The Impact of ZnO Nanofillers on the Mechanical and Anti-Corrosion Performances of Epoxy Composites
Abstract
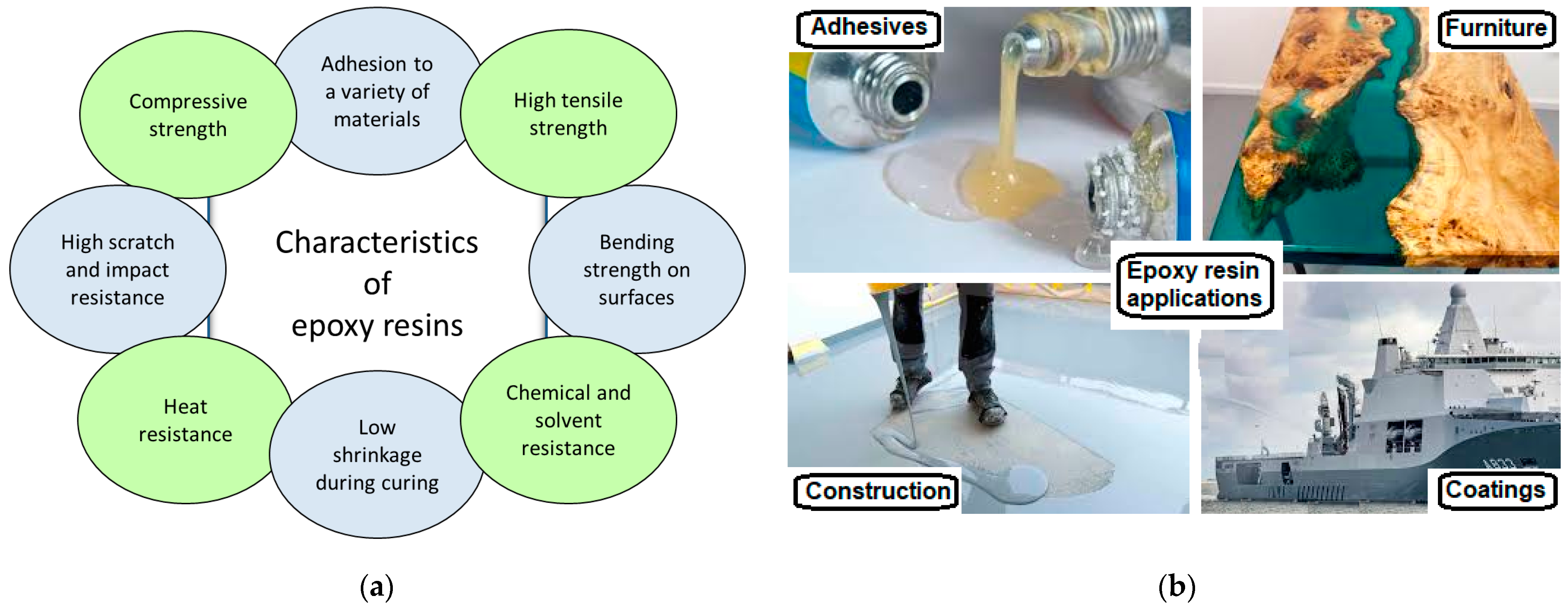
1. Introduction
2. Synthesis Methods of ZnO–Epoxy Nanocomposites
2.1. In Situ Polymerization
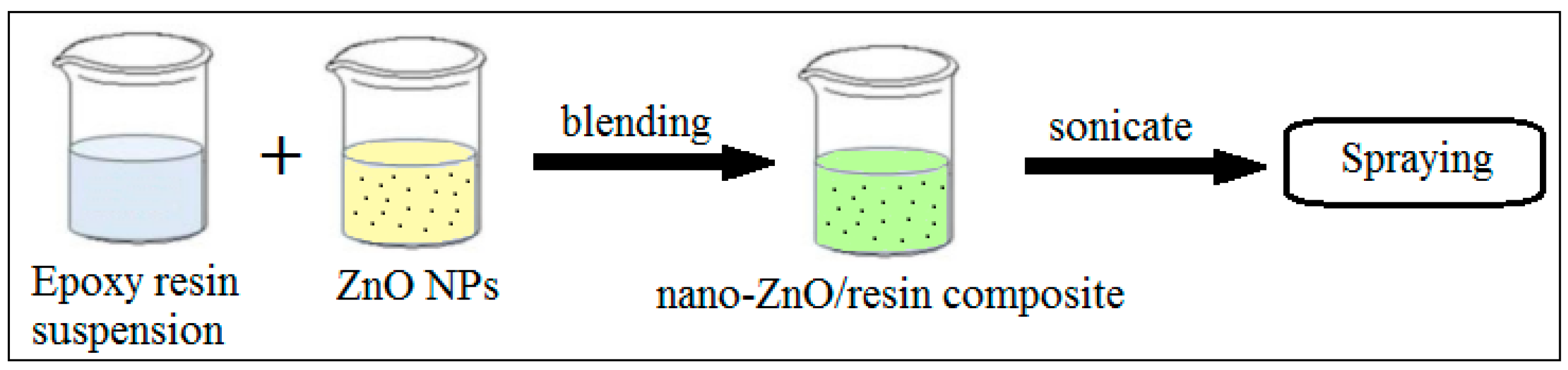
2.2. Solution Blending
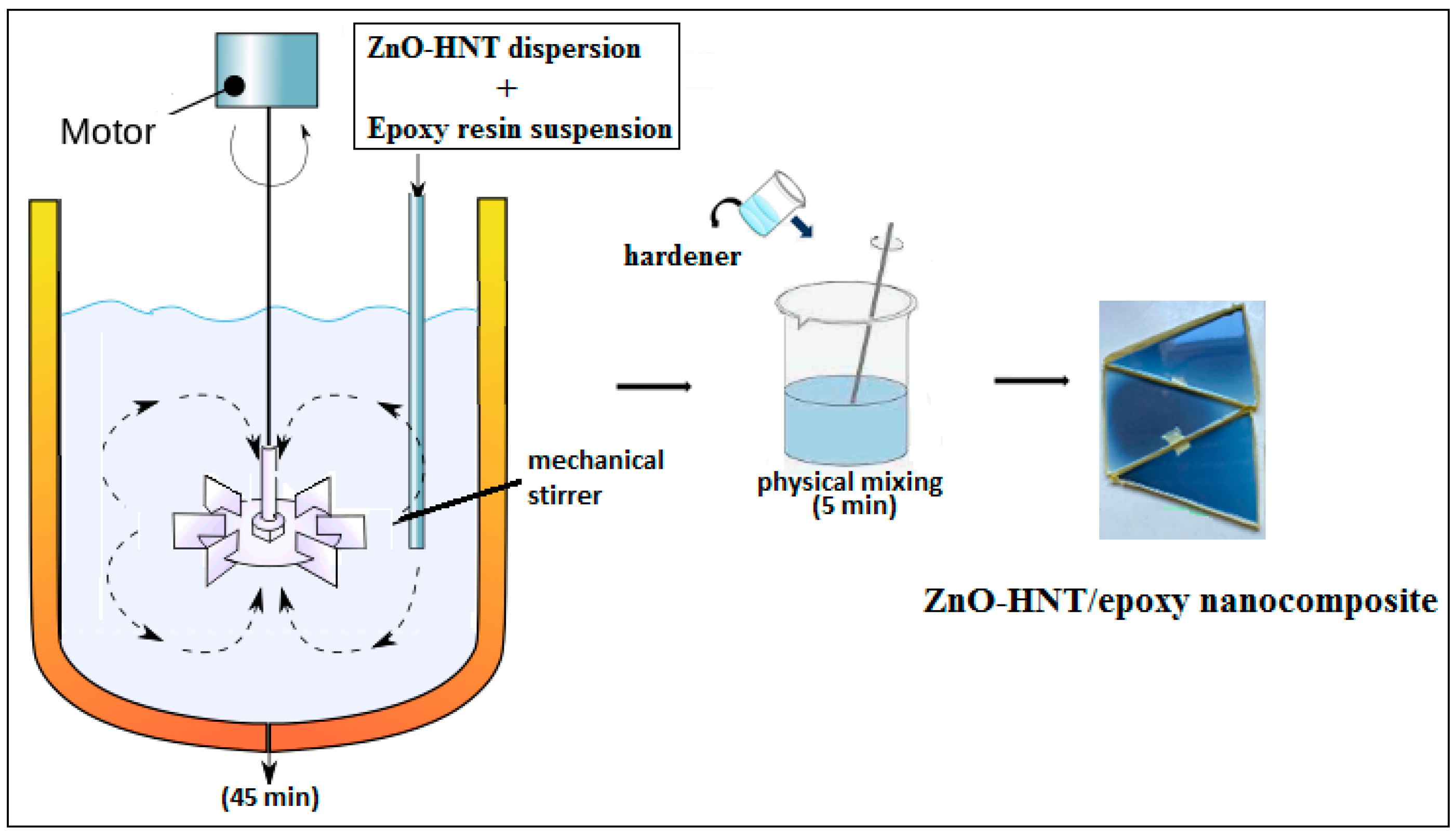
2.3. Mechanical Mixing
- ⮚
- Planetary Mills
- ⮚
- Three-Roll Mixers
- ⮚
- High-Speed Homogenizers
2.4. Dispersion of Nanofillers Using Ultrasound Energy
2.5. Stability Evaluation Methods
- ⮚
- Mechanical Stability Evaluation
- ⮚
- Thermal Stability Evaluation
- ⮚
- Environmental Stability Evaluation
- ⮚
- Dispersion Quality Evaluation
3. The Mechanical Properties of ZnO–Epoxy Nanocomposites
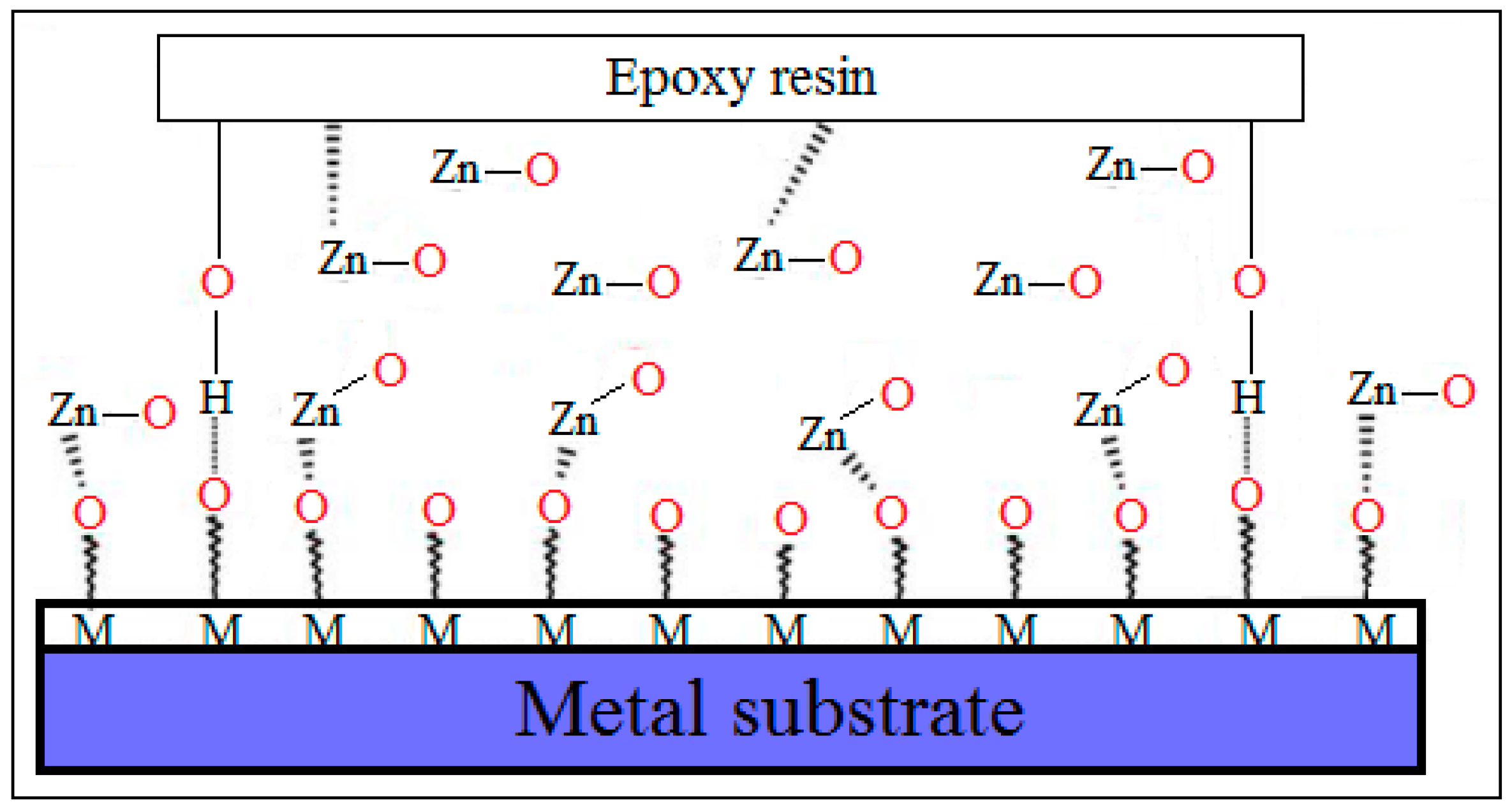
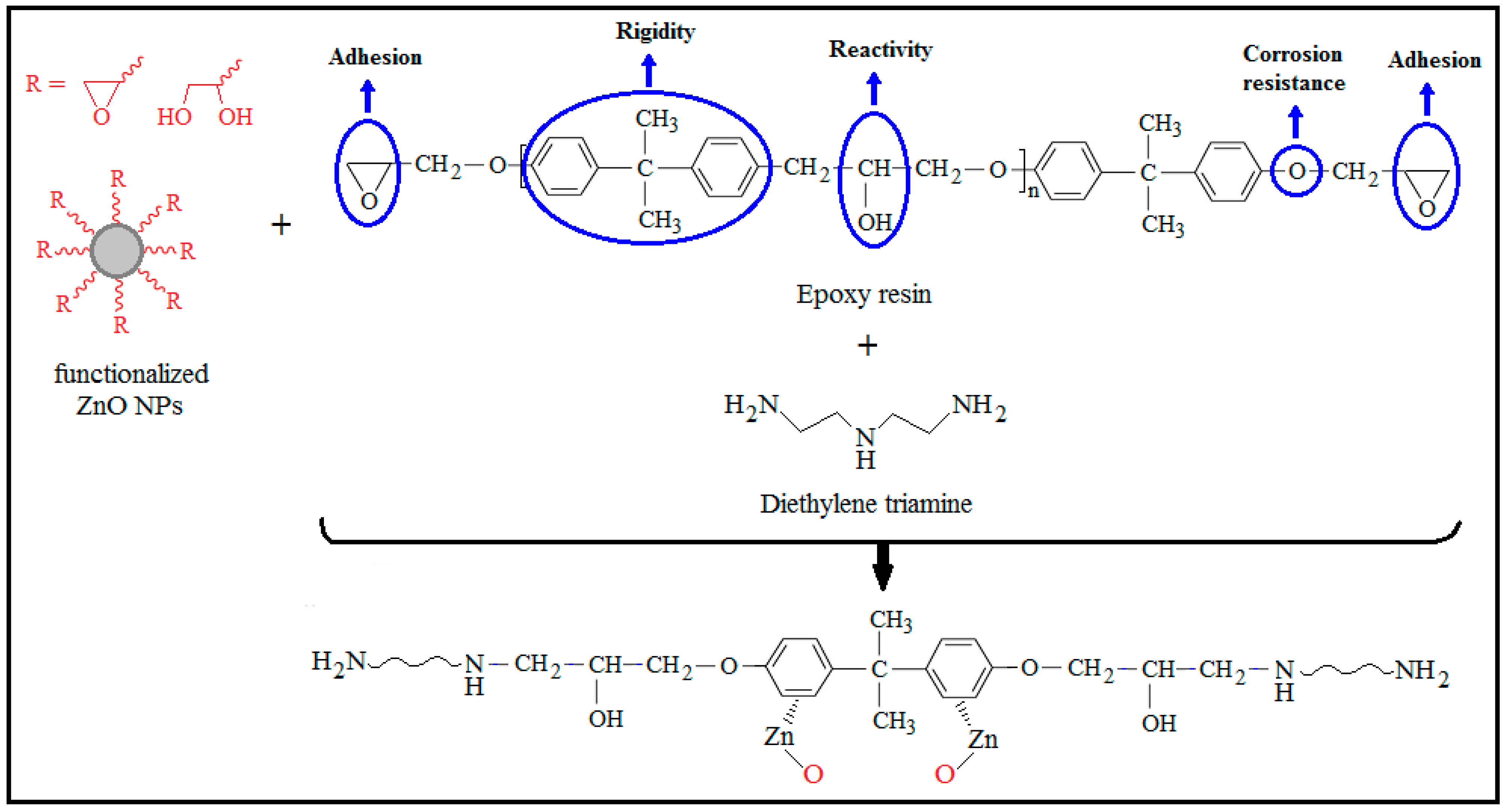
4. The Anti-Corrosion Performance of ZnO–Epoxy Nanocomposites
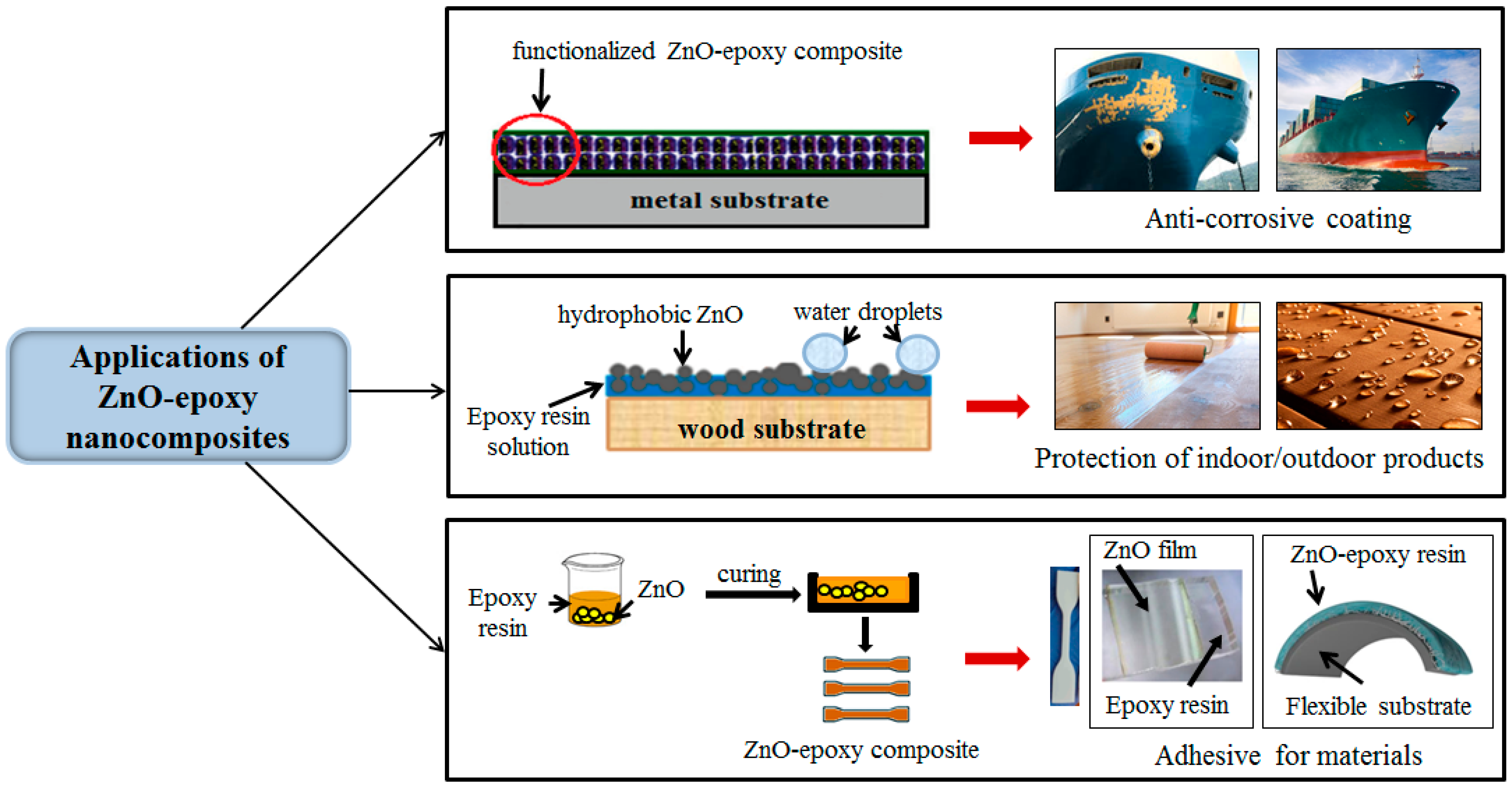
5. Applications of ZnO–Epoxy Nanocomposites
6. Current Challenges and Future Research Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Hasegawa, K.; Kamo, S.; Takagi, K.; Ma, W.; Takahara, A. Enhanced Adhesion Effect of Epoxy Resin on Metal Surfaces Using Polymer with Catechol and Epoxy Groups. ACS Appl. Polym. Mater. 2020, 2, 1500–1507. [Google Scholar] [CrossRef]
- Du, B.; Zhou, X.; Li, Q.; Liu, J.; Liu, Y.; Zeng, X.; Cheng, X.; Hu, H. Surface Treat Method to Improve the Adhesion between Stainless Steel and Resin: A Review. ACS Omega 2023, 8, 39984–40004. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kuwahara, R.; Tanaka, K. Effects of Chemistry of Silicon Surfaces on the Curing Process and Adhesive Strength for Epoxy Resin. ACS Appl. Polym. Mater. 2022, 4, 6038–6046. [Google Scholar] [CrossRef]
- Alonso-Villar, E.M.; Rivas, T.; Pozo-Antonio, J.S. Adhesives applied to granite cultural heritage: Effectiveness, harmful effects and reversibility. Constr. Build. Mater. 2019, 223, 951–964. [Google Scholar] [CrossRef]
- Sugiman, S.; Putra, I.K.P.; Setyawan, P.D. Effects of the media and ageing condition on the tensile properties and fracture toughness of epoxy resin. Polym. Degrad. Stab. 2016, 134, 311–321. [Google Scholar] [CrossRef]
- Amirbeygi, H.; Khosravi, H.; Tohidlou, E. Reinforcing effects of aminosilane-functionalized graphene on the tribological and mechanical behaviors of epoxy nanocomposites. Appl. Polym. Sci. 2019, 136, 47410. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Tastanova, L.; Akhmetova, M.; Apendina, A.; Orynbassar, R.; Lopukhova, M. The Influence of Pristine and Aminoacetic Acid-Treated Aluminum Nitride on the Structure, Curing Processes, and Properties of Epoxy Nanocomposites. J. Compos. Sci. 2023, 7, 482. [Google Scholar] [CrossRef]
- Zheng, S.; Lucas, P.A. Understanding Chemical Resistance in Epoxy System. Coat. World 2020, 29, 35–45. [Google Scholar]
- Ammar, S.; Ma, I.W.; Ramesh, K.; Ramesh, S. Chapter 2-Polymers-based nanocomposite coatings. In Nanomaterials-Based Coatings Fundamentals and Applications; Tri, P.N., Rtimi, S., Plamondon, C.M.O., Eds.; Elsevier: Amsterdam, Netherlands, 2019; pp. 9–39. [Google Scholar]
- Cerit, A.; Marti, M.E.; Soydal, U.; Kocaman, S.; Ahmetli, G. Effect of modification with various epoxide compounds on mechanical, thermal, and coating properties of epoxy resin. Int. J. Polym. Sci. 2016, 1, 4968365. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Gibson, G. Chapter 27-Epoxy Resins. In Brydson’s Plastics Materials, 8th ed.; Elsevier: Amsterdam, Netherlands, 2017; pp. 773–797. [Google Scholar]
- Ramakrishnan, T.; Mohan Gift, M.D.; Chitradevi, S.; Jegan, R.; Hency Jose, P.S.; Nagaraja, H.N.; Sharma, R.; Selvakumar, P.; Hailegiorgis, S.M. Study of Numerous Resins Used in Polymer Matrix Composite Materials. Adv. Compos. Mater. Automot. Appl. 2022, 2022, 1088926. [Google Scholar] [CrossRef]
- Sukanto, H.; Raharjo, W.W.; Ariawan, D.; Triyono, J.; Kaavesina, M. Epoxy resins thermosetting for mechanical engineering. Open Eng. 2021, 11, 797–814. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.A. Application of epoxy resins in building materials: Progress and prospects. Polym. Bull. 2022, 79, 1949–1975. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, S.; Li, W.; Zhang, S.; Li, K.; Nawaz, K.; Wang, P.; Yang, G.; Ragauskas, A.; Ozcan, S.; et al. Epoxy as Filler or Matrix for Polymer Composites. In Epoxy-Based Composites; IntechOpen: London, UK, 2022; pp. 1–18. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Chen, B.; Feng, Z.; Qin, J.; Wu, M.; Chen, L.; Chen, X.; Liang, L. High-performance naphthalene epoxy resins cured by catalyst for packaging materials. Mater. Today Commun. 2022, 33, 104483. [Google Scholar] [CrossRef]
- Rafique, I.; Kausar, A.; Anwar, Z.; Muhammad, B. Exploration of Epoxy Resins, Hardening Systems, and Epoxy/Carbon Nanotube Composite Designed for High Performance Materials: A Review. Polym.-Plast. Technol. Eng. 2016, 55, 312–333. [Google Scholar] [CrossRef]
- Song, J.; Xu, M.; Tan, C.; You, F.; Wang, X.; Zhou, S. Study on an Epoxy Resin System Used to Improve the Elasticity of Oil-Well Cement-Based Composites. Materials 2022, 15, 5258. [Google Scholar] [CrossRef]
- Razin, A.A.; Yari, H.; Ramezanzadeh, B. Stone-chipping and adhesion deterioration of automotive coating systems caused by outdoor weathering of underneath layers. J. Ind. Eng. Chem. 2015, 31, 291–300. [Google Scholar] [CrossRef]
- May, C. Epoxy Resins: Chemistry and Technology; Routledge: Abingdon, UK, 2018. [Google Scholar]
- Abdellaoui, H.; Raji, M.; Bouhfid, R.; el kacem Qaiss, A. Investigation of the deformation behavior of epoxy-based composite materials. In Failure Analysis in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Woodhead Publishing: Cambridge, UK, 2019; pp. 29–49. [Google Scholar]
- Bajpai, A.; Kadiyala, A.K.; Ó Brádaigh, C.M. Introduction to Epoxy/Synthetic Fiber Composites. In Handbook of Epoxy/Fiber Composites; Mavinkere Rangappa, S., Parameswaranpillai, J., Siengchin, S., Thomas, S., Eds.; Springer: Singapore, 2022; pp. 3–34. [Google Scholar] [CrossRef]
- Verma, A.; Baurai, K.; Sanjay, M.R.; Siengchin, S. Mechanical, microstructural, and thermal characterization insights of pyrolyzed carbon black from waste tires reinforced epoxy nanocomposites for coating application. Polym. Compos. 2020, 41, 338–349. [Google Scholar] [CrossRef]
- Scheiner, M.; Dickens, T.J.; Okoli, O. Progress towards self-healing polymers for composite structural applications. Polymer 2016, 83, 260–282. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Siengchin, S.; Pulikkalparambil, H.; Rangappa, S.M. Epoxy Composites: Fabrication, Characterization and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2021; pp. 1–448. [Google Scholar]
- Ou, B.L.; Wang, Y.W.; Lu, Y. A review on fundamentals and strategy of epoxy-resin-based anticorrosive coating materials. Polym.-Plast. Technol. Mater. 2021, 60, 601–625. [Google Scholar] [CrossRef]
- Zhang, M.X.; Zhao, X.Y.; Jia, H.; Xing, H.R.; Zhang, H.J.; Wang, X.Y.; Liu, C. Anticorrosion properties of modified basalt powder/epoxy resin coating. J. Coat. Technol. Res. 2022, 19, 1409–1420. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Yan, S.; Ni, C.; Yu, L.; Li, X. Epoxy composite coating with excellent anticorrosion and self-healing properties based on acrylate copolymers. Prog. Org. Coat. 2022, 172, 107098. [Google Scholar] [CrossRef]
- Appusamy, A.M.; Nanjappan, N.; Eswaran, P.; Subramanian, M. The effect of natural Gongura roselle fiber on the mechanical properties of 3D printed ABS and PLA composites. Polimery 2022, 67, 119–124. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Labesh Kumar, C.; Kandakodeeswaran, K.; Thangagiri, B.; Saxena Kuldeep, K.K.; Kishore, K.; Wagri, N.K.; Kumar, S. Investigation on mechanical properties of novel natural fiber-epoxy resin hybrid composites for engineering structural applications. Case Stud. Constr. Mater. 2023, 19, e02356. [Google Scholar] [CrossRef]
- Olayil, R.; Arumugaprabu, V.; Das, O.; Lenin Anselm, W.A. A Brief Review on Effect of Nano fillers on Performance of Composites. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1059, 012006. [Google Scholar] [CrossRef]
- Soni, S.K.; Thomas, B.; Thomas, S.B.; Tile, P.S.; Sakharwade, S.G. Carbon nanotubes as exceptional nanofillers in polymer and polymer/fiber nanocomposites: An extensive review. Mater. Today Commun. 2023, 37, 107358. [Google Scholar] [CrossRef]
- Al Sheheri, S.Z.; Al-Amshany, Z.M.; Al Sulami, Q.A.; Tashkandi, N.Y.; Hussein, M.A.; El-Shishtawy, R.M. The preparation of carbon nanofillers and their role on the performance of variable polymer nanocomposites. Des. Monomers Polym. 2019, 22, 8–53. [Google Scholar] [CrossRef]
- Rajeswari, N.; Sunitha, K.; Balasubramanian, K. Performance of nanofillers in epoxy resin for corrosion protection coating on metallic substrates. In Proceedings of the International Conference on Smart Technologies and Applied Research, Istanbul, Turkey, 16 January 2024; Volume 477, p. 00097. [Google Scholar]
- Bakkardouch, F.E.; Atmani, H.; El Khalloufi, M.; Jouaiti, A.; Laallam, L. Modified cellulose-based hybrid materials: Effect of ZnO and CuO nanoparticles on the thermal insulation property. Mater. Chem. Phys. 2021, 271, 124881. [Google Scholar] [CrossRef]
- Salas, A.; Jaramillo, A.F.; Palacio, D.A.; Díaz-Gómez, A.; Rojas, D.; Medina, C.; Pérez-Tijerina, E.; Solís-Pomar, F.; Meléndrez, M.F. Hybrid Materials Based on Nanoparticles Functionalized with Alkylsilanes Covalently Anchored to Epoxy Matrices. Polymers 2022, 14, 1579. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Heo, Y.-J.; Park, S.-J. Effect of morphology of calcium carbonate on toughness behavior and thermal stability of epoxy-based composites. Processes 2019, 7, 178. [Google Scholar] [CrossRef]
- Chen, J.; Xu, J.L.; Huang, J.; Dai, L.; Xue, M.S.; Luo, J.M. Corrosion resistance of T-ZnOw/PDMS-MAO composite coating on the sintered NdFeB magnet. J. Magn. Magn. Mater. 2021, 534, 168049. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, L.; Yang, Z.; Wang, L.; Gao, Z.; Shen, Q.; Fan, X.; Yang, H. Fabrication of Epoxy Composite Coatings with Micro-Nano Structure for Corrosion Resistance of Sintered NdFeB. Coatings 2023, 13, 1897. [Google Scholar] [CrossRef]
- Kasim, Y.Y.; Ali, G.G.; Younus, M.H. Irradiation Effects on The Sensitivity of ZnO Thin Films Synthesized on Glass Substrate by Sol-gel Method. Iraqi J. Sci. 2021, 62, 130. [Google Scholar] [CrossRef]
- Hu, C.; Li, Y.; Kong, Y.; Ding, Y. Preparation of poly (o-toluidine)/nano zno/epoxy composite coating and evaluation of its corrosion resistance properties. J. Synth. Met. 2016, 21, 62–70. [Google Scholar] [CrossRef]
- Yari, H.; Rostami, M. Enhanced weathering performance of epoxy/ZnO nanocomposite coatings via functionalization of ZnO UV blockers with amino and glycidoxy silane coupling agents. Prog. Org. Coat. 2020, 147, 105773. [Google Scholar] [CrossRef]
- Alam, M.A.; Samad, U.A.; Khan, R.; Alam, M.; Al-Zahrani, S.M. Anti-corrosive performance of epoxy coatings containing various nano-particles for splash zone applications. Korean J. Chem. Eng. 2017, 34, 2301–2310. [Google Scholar] [CrossRef]
- Jena, K.K.; Alhassan, S.M.; Arora, N. Facile and rapid synthesis of efficient epoxy-novolac acrylate/MWCNTs-APTES-ZnO hybrid coating films by UV irradiation: Thermo-mechanical, shape stability, swelling, hydrophobicity and antibacterial properties. Polymer 2019, 179, 121621. [Google Scholar] [CrossRef]
- Ponnamma, D.; Cabibihan, J.-J.; Rajan, M.; Pethaiah, S.S.; Deshmukh, K.; Gogoi, J.P.; Khadheer Pasha, S.K.; Basheer Ahamed, M.; Krishnegowda, J.; Chandrashekar, B.N.; et al. Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1210–1240. [Google Scholar] [CrossRef]
- Manigandan, S.; Manjari, G.J.N.; Manoj, K.; Gunasekar, P.; Devipriya, J.; Nithya, S. Functionalization of ZnO- Epoxy nanocomposite in glass fiber and Kevlar. Mater. Today Proc. 2019, 16, 1083–1089. [Google Scholar] [CrossRef]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef]
- Purcar, V.; Şomoghi, R.; Niţu, S.G.; Nicolae, C.-A.; Alexandrescu, E.; Gîfu, I.C.; Gabor, A.R.; Stroescu, H.; Ianchiş, R.; Căprărescu, S.; et al. The Effect of Different Coupling Agents on Nano-ZnO Materials Obtained via the Sol–Gel Process. Nanomaterials 2017, 7, 439. [Google Scholar] [CrossRef] [PubMed]
- Duraimurugan, J.; Kumar, G.S.; Venkatesh, M.; Maadeswaran, P.; Girija, E.K. Morphology and size controlled synthesis of zinc oxide nanostructures and their optical properties. J. Mater. Sci. Mater. Electron. 2018, 29, 9339–9346. [Google Scholar] [CrossRef]
- Javadi, E.; Ghaffari, M.; Bahlakeh, G.; Taheri, P. Photocatalytic, corrosion protection and adhesion properties of acrylic nanocomposite coating containing silane treated nano zinc oxide: A combined experimental and simulation study. Prog. Org. Coat. 2019, 135, 496–509. [Google Scholar] [CrossRef]
- Vagena, I.-A.; Gatou, M.-A.; Theocharous, G.; Pantelis, P.; Gazouli, M.; Pippa, N.; Gorgoulis, V.G.; Pavlatou, E.A.; Lagopati, N. Functionalized ZnO-Based Nanocomposites for Diverse Biological Applications: Current Trends and Future Perspectives. Nanomaterials 2024, 14, 397. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandy, M.; Qin, P.; Muneeswaran, T.; Rajivgandhi, G.; Quero, F.; Song, J.-M. Graphene-zinc oxide nanocomposites (G-ZnO NCs): Synthesis, characterization and their photocatalytic degradation of dye molecules. Mater. Sci. Eng. B 2020, 254, 114516. [Google Scholar] [CrossRef]
- Jin, C.; Cheng, Y.; Liu, W.; Lv, Y. Preparation of ZnO-GO nanocomposites and their properties. In Proceedings of the 7th International Conference on Green Materials and Environmental Engineering (GMEE2022), Changsha, China, 16–17 January 2022; Volume 341, p. 01001. [Google Scholar]
- Shaker, S.; Mohsin, A.K.; Edan, M. Preparation TiO2 and ZnO/TiO2 nanocomposites locally and use against Staphylococcus aureus. IOP Conf. Ser. Mater. Sci. Eng. 2020, 928, 072014. [Google Scholar] [CrossRef]
- Shinde, R.S.; Khairnar, S.D.; Patil, M.R.; Adole, V.A.; Koli, P.B.; Deshmane, V.V.; Halwar, D.K.; Shinde, R.A.; Pawar, T.B.; Jagdale, B.S.; et al. Synthesis and Characterization of ZnO/CuO Nanocomposites as an Efective Photocatalyst and Gas Sensor for Environmental Remediation. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1045–1066. [Google Scholar] [CrossRef]
- Wang, C.; Gao, Y.; Wang, L.; Li, P. Morphology regulation, structural, and photocatalytic properties of ZnO hierarchical microstructures synthesized by a simple hydrothermal method. Phys. Status Solidi A 2017, 214, 1600876. [Google Scholar] [CrossRef]
- Mohammadi, E.; Aliofkhazraei, M.; Hasanpoor, M.; Chipara, M. Hierarchical and Complex ZnO Nanostructures by Microwave-Assisted Synthesis: Morphologies, Growth Mechanism and Classification. Crit. Rev. Solid State Mater. Sci. 2018, 43, 475–541. [Google Scholar] [CrossRef]
- Tănase, M.A.; Soare, A.C.; Oancea, P.; Răducan, A.; Mihăescu, C.I.; Alexandrescu, E.; Petcu, C.; Diţu, L.M.; Ferbinteanu, M.; Cojocaru, B.; et al. Facile In Situ Synthesis of ZnO Flower-like Hierarchical Nanostructures by the Microwave Irradiation Method for Multifunctional Textile Coatings. Nanomaterials 2021, 11, 2574. [Google Scholar] [CrossRef]
- Apostoluk, A.; Zhu, Y.; Gautier, P.; Valette, A.; Bluet, J.-M.; Cornier, T.; Masenelli, B.; Daniele, S. Improved Visible Emission from ZnO Nanoparticles Synthesized via the Co-Precipitation Method. Materials 2023, 16, 5400. [Google Scholar] [CrossRef] [PubMed]
- Velayi, E.; Norouzbeigi, R. Single-step prepared hybrid ZnO/CuO nanopowders for water repellent and corrosion resistant coatings. Ceram. Int. 2019, 45, 16864–16872. [Google Scholar] [CrossRef]
- Karim, I.; Iqbal, F.; Ahmad, N.; Shakoor, A.; Naeem, J. Exploration of mechanical and structural properties of bitumen modified with polyethylene glycol and ZnO-nano particles. Polym. Polym. Compos. 2023, 31, 09673911231217838. [Google Scholar] [CrossRef]
- Fuseini, M.; Zaghloul, M.M.Y. Investigation of electrophoretic deposition of pani nano fibers as a manufacturing technology for corrosion protection. Prog. Org. Coat. 2022, 171, 107015. [Google Scholar] [CrossRef]
- Rohani, R.; Dzulkharnien, N.S.F.; Harun, N.H.; Ilias, I.A. Green Approaches, Potentials, and Applications of Zinc Oxide Nanoparticles in Surface Coatings and Films. Bioinorg. Chem. Appl. 2022, 2022, 3077747. [Google Scholar] [CrossRef] [PubMed]
- Elfadel, R.G.; Refat, H.M.; El-Wahab, H.A.; Salem, S.S.; Owda, M.E.; Abdel Reheim, M.A.M. Preparation of new surface coating based on modified oil-based polymers blended with ZnO and CuZnO NPs for steel protection. Sci. Rep. 2023, 13, 7268. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.S.; Mansoor, J.M.; Abdullah, H.W. Micro and Nano of ZnO Particles Effect on Some Mechanical and Thermal Properties of Epoxy Resin Composites. Tikrit J. Pure Sci. 2021, 26, 48–55. [Google Scholar] [CrossRef]
- Amrollahi, S.; Yari, H.; Rostami, M. Investigating the weathering performance of epoxy silicone nanocomposite coatings containing various loadings of Glycidoxypropyltrimethoxysilane-modified Zinc oxide nanoparticles. Prog. Org. Coat. 2022, 172, 107094. [Google Scholar] [CrossRef]
- Pourhashem, S.; Saba, F.; Duan, J.; Rashidi, A.; Guan, F.; Nezhad, E.G.; Hou, B. Polymer/Inorganic nanocomposite coatings with superior corrosion protection performance: A review. J. Ind. Eng. Chem. 2020, 88, 29–57. [Google Scholar] [CrossRef]
- Wei, H.; Xia, J.; Zhou, W.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K. Adhesion and cohesion of epoxy-based industrial composite coatings. Compos. B Eng. 2020, 193, 108035. [Google Scholar] [CrossRef]
- Madhup, M.K.; Shah, N.K.; Parekh, N.R. The Effect of Zinc Oxide Nanoparticles on Cohesive and Adhesive Bond of Epoxy/Amine Coating on Carbon Steel Substrate. IOSR J. Appl. Chem. 2017, 10, 47–58. [Google Scholar]
- Şomoghi, R.; Mihai, S.; Teodorescu, G.-M.; Vuluga, Z.; Gabor, A.R.; Nicolae, C.-A.; Trică, B.; Vătău, D.M.S.; Oancea, F.; Stănciulescu, C.M. Influence of HNT-ZnO Nanofillers on the Performance of Epoxy Resin Composites for Marine Applications. Coatings 2024, 14, 532. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Wu, J.; Chen, Z.; Zhang, X.; Li, M. Hydrothermal In-Situ Synthesis and Anti-Corrosion Performance of Zinc Oxide Hydroxyapatite Nanocomposite Anti-Corrosive Pigment. Coatings 2022, 12, 420. [Google Scholar] [CrossRef]
- Sari, M.G.; Saeb, M.R.; Shabanian, M.; Khaleghi, M.; Vahabi, H.; Vagner, C.; Zarrintaj, P.; Khalili, R.; Para, S.M.R.; Ramezanzadeh, B.; et al. Epoxy/starch-modified nano-zinc oxide transparent nanocomposite coatings: A showcase of superior curing behavior. Prog. Org. Coat. 2018, 115, 143–150. [Google Scholar] [CrossRef]
- Feichtenschlager, B.; Pabisch, S.; Svehla, J.; Peterlik, H.; Sajjad, M.; Koch, T.; Kickelbick, G. Epoxy Resin Nanocomposites: The Influence of Interface Modification on the Dispersion Structure—A Small-Angle-X-ray-Scattering Study. Surfaces 2020, 3, 664–682. [Google Scholar] [CrossRef]
- Verma, S.; Das, S.; Mohanty, S.; Nayak, S.K. Development of multifunctional polydimethylsiloxane (PDMS)-epoxy-zinc oxide nanocomposite coatings for marine applications. Polym. Adv. Technol. 2019, 30, 2275–2300. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, Y.; Feng, W. Three-dimensional interconnected networks for thermally conductive polymer composites: Design, preparation, properties, and mechanisms. Mater. Sci. Eng. R Rep. 2020, 142, 100580. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Li, Z.; Cao, Z.; Xie, Y.; Xue, M.; Zhao, J. Preparation of ZnO Nanoparticle/Acrylic Resin Superhydrophobic Coating via Blending Method and Its Wear Resistance and Antibacterial Properties. Materials 2021, 14, 3775. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Krishnamoorthy, A.; Tanna, A.; Kamathe, V.; Nagar, R.; Srinivasan, S. Recent Developments on the Synthesis of Nanocomposite Materials via Ball Milling Approach for Energy Storage Applications. Appl. Sci. 2022, 12, 9312. [Google Scholar] [CrossRef]
- Agubra, V.A.; Owuor, P.S.; Hosur, M.V. Influence of Nanoclay Dispersion Methods on the Mechanical Behavior of E-Glass/Epoxy Nanocomposites. Nanomaterials 2013, 3, 550–563. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Kamal, A.; Otabil, A.; Mamoun, O.; Liao, K. Graphene/Epoxy Nanocomposites for Improved Fracture Toughness: A Focused Review on Toughening Mechanism. Chem. Eng. J. Adv. 2023, 16, 100537. [Google Scholar] [CrossRef]
- Zanghellini, B.; Knaack, P.; Schörpf, S.; Semlitsch, K.-H.; Lichtenegger, H.C.; Praher, B.; Omastova, M.; Rennhofer, H. Solvent-Free Ultrasonic Dispersion of Nanofillers in Epoxy Matrix. Polymers 2021, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Q.; Cui, X.; Feng, X.; Teng, F.; Xu, M.; Su, W.; He, J. Study on the Mechanical and Toughness Behavior of Epoxy Nano-Composites with Zero-Dimensional and Two-Dimensional Nano-Fillers. Polymers 2022, 14, 3618. [Google Scholar] [CrossRef] [PubMed]
- Barra, G.; Guadagno, L.; Raimondo, M.; Santonicola, M.G.; Toto, E.; Vecchio Ciprioti, S. A Comprehensive Review on the Thermal Stability Assessment of Polymers and Composites for Aeronautics and Space Applications. Polymers 2023, 15, 3786. [Google Scholar] [CrossRef] [PubMed]
- Mailhot, B.; Morlat-Thérias, S.; Bussière, P.O.; Le Pluart, L.; Duchet, J.; Sautereau, H.; Gérard, J.F.; Gardette, J.L. Photoageing Behaviour of Epoxy Nanocomposites: Comparison between Spherical and Lamellar Nanofillers. Polym. Degrad. Stab. 2008, 93, 1786–1792. [Google Scholar] [CrossRef]
- Guloglu, G.E.; Altan, M.C. Moisture Absorption of Carbon/Epoxy Nanocomposites. J. Compos. Sci. 2020, 4, 21. [Google Scholar] [CrossRef]
- Johnsen, B.; Frømyr, T.; Thorvaldsen, T.; Olsen, T. Preparation and characterisation of epoxy/alumina polymer nanocomposites. Compos. Interfaces 2013, 20, 721–740. [Google Scholar] [CrossRef]
- Prasad, T.; Halder, S.; Goyat, M.S.; Dhar, S.S. Morphological dissimilarities of ZnO nanoparticles and its effect on thermo-physical behavior of epoxy composites. Polym. Compos. 2016, 39, 135–145. [Google Scholar] [CrossRef]
- Srikanth, C.; MADHU, G.M. Effect of nano CdO-ZnO content on structural, thermal, optical, mechanical and electrical properties of epoxy composites. J. Met. Mater. Miner. 2023, 33, 38–52. [Google Scholar] [CrossRef]
- Halder, S.; Prasad, T.; Khan, N.I.; Goyat, M.S.; Chauhan, S.R. Superior mechanical properties of poly vinyl alcohol-assisted ZnO nanoparticle reinforced epoxy composites. Mater. Chem. Phys. 2017, 192, 198–209. [Google Scholar] [CrossRef]
- Rashid, A.B.; Haque, M.; Mohaimenul Islam, S.M.; Rafi Uddin Labib, K.M. Nanotechnology-enhanced fiber-reinforced polymer composites: Recent advancements on processing techniques and applications. Heliyon 2024, 10, e24692. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.S.; Mansoor, J.M.; Abdullah, H.W.; Habeeb, A.A. Impact of ZnO Nanoparticles on Mechanical and Dielectric Properties of Epoxy Resin Composites. AIP Conf. Proc. 2023, 2475, 090013. [Google Scholar]
- Baghdadi, Y.N.; Youssef, L.; Bouhadir, K.; Harb, M.; Mustapha, S.; Patra, D.; Tehrani-Bagha, A.R. The Effects of Modified Zinc Oxide Nanoparticles on the Mechanical/Thermal Properties of Epoxy Resin. J. Appl. Polym. Sci. 2020, 137, 49330. [Google Scholar] [CrossRef]
- Mirmohammadi, S.M.; Jazani, O.M.; Ahangaran, F.; Khademi, M.H. Thermomechanical behavior of a novel hybrid epoxy/ZnO nanocomposite adhesive in structural bonding: Experimental analysis and ANN modeling. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133495. [Google Scholar] [CrossRef]
- Al-Lhaibi, S.A.; Al-Shabander, B.M. Study the effect of ZnO nanoparticles reinforced sawdust/epoxy composites on mechanical properties. Digest J. Nanomater. Biostruct. 2022, 17, 851–860. [Google Scholar] [CrossRef]
- Devaraju, A.; Sivasamy, P.; Loganathan, G.B. Mechanical properties of polymer composites with ZnO nano-particle. Mater. Today Proc. 2020, 22, 531–534. [Google Scholar] [CrossRef]
- Husna, O.N.; Ismail, M.C.; Mustapha, M. Mechanical and anticorrosive properties of epoxy containing modified graphene Oxide hybrids. Mater. Today Proc. 2020, 29, 94–99. [Google Scholar]
- Hassana, D.J.; Ali, N.A. Evaluation of Mechanical Properties for Epoxy reinforced with palm oil /Zinc oxide composites. Iraqi J. Sci. 2022, 20, 26–37. [Google Scholar] [CrossRef]
- Lorero, I.; Campo, M.; Del Rosario, G.; López, F.A.; Prolongo, S.G. New Manufacturing Process of Composites Reinforced with ZnO Nanoparticles Recycled from Alkaline Batteries. Polymers 2020, 12, 1619. [Google Scholar] [CrossRef]
- Ahmed, N.M.; Mohamed, M.G. Uplifting of anticorrosive coatings performance via TiO2/ZnO core–shell pigment for oil and gas pipelines protection. Sci. Rep. 2023, 13, 20121. [Google Scholar] [CrossRef]
- Mohan, A.C.; Renjanadevi, B. Effect of Zinc Oxide Nanoparticles on Mechanical Properties of Diglycidyl Ether of Bisphenol-A. J. Mater. Sci. Eng. 2016, 5, 291. [Google Scholar]
- Shubbar, S.D.A.; Diwan, M.A.; Kadhim, A.A.; Diwan, A.A. Influence of Zinc Oxide and Titanium Dioxide Nanoparticles on Kevlar/Epoxy Composites. Rev. Compos. Mater. Avancés 2023, 33, 165–173. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y.; Li, S.; Liu, X. Enhancement of Mechanical Properties and Bonding Properties of Flake-Zinc-Powder-Modified Epoxy Resin Composites. Polymers 2022, 14, 5323. [Google Scholar] [CrossRef] [PubMed]
- Thipperudrappa, S.; Kini, A.U.; Hiremath, A. Influence of zinc oxide nanoparticles on the mechanical and thermal responses of glass fiber-reinforced epoxy nanocomposites. Polym. Compos. 2020, 41, 174–181. [Google Scholar] [CrossRef]
- Smyth, K.; Elliott, M. Effects of changing salinity on the ecology of the marine environment. In Stressors in the Marine Environment Chapter: 9; Solan, M., Whiteley, N., Eds.; Open University Press: Berkshire, UK, 2016; pp. 161–174. [Google Scholar]
- Choqueuse, D.; Davies, P. Ageing of composites in underwater applications. In Ageing of Compos; Elsevier: Amsterdam, The Netherlands, 2008; pp. 467–498. [Google Scholar] [CrossRef]
- Dohare, S. Corrosion Protection and Modern Infrastructure. In Introduction to Corrosion-Basics and Advances; Singh, A., Ed.; IntechOpen: London, UK, 2023; p. 258. [Google Scholar]
- Summerscales, J. Materials selection for marine composites. In Marine Composites: Design and Performance; Pemberton, R., Summerscales, J., Graham-Jones, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–30. [Google Scholar] [CrossRef]
- Anwar, S.; Li, X. A review of high-quality epoxy resins for corrosion-resistant applications. J. Coat. Technol. Res. 2024, 21, 461–480. [Google Scholar] [CrossRef]
- Mihai, O.; Pantea, O.; Popovici, D.R.; Gheorghe, C.G. Evaluation of metal contents in correlation with phytosanitary treatments at vineyard. Rev. Chim. 2017, 68, 1387–1391. [Google Scholar] [CrossRef]
- Gheorghe, V.; Gheorghe, C.G.; Bondarev, A.; Somoghi, R. Ecotoxicity of o-Chlorobenzylidene Malononitrile (CBM) and Toxicological Risk Assessment for SCLP Biological Cultures (Saccharomyces sp., Chlorella sp., Lactobacillus sp., Paramecium sp.). Toxics 2023, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.W.; Johari, N.; Mazlan, S.A.; Syed Ahmad, S.N.A.; Arifin, R.; Hassan, N.A.; Johari, M.A.F. Superhydrophobic zinc oxide/epoxy coating prepared by a one-step approach for corrosion protection of carbon steel. J. Mater. Res. Technol. 2023, 25, 5751–5766. [Google Scholar] [CrossRef]
- Ibrahim, N.F.; Wan Abdullah, W.R.; Rooshde, M.S.; Mohd Ghazali, M.S.; Wan Nik, W.M.N. Corrosion Inhibition Properties of Epoxy-Zinc Oxide Nanocomposite Coating on Stainless Steel 316L. Solid State Phenom. 2020, 307, 285–290. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Wang, C.; Zhang, M.; Liu, P.; Wang, J. Fabrication of ZnO/epoxy resin superhydrophobic coating on AZ31 Magnesium Alloy. Chem. Eng. J. 2019, 368, 261–272. [Google Scholar] [CrossRef]
- Panaite, V.; Boiciuc, S.; Musat, V. ZnO Nanoparticles–Epoxy Resin Hybrid Nanocomposite with Anticorrosive and Antifouling Properties as Coatings for Naval Steel. Rev. Chim. 2015, 2, 213–218. [Google Scholar]
- Lv, K.; Pan, R.; Zhang, L.; Tian, Y.; Suia, Y.; Wan, D. Synergistically assembled graphene/ZnO composite to enhance anticorrosion performance of waterborne epoxy coatings. RSC Adv. 2022, 12, 9069–9076. [Google Scholar] [CrossRef]
- Othman, N.H.; Yahya, W.Z.N.; Che Ismail, M.; Mustapha, M.; Koi, Z.K. Highly dispersed graphene oxide–zinc oxide nanohybrids in epoxy coating with improved water barrier properties and corrosion resistance. J. Coat. Technol. Res. 2020, 17, 101–114. [Google Scholar] [CrossRef]
- Ibrahim, M.; Kannan, K.; Parangusan, H.; Eldeib, S.; Shehata, O.; Ismail, M.; Zarandah, R.; Sadasivuni, K.K. Enhanced Corrosion Protection of Epoxy/ZnO-NiO Nanocomposite Coatings on Steel. Coatings 2020, 10, 783. [Google Scholar] [CrossRef]
- Mahajan, A.G.; Deshpande, P.; Butee, S. Corrosion Protection of Mild Steel Using ZnO/NiO Pigment-Based Epoxy Coating. JOM 2024, 76, 612–621. [Google Scholar] [CrossRef]
- Qi, C.; Dam-Johansen, K.; Weinell, C.E.; Bi, H.; Wu, H. Enhanced anticorrosion performance of zinc rich epoxy coatings modified with stainless steel flakes. Prog. Org. Coat. 2022, 163, 106616. [Google Scholar] [CrossRef]
- Kabaoglu, E.; Karabork, F.; Balun Kayan, D.; Akdemir, A. Improvement of anti-corrosion performance (surface and near the cut edge) and mechanical properties of epoxy coatings modified with nano, micro and hybrid ZnO particles. J. Compos. Mater. 2023, 57, 451–463. [Google Scholar] [CrossRef]
- Jia, Z.; Hong, R. Anticorrosive and photocatalytic properties research of epoxy-silica organic–inorganic coating. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126647. [Google Scholar] [CrossRef]
- Baronins, J.; Antonov, M.; Abramovskis, V.; Rautmane, A.; Lapkovskis, V.; Bockovs, I.; Goel, S.; Thakur, V.K.; Shishkin, A. The Effect of Zinc Oxide on DLP Hybrid Composite Manufacturability and Mechanical-Chemical Resistance. Polymers 2023, 15, 4679. [Google Scholar] [CrossRef]
- Volkova, M.; Sondors, R.; Bugovecka, L.; Kons, A.; Avotina, L.; Andzane, J. Enhanced thermoelectric properties of self-assembling ZnO nanowire networks encapsulated in nonconductive polymers. Sci. Rep. 2023, 13, 21061. [Google Scholar] [CrossRef]
- Tuong, V.M.; Huyen, N.V.; Kien, N.T.; Dien, N.V. Durable Epoxy@ZnO Coating for Improvement of Hydrophobicity and Color Stability of Wood. Polymers 2019, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, R.; Liu, C.; Mo, J. Application of ZnO/epoxy resin superhydrophobic coating for buoyancy enhancement and drag reduction. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129714. [Google Scholar] [CrossRef]
- Lorero, I.; Campo, M.; Arribas, C.; Prolongo, M.G.; López, F.A.; Prolongo, S.G. Epoxy Composites Reinforced with ZnO from Waste Alkaline Batteries. Materials 2022, 15, 2842. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, F.; Wang, F.; Liu, L. Dual-action epoxy coating with anti-corrosion and antibacterial properties based on well-dispersed ZnO/basalt composite. Compos. Commun. 2023, 42, 101674. [Google Scholar] [CrossRef]
- Alam, M.A.; Samad, U.A.; Anis, A.; Alam, M.; Ubaidullah, M.; Al-Zahrani, S.M. Effects of SiO2 and ZnO Nanoparticles on Epoxy Coatings and Its Performance Investigation Using Thermal and Nanoindentation Technique. Polymers 2021, 13, 1490. [Google Scholar] [CrossRef]
- Roudpishi, M.M.; Farrash, S.M.H.; Shaterzadeh, A. Effect of zinc oxide nanoparticles on critical buckling load of glass/epoxy composites exposed to sunlight irradiation. Polym. Polym. Compos. 2023, 31, 09673911231202153. [Google Scholar]




| Methods | Advantages | Disadvantages |
|---|---|---|
| In situ polymerization |
|
|
| Solution blending |
|
|
| Mechanical mixing |
|
|
| Nanofiller Type | Nanofiller Property | Concentration of Nanofiller (wt. %) | Epoxy Resin Type | Mechanical Tests | Results | Refs. |
|---|---|---|---|---|---|---|
| ZnO | Commercial ZnO | 0.1, 0.3, 0.5, and 0.7 | Epoxy resin | Bending strength, flexural modulus, and stiffness | Mechanical properties were influenced by the nanofiller content. | [91] |
| ZnO | Commercial ZnO (particle size of ~100 nm) | 1, 2.5, and 5 | Bisphenol A (DGEBA) | Tensile strength | Composite with 2.5 wt. % ZnO filler showed the best tensile strength. | [92] |
| ZnO | Commercial ZnO | 0.5, 1, 2, and 5 | Resole resin | Tensile and lap shear tests | ZnO filler enhanced the mechanical properties of epoxy adhesives. | [93] |
| ZnO | Commercial ZnO (particle size of ~10–30 nm) | 0.1, 0.3, 0.5, 0.7, and 1 | Bisphenol A (DGEBA) | Flexural strength and hardness tests | Mechanical properties increased up to certain ZnO filler (0.5 wt. %) and then properties gradually decreased. | [94] |
| ZnO | Synthesized ZnO NPs | 0.1, 0.3, and 0.5 | Epoxy resin | Tensile, impact, and flexural properties | Composite with 0.5 wt. % ZnO filler presented more tensile, impact, and flexural strength. | [95] |
| ZnO-GO | Commercial ZnO | 0.001, 0.01, 0.1, and 0.5 | Bisphenol-A EPON 828 | Tensile strength, elongation at break, and Young’s modulus | Addition of a small amount of ZnO-GO (0.01 wt. %) improved the tensile strength and Young’s modulus of nanocomposite by 93%. | [96] |
| ZnO-OP | Commercial ZnO (99.8%) | 1, 3, 5, and 10 | Polymeric base material epoxy | Tensile, impact, hardness, and wear rate | Nanofillers improved the ductility and stiffness, and increased the impact strength of the epoxy nanocomposites. | [97] |
| ZnO | Recycled ZnO from spent alkaline batteries (average thickness of 35 nm) | 0, 2, 6. 10, and 30 | Bisphenol A | Stiffness and hardness tests | Utilization of 30 wt. % recycled ZnO NPs enhanced stiffness and hardness (82.3%) of epoxy composite. | [98] |
| ZnO-TiO2 | Commercial ZnO (white powder of purity 99%) | 29 | Bisphenol A | Impact resistance, ductility, and hardness | ZnO-TiO2 reinforced the epoxy matrix and improved the adhesion and elasticity of the coating film. | [99] |
| ZnO | Synthesized ZnO NPs | 1, 1.5, 2, 3, 4, 5 and 10 | Bisphenol A | Tensile strength and breaking force | Epoxy resin with 2 wt. % ZnO presented good tensile strength. | [100] |
| ZnO-TiO2 | Commercial ZnO (particle size of 150 nm) | 1, 2 and 4 | Bisphenol A | Tensile, flexural, and creep tests | Epoxy resin with 4 wt. % showed the highest flexural strength and good creep resistance. | [101] |
| Nanofiller Type | Nanofiller Property | Concentration of Nanofiller (wt. %) | Epoxy Resin Type | Corrosion Test | Results | Refs. |
|---|---|---|---|---|---|---|
| ZnO | Commercial ZnO (diameter < 50 nm) | 1 | Bisphenol A (DGEBA) | Immersion in 3.5 wt. % NaCl aqueous solution | Epoxy coating incorporating ZnO NPs presented great corrosion resistance with potential applications in the marine environment; corrosion rate—6.4971 × 10−6 mm/year (compared with control—0.07699 mm/year). | [111] |
| ZnO | Commercial ZnO (diameter < 100 nm) | 1, 3, 5, 7, and 10 | Bisphenol A and butyl glycidyl ether | Tested in 3.5 wt. % NaCl aqueous solution, at ambient temperature | Epoxy coating loaded with 3 wt. % ZnO NPs provided the best corrosion protection on metal substrate; corrosion rate—0.00047 mm/year, compared with control—0.05268 mm/year. | [112] |
| ZnO | Commercial ZnO (diameter of 300 nm) | 2, 3, and 4 | Epoxy resin (E51) | Immersion in 3.5 wt. % NaCl solution for 6 h and 12 h | Epoxy coatings presented different corrosion resistances as a function of the immersion time. | [113] |
| ZnO-GPTMS | Commercial ZnO (diameter ≤ 50 nm) | 1 | Bisphenol A (DGEBA) | Immersion in 5 wt. % aqueous NaCl solution over 30 days | Good level of anticorrosion resistance revealed by increasing the thickness of the nanocomposite coating. | [114] |
| ZnO-Gr | Commercial ZnO (99.99%) | 0.4 | Waterborne epoxy resin | Immersion in 3.5 wt. % NaCl solution for 7 days | Utilization of ZnO enhanced the dispersibility of graphene, improving the anticorrosive performance of epoxy coatings; impedance—200,530 Ω cm2. | [115] |
| ZnO-GO | Commercial ZnO | 0.001, 0.01, 0.1, and 0.5 | Bisphenol-A (EPON 828) | Exposed to 3.5 wt. % NaCl for 14 days | Epoxy nanocomposite with 0.1 wt. % ZnO-GO presented the most corrosion resistance with protection efficiencies of 61% (compared with 0.01% ZnO-GO—27% and 0.1 wt. % GO—40%). | [96] |
| ZnO-GO | Commercial ZnO (average diameter of 50 nm) | 0.1 | Bisphenol A (DGEBA, EPON 828) | Immersion in 3.5 wt. % NaCl salt solution | Utilization of ZnO-GO epoxy composite enhanced the anticorrosion property of the coating; impedance—2 × 1010 Ω cm2; protection efficiency 90%. | [116] |
| ZnO-TiO2 | Commercial ZnO (white powder of purity 99%) | 29 | Bisphenol A | Exposed to 3.5 wt. % NaCl for 28 days | Inclusion of ZnO-TiO2 into epoxy resin increased the durability and corrosion resistance of epoxy coatings; impedance—14,325 Ω cm2 after 28 days, compared with ZnO—7409 and TiO2—2104 Ω cm2. | [99] |
| ZnO-NiO | Synthesized ZnO NPs (powder) | 1 | Bisphenol A | Immersion in 3.5 wt. % NaCl solution | Incorporation of ZnO-NiO into epoxy nanocomposite improved the anti-corrosion performance of coating; corrosion rate—2.47 mpy (milli-inches per year), compared with control—43.99 mpy. | [117] |
| ZnO-NiO | Commercial ZnO | 1 | Bisphenol A | Tested in 3.5 wt. % NaCl | Utilization of ZnO-NiO in epoxy matrix enhanced the corrosion resistance of coating; corrosion rate—0.02 mpy, compared with control—13.54 mpy. | [118] |
| ZnO-HAP | Commercial ZnO | 80, 20 and 50 | Epoxy resin (E51) | Tested in 3.5 wt. % NaCl solution for 24 and 72 h | Epoxy resin coating with 65 wt. % ZnO presented the greatest anti-corrosion performance; resistance—31,432 Ω cm2, compared with control—688.9 Ω cm2, after 72 h. | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şomoghi, R.; Semenescu, A.; Pasăre, V.; Chivu, O.R.; Nițoi, D.F.; Marcu, D.F.; Florea, B. The Impact of ZnO Nanofillers on the Mechanical and Anti-Corrosion Performances of Epoxy Composites. Polymers 2024, 16, 2054. https://doi.org/10.3390/polym16142054
Şomoghi R, Semenescu A, Pasăre V, Chivu OR, Nițoi DF, Marcu DF, Florea B. The Impact of ZnO Nanofillers on the Mechanical and Anti-Corrosion Performances of Epoxy Composites. Polymers. 2024; 16(14):2054. https://doi.org/10.3390/polym16142054
Chicago/Turabian StyleŞomoghi, Raluca, Augustin Semenescu, Vili Pasăre, Oana Roxana Chivu, Dan Florin Nițoi, Dragoş Florin Marcu, and Bogdan Florea. 2024. "The Impact of ZnO Nanofillers on the Mechanical and Anti-Corrosion Performances of Epoxy Composites" Polymers 16, no. 14: 2054. https://doi.org/10.3390/polym16142054
APA StyleŞomoghi, R., Semenescu, A., Pasăre, V., Chivu, O. R., Nițoi, D. F., Marcu, D. F., & Florea, B. (2024). The Impact of ZnO Nanofillers on the Mechanical and Anti-Corrosion Performances of Epoxy Composites. Polymers, 16(14), 2054. https://doi.org/10.3390/polym16142054








