Single/Multi-Network Conductive Hydrogels—A Review
Abstract
:1. Introduction
2. Conductive Hydrogels
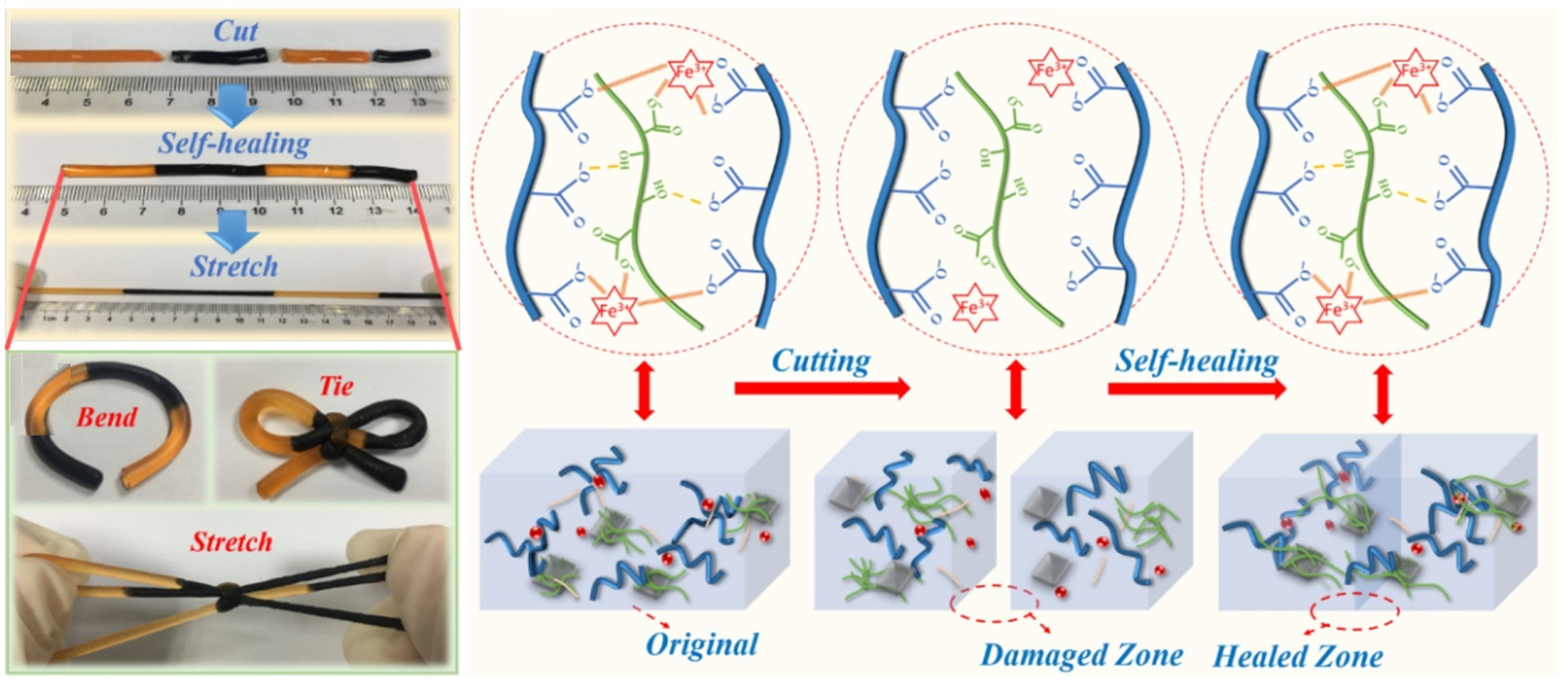
2.1. Self-Healing Conductive Hydrogels
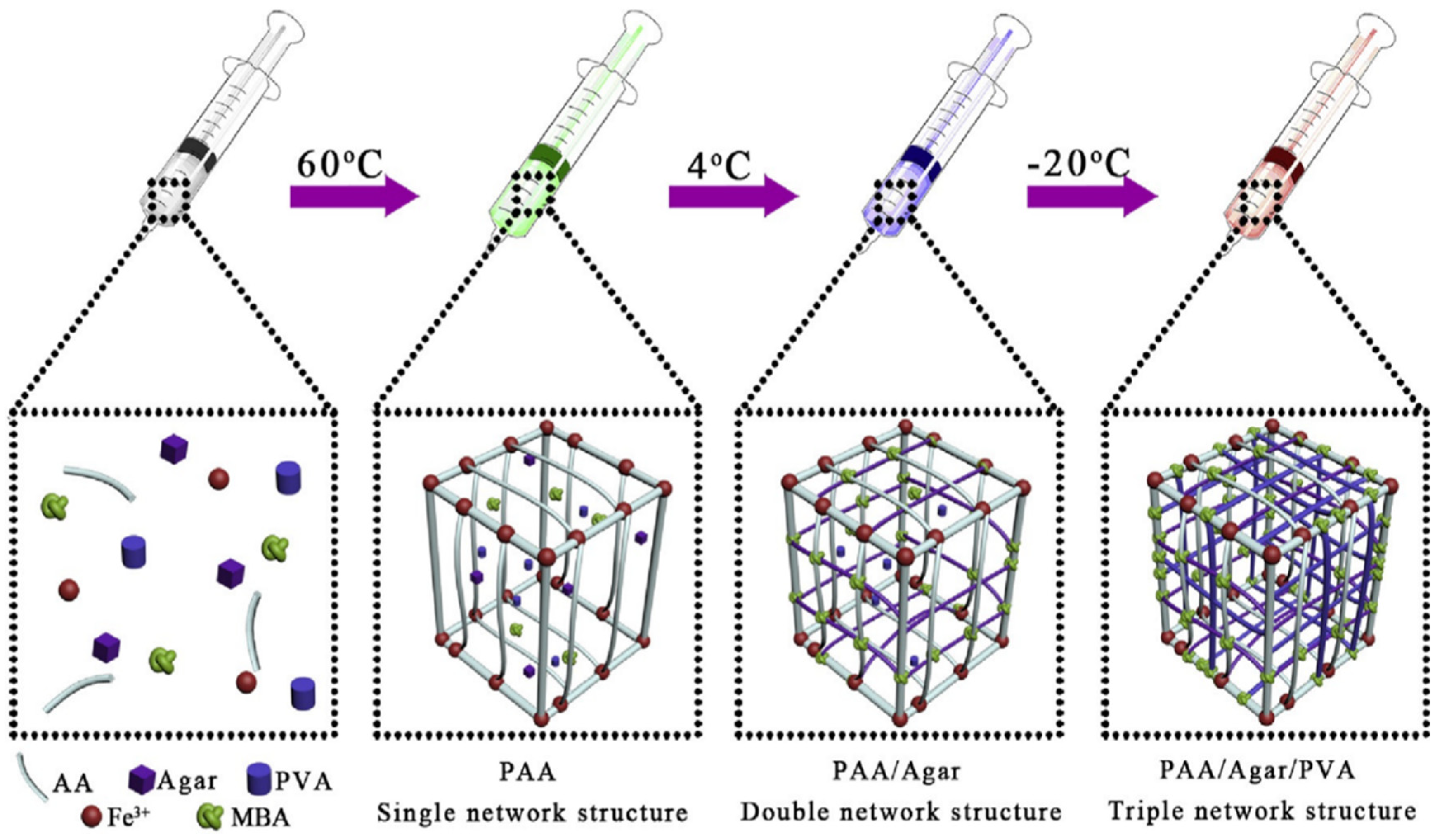
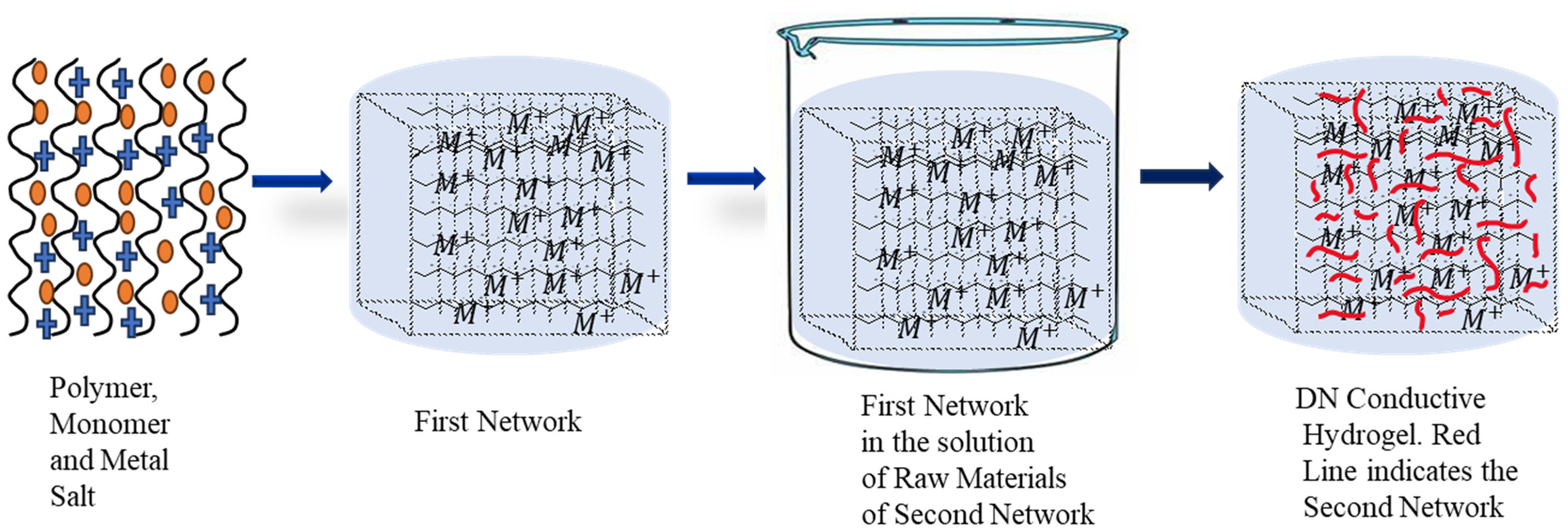
2.2. Double/Triple Network Conductive Hydrogels
2.3. Natural Polymer Based Conductive Hydrogels
2.4. Stimuli-Responsive Conductive Hydrogels
2.5. Gamma Radiation-Induced Conductive Hydrogels
2.6. Three-Dimensional Printable Conductive Hydrogels
3. Current Challenges and Future Prospects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | 3-dimension |
| AAm | Acrylamide |
| AMPS | Poly (2-acrylamido-2-methylpropanesulfonic acid |
| APTAC | (3-Acrylamidopropyl) trimethylammonium chloride |
| BEDT-TTF | Bis(ethylenedithiolo) tetrathiafulvalene |
| CAD | Computer-aided design |
| CHs | Conductive hydrogels |
| CMC | Carboxymethyl cellulose |
| CNPs | Carbon nanoparticles |
| CNT | Carbon nanotube |
| CS | Chitosan |
| DADMAC | diallyldimethylammonium chloride |
| DIW | Direct-ink-write |
| DN | Double network |
| EGaIn | Eutectic gallium indium |
| GN | Graphene |
| GO | Graphene oxides |
| HA | Hyaluronic acid |
| NIPAM | N-isopropylacrylamide |
| NIR | Near-infrared light |
| PAA | Polyacrylic acid |
| PANI | Polyaniline |
| PC | Polycarbazole |
| PEDOT: PSS | Poly(3,4-ethylenedioxythiophene: polystyrene sulfonate) |
| PEGDA | Polyethylene glycol diacrylate |
| PPV | Phenylene vinylene |
| PPy | Polypyrrole |
| PTh | Polythiophene |
| PVA | Poly (vinyl alcohol) |
| TCNQ | 7,7,8,8-tetracyanoquinodimethane |
| TMTSF | Tetramethyl-tetraselenafulvalene |
| TN | Triple network |
| TOCNFs | TEMPO-oxidized cellulose nanofibers |
| TTE | Tetrathiafulvalene |
References
- Dong, H.; Zhang, L.X.; Xu, H.; Yin, Y.Y.; Zhao, X.B.; Bie, L.J. H-Bonding Interactions Enable a 3D Pillared Cobalt (II) Coordination Polymer for Touchless Finger Moisture Detection. Tungsten 2023, 5, 109–117. [Google Scholar] [CrossRef]
- Jin, H.Z.; Qiu, C.X.; Li, Y.S.; Liu, B.; Liu, J.Y.; Chen, Q.; Lu, X.F.; Li, C.X.; Wang, Q.K. Structural and Functional Design of Geopolymer Adsorbents: A Review. Tungsten 2024, 6, 48–76. [Google Scholar] [CrossRef]
- Goyal, M.; Singh, K.; Bhatnagar, N. Conductive Polymers: A Multipurpose Material for Protecting Coating. Prog. Org. Coat. 2024, 187, 108083. [Google Scholar] [CrossRef]
- Liu, K.; Wei, S.; Song, L.; Liu, H.; Wang, T. Conductive Hydrogels—A Novel Material: Recent Advances and Future Perspectives. J. Agric. Food Chem. 2020, 68, 7269–7280. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, N.; Kimura, H.; Inoue, A. Microstructures and Mechanical Properties of Highly Electrically Conductive Cu0.5, Cu1 and Cu2at% Zr Alloy Wires. Mater. Trans. 2013, 54, 176–183. [Google Scholar] [CrossRef]
- Zhu, T.; Ni, Y.; Biesold, G.M.; Cheng, Y.; Ge, M.; Li, H.; Huang, J.; Lin, Z.; Lai, Y. Recent Advances in Conductive Hydrogels: Classifications, Properties, and Applications. Chem. Soc. Rev. 2022, 52, 473–509. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhai, H.; Hu, P.; Jiang, H. The Stoichiometry of TCNQ-Based Organic Charge-Transfer Cocrystals. Crystals 2020, 10, 993. [Google Scholar] [CrossRef]
- Otsubo, T.; Takimiya, K. Recent Synthetic Advances of Tetrathiafulvalene-Based Organic Conductors. Bull. Chem. Soc. Jpn. 2004, 77, 43–58. [Google Scholar] [CrossRef]
- Guarino, V.; Alvarez-Perez, M.A.; Borriello, A.; Napolitano, T.; Ambrosio, L. Conductive PANi/PEGDA Macroporous Hydrogels For Nerve Regeneration. Adv. Healthc. Mater. 2013, 2, 218–227. [Google Scholar] [CrossRef]
- Ren, K.; Cheng, Y.; Huang, C.; Chen, R.; Wang, Z.; Wei, J. Self-Healing Conductive Hydrogels Based on Alginate, Gelatin and Polypyrrole Serve as a Repairable Circuit and a Mechanical Sensor. J. Mater. Chem. B 2019, 7, 5704–5712. [Google Scholar] [CrossRef]
- Yang, T.; Yang, M.; Xu, C.; Yang, K.; Su, Y.; Ye, Y.; Dou, L.; Yang, Q.; Ke, W.; Wang, B.; et al. PEDOT:PSS Hydrogels with High Conductivity and Biocompatibility for in Situ Cell Sensing. J. Mater. Chem. B 2023, 11, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Pattavarakorn, D.; Youngta, P.; Jaesrichai, S.; Thongbor, S.; Chaimongkol, P. Electroactive Performances of Conductive Polythiophene/Hydrogel Hybrid Artificial Muscle. Energy Procedia 2013, 34, 673–681. [Google Scholar] [CrossRef]
- Niamlang, S.; Sirivat, A. Electrically Controlled Release of Salicylic Acid from Poly(p-Phenylene Vinylene)/Polyacrylamide Hydrogels. Int. J. Pharm. 2009, 371, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, W.; Petcharoen, K.; Paradee, N.; Lerdwijitjarud, W.; Sirivat, A. Electrically Responsive Materials Based on Polycarbazole/Sodium Alginate Hydrogel Blend for Soft and Flexible Actuator Application. Carbohydr. Polym. 2016, 151, 213–222. [Google Scholar] [CrossRef]
- Keplinger, C.; Sun, J.Y.; Foo, C.C.; Rothemund, P.; Whitesides, G.M.; Suo, Z. Stretchable, Transparent, Ionic Conductors. Science 2013, 341, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically Conductive Hydrogels for Flexible Energy Storage Systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Kim, C.C.; Lee, H.H.; Oh, K.H.; Sun, J.Y. Highly Stretchable, Transparent Ionic Touch Panel. Science 2016, 353, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, H.; Lai, J.; Yan, B.; Liu, H.; Jin, X.; Ma, A.; Zhang, G.; Zhao, W.; Chen, W. Extremely Stretchable and Electrically Conductive Hydrogels with Dually Synergistic Networks for Wearable Strain Sensors. J. Mater. Chem. C 2018, 6, 9200–9207. [Google Scholar] [CrossRef]
- Grancarić, A.M.; Jerković, I.; Koncar, V.; Cochrane, C.; Kelly, F.M.; Soulat, D.; Legrand, X. Conductive Polymers for Smart Textile Applications. J. Ind. Text. 2018, 48, 612–642. [Google Scholar] [CrossRef]
- Pérez-Martínez, C.J.; Morales Chávez, S.D.; Del Castillo-Castro, T.; Lara Ceniceros, T.E.; Castillo-Ortega, M.M.; Rodríguez-Félix, D.E.; Gálvez Ruiz, J.C. Electroconductive Nanocomposite Hydrogel for Pulsatile Drug Release. React. Funct. Polym. 2016, 100, 12–17. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D Printing of Electrically Conductive Hydrogels for Tissue Engineering and Biosensors—A Review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nonoyama, T.; Gong, J.P. Double-Network Hydrogel and Its Potential Biomedical Application: A Review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2015, 229, 853–863. [Google Scholar] [CrossRef]
- Cong, J.; Fan, Z.; Pan, S.; Tian, J.; Lian, W.; Li, S.; Wang, S.; Zheng, D.; Miao, C.; Ding, W.; et al. Polyacrylamide/Chitosan-Based Conductive Double Network Hydrogels with Outstanding Electrical and Mechanical Performance at Low Temperatures. ACS Appl. Mater. Interfaces 2021, 13, 34942–34953. [Google Scholar] [CrossRef]
- Andrade, J.R.; Raphael, E.; Pawlicka, A. Plasticized Pectin-Based Gel Electrolytes. Electrochim. Acta 2009, 54, 6479–6483. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, D.; Gan, W.; Zhang, Z. A ZnO/PVA/PAADDA Composite Electrode for Rechargeable Zinc-Air Battery. Ionics 2017, 23, 3469–3477. [Google Scholar] [CrossRef]
- Sharma, K.; Kaith, B.S.; Kumar, V.; Kalia, S.; Kumar, V.; Swart, H.C. Synthesis and Biodegradation Studies of Gamma Irradiated Electrically Conductive Hydrogels. Polym. Degrad. Stab. 2014, 107, 166–177. [Google Scholar] [CrossRef]
- Martinez, M.V.; Bruno, M.M.; Miras, M.C.; Barbero, C.A. Electroactive Polymers Made by Loading Redox Ions inside Crosslinked Polymeric Hydrogels. Effects of Hydrophobic Interactions and Solvent Dynamics. Electrochim. Acta 2016, 219, 363–376. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Yan, P.; Hou, G.; Li, G. Two 7,7,8,8-Tetracyanoquinodimethane Lead and Zinc Complexes Featuring 3D and 0D Structure: Synthesis, Structure and Electrochemical Properties. Inorg. Chim. Acta 2014, 413, 32–37. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, D.; Zhang, G.; Zhu, D. Tetrathiafulvalene (TTF)-Based Gelators: Stimuli Responsive Gels and Conducting Nanostructures. Sci. China Chem. 2011, 54, 596–602. [Google Scholar] [CrossRef]
- Blundell, T.J.; Morritt, A.L.; Rusbridge, E.K.; Quibell, L.; Oakes, J.; Akutsu, H.; Nakazawa, Y.; Imajo, S.; Kadoya, T.; Yamada, J.I.; et al. Molecular Conductors from Bis(Ethylenedithio)Tetrathiafulvalene with Tris(Oxalato)Gallate and Tris(Oxalato)Iridate. Mater. Adv. 2022, 3, 4724–4735. [Google Scholar] [CrossRef]
- Tang, S.J.; Wang, A.T.; Lin, S.Y.; Huang, K.Y.; Yang, C.C.; Yeh, J.M.; Chiu, K.C. Polymerization of Aniline under Various Concentrations of APS and HCl. Polym. J. 2011, 43, 667–675. [Google Scholar] [CrossRef]
- Bi, S.; Hou, L.; Lu, Y. Multifunctional Sodium Alginate Fabric Based on Reduced Graphene Oxide and Polypyrrole for Wearable Closed-Loop Point-of-Care Application. Chem. Eng. J. 2021, 406, 126778. [Google Scholar] [CrossRef]
- Goyal, M.; Bhatnagar, N. Conductive Polymer-Based Coating Layer on Copper Current Collector for Enhanced Performance of Li-Ion Battery. J. Appl. Polym. Sci. 2022, 139, e53213. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From Fundamental Perspectives to Applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Banerjee, J.; Dutta, K. A Short Overview on the Synthesis, Properties and Major Applications of Poly(p-Phenylene Vinylene). Chem. Pap. 2021, 75, 5139–5151. [Google Scholar] [CrossRef]
- Nayana, V.; Kandasubramanian, B. Polycarbazole and Its Derivatives: Progress, Synthesis, and Applications. J. Polym. Res. 2020, 27, 285. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional Conductive Hydrogels and Their Applications as Smart Wearable Devices. J. Mater. Chem. B 2021, 9, 2561–2583. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Qing, X.; Zhou, H.; Shen, C.; Wang, J.; Lu, Y. Mechanically Strong Conducting Hydrogels with Special Double-Network Structure. Synth. Met. 2010, 160, 791–796. [Google Scholar] [CrossRef]
- Song, Y.; Niu, L.; Ma, P.; Li, X.; Feng, J.; Liu, Z. Rapid Preparation of Antifreezing Conductive Hydrogels for Flexible Strain Sensors and Supercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 10006–10017. [Google Scholar] [CrossRef]
- Bai, Y.; Yan, S.; Wang, Y.; Wang, Q.; Duan, X. Facile Preparation of a Self-Adhesive Conductive Hydrogel with Long-Term Usability. ACS Appl. Mater. Interfaces 2023, 15, 48744–48753. [Google Scholar] [CrossRef]
- Khan, M.; Shah, L.A.; Ara, L.; Ullah, R.; Yoo, H.M. Micelle-Micelle Cross-Linked Highly Stretchable Conductive Hydrogels for Potential Applications of Strain and Electronic Skin Sensors. Chem. Mater. 2023, 35, 5582–5592. [Google Scholar] [CrossRef]
- Lin, X.; Yang, X.; Li, P.; Xu, Z.; Zhao, L.; Mu, C.; Li, D.; Ge, L. Antibacterial Conductive Collagen-Based Hydrogels for Accelerated Full-Thickness Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 22817–22829. [Google Scholar] [CrossRef] [PubMed]
- Patnam, H.; Graham, S.A.; Manchi, P.; Paranjape, M.V.; Yu, J.S. Single-Electrode Triboelectric Nanogenerators Based on Ionic Conductive Hydrogel for Mechanical Energy Harvester and Smart Touch Sensor Applications. ACS Appl. Mater. Interfaces 2023, 15, 16768–16777. [Google Scholar] [CrossRef] [PubMed]
- Prameswati, A.; Nurmaulia Entifar, S.A.; Han, J.W.; Wibowo, A.F.; Kim, J.H.; Sembiring, Y.S.B.; Park, J.; Lee, J.; Lee, A.Y.; Song, M.H.; et al. Self-Healable Conductive Hydrogels with High Stretchability and Ultralow Hysteresis for Soft Electronics. ACS Appl. Mater. Interfaces 2023, 15, 24648–24657. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, T.; Zhang, Z.; Wang, H.; Lu, S. Tremella Polysaccharide-Based Conductive Hydrogel with Anti-Freezing and Self-Healing Ability for Motion Monitoring and Intelligent Interaction. Int. J. Biol. Macromol. 2023, 248, 125987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, W.; Dai, Y.; Wu, C.; Li, B.; Guo, X.; Liao, B.; Zeng, W. Ultrasensitive, Flexible and Dual Strain-Temperature Sensor Based on Ionic-Conductive Composite Hydrogel for Wearable Applications. Compos. Part A Appl. Sci. Manuf. 2023, 171, 107572. [Google Scholar] [CrossRef]
- Li, Z.; Yin, F.; He, W.; Hang, T.; Li, Z.; Zheng, J.; Li, X.; Jiang, S.; Chen, Y. Anti-Freezing, Recoverable and Transparent Conductive Hydrogels Co-Reinforced by Ethylene Glycol as Flexible Sensors for Human Motion Monitoring. Int. J. Biol. Macromol. 2023, 230, 123117. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Peng, K.; Lin, L.; Yang, J.; Cheng, Z.; Gan, Q.; Chen, Y.; Feng, C. A Conductive Hydrogel Based on Nature Polymer Agar with Self-Healing Ability and Stretchability for Flexible Sensors. Chem. Eng. J. 2023, 454, 139843. [Google Scholar] [CrossRef]
- Ullah, R.; Shah, L.A.; Khan, M.; Ara, L. Guar Gum Reinforced Conductive Hydrogel for Strain Sensing and Electronic Devices. Int. J. Biol. Macromol. 2023, 246, 125666. [Google Scholar] [CrossRef]
- Shen, K.; Liu, Z.; Xie, R.; Zhang, Y.; Yang, Y.; Zhao, X.; Zhang, Y.; Yang, A.; Cheng, Y. Nanocomposite Conductive Hydrogels with Robust Elasticity and Multifunctional Responsiveness for Flexible Sensing and Wound Monitoring. Mater. Horiz. 2023, 10, 2096–2108. [Google Scholar] [CrossRef]
- Zhang, X. Dry and Frost Resistance Conductive Hydrogels Based on Carbon Nanotubes Hybrids for Use as Flexible Strain Sensor. Sens. Actuators A Phys. 2023, 350, 114143. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Guo, Z.; Zhang, C.; Tan, H.; Gong, G.; Yu, M.; Xu, L. Fully Physical Crosslinked BSA-Based Conductive Hydrogels with High Strength and Fast Self-Recovery for Human Motion and Wireless Electrocardiogram Sensing. Int. J. Biol. Macromol. 2023, 230, 123195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, L.; Lin, X.; Lu, J.; Huang, Z.; Sun, P.; Zhang, Y.; Xu, X.; Li, Q.; Liu, H. Dual-Network Polyvinyl Alcohol/Polyacrylamide/Xanthan Gum Ionic Conductive Hydrogels for Flexible Electronic Devices. Int. J. Biol. Macromol. 2023, 233, 123573. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Garcia, J.; Leahy, L.M.; Song, R.; Mullarkey, D.; Fei, B.; Dervan, A.; Shvets, I.V.; Stamenov, P.; Wang, W.; et al. 3D Printing of Multifunctional Conductive Polymer Composite Hydrogels. Adv. Funct. Mater. 2023, 33, 2214196. [Google Scholar] [CrossRef]
- Jia, M.; Chen, Q.; Chen, K.; Zhang, X.; Feng, H.; Feng, C.; Li, X.; Zhang, D. Muscle-Inspired MXene-Based Conductive Hydrogel by Magnetic Induced for Flexible Multifunctional Sensors. Eur. Polym. J. 2024, 214, 113149. [Google Scholar] [CrossRef]
- Hou, M.; Yu, M.; Liu, W.; Zhang, H.; Wang, Z.; Du, J.; Xu, L.; Li, N.; Xu, J. Mxene Hybrid Conductive Hydrogels with Mechanical Flexibility, Frost-Resistance, Photothermoelectric Conversion Characteristics and Their Multiple Applications in Sensing. Chem. Eng. J. 2024, 483, 149299. [Google Scholar] [CrossRef]
- Hu, M.; Qiu, L.; Huang, Y.; Wang, D.; Li, J.; Liang, C.; Wu, G.; Peng, F. An Adhesive, Low Swelling and Conductive Tri-Network Hydrogel for Wearable Electronic Devices. J. Mater. Chem. C 2024, 12, 8534–8544. [Google Scholar] [CrossRef]
- Khan, M.; Rahman, T.U.; Sher, M.; Shah, L.A.; Md Akil, H.; Fu, J.; Yoo, H.M. Flexible Ionic Conductive Hydrogels with Wrinkled Texture for Flexible Strain Transducer with Language Identifying Diversity. Chem. Mater. 2024, 36, 4703–4713. [Google Scholar] [CrossRef]
- Mogli, G.; Reina, M.; Chiappone, A.; Lamberti, A.; Pirri, C.F.; Roppolo, I.; Stassi, S. Self-Powered Integrated Tactile Sensing System Based on Ultrastretchable, Self-Healing and 3D Printable Ionic Conductive Hydrogel. Adv. Funct. Mater. 2024, 34, 2307133. [Google Scholar] [CrossRef]
- Chen, M.; Liu, H.; Chen, X.; Kang, L.; Yao, X.; Tan, L.; Zhu, W.; Yu, J.; Qin, X.; Wu, D. A Novel Multifunction of Wearable Ionic Conductive Hydrogel Sensor for Promoting Infected Wound Healing. Appl. Mater. Today 2024, 39, 102298. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Liu, W.; Yu, D.; Song, Z.; Li, G.; Liu, X.; Wang, H.; Ge, S. Using Chitosan Nanofibers to Simultaneously Improve the Toughness and Sensing Performance of Chitosan-Based Ionic Conductive Hydrogels. Int. J. Biol. Macromol. 2024, 260, 129272. [Google Scholar] [CrossRef]
- Aycan, D.; Karaca, F.; Koca, A.; Alemdar, N. Electro-Stimulated Drug Release by Methacrylated Hyaluronic Acid-Based Conductive Hydrogel with Enhanced Mechanical Properties. Int. J. Biol. Macromol. 2023, 231, 123297. [Google Scholar] [CrossRef]
- Zaman, S.u.; Mushtaq, B.; Ahmad, F.; Ahmad, S.; Rasheed, A.; Nawab, Y. Development of Conductive Cotton Non-Woven Alginate Hydrogel Composite for Smart Textiles. J. Polym. Environ. 2023, 31, 3998–4006. [Google Scholar] [CrossRef]
- Taylor, D.L.; in het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hsu, S.H. Synthesis and Biomedical Applications of Self-Healing Hydrogels. Front. Chem. 2018, 6, 449. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Z.; Chandrasekaran, S.; Liu, Y.; Wu, M. Self-Healing, Antibacterial, and Conductive Double Network Hydrogel for Strain Sensors. Carbohydr. Polym. 2023, 303, 120468. [Google Scholar] [CrossRef]
- Zheng, C.; Lu, K.; Lu, Y.; Zhu, S.; Yue, Y.; Xu, X.; Mei, C.; Xiao, H.; Wu, Q.; Han, J. A Stretchable, Self-Healing Conductive Hydrogels Based on Nanocellulose Supported Graphene towards Wearable Monitoring of Human Motion. Carbohydr. Polym. 2020, 250, 116905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, T.; Xie, X.; Song, G.; Ma, X.; Mu, Q.; Huang, Z.; Liu, X.; Sun, C.; Xu, W. Advances and Challenges in Conductive Hydrogels: From Properties to Applications. Eur. Polym. J. 2022, 177, 111454. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Gong, X.; Chang, Y.; Zheng, J. Highly Conductive and Tough Double-network Hydrogels for Smart Electronics. SmartMat 2024, 5, e1160. [Google Scholar] [CrossRef]
- Dai, S.; Wang, S.; Dong, X.; Xu, X.; Cao, X.; Chen, Y.; Zhou, X.; Ding, J.; Yuan, N. A Transparent, Tough Self-Healing Hydrogel Based on a Dual Physically and Chemically Triple Crosslinked Network. J. Mater. Chem. C 2019, 7, 14581–14587. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, F.; Pang, B.; Qin, X.; Feng, S. Triple Network Hydrogels (TN Gels) Prepared by a One-Pot, Two-Step Method with High Mechanical Properties. RSC Adv. 2018, 8, 6789–6797. [Google Scholar] [CrossRef]
- Argun, A.; Can, V.; Altun, U.; Okay, O. Nonionic Double and Triple Network Hydrogels of High Mechanical Strength. Macromolecules 2014, 47, 6430–6440. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, J.; Hou, J.; Wang, Z.; Wu, J.; Meng, G.; Liu, Z.; Guo, X. A Novel Design Strategy for Triple-Network Structure Hydrogels with High-Strength, Tough and Self-Healing Properties. Polymer 2018, 135, 16–24. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of Double Network Hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super Tough Double Network Hydrogels and Their Application as Biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive Polymers: Towards a Smart Biomaterial for Tissue Engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, F.; Wu, J. Physically Crosslinked Hydrogels from Polysaccharides Prepared by Freeze-Thaw Technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Rowley, J.A.; Madlambayan, G.; Mooney, D.J. Alginate Hydrogels as Synthetic Extracellular Matrix Materials. Biomaterials 1999, 20, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Park, J.M.; Oh, M.S.; Nguyen, T.L.; Shin, H.; Kim, J.S.; Kim, D.; Park, H.S.; Kim, J. Superstrong, Superstiff, and Conductive Alginate Hydrogels. Nat. Commun. 2022, 13, 3019. [Google Scholar] [CrossRef]
- Shi, X.W.; Qiu, L.; Nie, Z.; Xiao, L.; Payne, G.F.; Du, Y. Protein Addressing on Patterned Microchip by Coupling Chitosan Electrodeposition and “electro-Click” Chemistry. Biofabrication 2013, 5, 041001. [Google Scholar] [CrossRef]
- Shi, Z.; Gao, X.; Ullah, M.W.; Li, S.; Wang, Q.; Yang, G. Electroconductive Natural Polymer-Based Hydrogels. Biomaterials 2016, 111, 40–54. [Google Scholar] [CrossRef]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef]
- Liang, X.; Qu, B.; Li, J.; Xiao, H.; He, B.; Qian, L. Preparation of Cellulose-Based Conductive Hydrogels with Ionic Liquid. React. Funct. Polym. 2015, 86, 1–6. [Google Scholar] [CrossRef]
- Sun, X.; Liang, Y.; Ye, L.; Liang, H. An Extremely Tough and Ionic Conductive Natural-Polymer-Based Double Network Hydrogel. J. Mater. Chem. B 2021, 9, 7751–7759. [Google Scholar] [CrossRef]
- Pyarasani, R.D.; Jayaramudu, T.; John, A. Polyaniline-Based Conducting Hydrogels. J. Mater. Sci. 2019, 54, 974–996. [Google Scholar] [CrossRef]
- Nada, A.A.; Andicsová, A.E.; Mosnáček, J. Irreversible and Self-Healing Electrically Conductive Hydrogels Made of Bio-Based Polymers. Int. J. Mol. Sci. 2022, 23, 842. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, R.; Guo, B. Stimuli-Responsive Conductive Hydrogels: Design, Properties, and Applications. Mater. Chem. Front. 2021, 5, 2092–2123. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, C.; Peng, L.; Yu, G. Conductive “Smart” Hybrid Hydrogels with PNIPAM and Nanostructured Conductive Polymers. Adv. Funct. Mater. 2015, 25, 1219–1225. [Google Scholar] [CrossRef]
- Zheng, M.; Cai, K.; Chen, M.; Zhu, Y.; Zhang, L.; Zheng, B. PH-Responsive Poly(Gellan Gum-Co-Acrylamide-Co-Acrylic Acid) Hydrogel: Synthesis, and Its Application for Organic Dye Removal. Int. J. Biol. Macromol. 2020, 153, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, W.; Wang, A. A PH Sensitive Carboxymethyl Cellulose-g-poly (Acrylic Acid)/Polyvinylpyrrolidone/Sodium Alginate Composite Hydrogel Bead for the Controlled Release of Diclofenac. J. Control. Release 2015, 213, e91–e92. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Wang, Z.; Zhu, X.; Liu, J.; Min, X.; Cao, T.; Fan, X. Smart Composite Hydrogels with PH-Responsiveness and Electrical Conductivity for Flexible Sensors and Logic Gates. Polymers 2019, 11, 1564. [Google Scholar] [CrossRef]
- Zhang, B.; He, J.; Shi, M.; Liang, Y.; Guo, B. Injectable Self-Healing Supramolecular Hydrogels with Conductivity and Photo-Thermal Antibacterial Activity to Enhance Complete Skin Regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Ren, J.; Qu, X. 3D Graphene Oxide-Polymer Hydrogel: Near-Infrared Light-Triggered Active Scaffold for Reversible Cell Capture and on-Demand Release. Adv. Mater. 2013, 25, 6737–6743. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Xiao, Y.; Gao, G.; Liu, S.; Zhang, J.; Fu, J. Electric Field Actuation of Tough Electroactive Hydrogels Cross-Linked by Functional Triblock Copolymer Micelles. ACS Appl. Mater. Interfaces 2016, 8, 26326–26331. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Duan, L.; Gao, G. Ultra-Stretchable Wearable Strain Sensors Based on Skin-Inspired Adhesive, Tough and Conductive Hydrogels. Chem. Eng. J. 2019, 365, 10–19. [Google Scholar] [CrossRef]
- Liu, J.; Tan, C.S.Y.; Yu, Z.; Li, N.; Abell, C.; Scherman, O.A. Tough Supramolecular Polymer Networks with Extreme Stretchability and Fast Room-Temperature Self-Healing. Adv. Mater. 2017, 29, 1605325. [Google Scholar] [CrossRef]
- Deng, Z.; Hu, T.; Lei, Q.; He, J.; Ma, P.X.; Guo, B. Stimuli-Responsive Conductive Nanocomposite Hydrogels with High Stretchability, Self-Healing, Adhesiveness, and 3D Printability for Human Motion Sensing. ACS Appl. Mater. Interfaces 2019, 11, 6796–6808. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable Antibacterial Conductive Hydrogels with Dual Response to an Electric Field and PH for Localized “Smart” Drug Release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Huang, H.; Han, L.; Fu, X.; Wang, Y.; Yang, Z.; Pan, L.; Xu, M. Multiple Stimuli Responsive and Identifiable Zwitterionic Ionic Conductive Hydrogel for Bionic Electronic Skin. Adv. Electron. Mater. 2020, 6, 2000239. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Shi, X.; Han, D.; Liang, F. A Thermally Responsive Host–Guest Conductive Hydrogel with Self-Healing Properties. Mater. Chem. Front. 2018, 2, 2212–2219. [Google Scholar] [CrossRef]
- Li, R.; Ren, J.; Li, M.; Zhang, M.; Li, Y.; Yang, W. Self-Healing, Self-Adhesive, Stretchable and Flexible Conductive Hydrogels for High-Performance Strain Sensors. Soft Matter 2023, 19, 5723–5736. [Google Scholar] [CrossRef]
- Bhuyan, M.M.; Okabe, H.; Hidaka, Y.; Dafader, N.C.; Rahman, N.; Hara, K. Synthesis of Pectin-N, N-Dimethyl Acrylamide Hydrogel by Gamma Radiation and Application in Drug Delivery (in Vitro). J. Macromol. Sci. Part A 2018, 55, 369–376. [Google Scholar] [CrossRef]
- Kattan, M.; Alkassiri, H.; Daher, Y. Dimethyl Sulfoxide, Diethyl Fumarate Solution for High Dose Dosimetry. Int. J. Radiat. Res. 2015, 13, 373–378. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, W.; Yan, K.; Pan, L.; Yu, G. Doping Engineering of Conductive Polymer Hydrogels and Their Application in Advanced Sensor Technologies. Chem. Sci. 2019, 10, 6232–6244. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Park, J.-S.; Kim, Y.-A.; Yang, S.-J.; Jeong, S.-I.; Lee, J.-Y.; Lim, Y.-M. Gamma Ray-Induced Polymerization and Cross-Linking for Optimization of PPy/PVP Hydrogel as Biomaterial. Polymers 2020, 12, 111. [Google Scholar] [CrossRef]
- Chang, S.; Wang, B.; Liu, Y.; Li, Z.; Hu, X.; Zhang, X.; Zhang, H. Radiation-Assistant Preparation of Highly Conductive, Transparent and Self-Healing Hydrogels with Triple-Network Structure. Polymer 2020, 188, 122156. [Google Scholar] [CrossRef]
- Yang, M.; Guo, W.; Liu, S.; Zhang, B.; Chen, Y.; Wang, Y. Highly Stretchable Gamma-Irradiated Poly (Vinyl Alcohol)/Tannic Acid Composite Hydrogels with Superior Transparency and Antibacterial Activity. J. Polym. Res. 2021, 28, 412. [Google Scholar] [CrossRef]
- Haque, S.K.; Bhuyan, M.M.; Jeong, J.H. Radiation-Induced Hydrogel for Water Treatment. Gels. 2024, 10, 375. [Google Scholar] [CrossRef]
- Kodama, H. Automatic Method for Fabricating a Three-Dimensional Plastic Model with Photo-Hardening Polymer. Rev. Sci. Instrum. 1981, 52, 1770–1773. [Google Scholar] [CrossRef]
- Ramli, R.N.; Lee, C.K.; Kassim, M.A. Extraction and Characterization of Starch from Microalgae and Comparison with Commercial Corn Starch. IOP Conf. Ser. Mater. Sci. Eng. 2020, 716, 012012. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, K.; Shin, M. Recent Advances in 3D Printable Conductive Hydrogel Inks for Neural Engineering. Nano Converg. 2023, 10, 41. [Google Scholar] [CrossRef]
- Neiman, J.A.S.; Raman, R.; Chan, V.; Rhoads, M.G.; Sam, M.; Raredon, B.; Velazquez, J.J.; Dyer, R.L.; Bashir, R.; Hammond, P.T.; et al. Photopatterning of Hydrogel Scaffolds Coupled to Filter Materials Using Stereolithography for Perfused 3D Culture of Hepatocytes. Biotechnol. Bioeng. 2015, 112, 777–787. [Google Scholar] [CrossRef]
- Burke, G.; Devine, D.M.; Major, I. Effect of Stereolithography 3d Printing on the Properties of Pegdma Hydrogels. Polymers 2020, 12, 2015. [Google Scholar] [CrossRef]
- Song, Y.; Wang, B.; Altemose, P.; Kowall, C.; Li, L. 3D-Printed Membranes with a Zwitterionic Hydrogel Coating for More Robust Oil-Water Separation. Ind. Eng. Chem. Res. 2020, 59, 21058–21065. [Google Scholar] [CrossRef]
- Joas, S.; Tovar, G.E.M.; Celik, O.; Bonten, C.; Southan, A. Extrusion-Based 3d Printing of Poly(Ethylene Glycol) Diacrylate Hydrogels Containing Positively and Negatively Charged Groups. Gels 2018, 4, 69. [Google Scholar] [CrossRef]
- Han, C.; Wang, X.; Ni, Z.; Ni, Y.; Huan, W.; Lv, Y.; Bai, S. Effects of Nanocellulose on Alginate/Gelatin Bio-Inks for Extrusion-Based 3D Printing. BioResources 2020, 15, 7357–7373. [Google Scholar] [CrossRef]
- Allencherry, J.; Pradeep, N.; Shrivastava, R.; Joy, L.; Imbriacco, F.; Özel, T. Investigation of Hydrogel and Gelatin Bath Formulations for Extrusion-Based 3D Bioprinting Using Deep Learning. Procedia CIRP 2022, 110, 362–367. [Google Scholar] [CrossRef]
- Boland, T.; Tao, X.; Damon, B.J.; Manley, B.; Kesari, P.; Jalota, S.; Bhaduri, S. Drop-on-Demand Printing of Cells and Materials for Designer Tissue Constructs. Mater. Sci. Eng. C 2007, 27, 372–376. [Google Scholar] [CrossRef]
- Tao, O.; Kort-Mascort, J.; Lin, Y.; Pham, H.M.; Charbonneau, A.M.; ElKashty, O.A.; Kinsella, J.M.; Tran, S.D. The Applications of 3D Printing for Craniofacial Tissue Engineering. Micromachines 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Cabral, J.D.; Saunderson, S.; Ali, M.A. 3D Printing of Chitooligosaccharide-Polyethylene Glycol Diacrylate Hydrogel Inks for Bone Tissue Regeneration. J. Biomed. Mater. Res. Part A 2023, 111, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Adamkiewicz, M.; Rubinsky, B. Cryogenic 3D Printing for Tissue Engineering. Cryobiology 2015, 71, 518–521. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X. Fluid and Cell Behaviors along a 3D Printed Alginate/Gelatin/Fibrin Channel. Biotechnol. Bioeng. 2015, 112, 1683–1695. [Google Scholar] [CrossRef]
- Cidonio, G.; Glinka, M.; Dawson, J.I.; Oreffo, R.O.C. The Cell in the Ink: Improving Biofabrication by Printing Stem Cells for Skeletal Regenerative Medicine. Biomaterials 2019, 209, 10–24. [Google Scholar] [CrossRef]
- Chekini, M.; Krivoshapkina, E.; Shkodenko, L.; Koshel, E.; Shestovskaya, M.; Dukhinova, M.; Kheiri, S.; Khuu, N.; Kumacheva, E. Nanocolloidal Hydrogel with Sensing and Antibacterial Activities Governed by Iron Ion Sequestration. Chem. Mater. 2020, 32, 10066–10075. [Google Scholar] [CrossRef]
- Lian, Q.; Jiao, T.; Zhao, T.; Wang, H.; Yang, S.; Li, D. 3D Bioprinted Skin Substitutes for Accelerated Wound Healing and Reduced Scar. J. Bionic Eng. 2021, 18, 900–914. [Google Scholar] [CrossRef]
- Ku, J.K.; Lee, K.G.; Ghim, M.S.; Kim, Y.K.; Park, S.H.; Park, Y.; Cho, Y.S.; Lee, B.K. Onlay-Graft of 3D Printed Kagome-Structure PCL Scaffold Incorporated with RhBMP-2 Based on Hyaluronic Acid Hydrogel. Biomed. Mater. 2021, 16, 055004. [Google Scholar] [CrossRef]
- Han, D.; Farino, C.; Yang, C.; Scott, T.; Browe, D.; Choi, W.; Freeman, J.W.; Lee, H. Soft Robotic Manipulation and Locomotion with a 3D Printed Electroactive Hydrogel. ACS Appl. Mater. Interfaces 2018, 10, 17512–17518. [Google Scholar] [CrossRef]
- Mohammadi, S.; Keshvari, H.; Eskandari, M.; Faghihi, S. Graphene Oxide–Enriched Double Network Hydrogel with Tunable Physico-Mechanical Properties and Performance. React. Funct. Polym. 2016, 106, 120–131. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel Bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef]
- Sunwoo, S.H.; Han, S.I.; Joo, H.; Cha, G.D.; Kim, D.; Choi, S.H.; Hyeon, T.; Kim, D.H. Advances in Soft Bioelectronics for Brain Research and Clinical Neuroengineering. Matter 2020, 3, 1923–1947. [Google Scholar] [CrossRef]
- Yao, B.; Wang, H.; Zhou, Q.; Wu, M.; Zhang, M.; Li, C.; Shi, G. Ultrahigh-Conductivity Polymer Hydrogels with Arbitrary Structures. Adv. Mater. 2017, 29, 1700974. [Google Scholar] [CrossRef]
- Heo, D.N.; Lee, S.J.; Timsina, R.; Qiu, X.; Castro, N.J.; Zhang, L.G. Development of 3D Printable Conductive Hydrogel with Crystallized PEDOT:PSS for Neural Tissue Engineering. Mater. Sci. Eng. C 2019, 99, 582–590. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Chen, S.; Lei, T.; Kim, Y.; Niu, S.; Wang, H.; Wang, X.; Foudeh, A.M.; Tok, J.B.H.; et al. Soft and Elastic Hydrogel-Based Microelectronics for Localized Low-Voltage Neuromodulation. Nat. Biomed. Eng. 2019, 3, 58–68. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS Hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D Printing of Conducting Polymers. Nat. Commun. 2020, 11, 4–11. [Google Scholar] [CrossRef]
- Zhu, K.; Shin, S.R.; van Kempen, T.; Li, Y.C.; Ponraj, V.; Nasajpour, A.; Mandla, S.; Hu, N.; Liu, X.; Leijten, J.; et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv. Funct. Mater. 2017, 27, 1605352. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological Strategies for Engineering Complex Tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef]
- Jin, S.; Kim, Y.; Son, D. Hydrogel Ink for On-Skin Direct Writing of Electronics. Gels 2022, 8, 336. [Google Scholar] [CrossRef]
- Liao, M.; Liao, H.; Ye, J.; Wan, P.; Zhang, L. Polyvinyl Alcohol-Stabilized Liquid Metal Hydrogel for Wearable Transient Epidermal Sensors. ACS Appl. Mater. Interfaces 2019, 11, 47358–47364. [Google Scholar] [CrossRef]
- Ryu, S.; Chou, J.B.; Lee, K.; Lee, D.; Hong, S.H.; Zhao, R.; Lee, H.; Kim, S.G. Direct Insulation-to-Conduction Transformation of Adhesive Catecholamine for Simultaneous Increases of Electrical Conductivity and Mechanical Strength of CNT Fibers. Adv. Mater. 2015, 27, 3250–3255. [Google Scholar] [CrossRef]
- Choe, G.; Oh, S.; Seok, J.M.; Park, S.A.; Lee, J.Y. Graphene Oxide/Alginate Composites as Novel Bioinks for Three-Dimensional Mesenchymal Stem Cell Printing and Bone Regeneration Applications. Nanoscale 2019, 11, 23275–23285. [Google Scholar] [CrossRef]
- Su, G.; Cao, J.; Zhang, X.; Zhang, Y.; Yin, S.; Jia, L.; Guo, Q.; Zhang, X.; Zhang, J.; Zhou, T. Human-Tissue-Inspired Anti-Fatigue-Fracture Hydrogel for a Sensitive Wide-Range Human-Machine Interface. J. Mater. Chem. A 2020, 8, 2074–2082. [Google Scholar] [CrossRef]
- Chen, L.; Kang, Q.; Tao, W. Pore-Scale Study of Reactive Transport Processes in Catalyst Layer Agglomerates of Proton Exchange Membrane Fuel Cells. Electrochim. Acta 2019, 306, 454–465. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Wang, X.; Wang, Z.; Li, M.; Hu, X.; Wang, Y.; Liu, H.; Wang, Y. 3D Printed Dual Network Cross-Linked Hydrogel Electrolytes for High Area Capacity Flexible Zinc Ion Micro-Batteries. Chem. Eng. J. 2024, 490, 151523. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, F.; Zhang, Y.Z.; Li, L.; Gao, S.Y.; Yang, X.L.; Wang, S.; Chen, P.F.; Lai, W.Y. 3D Printable Conductive Polymer Hydrogels with Ultra-High Conductivity and Superior Stretchability for Free-Standing Elastic All-Gel Supercapacitors. Chem. Eng. J. 2022, 450, 138311. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, X.; Liu, C.; Li, Z.; Liu, C.; Shi, X.; Li, Z.; Mei, C.; Li, M.-C. 3D Printed MXene-Based Films and Cellulose Nanofiber Reinforced Hydrogel Electrolyte to Enable High-Performance Flexible Supercapacitors. J. Mater. Chem. A 2024, 12, 3734–3744. [Google Scholar] [CrossRef]
- Bao, X.; Meng, J.; Tan, Z.; Zhang, C.; Li, L.; Liu, T. Direct-Ink-Write 3D Printing of Highly-Stretchable Polyaniline Gel with Hierarchical Conducting Network for Customized Wearable Strain Sensors. Chem. Eng. J. 2024, 491, 151918. [Google Scholar] [CrossRef]
- Advincula, R.C.; Dizon, J.R.C.; Caldona, E.B.; Viers, R.A.; Siacor, F.D.C.; Maalihan, R.D.; Espera, A.H. On the Progress of 3D-Printed Hydrogels for Tissue Engineering. MRS Commun. 2021, 11, 539–553. [Google Scholar] [CrossRef]
- Esmaeely Neisiany, R.; Enayati, M.S.; Sajkiewicz, P.; Pahlevanneshan, Z.; Ramakrishna, S. Insight Into the Current Directions in Functionalized Nanocomposite Hydrogels. Front. Mater. 2020, 7, 25. [Google Scholar] [CrossRef]
- Xu, Z.; Qiao, X.; Tao, R.; Li, Y.; Zhao, S.; Cai, Y.; Luo, X. A Wearable Sensor Based on Multifunctional Conductive Hydrogel for Simultaneous Accurate PH and Tyrosine Monitoring in Sweat. Biosens. Bioelectron. 2023, 234, 115360. [Google Scholar] [CrossRef]
- Guo, B.; Wu, Y.; He, S.; Wang, C.; Yao, M.; Yu, Q.; Wu, X.; Yu, C.; Liu, M.; Liang, L.; et al. Anisotropic and Super-Strong Conductive Hydrogels Enabled by Mechanical Stretching Combined with the Hofmeister Effect. J. Mater. Chem. A 2023, 11, 8038–8047. [Google Scholar] [CrossRef]
- Dong, R.; Zhao, X.; Guo, B.; Ma, P.X. Self-Healing Conductive Injectable Hydrogels with Antibacterial Activity as Cell Delivery Carrier for Cardiac Cell Therapy. ACS Appl. Mater. Interfaces 2016, 8, 17138–17150. [Google Scholar] [CrossRef]
- Liu, S.; Kang, M.; Li, K.; Yao, F.; Oderinde, O.; Fu, G.; Xu, L. Polysaccharide-Templated Preparation of Mechanically-Tough, Conductive and Self-Healing Hydrogels. Chem. Eng. J. 2018, 334, 2222–2230. [Google Scholar] [CrossRef]
- Liu, S.; Oderinde, O.; Hussain, I.; Yao, F.; Fu, G. Dual Ionic Cross-Linked Double Network Hydrogel with Self-Healing, Conductive, and Force Sensitive Properties. Polymer 2018, 144, 111–120. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, X.; Thomas, A.K.; Patsis, P.A.; Kurth, T.; Kräter, M.; Eckert, K.; Bornhäuser, M.; Zhang, Y. Noncovalently Assembled Electroconductive Hydrogel. ACS Appl. Mater. Interfaces 2018, 10, 14418–14425. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, F.; Chen, X.; Wang, X.W.; Zhang, W.B.; Peng, J.; Li, J.; Zhai, M. Stretchable, Conductive, and Self-Healing Hydrogel with Super Metal Adhesion. Chem. Mater. 2018, 30, 4289–4297. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional Stimuli-Responsive Hydrogels with Self-Healing, High Conductivity, and Rapid Recovery through Host-Guest Interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Hur, J.; Im, K.; Kim, S.W.; Kim, J.; Chung, D.Y.; Kim, T.H.; Jo, K.H.; Hahn, J.H.; Bao, Z.; Hwang, S.; et al. Polypyrrole/Agarose-Based Electronically Conductive and Reversibly Restorable Hydrogel. ACS Nano 2014, 8, 10066–10076. [Google Scholar] [CrossRef]
- Talukder, M.M.; Rahman Khan, M.M.; Amin, M.K. A Review on Polyaniline (PANI) Based Nanocomposites for Water Purification. S. Afr. J. Chem. Eng. 2023, 44, 276–282. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Liu, H.; Li, D.; Fu, S.; Jin, K.; Cheng, Y.; Fu, Z.; Xing, F.; Tian, Y. Tough, High Conductivity Pectin Polysaccharide-Based Hydrogel for Strain Sensing and Real-Time Information Transmission. Int. J. Biol. Macromol. 2024, 257, 128757. [Google Scholar] [CrossRef]
- Chelfouh, N.; Coquil, G.; Rousselot, S.; Foran, G.; Briqueleur, E.; Shoghi, F.; Caradant, L.; Dollé, M. Apple Pectin-Based Hydrogel Electrolyte for Energy Storage Applications. ACS Sustain. Chem. Eng. 2022, 10, 15802–15812. [Google Scholar] [CrossRef]




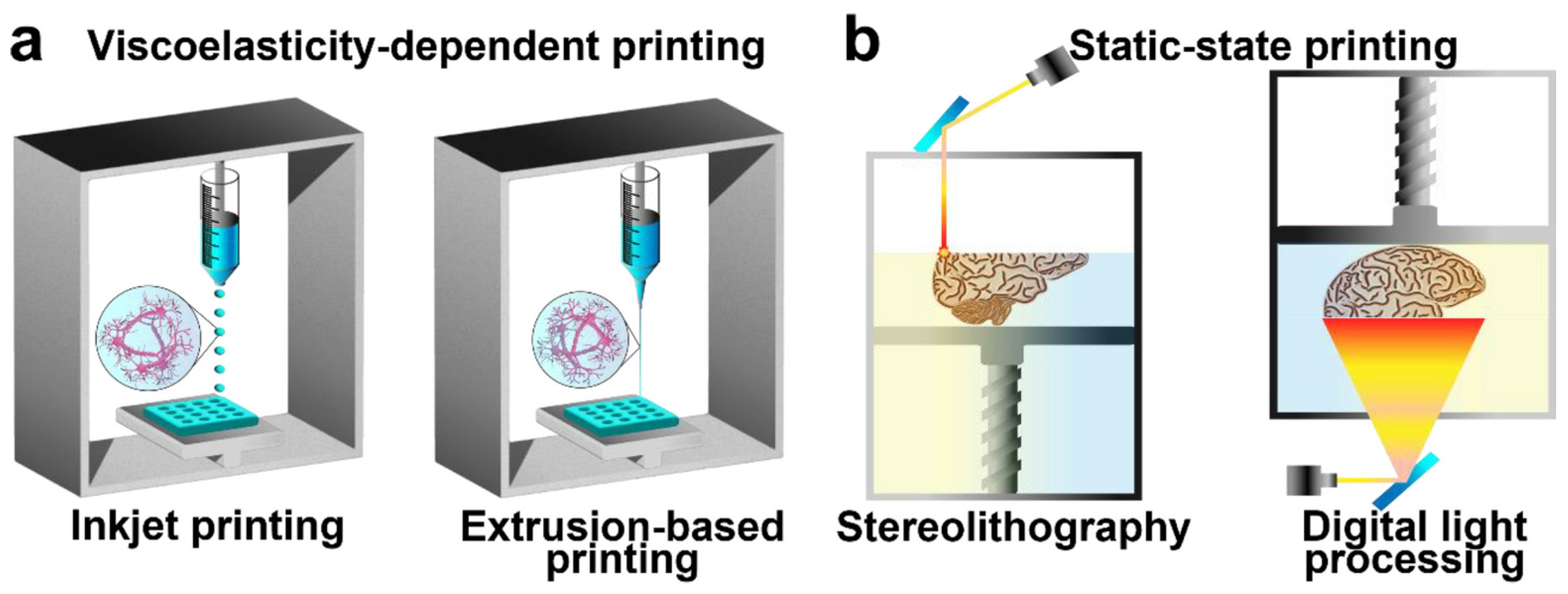
| S.N | Conductive Organic Compounds /Polymers | Structure | Properties | Reference | |
|---|---|---|---|---|---|
| 1 | TCNQ | 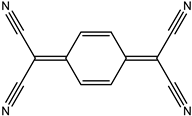 |
| [28] | |
| 2 | TTE |  |
| [29] | |
| 3 | BEDT-TTF |  |
| [30] | |
| 4 | PANI | 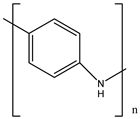 |
| [31] | |
| 5 | PPy | 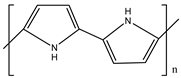 |
| [32] | |
| 6 | PEDOT |  | PEDOT: PSS |
| [33] |
| 7 | PSS | 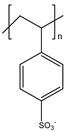 | |||
| 8 | PTh | 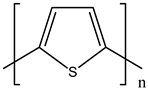 |
| [34] | |
| 9 | PPV | 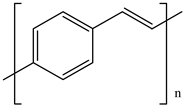 |
| [35] | |
| 10 | PC | 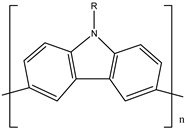 |
| [36] | |
| S.N. | Conductive Hydrogels | Notable Properties | Field of Application | Reference |
|---|---|---|---|---|
| 1 | Carboxymethyl cellulose/poly(acrylic acid/Fe3+/LiCl |
| Flexible electronics | [39] |
| 2 | PVA/PA/PDA |
| Wearable bioelectronic sensors | [40] |
| 3 | Acrylamide/Lauryl methacrylate/L-glutamic acid |
| Wearable strain sensors | [41] |
| 4 | ε-PL-SH/PPy (CHLY Collagen based hydrogel) |
|
| [42] |
| 5 | PVA/CMC/LiCl |
| Touch sensor | [43] |
| 6 | PVA/PEDOT:PSS/NaCl |
| Wearable sensors | [44] |
| 7 | PVA/TEF/SF |
| Wearable sensors | [45] |
| 8 | NIPAM/co-MBAA/AM with ionic LiCl and glycerol |
|
| [46] |
| 9 | PVA/EG(ethylene glycol) with metal ion MgCl2 |
| Flexible strain sensor | [47] |
| 10 | Agar/Borax/MXene |
| Flexible strain sensor | [48] |
| 11 | AAm/co-Butyl acrylate/Gaur Gum |
|
| [49] |
| 12 | P(AM-APBA)XLG/CNTs |
|
| [50] |
| 13 | PAM/SA/CNTs with silica |
|
| [51] |
| 14 | Bovine serum albumin-MA-PPy/P(AM-co-AA)/Fe3+ |
|
| [52] |
| 15 | PVA/PAAm/XG (xanthum gum)/Zn2+ |
|
| [53] |
| 16 | PEDOT:PSS/CNTs |
|
| [54] |
| 17 | PAAm/PVA/PDA-Fe3O4-MXene |
|
| [55] |
| 18 | KMGHCa (K-MXene/GG/HEAA/CaCl2) |
|
| [56] |
| 19 | AG/SBMA/PPy with Fe3+ |
|
| [57] |
| 20 | P123(Pluronic)/LAD/TMAx |
|
| [58] |
| 21 | PVA/AAc/NaCl |
|
| [59] |
| 22 | Chitosan(CS)/tannic acid(TA)/PAA (QCMCS hydrogel) |
|
| [60] |
| 23 | CS/CSF1.5-PAA-Fe3+-G hydrogel |
|
| [61] |
| 24 | HA/MA-rGO-PANI |
| Drug delivery | [62] |
| 25 | SA(sodium alginate)/CaCl2/AgNO3 | High surface resistivity | Textile applications | [63] |
| Parameter | Results | |
|---|---|---|
| Stretchability | ~850% | |
| Viscoelasticity (storage modulus) | of 32 kPa | |
| Mechanical strength | Compression strength | 2.54 MPa |
| Tensile strength | 0.32 MPa | |
| Electrical conductivity | ~2.5 S m−1 | |
| Healing efficiency | 96.7% within 12 h | |
| S.N | Hydrogel | Conductance | Application | Reference |
|---|---|---|---|---|
| 1 | Self-Healing Conductive Injectable hydrogels | 2.25–3.5 × 10−3 S cm−1 | Wound dressing and cutaneous, wound healing | [152] |
| 2 | Polysaccharide-templated conductive and self-healing hydrogel | 1.52 × 10−3 S cm−1 | Circuit | [153] |
| 3 | Dual ionic cross-linked double network hydrogel | 1.6–6.2 × 10−3 S cm−1 | Self-repaired circuit | [154] |
| 4 | Non-covalently Assembled Electroconductive hydrogel | ~8–~16 × 10−3 S cm−1 | Tissue engineering | [155] |
| 5 | Hydrogel with Super Metal Adhesion | 1.05 × 10−2 S cm−1 | Adhesive | [156] |
| 6 | Human Motion Sensing hydrogel | 1.3–1.9 × 10−3 S cm−1 | Human motion | [97] |
| 7 | Multifunctional Stimuli-Responsive hydrogels | 3.5 × 10−2 S cm−1 | Sensors, human motion sensing | [157] |
| 8 | Polypyrrole/Agarose-Based conductive hydrogel | 1.91 × 10−6 −1.95 × 10−1 S cm−1 | Patterning and self-repaired circuit | [158] |
| First Network | Second Network | |
|---|---|---|
| A | Hyaluronic acid + FeCl3 + Acrylic acid | PANI |
| B | Pectin + Hyaluronic acid + NaIO4 | PANI + NIPAM |
| C | Pectin + Hyaluronic acid + LiCl | NIPAM + Acrylic acid |
| D | Hyaluronic acid + Alginate + CaO | Tetracyanoquinodimethane + PANI |
| E | 2-acrylamido-2-methylpropanesulfonic acid + Na2SO4 + PANI | Graphene + NIPAM |
| F | Hyaluronic acid + Fe2(SO4)3 + Polythiophene | Graphene + Acrylic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, N.; Bhuyan, M.M.; Jeong, J.-H. Single/Multi-Network Conductive Hydrogels—A Review. Polymers 2024, 16, 2030. https://doi.org/10.3390/polym16142030
Hasan N, Bhuyan MM, Jeong J-H. Single/Multi-Network Conductive Hydrogels—A Review. Polymers. 2024; 16(14):2030. https://doi.org/10.3390/polym16142030
Chicago/Turabian StyleHasan, Nahid, Md Murshed Bhuyan, and Jae-Ho Jeong. 2024. "Single/Multi-Network Conductive Hydrogels—A Review" Polymers 16, no. 14: 2030. https://doi.org/10.3390/polym16142030






