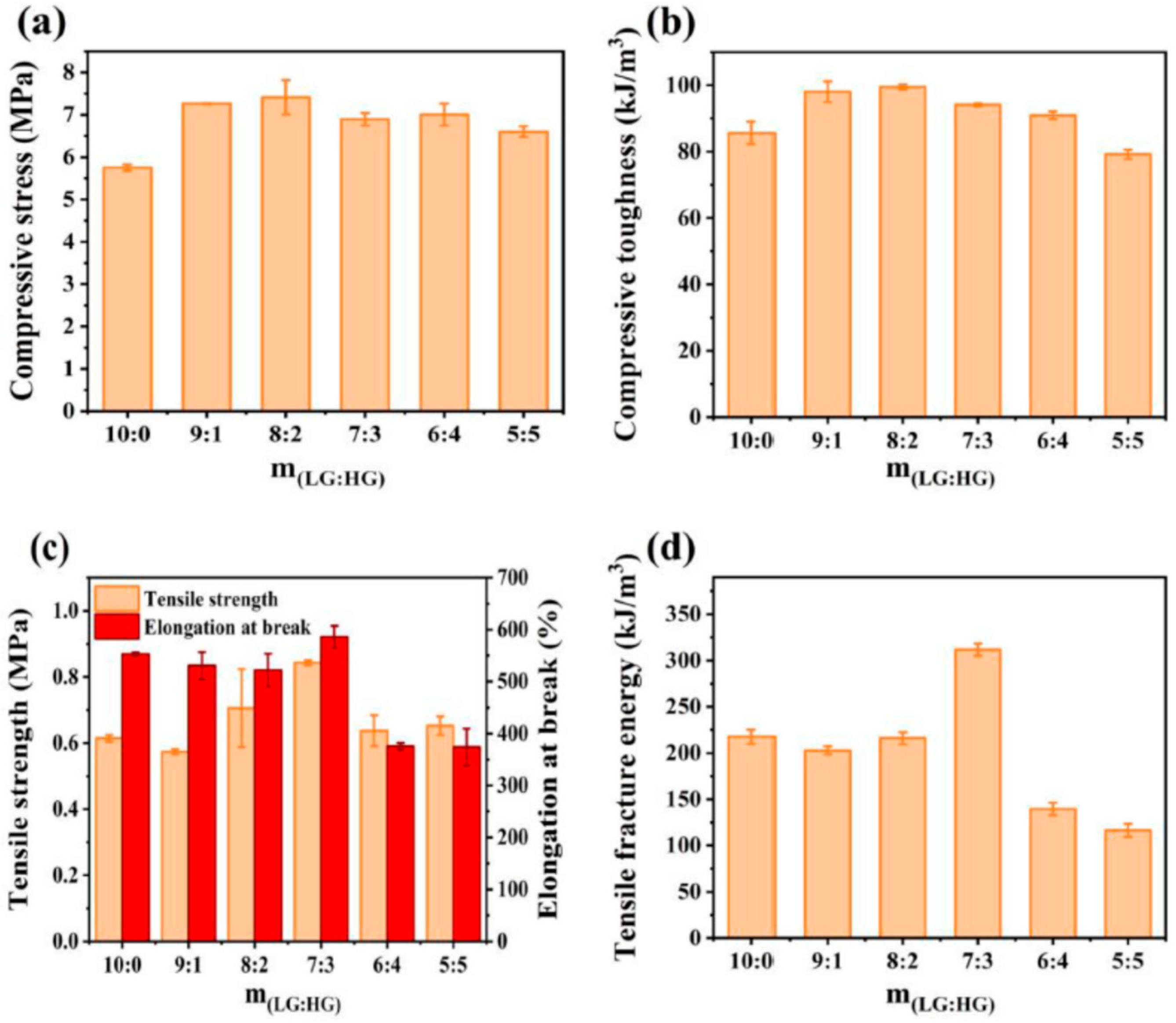
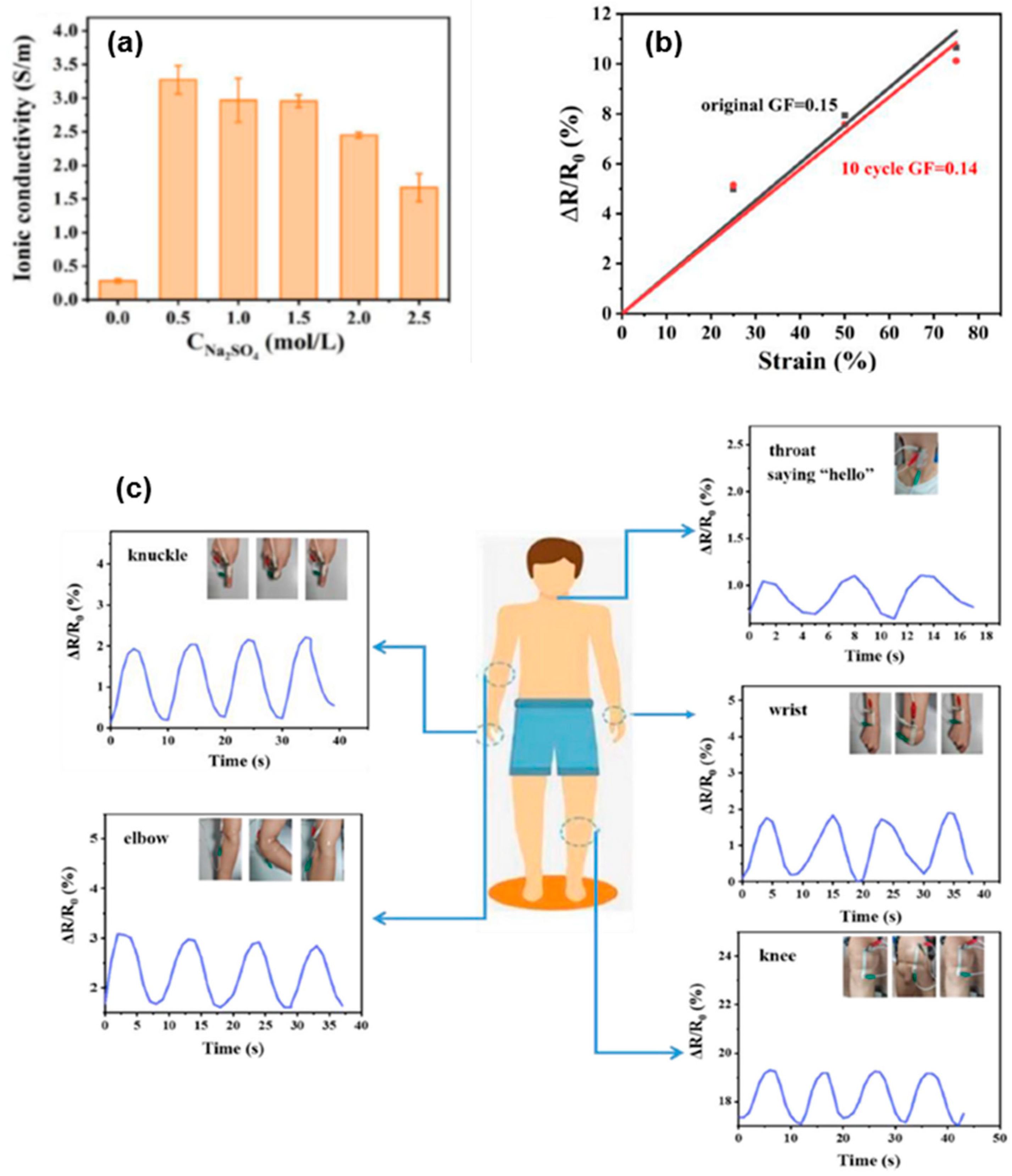




| Method | Acquired Information |
|---|---|
| Scanning Electron Microscopy (SEM) |
|
| Mechanical Properties |
|
| Rheology |
|
| Fourier-Transform Infrared (FTIR) Spectroscopy |
|
| Thermo-Gravimetric Analysis (TGA) |
|
| Differential Scanning Calorimetry (DSC) | thermal transition behavior and associated enthalpy for each process
|
| Dynamic Mechanical Analysis (DMA) |
|
| Small-Angle Neutron Scattering (SANS) |
|
| Atomic Force Microscopy |
|
| Swelling Behavior |
|
| X-Ray Diffraction |
|
| Sol–Gel Analysis |
|
| Contact Angle Analysis |
|
| Biocompatible Tests |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bercea, M. Recent Advances in Poly(vinyl alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. https://doi.org/10.3390/polym16142021
Bercea M. Recent Advances in Poly(vinyl alcohol)-Based Hydrogels. Polymers. 2024; 16(14):2021. https://doi.org/10.3390/polym16142021
Chicago/Turabian StyleBercea, Maria. 2024. "Recent Advances in Poly(vinyl alcohol)-Based Hydrogels" Polymers 16, no. 14: 2021. https://doi.org/10.3390/polym16142021
APA StyleBercea, M. (2024). Recent Advances in Poly(vinyl alcohol)-Based Hydrogels. Polymers, 16(14), 2021. https://doi.org/10.3390/polym16142021







