Effect of Additives on Thermal Degradation and Crack Propagation Properties of Recycled Polyethylene Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Experimental Methodologies
2.2.1. Notched Crack Ligament Stress (NCLS)
2.2.2. Un-Notched, Constant Ligament Stress Crack (UCLS)
2.2.3. Oxidative Induction Time (OIT)
2.2.4. Differential Scanning Calorimeter (DSC)
3. Results and Discussion
3.1. Oxidative Induction Time (OIT)
3.2. Notched Crack Ligament Stress (NCLS)
3.3. Un-Notched Crack Ligament Stress (UCLS)
3.4. Differential Scanning Calorimetry (DSC)
4. Conclusions
- -
- A compatibilizer;
- -
- Antioxidants;
- -
- Carbon black.
- For thermal degradation, the mixing of carbon black and antioxidants, or antioxidants and compatibilizers, multiplied the Oxidative Induction Time (OIT) by a factor of 7 compared to the reference blend without any additives.
- The addition of carbon black negatively impacts stress cracking, while adding an antioxidant with a compatibilizer improved the time before failure in the NCLS.
- No matter which additive was used, UCLS decreased compared to the reference blend.
- This study showed that the addition of a compatibilizer decreases the degree of crystallinity; however, the addition of carbon black and antioxidants increases the degree of crystallinity.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Subramanian, P.M. Plastics recycling and waste management in the US. Resour. Conserv. Recycl. 2000, 28, 253–263. [Google Scholar] [CrossRef]
- Aznar, M.P.; Caballero, M.A.; Sancho, J.A.; Francés, E. Plastic waste elimination by co-gasification with coal and biomass in fluidized bed with air in pilot plant. Fuel Process. Technol. 2006, 87, 409–420. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2020, 42, e2000415. [Google Scholar] [CrossRef] [PubMed]
- Plastics Pipe Institute. Handbook of PE Pipe|HDPE Handbook, 2nd ed.; Plastics Pipe Institute: Irving, TX, USA, 2008. [Google Scholar]
- AASHTO-M 294; Standard Specification for Corrugated Polyethylene Pipe, 300- to 1500-mm (12- to 60-in.) Diameter. American Association of State Highway and Transportation Officials: Washinghton, DC, USA, 2018.
- Pospiech, D. Influencing the interface in polymer blends by compatibilization with block copolymers. Polym. Surf. Interfaces Charact. Modif. Appl. 2008, 275–298. [Google Scholar]
- Hoff, A.; Jacobsson, S. Thermo-oxidative degradation of low-density polyethylene close to industrial processing conditions. J. Appl. Polym. Sci. 1981, 26, 3409. [Google Scholar] [CrossRef]
- Lamtai, A.; Elkoun, S.; Robert, M.; Mighri, F.; Diez, C. Mechanical Recycling of Thermoplastics: A Review of Key Issues. Waste 2023, 1, 860–883. [Google Scholar] [CrossRef]
- Pinheiro, L.; Chinelatto, M.; Canevarolo, S. The role of chain scission and chain branching in high density polyethylene during thermo-mechanical degradation. Polym. Degrad. Stab. 2004, 86, 445–453. [Google Scholar] [CrossRef]
- Cuadri, A.; Martín-Alfonso, J. The effect of thermal and thermo-oxidative degradation conditions on rheological, chemical and thermal properties of HDPE. Polym. Degrad. Stab. 2017, 141, 11–18. [Google Scholar] [CrossRef]
- Liu, W.C.; Halley, P.J.; Gilbert, R.G. Mechanism of degradation of starch, a highly branched polymer, during extrusion. Macromolecules 2010, 43, 2855. [Google Scholar] [CrossRef]
- Moncada, A.I.; Huang, W.; Horstman, N. Handbook of Industrial Polyethylene Technology; Scivener Publishing LLC.: Midland, TX, USA, 2016; pp. 715–750. [Google Scholar]
- Kim, K.J.; Kim, B.K. Crosslinking of HDPE during reactive extrusion: Rheology, thermal, and mechanical properties. J. Appl. Polym. Sci. 1993, 48, 981–986. [Google Scholar] [CrossRef]
- Sánchez, C.G.; Martínez-Aguirre, A.; Pérez-García, B.; Acosta, J.; Fonseca-Valero, C.; De La Orden, M.U.; Sánchez, C.; Martínez Urreaga, J. Enhancement of mechanical properties of waste-sourced biocomposites through peroxide induced crosslinking. Compos. Part A 2016, 80, 285. [Google Scholar] [CrossRef]
- Rosales-Jasso, A.; Allen, N.S.; Sasaki, M. Evaluation of novel 4,4-dimethyloxazolidine derivatives as thermal and UV stabilisers in linear low density polyethylene (LLDPE) film. Polym. Degrad. Stab. 1999, 64, 277. [Google Scholar] [CrossRef]
- Iyer, K.A.; Zhang, L.; Torkelson, J.M. Direct Use of Natural Antioxidant-rich Agro-wastes as Thermal Stabilizer for Polymer: Processing and Recycling. ACS Sustain. Chem. Eng. 2015, 4, 881–889. [Google Scholar] [CrossRef]
- Peña, J.; Allen, N.; Edge, M.; Liauw, C.; Valange, B. Studies of synergism between carbon black and stabilisers in LDPE photodegradation. Polym. Degrad. Stab. 2001, 72, 259–270. [Google Scholar] [CrossRef]
- Deveci, S.; Antony, N.; Eryigit, B. Effect of carbon black distribution on the properties of polyethylene pipes—Part 1: Degradation of post yield mechanical properties and fracture surface analyses. Polym. Degrad. Stab. 2018, 148, 75–85. [Google Scholar] [CrossRef]
- La Mantia, F.P. Recycled plastics: Additives and their effects on properties. In Plastics Additives: An A-Z Reference; Pritchard, G., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 535–543. [Google Scholar] [CrossRef]
- Aid, S. Etude de la Miscibilité des Polymères par la Méthode de Coalescence des Grains en vue du Recyclage des DEEE par Rotomoulage; ENSAM: Paris, France, 2017. [Google Scholar]
- Karaagac, E.; Koch, T.; Archodoulaki, V.-M. The effect of PP contamination in recycled high-density polyethylene (rPE-HD) from post-consumer bottle waste and their compatibilization with olefin block copolymer (OBC). Waste Manag. 2020, 119, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Gall, M.; Schweighuber, A.; Buchberger, W.; Lang, R.W. Plastic Bottle Cap Recycling—Characterization of Recyclate Composition and Opportunities for Design for Circularity. Sustainability 2020, 12, 10378. [Google Scholar] [CrossRef]
- Adib, A.; Domínguez, C.; Rodríguez, J.; Martín, C.; García, R.A. The effect of microstructure on the slow crack growth resistance in polyethylene resins. Polym. Eng. Sci. 2015, 55, 1018–1023. [Google Scholar] [CrossRef]
- Messiha, M.; Frank, A.; Koch, T.; Arbeiter, F.; Pinter, G. Effect of polyethylene and polypropylene cross-contamination on slow crack growth resistance. Int. J. Polym. Anal. Charact. 2020, 25, 649–666. [Google Scholar] [CrossRef]
- ASTM F2136-18; Standard Test Method for Notched, Constant Ligament-Stress (NCLS) Test to Determine Slow-Crack-Growth Resistance of HDPE Resins or HDPE Corrugated Pipe. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM F3181-16; Standard Test Method for The Un-notched, Constant Ligament Stress Crack Test (UCLS) for HDPE Materials Containing Post- Consumer Recycled HDPE. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D3895-14; Standard Test Method for Oxidative-Induction Time of Polyolefins by Differential Scanning Calorimetry. ASTM International: West Conshohocken, PA, USA, 2019.
- Wong, W.-K.; Hsuan, Y.G. Interaction between carbon black and antioxidants in high-density polyethylene pipe resin. Transp. Res. Rec. 2012, 2310, 137–144. [Google Scholar] [CrossRef]
- Darweesh, M.H.; Stoll, B.; El-Taweel, S.H. Compatibilization of polypropylene/high-density polyethylene blends using poly(propylene-co-ethylene). J. Appl. Polym. Sci. 2023, 140, e53687. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/app.53687 (accessed on 12 February 2024). [CrossRef]
- Phease, T.; Billingham, N.; Bigger, S. The effect of carbon black on the oxidative induction time of medium-density polyethylene. Polymer 2000, 41, 9123–9130. [Google Scholar] [CrossRef]
- Rwei, S.; Manas-Zloczower, I.; Feke, D.L. Analysis of dispersion of carbon black in polymeric melts and its effect on compound properties. Polym. Eng. Sci. 1992, 32, 130–135. [Google Scholar] [CrossRef]
- Pircheraghi, G.; Sarafpour, A.; Rashedi, R.; Afzali, K.; Adibfar, M. Correlation between Rheological and Mechanical Properties of Black PE100 Compounds—Effect of Carbon Black Masterbatch; Department of Materials Science and Engineering, Sharif University of Technology: Tehran, Iran, 2017. [Google Scholar]
- Munson, D. Pennsylvania Edge Notched Tensile Resistance of High Density Polyethylene Butt Fusion Joints; Report No: 3002003089; Electric Power Research Institute: Palo Alto, CA, USA, 2016. [Google Scholar]
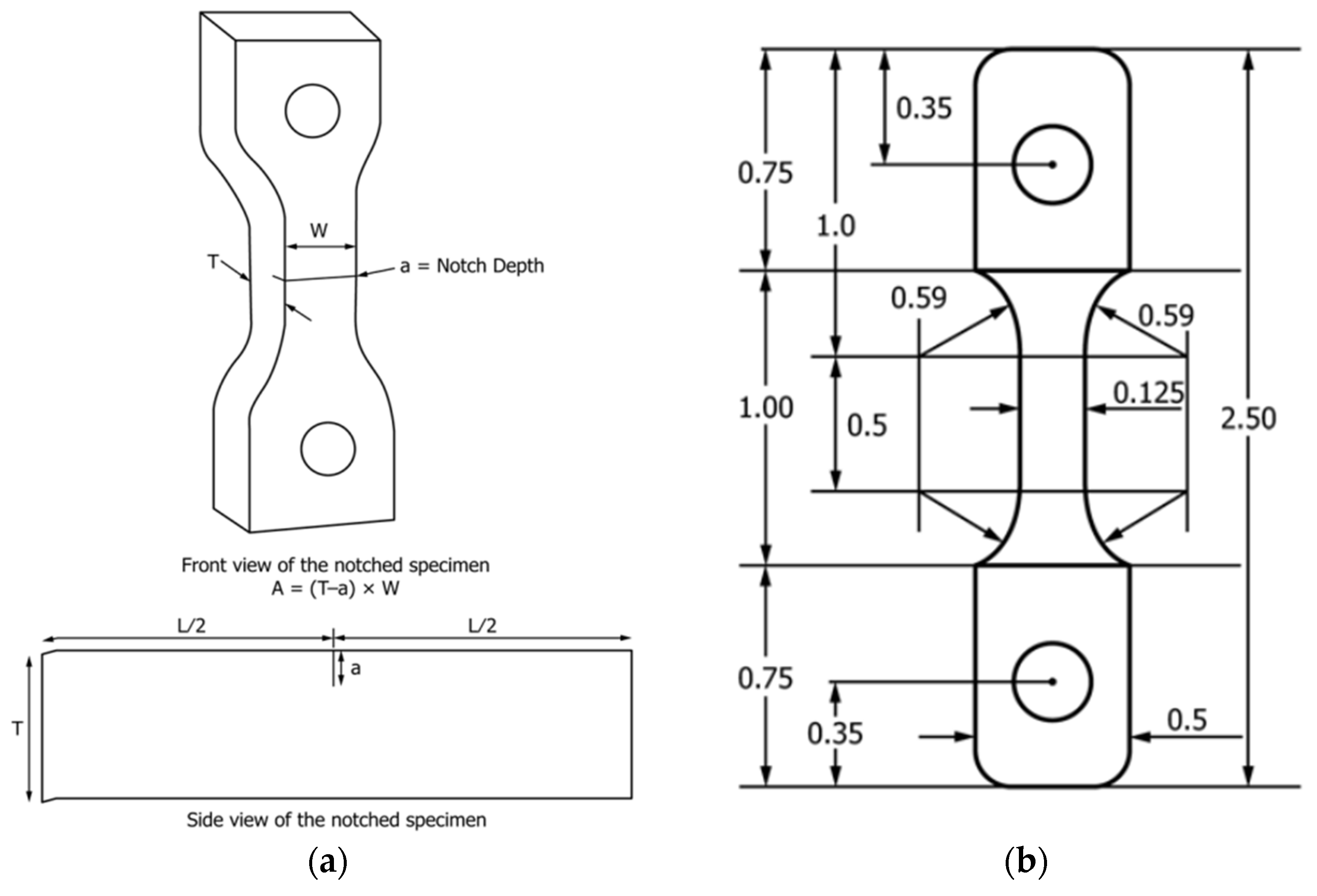
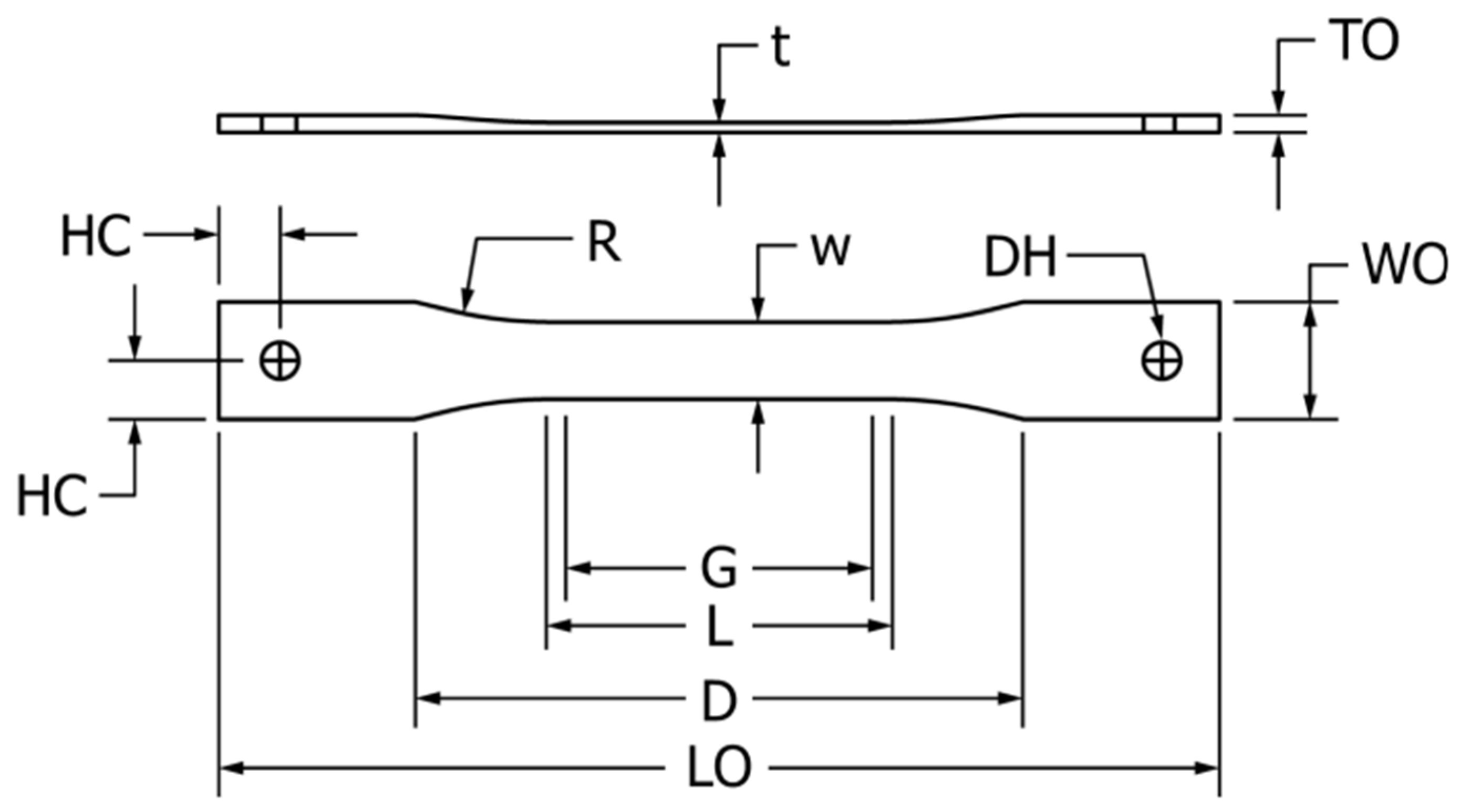
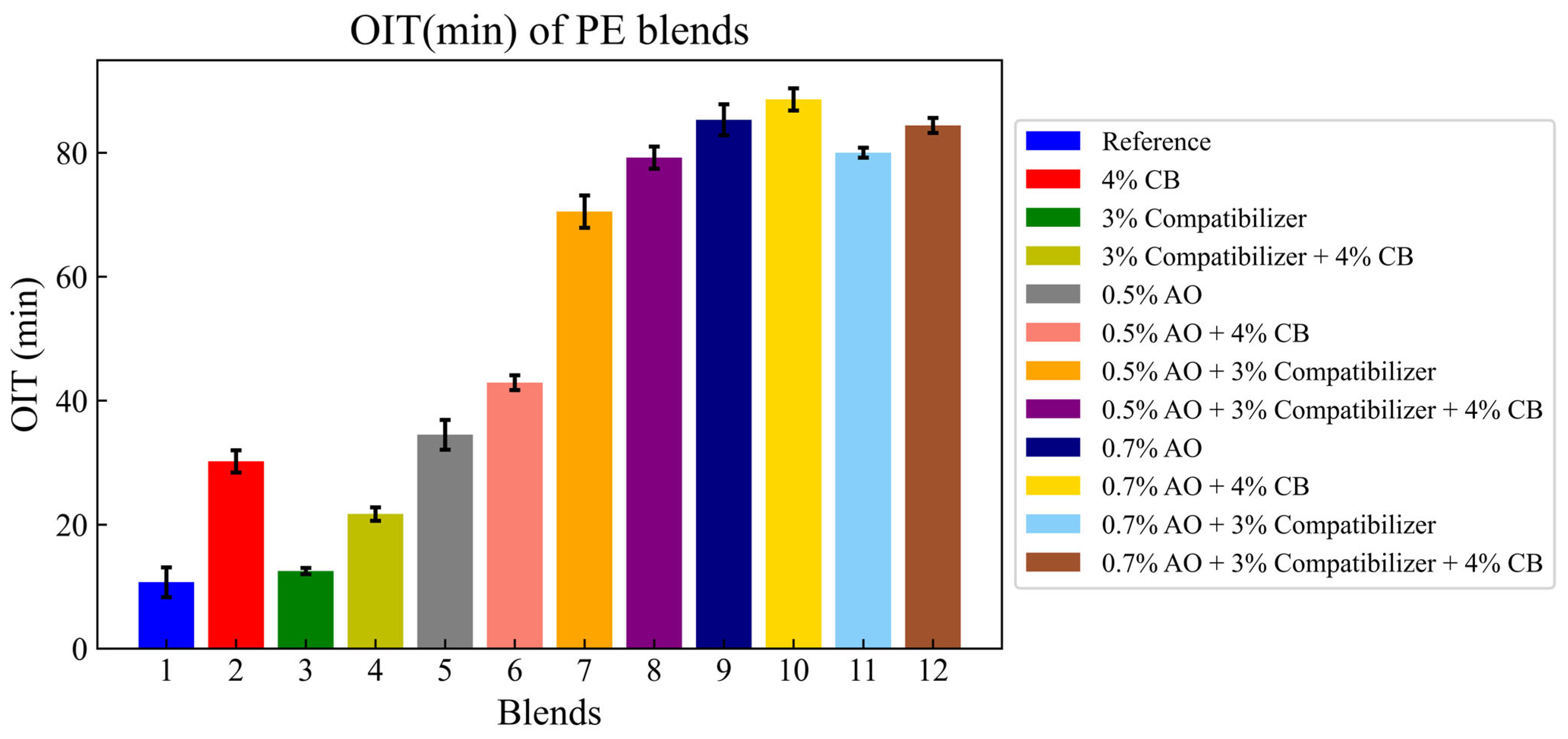


| Composition | Rate (%) |
|---|---|
| rHMWPE | 65 |
| rHDPE | 35 |
| Polymer Type | MFI (g/10 min) 190 °C/2.16 kg | MFI (g/10 min) 190 °C/21.6 kg | Density (g/cm3) |
|---|---|---|---|
| HDPE | 0.5 | 3–5 | 0.951 |
| HMW | 0.1 | - | 0.948 |
| Antioxidant | 1.4 | - | 0.862 |
| Compatibilizer | - | - | 0.47 |
| Carbon black | - | 6–13 | - |
| Additives | Levels | Quantities (wt %) |
|---|---|---|
| Antioxidant | 3 | 0 0.5 0.7 |
| Compatibilizer | 2 | 0 3 |
| Carbon black | 2 | 0 4 |
| Number of Blends | Antioxidant (wt %) | Compatibilizer (wt %) | Carbon Black (wt %) |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 4 |
| 3 | 0 | 3 | 0 |
| 4 | 0 | 3 | 4 |
| 5 | 0.5 | 0 | 0 |
| 6 | 0.5 | 0 | 4 |
| 7 | 0.5 | 3 | 0 |
| 8 | 0.5 | 3 | 4 |
| 9 | 0.7 | 0 | 0 |
| 10 | 0.7 | 0 | 4 |
| 11 | 0.7 | 3 | 0 |
| 12 | 0.7 | 3 | 4 |
| T (°C) | 200 | 210 | 215 | 225 | 235 | 220 |
| Zone | 1 | 2 | 3 | 4 | 5–7 | 7–12 |
| Sample Number | Degree of Crystallinity (%) |
|---|---|
| 1 (Reference) | 55 |
| 2 | 57 |
| 3 | 39 |
| 4 | 48 |
| 5 | 57 |
| 6 | 58 |
| 7 | 55 |
| 8 | 52 |
| 9 | 59 |
| 10 | 58 |
| 11 | 57 |
| 12 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharmoudi, H.; Lamtai, A.; Elkoun, S.; Robert, M.; Diez, C. Effect of Additives on Thermal Degradation and Crack Propagation Properties of Recycled Polyethylene Blends. Polymers 2024, 16, 2060. https://doi.org/10.3390/polym16142060
Kharmoudi H, Lamtai A, Elkoun S, Robert M, Diez C. Effect of Additives on Thermal Degradation and Crack Propagation Properties of Recycled Polyethylene Blends. Polymers. 2024; 16(14):2060. https://doi.org/10.3390/polym16142060
Chicago/Turabian StyleKharmoudi, Hniya, Alae Lamtai, Said Elkoun, Mathieu Robert, and Carl Diez. 2024. "Effect of Additives on Thermal Degradation and Crack Propagation Properties of Recycled Polyethylene Blends" Polymers 16, no. 14: 2060. https://doi.org/10.3390/polym16142060







