Tailoring Optical Performance of Polyvinyl Alcohol/Crystal Violet Band-Pass Filters via Solvent Features
Abstract
1. Introduction
2. Materials and Methods
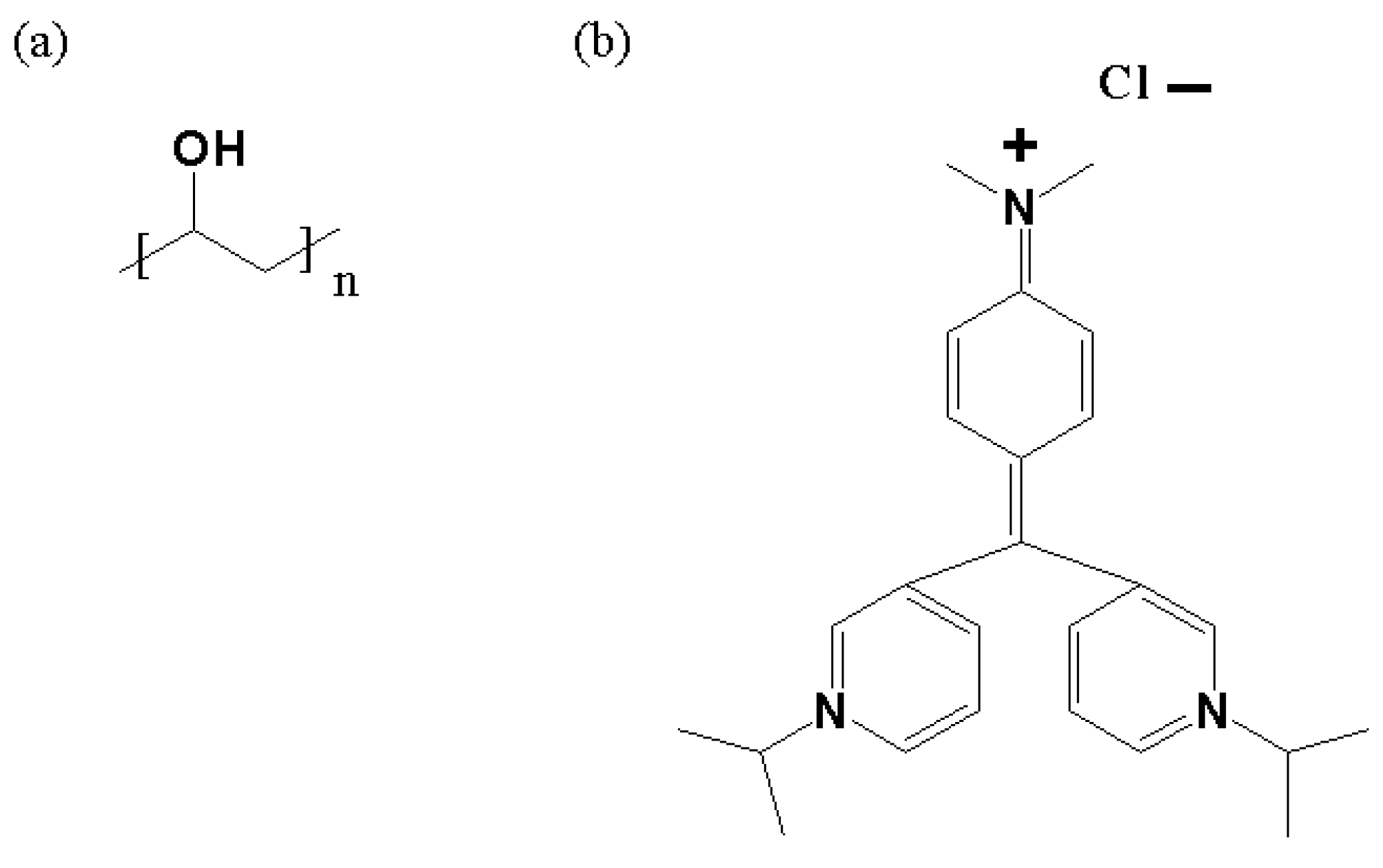
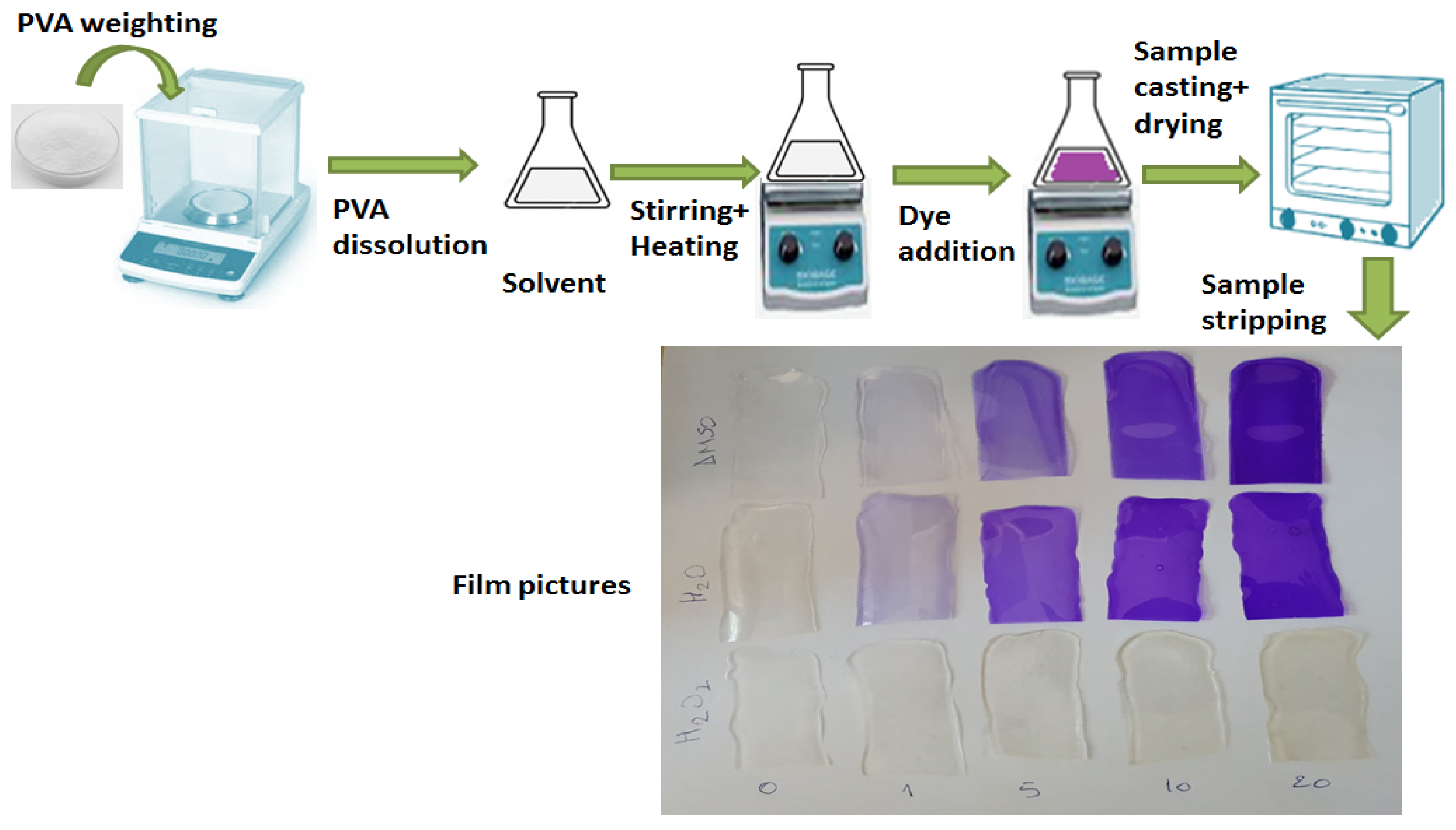
2.1. Materials and Sample Preparation
2.2. Characterization Methods
3. Results and Discussion
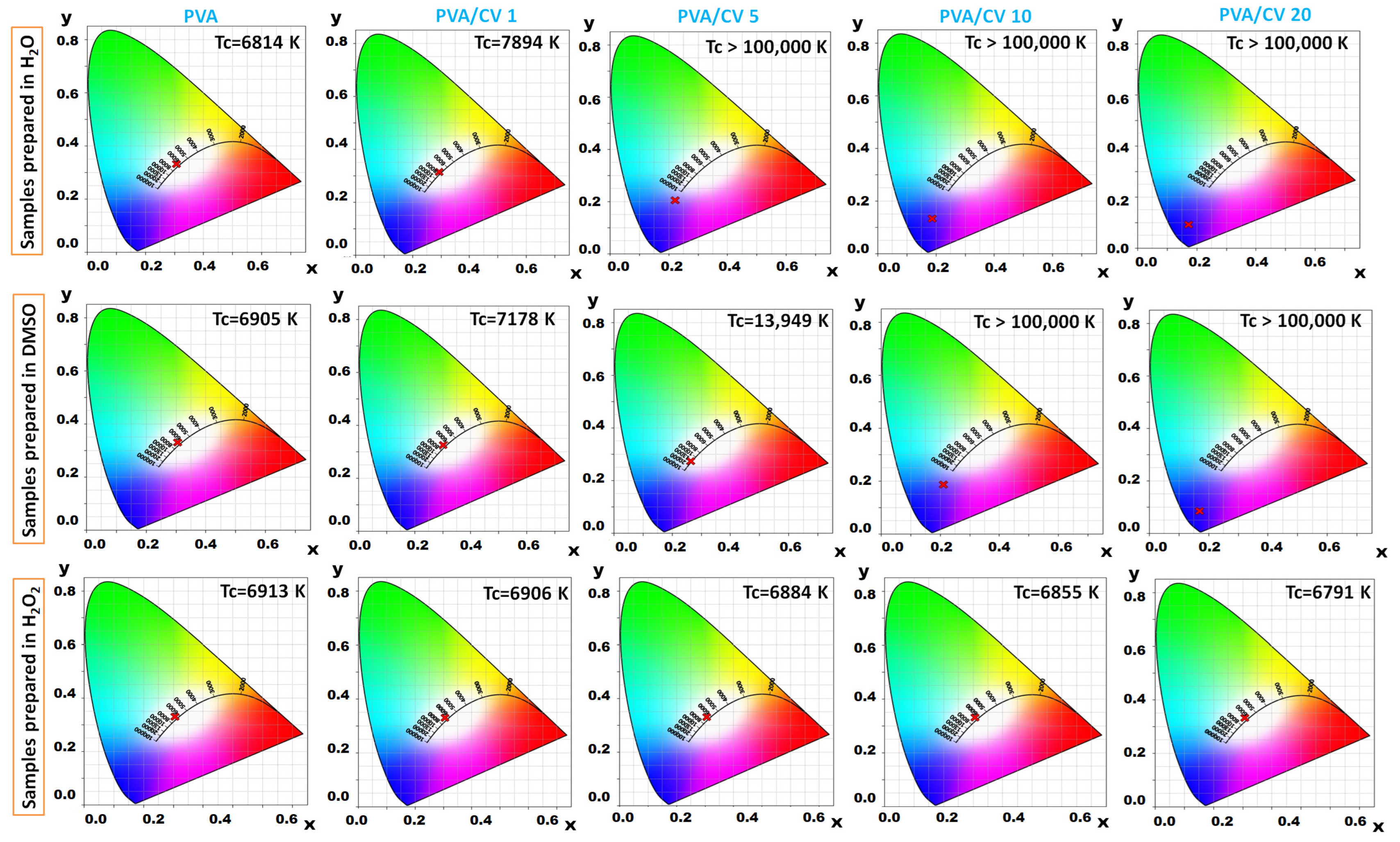
3.1. Colorimetry Analysis
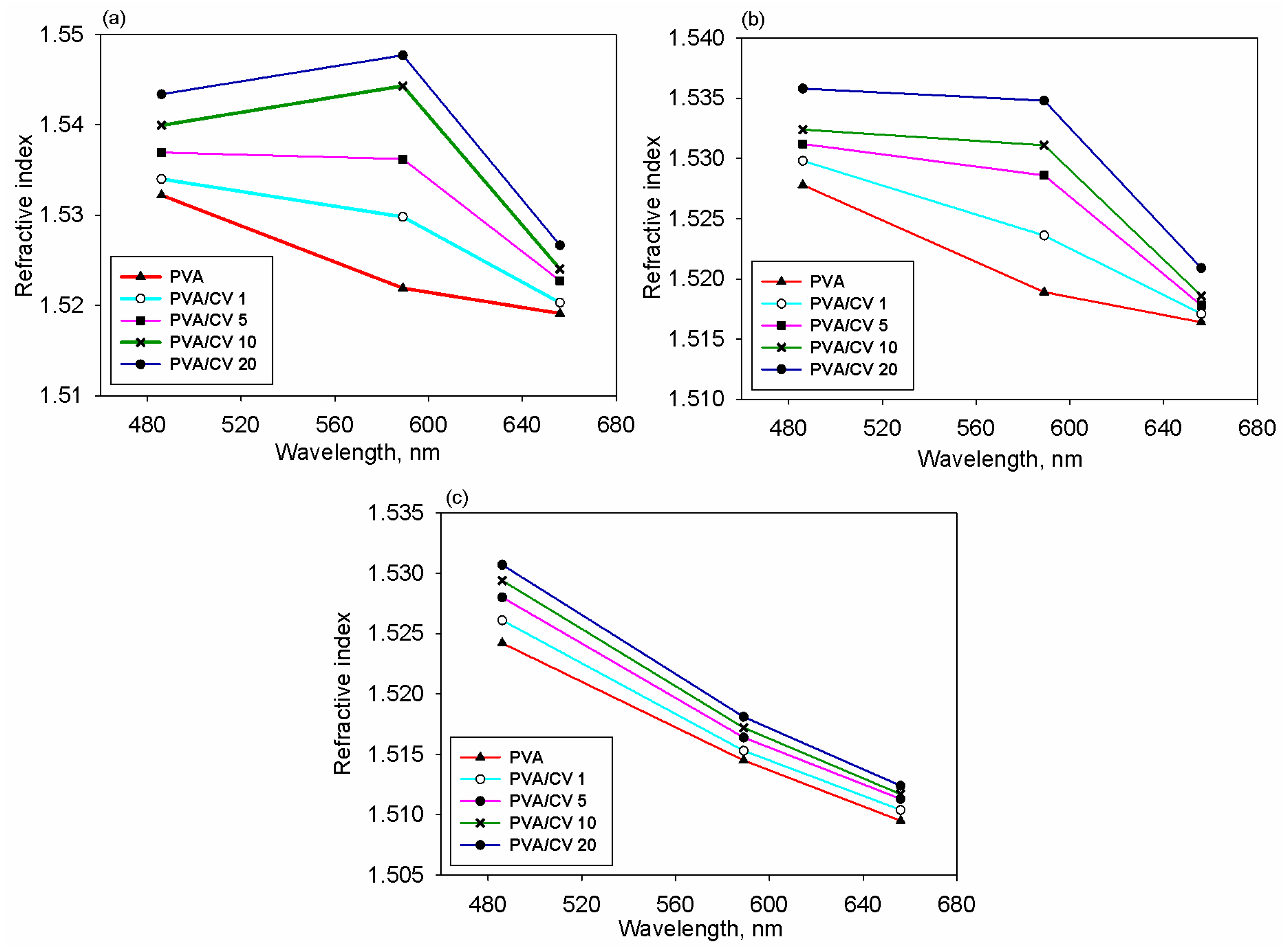
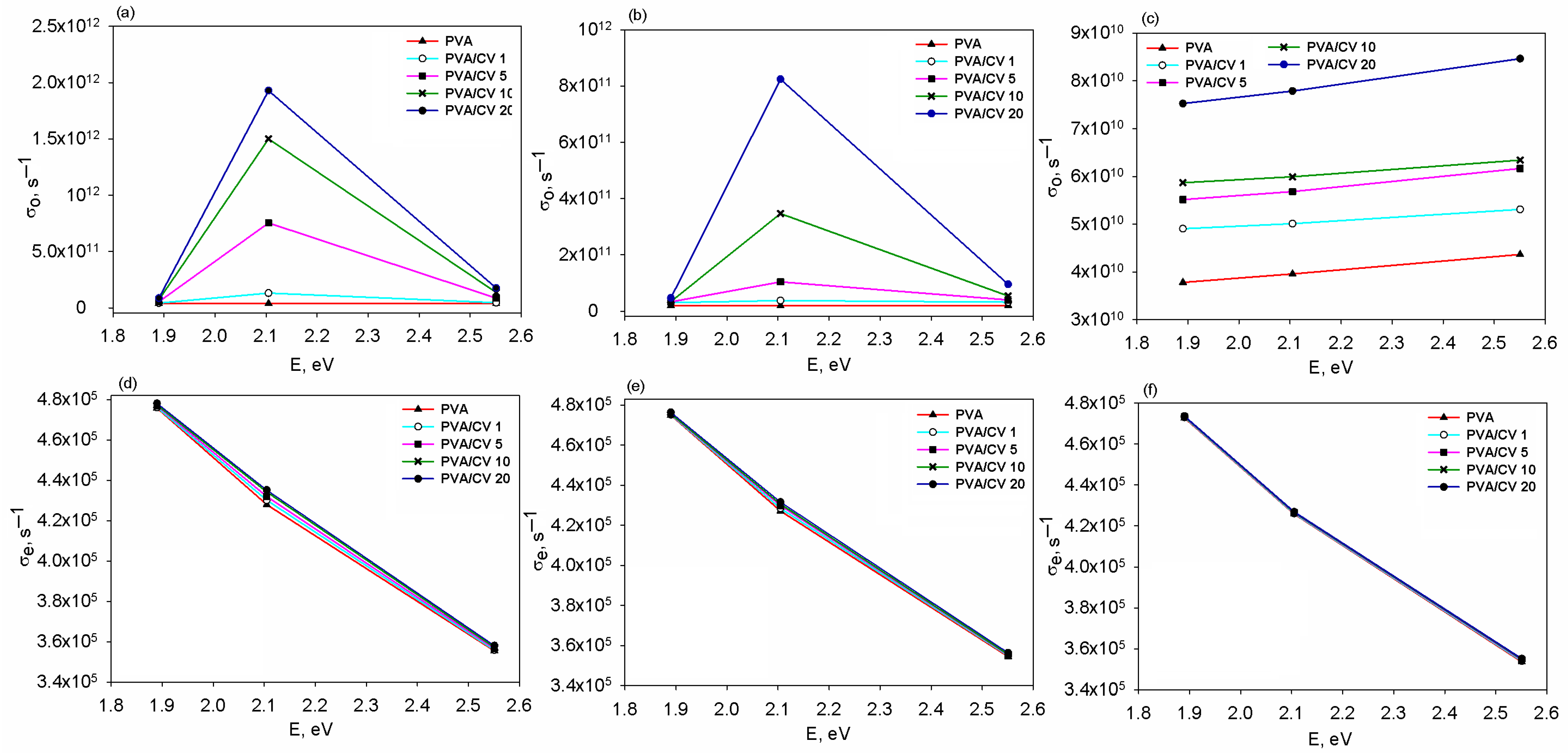
3.2. Refractive Index Dispersion and Optical Conductivity
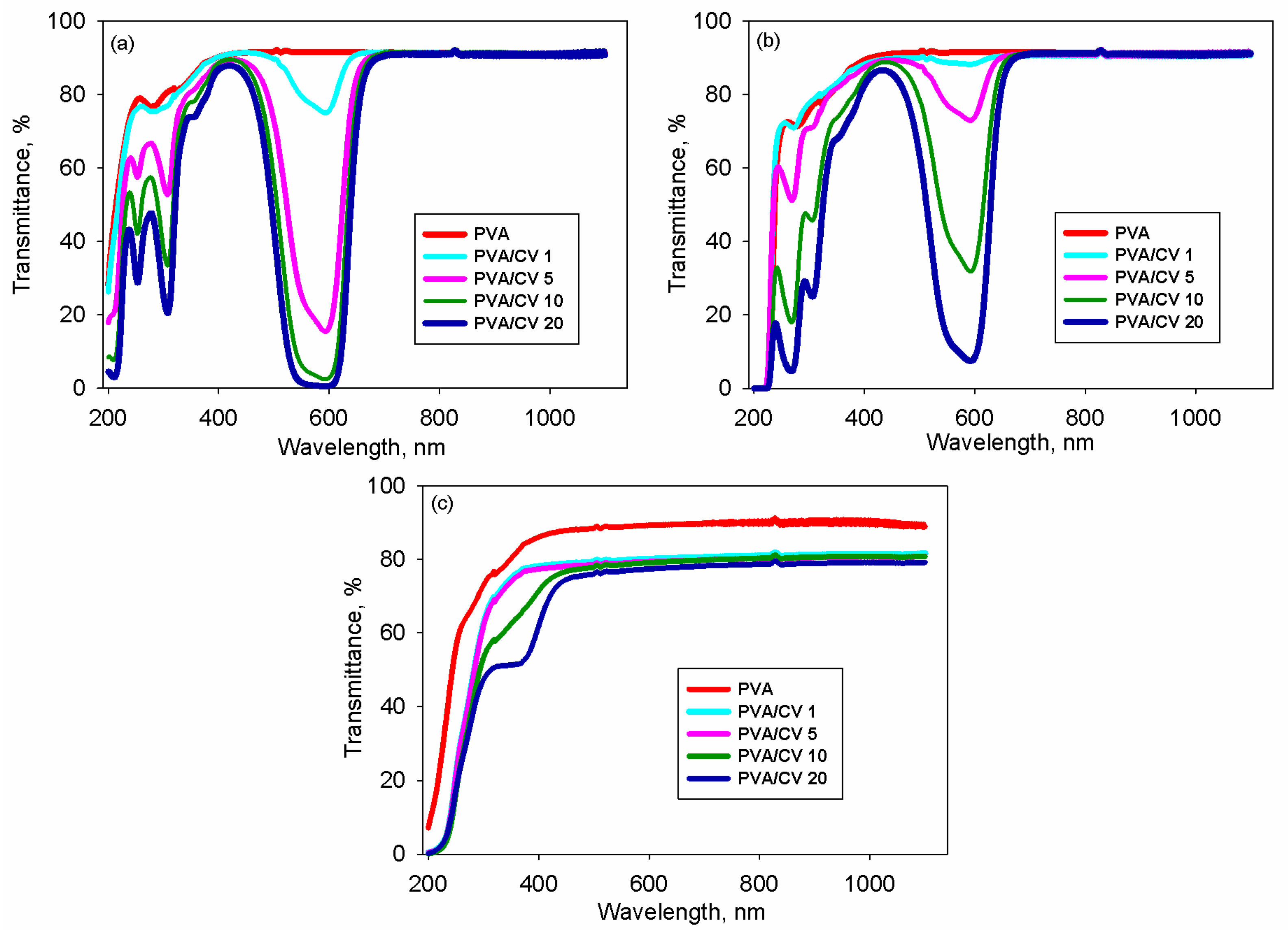
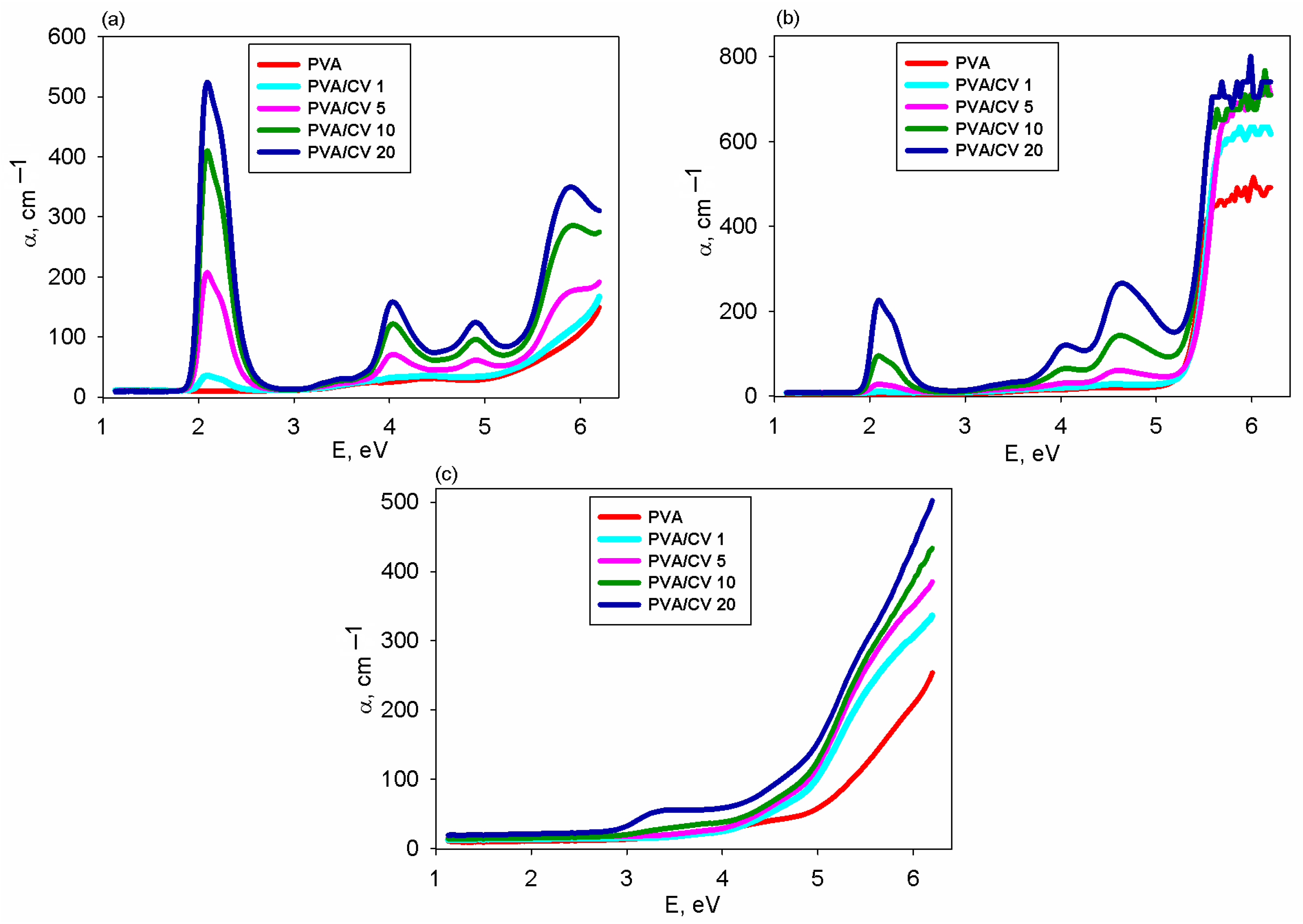
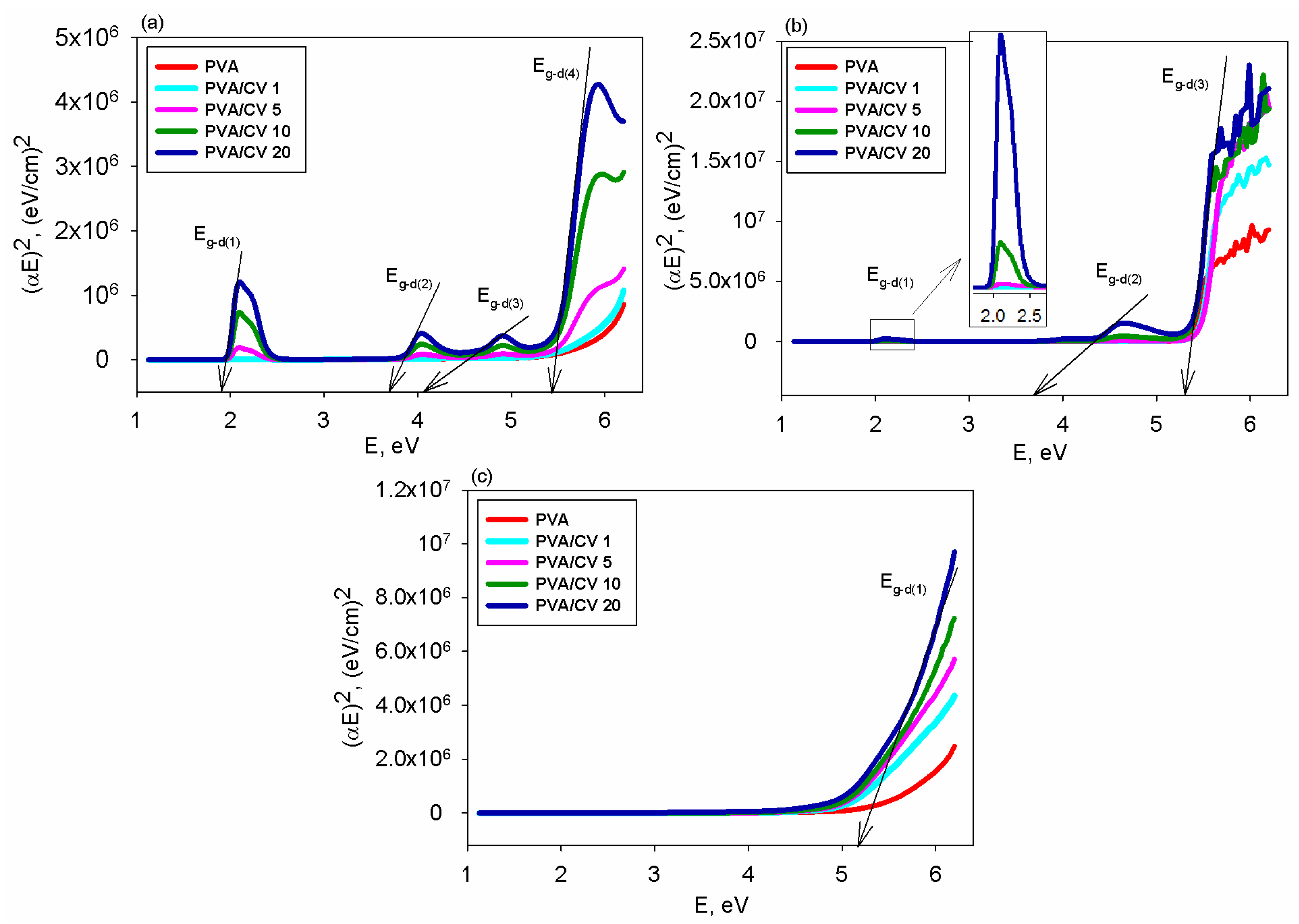
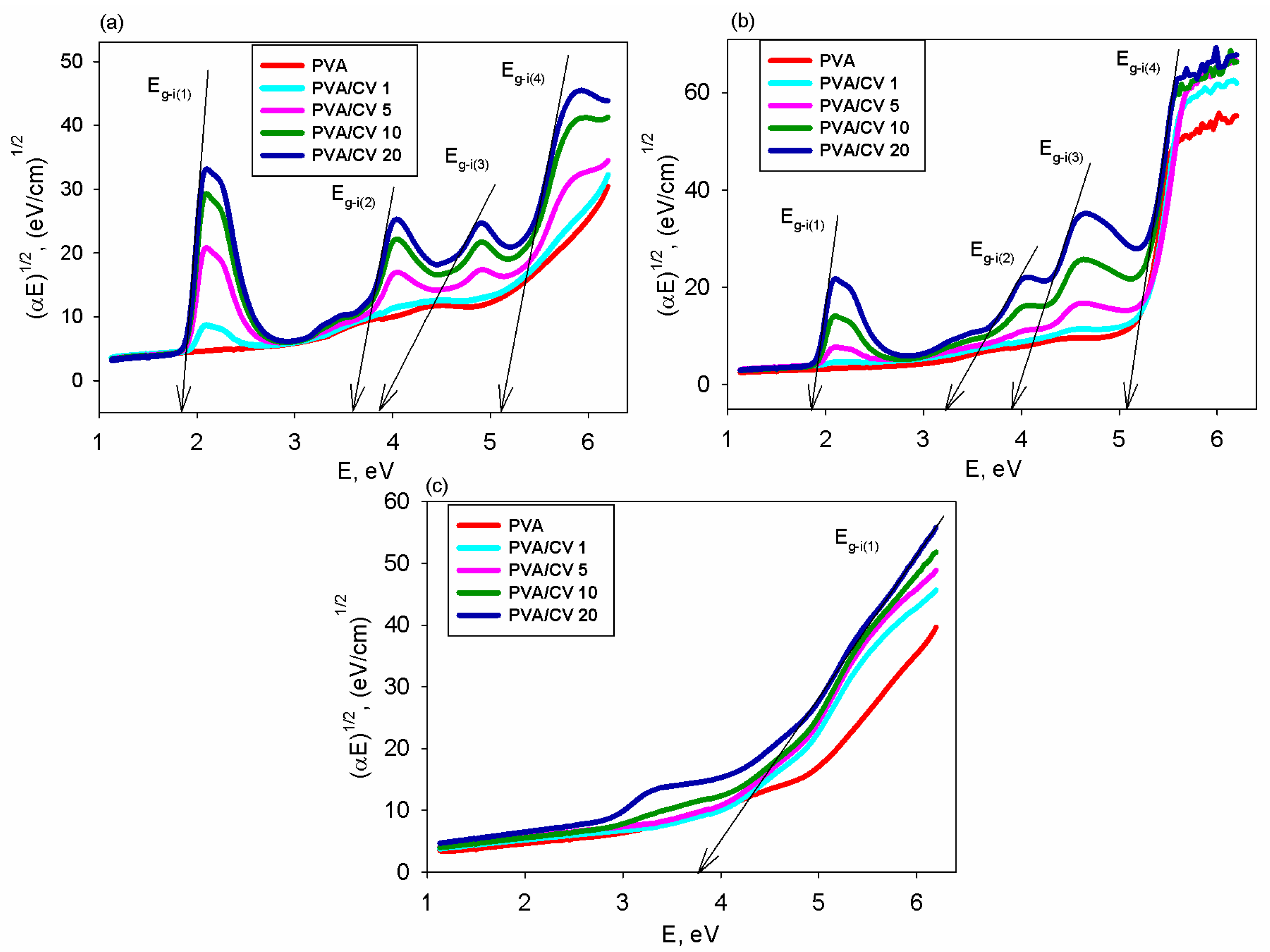
3.3. Transmittance and Band Gap Energy
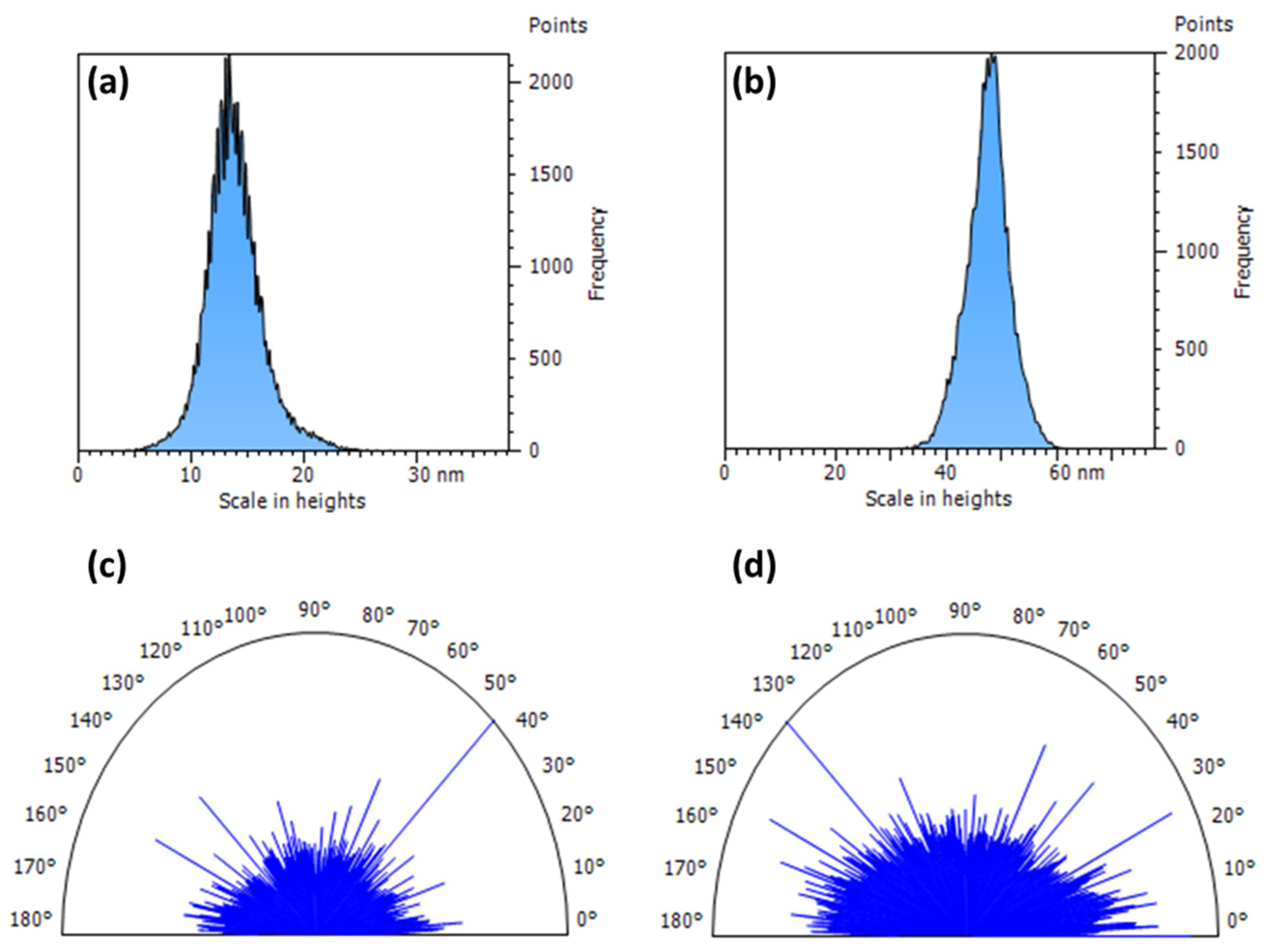
3.4. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macleod, H.A. Thin-Film Optical Filters, 5th ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781315270494. [Google Scholar]
- Kochergin, V. Omnidirectional Optical Filters. In Omnidirectional Optical Filters; Springer Nature: Berlin, Germany, 2003. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, W.; Yang, R.; Yang, L.; Wang, F.; Guo, J.; Xu, Z.; Feng, Q.; Wang, Y.; Hu, Q. 16-channel flexible optical passband filter array for CDCF ROADM. Opt. Commun. 2019, 450, 61–66. [Google Scholar] [CrossRef]
- Ge, P.; Ling, X.; Wang, J.; Liang, X.; Li, S.; Zhao, C. Optical Filter Bank Modeling and Design for Multi-Color Visible Light Communications. IEEE Photonics J. 2021, 13, 7901219. [Google Scholar] [CrossRef]
- Ghosh, B.; Mandal, S. Fiber Bragg grating-based optical filters for high-resolution sensing: A comprehensive analysis. Results Opt. 2023, 12, 100441. [Google Scholar] [CrossRef]
- Jen, Y.-J.; Lin, M.-J. Design and Fabrication of a Narrow Bandpass Filter with Low Dependence on Angle of Incidence. Coatings 2018, 8, 231. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Magkiriadou, S.; Choi, T.M.; Kim, Y.; Manoharan, V.N. Full-Spectrum Photonic Pigments with Non-iridescent Structural Colors through Colloidal Assembly. Angew. Chem. Int. Ed. 2014, 53, 2899–2903. [Google Scholar] [CrossRef]
- Ding, Y.; Pu, M.; Liu, L.; Xu, J.; Peucheret, C.; Zhang, X.; Huang, D.; Ou, H. Bandwidth and wavelength-tunable optical bandpass filter based on silicon microring-MZI structure. Opt. Express 2011, 19, 6462. [Google Scholar] [CrossRef]
- Madsen, C.K. Efficient architectures for exactly realizing optical filters with optimum bandpass designs. IEEE Photonics Technol. Lett. 1998, 10, 1136–1138. [Google Scholar] [CrossRef]
- Chu, Y.M.; Chiang, K.S.; Liu, Q. Widely tunable optical bandpass filter by use of polymer long-period waveguide gratings. Appl. Opt. 2006, 45, 2755. [Google Scholar] [CrossRef]
- Chan, E.H.W.; Alameh, K.E.; Minasian, R.A. Photonic bandpass filters with high skirt selectivity and stopband attenuation. J. Light. Technol. 2002, 20, 1962–1967. [Google Scholar] [CrossRef]
- Kischkat, J.; Peters, S.; Semtsiv, M.P.; Wegner, T.; Elagin, M.; Monastyrskyi, G.; Flores, Y.; Kurlov, S.; Masselink, W.T. Ultra-narrow angle-tunable Fabry–Perot bandpass interference filter for use as tuning element in infrared lasers. Infrared Phys. Technol. 2014, 67, 432–435. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thangaraju, B.; Khalaf, O.I.; Alotaibi, Y.; Alghamdi, S. Design and Synthesis of Multi-Mode Bandpass Filter for Wireless Applications. Electronics 2021, 10, 2853. [Google Scholar] [CrossRef]
- Pust, O. Innovative Filter Solutions for Hyperspectral Imaging. Opt. Photonik 2016, 11, 24–27. [Google Scholar] [CrossRef]
- Monti, A.; Alu, A.; Toscano, A.; Bilotti, F. Design of High-Q Passband Filters Implemented Through Multipolar All-Dielectric Metasurfaces. IEEE Trans. Antennas Propag. 2021, 69, 5142–5147. [Google Scholar] [CrossRef]
- Worth, B.; Lee, K.M.; Tondiglia, V.P.; Myers, J.; Mou, S.; White, T.J. Dynamic, infrared bandpass filters prepared from polymer-stabilized cholesteric liquid crystals. Appl. Opt. 2016, 55, 7134. [Google Scholar] [CrossRef]
- Kedawat, G.; Gupta, B.K.; Kumar, P.; Dwivedi, J.; Kumar, A.; Agrawal, N.K.; Kumar, S.S.; Vijay, Y.K. Fabrication of a Flexible UV Band-Pass Filter Using Surface Plasmon Metal–Polymer Nanocomposite Films for Promising Laser Applications. ACS Appl. Mater. Interfaces 2014, 6, 8407–8414. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, L.; Zhao, C.; Sakai, R.; Mikami, Y.; Feng, T.; Chen, C.; Liu, W.; Yoshioka, H.; Li, Z.; et al. Tunable and flexible deep-ultraviolet bandpass filters based on micro- and nanoparticle/polydimethylsiloxane hybrid membranes. Opt. Mater. 2021, 115, 111073. [Google Scholar] [CrossRef]
- El-Gamal, S.; Ismail, A.M. Electrical and optical properties of novel brilliant cresyl blue dye-doped poly(methyl methacrylate) as selective cut-off laser filters. Polym. Int. 2020, 69, 1308–1318. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, M. NIR band-pass filters for CMOS image sensors constructed with NIR absorbing dyes and plasmonic nanoparticles. Opt. Express 2022, 30, 44533. [Google Scholar] [CrossRef]
- Nechifor, C.D.; Postolache, M.; Albu, R.M.; Barzic, A.I.; Dorohoi, D.O. Induced birefringence of rubbed and stretched polyvinyl alcohol foils as alignment layers for nematic molecules. Polym. Adv. Technol. 2019, 30, 2143–2152. [Google Scholar] [CrossRef]
- Das, H.; Rabha, S.; Bhattacharjee, S.; Datta, P. Low-Pass Filter Characteristics of Zns: Cu Quantum Dots Embedded in Polyvinyl Alcohol (PVA). Int. J. Innov. Res. Dev. 2012, 1, 48–55. [Google Scholar]
- Bisen, R.; Tripathi, J.; Sharma, A.; Khare, A.; Kumar, Y.; Tripathi, S. Optical behaviour of coumarin dye in PVA and PMMA film matrices. Vacuum 2018, 152, 65–69. [Google Scholar] [CrossRef]
- Renjini, R.; Mitty, G.; Nampoori, V.P.N.; Mathew, S. Xanthene dye-doped PVA based thin-film optical filter characteristics and its green laser beam blocking. In Polymeric and Nanostructured Materials. Synthesis, Properties, and Advanced Applications; Thankappan, A., Kalarikkal, N., Thomas, S., Padinjakkara, A., Eds.; Apple Academic Press: Oakville, ON, Canada, 2019; pp. 127–134. [Google Scholar]
- Ali, F.M.; Yahia, I.S.; Sayed, M.A. Synthesis and optimization of a novel polymer: Dye composite (PVA:MV-6B) films for band-stop optical filters. Optik 2019, 192, 162902. [Google Scholar] [CrossRef]
- Durgesh; Kumar, R.; Sharma, P.K.; Sharma, A. Optical analysis of Nic-Ags based nanocomposite films for promising application as ultraviolet region bandpass filters. Mater. Sci. Eng. B 2022, 276, 115560. [Google Scholar] [CrossRef]
- El-Bashir, S.M.; Yahia, I.S.; Binhussain, M.A.; AlSalhi, M.S. Designing of PVA/Rose Bengal long-pass optical window applications. Results Phys. 2017, 7, 1238–1244. [Google Scholar] [CrossRef]
- Zelinschi, C.B.; Stoica, I.; Dorohoi, D.O. Changes in morphology and optical properties of polyvinyl alcohol foils induced by Congo red dye concentration and stretching degree. J. Polym. Eng. 2014, 34, 345–351. [Google Scholar] [CrossRef]
- Dorohoi, D.O.; Dumitrascu, L.; Dumitrascu, I. Order degree of polyvinyl alcohol (PVA) films estimated by a spectral method. Mater. Plast. 2008, 45, 106–108. [Google Scholar]
- Albu, R.M.; Stoica, I.; Barzic, A.I.; Postolache, M.; Angheluta, M.D.; Dorohoi, D.O. Effect of mechanical treatments on orientation behavior and spectral properties of azoderivative dyes incorporated in poly(vinyl alcohol) films. Polym. Eng. Sci. 2021, 61, 2453–2465. [Google Scholar] [CrossRef]
- Ghoshal, D.; Bhattacharya, D.; Mondal, D.; Das, S.; Bose, N.; Basu, M. Methylene Blue/PVA composite film for flexible, wide-scale UV–VIS laser cut-off filter. Mater. Res. Express 2019, 6, 075332. [Google Scholar] [CrossRef]
- Obeed, H.H.; Mohammad, R.K.; Al-Aaraji, H.H.; Tahir, K.J.; Hussein, B.M.; Ridha, N.J.; Alosfur, F.K.M.; Amadlool, R. Study of the optical properties of crystal violet doped PVA. AIP Conf. Proc. 2022, 2547, 030021. [Google Scholar]
- Kumar, V.; Sreelekshmi, K.R.; Shikha, K.T. Ancy Smitha Alex, Praveen Kumar Solasa, Refractometric Determination for Hydrogen Peroxide in Aqueous Solution-A Green Alternate to Iodometric Method. Anal. Chem. Indian J. 2021, 21, 162. [Google Scholar]
- LeBel, R.G.; Goring, D.A.I. Density, Viscosity, Refractive Index, and Hygroscopicity of Mixtures of Water and Dimethyl Sulfoxide. J. Chem. Eng. Data 1962, 7, 100–101. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters a User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Goodman, T.M. International standards for colour. In Colour Design; Elsevier: Amsterdam, The Netherlands, 2012; pp. 417–452. [Google Scholar]
- Choudhury, A.K.R. Using instruments to quantify colour. In Principles of Colour and Appearance Measurement; Elsevier: Amsterdam, The Netherlands, 2014; pp. 270–317. [Google Scholar]
- Farrugia, K.J.; Savage, K.A.; Bandey, H.; Ciuksza, T.; Nic Daéid, N. Chemical enhancement of footwear impressions in blood on fabric—Part 2: Peroxidase reagents. Sci. Justice 2011, 51, 110–121. [Google Scholar] [CrossRef]
- Kenny, P.W. Hydrogen-Bond Donors in Drug Design. J. Med. Chem. 2022, 65, 14261–14275. [Google Scholar] [CrossRef]
- Watanabe, S.; Oyaizu, K. Designing Ultrahigh-Refractive-Index Amorphous Poly(phenylene sulfide)s Based on Dense Intermolecular Hydrogen-Bond Networks. Macromolecules 2022, 55, 2252–2259. [Google Scholar] [CrossRef]
- Watanabe, S.; Takayama, T.; Oyaizu, K. Transcending the Trade-off in Refractive Index and Abbe Number for Highly Refractive Polymers: Synergistic Effect of Polarizable Skeletons and Robust Hydrogen Bonds. ACS Polym. Au 2022, 2, 458–466. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Branco, K.P.; Baptista, M.S.; Indig, G.L. Solvent and concentration effects on the visible spectra of tri-para-dialkylamino-substituted triarylmethane dyes in liquid solutions. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2002, 58, 2971–2982. [Google Scholar] [CrossRef]
- Xiang, A.; Lv, C.; Zhou, H. Changes in Crystallization Behaviors of Poly(Vinyl Alcohol) Induced by Water Content. J. Vinyl Addit. Technol. 2020, 26, 613–622. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Kang, J.; Chen, J.; Cao, Y. Water absorption dependence of the formation of poly(vinyl alcohol)-iodine complexes for poly(vinyl alcohol) films. RSC Adv. 2021, 11, 28785–28796. [Google Scholar] [CrossRef]
- Simone, E.A.; Dziubla, T.D.; Muzykantov, V.R. Polymeric carriers: Role of geometry in drug delivery. Expert Opin. Drug Deliv. 2008, 5, 1283–1300. [Google Scholar] [CrossRef]
- Higashihara, T.; Ueda, M. Recent Progress in High Refractive Index Polymers. Macromolecules 2015, 48, 1915–1929. [Google Scholar] [CrossRef]
- Cai, Y.; Hernandez, T.S.; Yeang, A.L.; Strand, M.T.; Yavitt, F.M.; Abraham, E.; McGehee, M.D. Gel polymer electrolyte for reversible metal electrodeposition dynamic windows enables dual-working electrodes for faster switching and reflectivity control. Front. Nanotechnol. 2022, 4, 1083247. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.N.; Ding, W.; Shi, B. Preparation of oxidised polyvinyl alcohol using hydrogen peroxide and its application for collagen modification. J. Soc. Leather Technol. Chem. 2019, 103, 14–20. [Google Scholar]
- Hamad, D.; Mehrvar, M.; Dhib, R. Experimental study of polyvinyl alcohol degradation in aqueous solution by UV/H2O2 process. Polym. Degrad. Stab. 2014, 103, 75–82. [Google Scholar] [CrossRef]
- Bouzidi, A.; Omri, K.; Jilani, W.; Guermazi, H.; Yahia, I.S. Influence of TiO2 Incorporation on the Microstructure, Optical, and Dielectric Properties of TiO2/Epoxy Composites. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1114–1126. [Google Scholar] [CrossRef]
- Dmitrieva, N.G.; Bocharnikova, E.N.; Ezhov, D.M. Induced Absorption Spectra of a Crystal Violet Dye. Russ. Phys. J. 2022, 64, 2089–2095. [Google Scholar] [CrossRef]
- Klein, J.; Kampermann, L.; Mockenhaupt, B.; Behrens, M.; Strunk, J.; Bacher, G. Limitations of the Tauc Plot Method. Adv. Funct. Mater. 2023, 33, 2304523. [Google Scholar] [CrossRef]
- Zanatta, A.R. Revisiting the optical bandgap of semiconductors and the proposal of a unified methodology to its determination. Sci. Rep. 2019, 9, 11225. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties and Electronic Structure of Amorphous Semiconductors. In Optical Properties of Solids; Springer: Boston, MA, USA, 1969; pp. 123–136. ISBN 9781139094337. [Google Scholar]
- Haryński, Ł.; Olejnik, A.; Grochowska, K.; Siuzdak, K. A facile method for Tauc exponent and corresponding electronic transitions determination in semiconductors directly from UV–Vis spectroscopy data. Opt. Mater. 2022, 127, 112205. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Moss, T.S. A Relationship between the Refractive Index and the Infra-Red Threshold of Sensitivity for Photoconductors. Proc. Phys. Soc. Sect. B 1950, 63, 167. [Google Scholar] [CrossRef]
- Steiner, T. The Hydrogen Bond in the Solid State. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Laurence, C.; Berthelot, M. Observations on the strength of hydrogen bonding. Perspect. Drug Discov. Des. 2000, 18, 39–60. [Google Scholar] [CrossRef]
- Bennett, H.E.; Porteus, J.O. Relation Between Surface Roughness and Specular Reflectance at Normal Incidence. J. Opt. Soc. Am. 1961, 51, 123. [Google Scholar] [CrossRef]
- Stoica, I.; Barzic, A.I.; Hulubei, C. The impact of rubbing fabric type on surface roughness and tribological properties of some semi-alicyclic polyimides evaluated from atomic force measurements. Appl. Surf. Sci. 2013, 268, 442–449. [Google Scholar] [CrossRef]
- Jain, N.; Singh, V.K.; Chauhan, S. A review on mechanical and water absorption properties of polyvinyl alcohol based composites/films. J. Mech. Behav. Mater. 2017, 26, 213–222. [Google Scholar] [CrossRef]
- Maruyama, K.; Akahoshi, H.; Kobayashi, M.; Tanizaki, Y. Assignment of Conjugate Double Bond Systems Produced in Heated PVA Film by Absorption and Excitation Spectra. Bull. Chem. Soc. Jpn. 1985, 58, 2923–2928. [Google Scholar] [CrossRef]
- Sau, S.; Pandit, S.; Kundu, S. Crosslinked poly (vinyl alcohol): Structural, optical and mechanical properties. Surf. Interfaces 2021, 25, 101198. [Google Scholar] [CrossRef]


| Solvent | Refractive Index * | Hansen Solubility Parameters, MPa1/2 ** | Calculated Parameters from Simulations | ||||
|---|---|---|---|---|---|---|---|
| δd | δp | δh | Polarizability, Å3 | Refractivity, Å3 | Dipole Moment, D | ||
| H2O | 1.33 | 15.60 | 16.00 | 42.30 | 1.41 | 3.36 | 1.74 |
| DMSO | 1.48 | 18.40 | 16.40 | 10.20 | 5.01 | 20.56 | 4.48 |
| H2O2 | 1.41 | 15.50 | 12.20 | 42.70 | 2.05 | 4.56 | 2.71 |
| System | CV Content, UI | X | Y | Z | L* | a* | b* |
|---|---|---|---|---|---|---|---|
| PVA/CV/H2O | 0 | 4154.3712 | 4497.5076 | 4898.5273 | 96.6582 | 0.0918 | −0.3890 |
| 1 | 3730.7950 | 4000.1610 | 4980.4176 | 92.3423 | 1.5930 | −8.9088 | |
| 5 | 1814.8195 | 1679.8231 | 4697.2185 | 65.1322 | 18.8166 | −52.0406 | |
| 10 | 1219.2217 | 869.2384 | 4440.1880 | 49.1356 | 42.0053 | −76.0544 | |
| 20 | 979.0900 | 526.7763 | 4185.5423 | 39.1207 | 62.4166 | −89.6497 | |
| PVA/CV/DMSO | 0 | 4218.4243 | 4566.2714 | 5035.3936 | 97.2295 | 0.1129 | −1.2001 |
| 1 | 4096.6821 | 4423.2108 | 5065.6116 | 96.0344 | 0.5219 | −3.6526 | |
| 5 | 2750.4957 | 2853.5661 | 4827.5368 | 80.8058 | 6.0438 | −26.7759 | |
| 10 | 1612.6946 | 1431.3740 | 4614.9630 | 60.9172 | 22.7616 | −58.1811 | |
| 20 | 903.3637 | 453.7775 | 3975.3192 | 36.4469 | 65.9984 | −91.1144 | |
| PVA/CV/H2O2 | 0 | 4188.7733 | 4533.7303 | 5005.2761 | 96.9599 | 0.1286 | −1.2725 |
| 1 | 4176.8625 | 4520.4744 | 4987.0259 | 96.8497 | 0.1415 | −1.2240 | |
| 5 | 4214.3799 | 4563.5649 | 5015.9421 | 97.2071 | 0.0533 | −0.9854 | |
| 10 | 4192.7908 | 4541.2413 | 4970.4316 | 97.0222 | 0.0155 | −0.7091 | |
| 20 | 4179.0709 | 4530.4436 | 4908.6201 | 96.9326 | −0.1301 | −0.0495 |
| System | CV Content, UI | Eg-d(1) (eV) | Eg-d(2) (eV) | Eg-d(3) (eV) | Eg-d(4) (eV) | Eg-i(1) (eV) | Eg-i(2) (eV) | Eg-i(3) (eV) | Eg-i(4) (eV) |
|---|---|---|---|---|---|---|---|---|---|
| PVA/CV/H2O | 0 | - | - | - | 5.75 | - | - | - | 5.46 |
| 1 | - | - | - | 5.64 | - | - | - | 5.34 | |
| 5 | 1.93 | 3.72 | 4.29 | 5.56 | 1.90 | 3.67 | 4.15 | 5.27 | |
| 10 | 1.88 | 3.75 | 4.18 | 5.47 | 1.88 | 3.62 | 4.02 | 5.16 | |
| 20 | 1.90 | 3.68 | 4.07 | 5.42 | 1.82 | 3.57 | 3.85 | 5.11 | |
| PVA/CV/DMSO | 0 | - | - | 5.28 | - | - | - | 5.24 | - |
| 1 | - | - | 5.32 | - | - | - | 5.20 | - | |
| 5 | 1.97 | 4.12 | 5.43 | - | 1.93 | 4.10 | 5.16 | - | |
| 10 | 1.93 | 3.84 | 5.49 | - | 1.89 | 3.39 | 5.10 | - | |
| 20 | 1.85 | 3.66 | 5.41 | - | 1.86 | 3.22 | 5.06 | - | |
| PVA/CV/H2O2 | 0 | 5.85 | - | - | - | 5.68 | - | - | - |
| 1 | 5.56 | - | - | - | 5.51 | - | - | - | |
| 5 | 5.42 | - | - | - | 5.38 | - | - | - | |
| 10 | 5.28 | - | - | - | 5.25 | - | - | - | |
| 20 | 5.09 | - | - | - | 5.16 | - | - | - |
| System | CV Content, UI | Sq, nm | Sdr % | Sku | Stdi | Str |
|---|---|---|---|---|---|---|
| PVA/CV/H2O | 0 | 2.5 | 0.072 | 5.585 | 0.689 | 0.726 |
| 20 | 3.9 | 0.583 | 4.287 | 0.812 | 0.750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albu, R.M.; Stoica, I.; Nica, S.L.; Soroceanu, M.; Barzic, A.I. Tailoring Optical Performance of Polyvinyl Alcohol/Crystal Violet Band-Pass Filters via Solvent Features. Polymers 2024, 16, 3288. https://doi.org/10.3390/polym16233288
Albu RM, Stoica I, Nica SL, Soroceanu M, Barzic AI. Tailoring Optical Performance of Polyvinyl Alcohol/Crystal Violet Band-Pass Filters via Solvent Features. Polymers. 2024; 16(23):3288. https://doi.org/10.3390/polym16233288
Chicago/Turabian StyleAlbu, Raluca Marinica, Iuliana Stoica, Simona Luminita Nica, Marius Soroceanu, and Andreea Irina Barzic. 2024. "Tailoring Optical Performance of Polyvinyl Alcohol/Crystal Violet Band-Pass Filters via Solvent Features" Polymers 16, no. 23: 3288. https://doi.org/10.3390/polym16233288
APA StyleAlbu, R. M., Stoica, I., Nica, S. L., Soroceanu, M., & Barzic, A. I. (2024). Tailoring Optical Performance of Polyvinyl Alcohol/Crystal Violet Band-Pass Filters via Solvent Features. Polymers, 16(23), 3288. https://doi.org/10.3390/polym16233288







