High-Value and Environmentally Friendly Recycling Method for Coal-Based Solid Waste Based on Polyurethane Composite Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
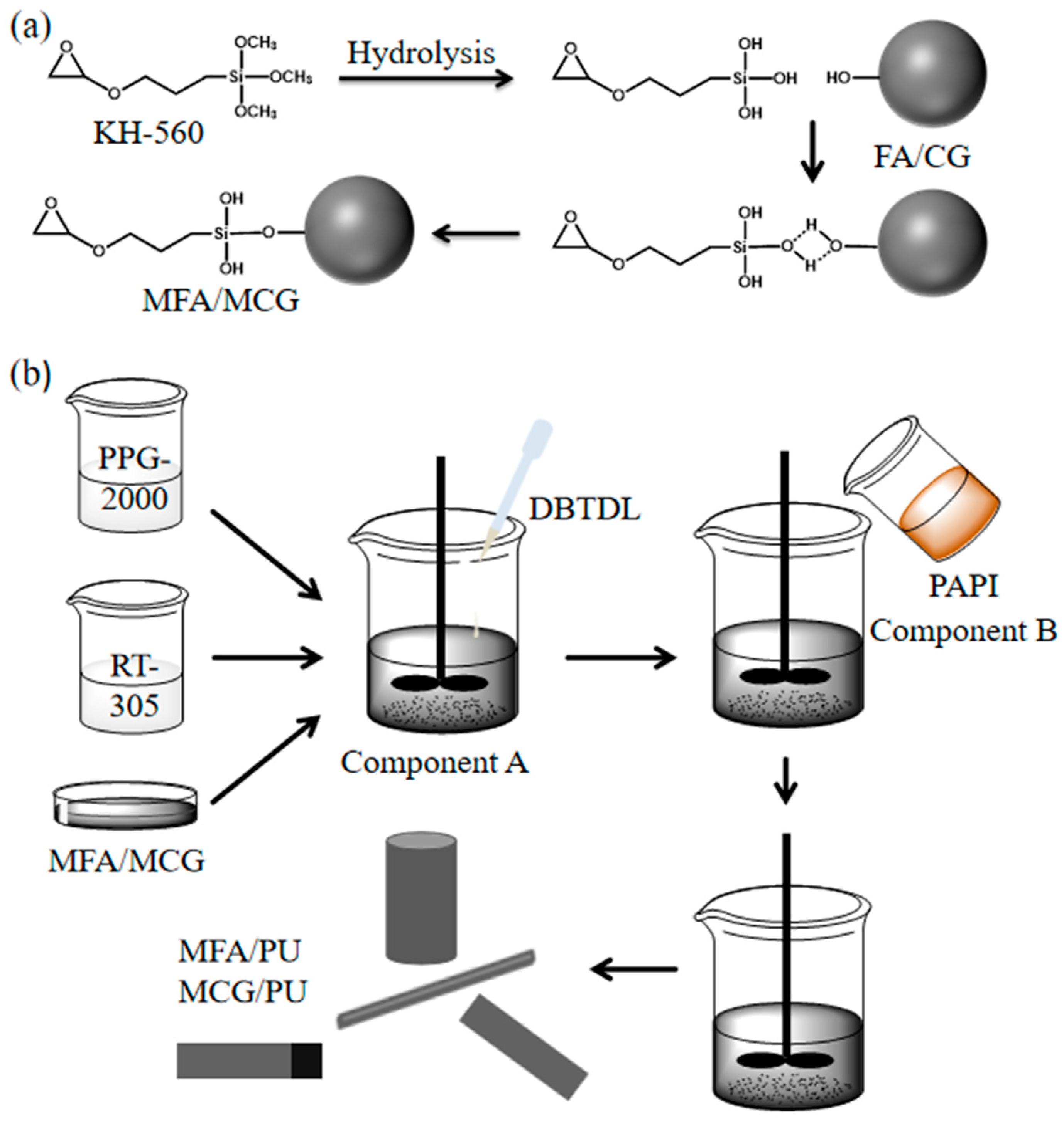
2.2. Surface Modification of FA and CG Powder
2.3. Preparation of PU Composites
2.4. Testing and Characterization
3. Results and Discussion
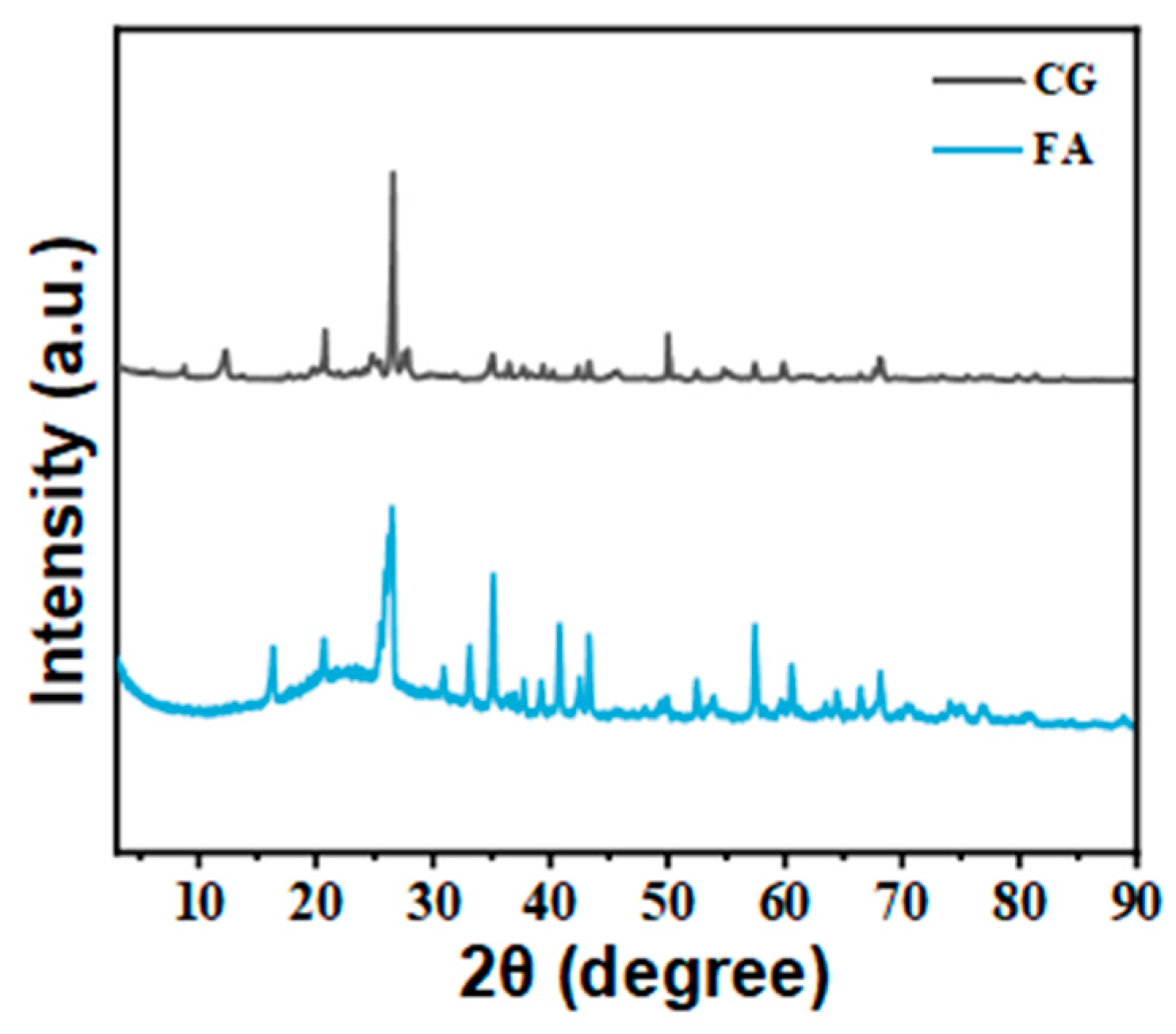
3.1. Composition and Morphological Analyses of FA and CG
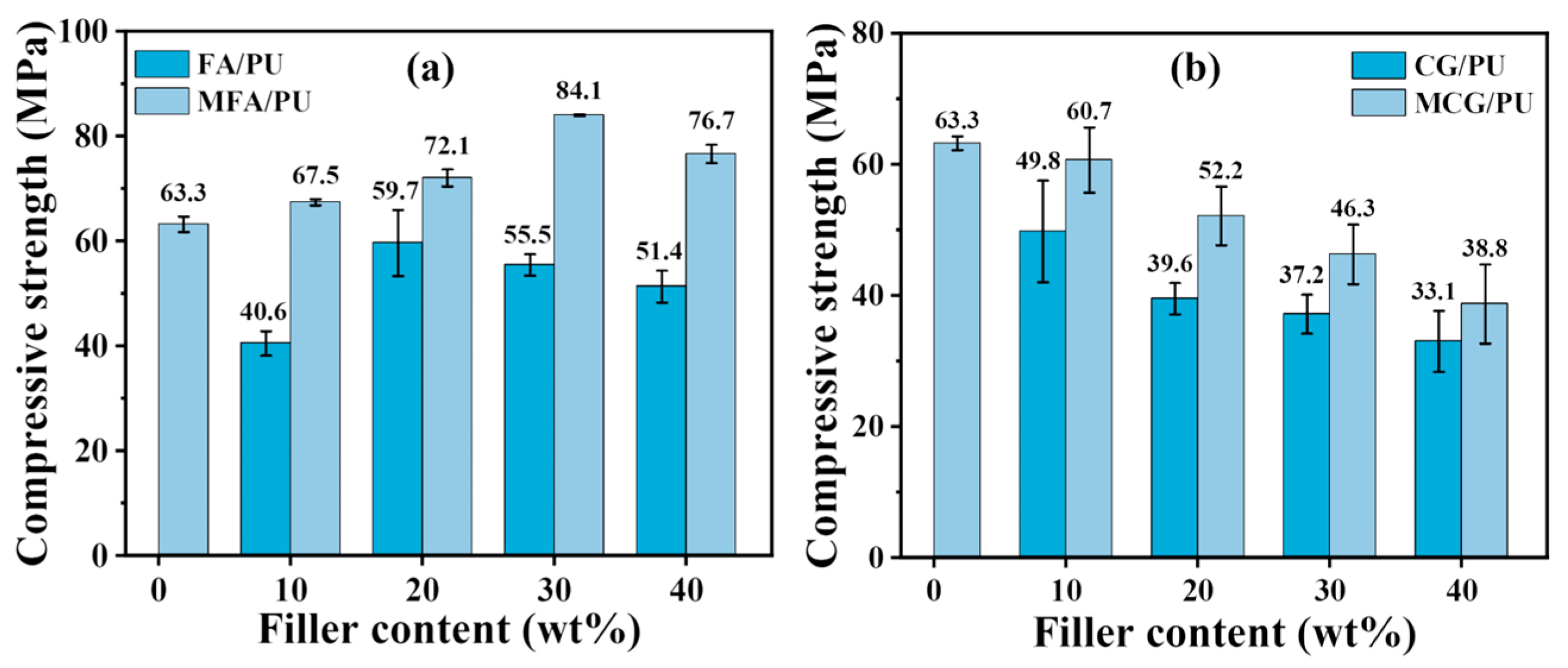
3.2. Compressive Strength of Composites
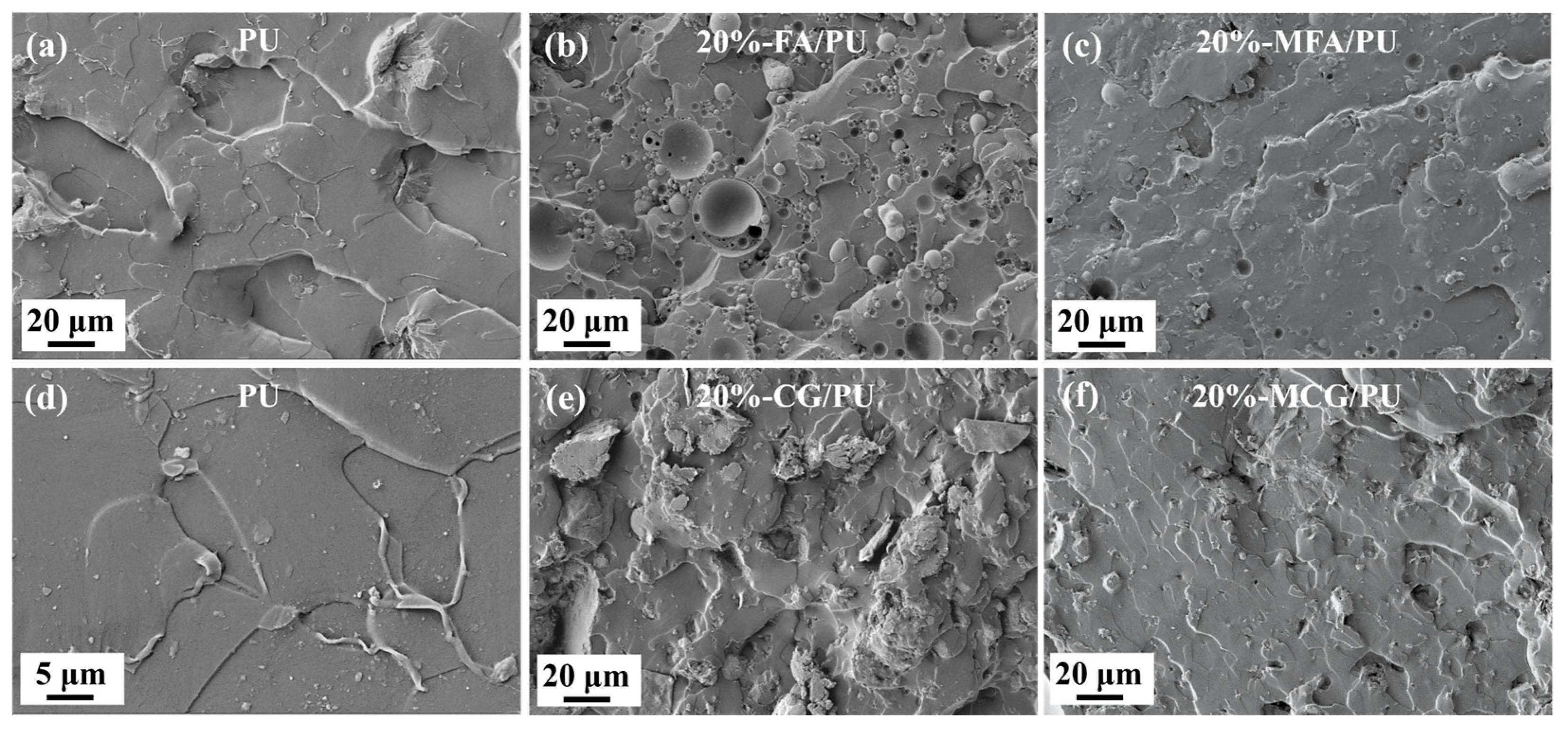
3.3. Analysis of the Cross-Section Morphologies of Composites
3.4. Limiting Oxygen Index
3.5. Thermal Stability
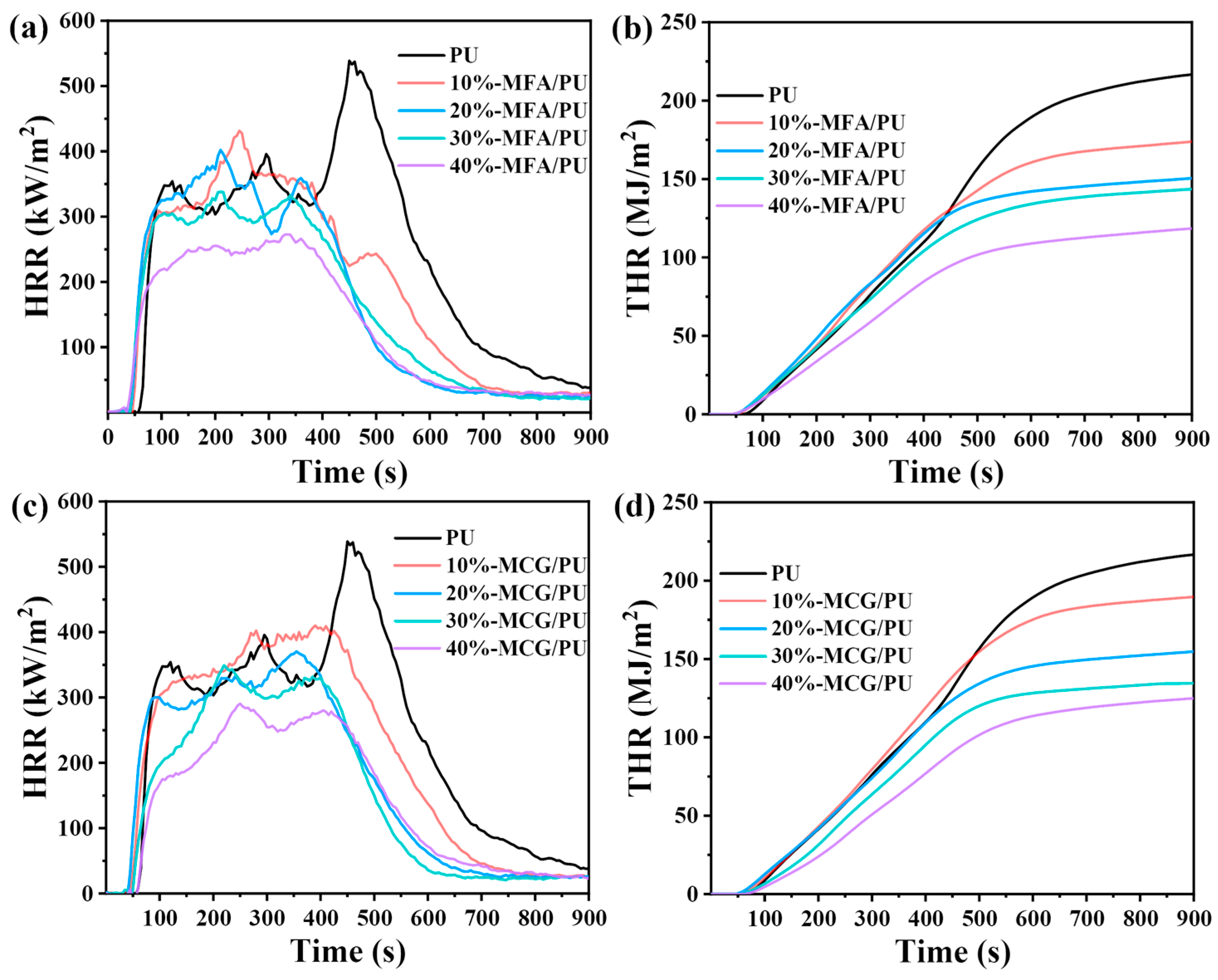
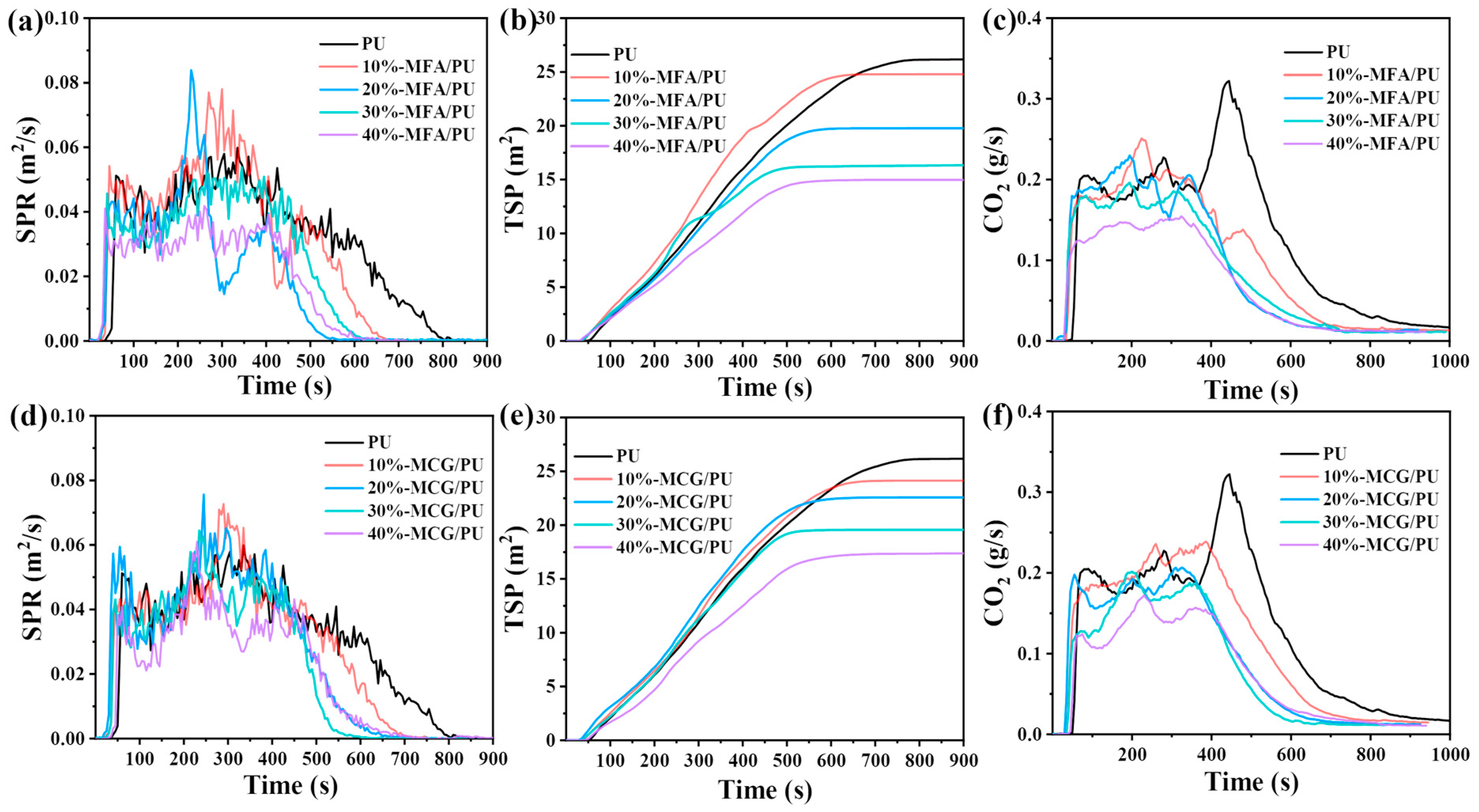
3.6. Cone Calorimetric Test
3.7. Carbon Residue Analysis
3.8. Flame-Retardant Mechanism
3.9. Water Quality Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Wang, Y.; Zhao, S.; Hu, H.; Cao, C.; Li, A.; Yu, Y.; Yao, H. A review on current status of the co-combustion technology of organic solid waste (OSW) and coal in China. Energ. Fuel. 2020, 34, 15448–15487. [Google Scholar] [CrossRef]
- Gao, S.; Meegoda, J.N.; Hu, L. Microscopic Modeling of Air Migration during Air Sparging. J. Hazard. Toxic Radioact. Waste 2011, 15, 70–79. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean 2019, 239, 117946. [Google Scholar] [CrossRef]
- Li, D.; Wu, D.; Xu, F.; Lai, J.; Shao, L. Literature overview of Chinese research in the field of better coal utilization. J. Clean Prod. 2018, 185, 959–980. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, Q.; Liu, H.; Gao, S. Utilization of coal gangue sand in structural concrete as fine aggregate towards sustainable production. Constr. Build. Mater. 2024, 417, 135264. [Google Scholar] [CrossRef]
- Luo, L.; Li, K.; Fu, W.; Liu, C.; Yang, S. Preparation, characteristics and mechanisms of the composite sintered bricks produced from shale, sewage sludge, coal gangue powder and iron ore tailings. Constr. Build. Mater. 2020, 232, 117250. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, G.; Guo, L.; Zhou, L.; Yuan, K. Utilization of coal gangue as coarse aggregates in structural concrete. Constr. Build. Mater. 2021, 268, 121212. [Google Scholar] [CrossRef]
- Zhu, B.; Gao, Y.; Hao, H.; Ji, G.; Yang, C.; Wang, F.; Su, J.; Wu, X.; Song, X.; Ma, L.; et al. One-pot synthesis of coal gangue-derived NiCG composite for enhancing microwave absorption. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131305. [Google Scholar] [CrossRef]
- Xiao, J.; Li, F.; Zhong, Q.; Bao, H.; Wang, B.; Huang, J.; Zhang, Y. Separation of aluminum and silica from coal gangue by elevated temperature acid leaching for the preparation of alumina and SiC. Hydrometallurgy 2015, 155, 118–124. [Google Scholar] [CrossRef]
- Sobczak, M. Biodegradable Polyurethane Elastomers for Biomedical Applications—Synthesis Methods and Properties. Polym. Plast. Technol. Mater. 2015, 54, 155–172. [Google Scholar] [CrossRef]
- Sklenicková, K.; Abbrent, S.; Halecky, M.; Kocí, V.; Benes, H. Biodegradability and ecotoxicity of polyurethane foams: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 157–202. [Google Scholar] [CrossRef]
- Kausar, A. Polyurethane nanocomposite coatings: State of the art and perspectives. Polym. Int. 2018, 67, 1470–1477. [Google Scholar] [CrossRef]
- Golling, F.; Pires, R.; Hecking, A.; Weikard, J.; Richter, F.; Danielmeier, K.; Dijkstra, D. Polyurethanes for coatings and adhesives—Chemistry and applications. Polym. Int. 2019, 68, 848–855. [Google Scholar] [CrossRef]
- Visakh, P.; Semkin, A.; Rezaev, I.; Fateev, A. Review on soft polyurethane flame retardant. Constr. Build. Mater. 2019, 227, 116673. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Wu, J.; Li, X.; Su, X.; Zhao, Z.; Jiang, H.; Zou, M. Microporous Polyurethane Elastomer’s Comprehensive Properties Affected by Flame Retardant EG/APP. J. Phys. Conf. Ser. 2023, 2660, 012003. [Google Scholar] [CrossRef]
- Sun, J.; Hu, Y.; Guan, M.; Hou, Y.; Zhang, S.; Liu, X.; Tang, G. Research progress in the application of bulk solid waste in the field of flame retardation. J. Environ. Chem. Eng. 2023, 11, 111505. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, X.; Wu, M.; Zhao, Y.; Yu, C. Effects of different catalysts on the structure and properties of polyurethane/water glass grouting materials. J. Appl. Polym. Sci. 2018, 135, 46460. [Google Scholar] [CrossRef]
- Yu, X.; Liu, L.; Wang, Y.; Bai, G.; Zhang, Y.; Diab, A. Effects of Foaming and Drainage Behavior on Structure and Properties of Polyurethane/Water Glass (PU/WG) Grouting Materials for Coal Mines. Adv. Civ. Eng 2021, 2021, 5868654. [Google Scholar] [CrossRef]
- Xu, S.; Liu, J.; Liu, X.; Li, H.; Gu, X.; Sun, J.; Zhang, S. Preparation of Ni-Fe layered double hydroxides and its application in thermoplastic polyurethane with flame retardancy and smoke suppression. Polym. Degrad. Stab. 2022, 202, 110043. [Google Scholar] [CrossRef]
- Zhang, B.; Fan, Z.; Hu, S.; Shen, Z.; Ma, H. Mechanical response of the fly ash cenospheres/polyurethane syntactic foams fabricated through infiltration process. Constr. Build. Mater. 2019, 206, 552–559. [Google Scholar] [CrossRef]
- Qin, C.; Lu, W.; He, Z.; Qi, G.; Li, J.; Hu, X. Effect of Silane Treatment on Mechanical Properties of Polyurethane/Mesoscopic Fly Ash Composites. Polymers 2019, 11, 11040741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, W.; Yang, K.; Yu, W.; Zhu, F.; Zheng, Q. The Influence of Fly Ash on the Foaming Behavior and Flame Retardancy of Polyurethane Grouting Materials. Polymers 2022, 14, 1113. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liu, C.; Zhou, K.; Xuan, X.; Shi, C. Synergistic effect between solid wastes and intumescent flame retardant on flammability and smoke suppression of thermoplastic polyurethane composites. Polym. Adv. Technol. 2019, 31, 4–14. [Google Scholar] [CrossRef]
- Hull, T.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 96, 1462–1469. [Google Scholar] [CrossRef]
- Agrawal, A.; Kaur, R.; Walia, R. Investigation on flammability of rigid polyurethane foam-mineral fillers composite. Fire Mater. 2019, 43, 917–927. [Google Scholar] [CrossRef]
- GB/T 2567-2008; Test methods for properties of resin casting body. Standardization Administration of China: Beijing, China, 2008.
- GB/T 2406.2-2009; Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. Standardization Administration of China: Beijing, China, 2009.
- GB/T 5750.1-2023; Standard Examination Methods for Drinking Water—Part 1: General Principles. State Administration for Market Regulation and Standardization Administration of China: Beijing, China, 2023.
- GB 5749-2022; Standards for drinking water quality. State Administration for Market Regulation and Standardization Administration of China: Beijing, China, 2022.
- Shao, S.; Ma, B.; Wang, C.; Chen, Y. Thermal behavior and chemical reactivity of coal gangue during pyrolysis and combustion. Fuel 2023, 331, 125927. [Google Scholar] [CrossRef]
- Dong, L.; Liang, X.; Song, Q.; Gao, G.; Song, L.; Shu, Y.; Shu, X. Study on Al2O3 extraction from activated coal gangue under different calcination atmospheres. J. Therm. Sci. 2017, 26, 570–576. [Google Scholar] [CrossRef]
- Liu, F.; Xie, M.; Yu, G.; Ke, C.; Zhao, H. Study on Calcination Catalysis and the Desilication Mechanism for Coal Gangue. ACS Sustain. Chem. Eng. 2021, 9, 10318–10325. [Google Scholar] [CrossRef]
- Sertsova, A.; Marakulin, S.; Yurtov, E.V. Metal Compound Nanoparticles: Flame Retardants for Polymer Composites. Russ. J. Gen. Chem. 2017, 87, 1395–1402. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, J.; Zhang, J.; Li, H.; Zhang, S.; Sun, J.; Xin, F.; Liu, F.; Qin, Z.; Tang, W. TiO2/SiO2/kaolinite hybrid filler to improve the flame retardancy, smoke suppression and anti-aging characteristics of epoxy resin. Mater. Chem. Phys. 2022, 277, 125576. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Liao, W.; Gu, J.; Li, D. A new intumescent flame-retardant: Preparation, surface modification, and its application in polypropylene. Polym. Adv. Technol. 2008, 19, 1055–1061. [Google Scholar] [CrossRef]
- Tsai, K. Orientation effect on cone calorimeter test results to assess fire hazard of materials. J. Hazard. Mater. 2009, 172, 763–772. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Jiao, C. Smoke suppression properties of ferrite yellow on flame retardant thermoplastic polyurethane based on ammonium polyphosphate. J. Hazard. Mater. 2014, 266, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Chen, X. Flammability and thermal degradation of intumescent flame-retardant polypropylene composites. Polym. Eng. Sci. 2010, 50, 767–772. [Google Scholar] [CrossRef]
- Razak, J.; Akil, H.; Ong, H. Effect of inorganic fillers on the flammability behavior of polypropylene composites. J. Thermoplast. Compos. Mater. 2007, 20, 195–205. [Google Scholar] [CrossRef]
- Dong, Y.; Gui, Z.; Hu, Y.; Wu, Y.; Jiang, S. The influence of titanate nanotube on the improved thermal properties and the smoke suppression in poly(methyl methacrylate). J. Hazard. Mater. 2012, 209-210, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Hull, T.; Stec, A.; Nazare, S. Fire Retardant Effects of Polymer Nanocomposites. J. Nanosci. Nanotechnol. 2009, 9, 4478–4486. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bai, Z.; Xu, X.; Guo, J.; Chen, X.; Hsu, S.; Lu, Z.; Wu, H. Phosphonitrile decorating expandable graphite as a high-efficient flame retardant for rigid polyurethane foams. Polymer 2023, 283, 126268. [Google Scholar] [CrossRef]
- Chow, J.; Chai, W.; Yeh, C.; Chuang, F. Recycling and Application Characteristics of Fly Ash from Municipal Solid Waste Incinerator Blended with Polyurethane Foam. Environ. Eng. Sci. 2008, 25, 461–473. [Google Scholar] [CrossRef]




| Sample | PPG-200 (g) | RT-305 (g) | PAPI (g) | DBTDL (g) | MFA (g) | MCG (g) |
|---|---|---|---|---|---|---|
| PU | 23.75 | 38.75 | 37.5 | 0.15 | 0 | 0 |
| 10%-MFA/PU | 21.37 | 34.88 | 33.75 | 0.15 | 10 | 0 |
| 20%-MFA/PU | 19 | 31 | 30 | 0.15 | 20 | 0 |
| 30%-MFA/PU | 16.63 | 27.12 | 26.25 | 0.15 | 30 | 0 |
| 40%-MFA/PU | 14.25 | 23.25 | 22.5 | 0.15 | 40 | 0 |
| 10%-MCG/PU | 21.37 | 34.88 | 33.75 | 0.15 | 0 | 10 |
| 20%-MCG/PU | 19 | 31 | 30 | 0.15 | 0 | 20 |
| 30%-MCG/PU | 16.63 | 27.12 | 26.25 | 0.15 | 0 | 30 |
| 40%-MCG/PU | 14.25 | 23.25 | 22.5 | 0.15 | 0 | 40 |
| Sample | Noncrystalline (%) | Quartz (%) | Kaolin (%) | Illite (%) | Plagioclase (%) | Potassium feldspar (%) | Chlorite (%) | Mullite (%) |
|---|---|---|---|---|---|---|---|---|
| CG | 22.6 | 26.9 | 19.3 | 12.9 | 8.4 | 5.0 | 4.9 | — |
| FA | 69.7 | 7.5 | — | — | — | — | — | 22.8 |
| Sample | SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | CaO (%) | K2O (%) | SO3 (%) | Other (%) | Ignition Loss (%) |
|---|---|---|---|---|---|---|---|---|
| FA | 54.79 | 31.43 | 4.19 | 3.06 | 2.42 | 0.82 | 3.29 | — |
| CG | 54.81 | 21.95 | 4.82 | 1.20 | 3.21 | 0.24 | 4.07 | 9.7 |
| Sample | PU | MFA/PU | MCG/PU | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Filler content (wt%) | — | 10 | 20 | 30 | 40 | 10 | 20 | 30 | 40 |
| LOI (%) | 19 | 22 | 25 | 29 | 28 | 21 | 22 | 23.5 | 22 |
| Sample | PHRR (kW/m2) | THR (kW/m2) | TSP (MJ/m2) | Residue (%) |
|---|---|---|---|---|
| PU | 538.68 | 266.6 | 26.2 | 10.7 |
| 10%-MFA/PU | 429.44 | 182.5 | 24.8 | 16.9 |
| 20%-MFA/PU | 402.02 | 151.0 | 19.7 | 29.2 |
| 30%-MFA/PU | 337.87 | 145.6 | 16.3 | 37.8 |
| 40%-MFA/PU | 272.65 | 119.7 | 14.9 | 46.0 |
| 10%-MCG/PU | 410.03 | 190.8 | 24.1 | 19.3 |
| 20%-MCG/PU | 370.43 | 155.4 | 22.6 | 26.1 |
| 30%-MCG/PU | 348.99 | 134.5 | 19.6 | 35.7 |
| 40%-MCG/PU | 290.50 | 125.9 | 17.4 | 43.9 |
| Testing Items | Domestic Water | Water with 30%-FA/PU | Water with 30%-CG/PU | Standard Limit [29] |
|---|---|---|---|---|
| Total hardness (mg/L) | 2.13 | 2.57 | 2.18 | 450 |
| Sulfate ions (mg/L) | 62.5 | 69.2 | 68.1 | 250 |
| Chloride (mg/L) | 17.5 | 17.9 | 18.5 | 250 |
| Zn (mg/L) | 3.35 × 10−3 | 3.84 × 10−3 | 4.77 × 10−3 | 1.0 |
| Pb (mg/L) | 1.01 × 10−5 | 1.90 × 10−5 | 1.10 × 10−5 | 0.01 |
| Hg (mg/L) | ND | ND | ND | 0.001 |
| As (mg/L) | 8.38 × 10−4 | 1.04 × 10−3 | 1.01 × 10−3 | 0.01 |
| Cd (mg/L) | ND | ND | ND | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, Y.; Li, M.; Zhang, S.; Jia, L.; Zhu, F.; Yu, W. High-Value and Environmentally Friendly Recycling Method for Coal-Based Solid Waste Based on Polyurethane Composite Materials. Polymers 2024, 16, 2044. https://doi.org/10.3390/polym16142044
Li X, Liu Y, Li M, Zhang S, Jia L, Zhu F, Yu W. High-Value and Environmentally Friendly Recycling Method for Coal-Based Solid Waste Based on Polyurethane Composite Materials. Polymers. 2024; 16(14):2044. https://doi.org/10.3390/polym16142044
Chicago/Turabian StyleLi, Xu, Yang Liu, Mingyi Li, Sitong Zhang, Lan Jia, Fengbo Zhu, and Wenwen Yu. 2024. "High-Value and Environmentally Friendly Recycling Method for Coal-Based Solid Waste Based on Polyurethane Composite Materials" Polymers 16, no. 14: 2044. https://doi.org/10.3390/polym16142044
APA StyleLi, X., Liu, Y., Li, M., Zhang, S., Jia, L., Zhu, F., & Yu, W. (2024). High-Value and Environmentally Friendly Recycling Method for Coal-Based Solid Waste Based on Polyurethane Composite Materials. Polymers, 16(14), 2044. https://doi.org/10.3390/polym16142044








