Alginate Extracted from Azotobacter chroococcum Loaded in Selenium Nanoparticles: Insight on Characterization, Antifungal and Anticancer Activities
Abstract
1. Introduction
2. Material and Methods
2.1. Azotobacter spp. Isolation
2.2. Azotobacter spp. Identification
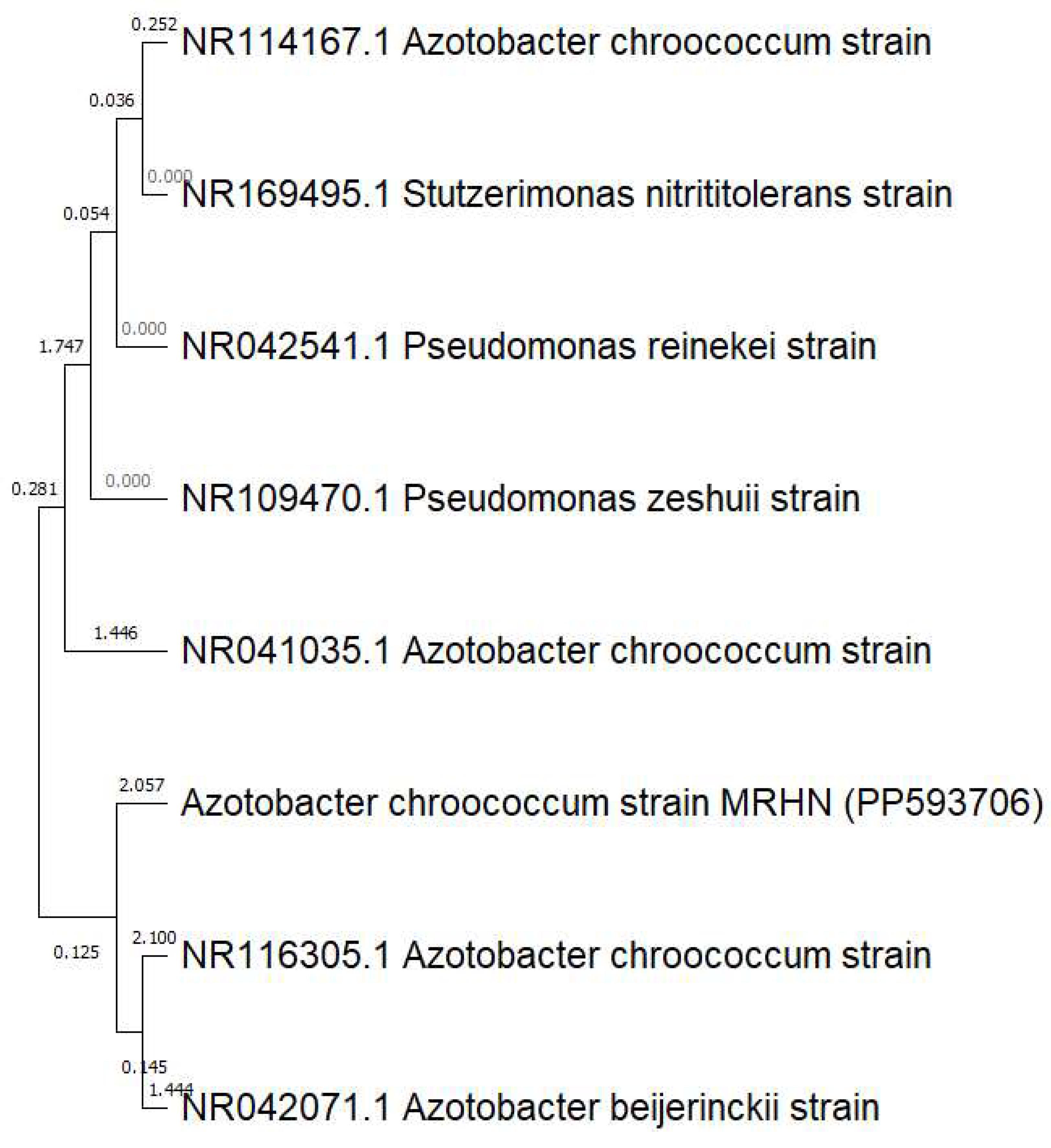
2.3. Phylogenetic Analysis
2.4. Alginate Extraction
2.5. Synthesis of Selenium Nanoparticles
2.6. Loading Alginate with Selenium Nanoparticles (Alg-Se-NCMs)
2.7. Characterizations of Alginate
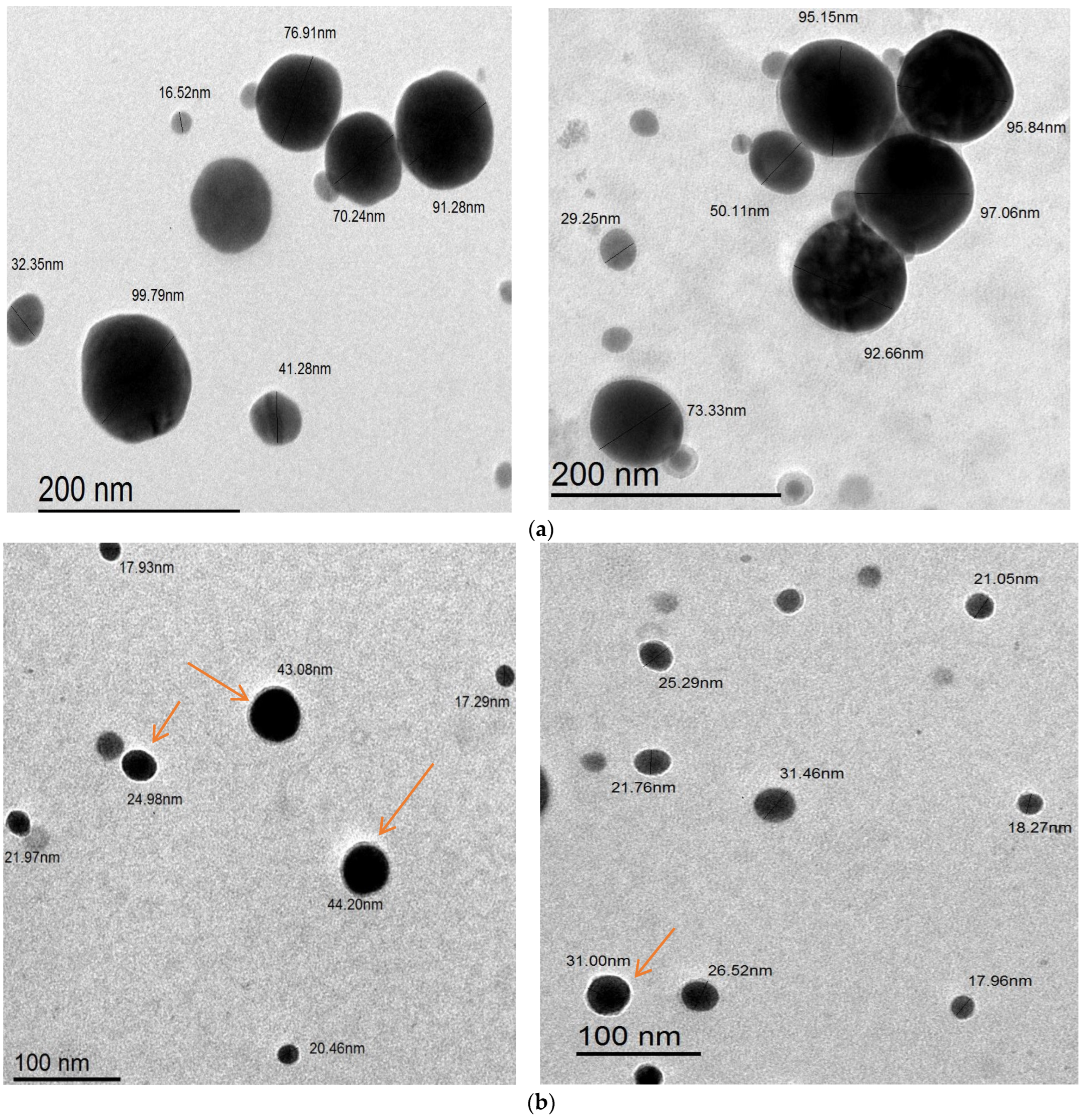
2.8. Characterizations of As/Se-NPs and Alg-Se-NCMs
2.9. Anticancer Activities
2.10. Assessment of the Antifungal Activity of As/Se-NPs and Alg-Se-NCMs
2.11. Data Analysis
3. Results and Discussion
3.1. Bacterial Identification and Phylogenetic Analysis
3.2. Alginate Characterization
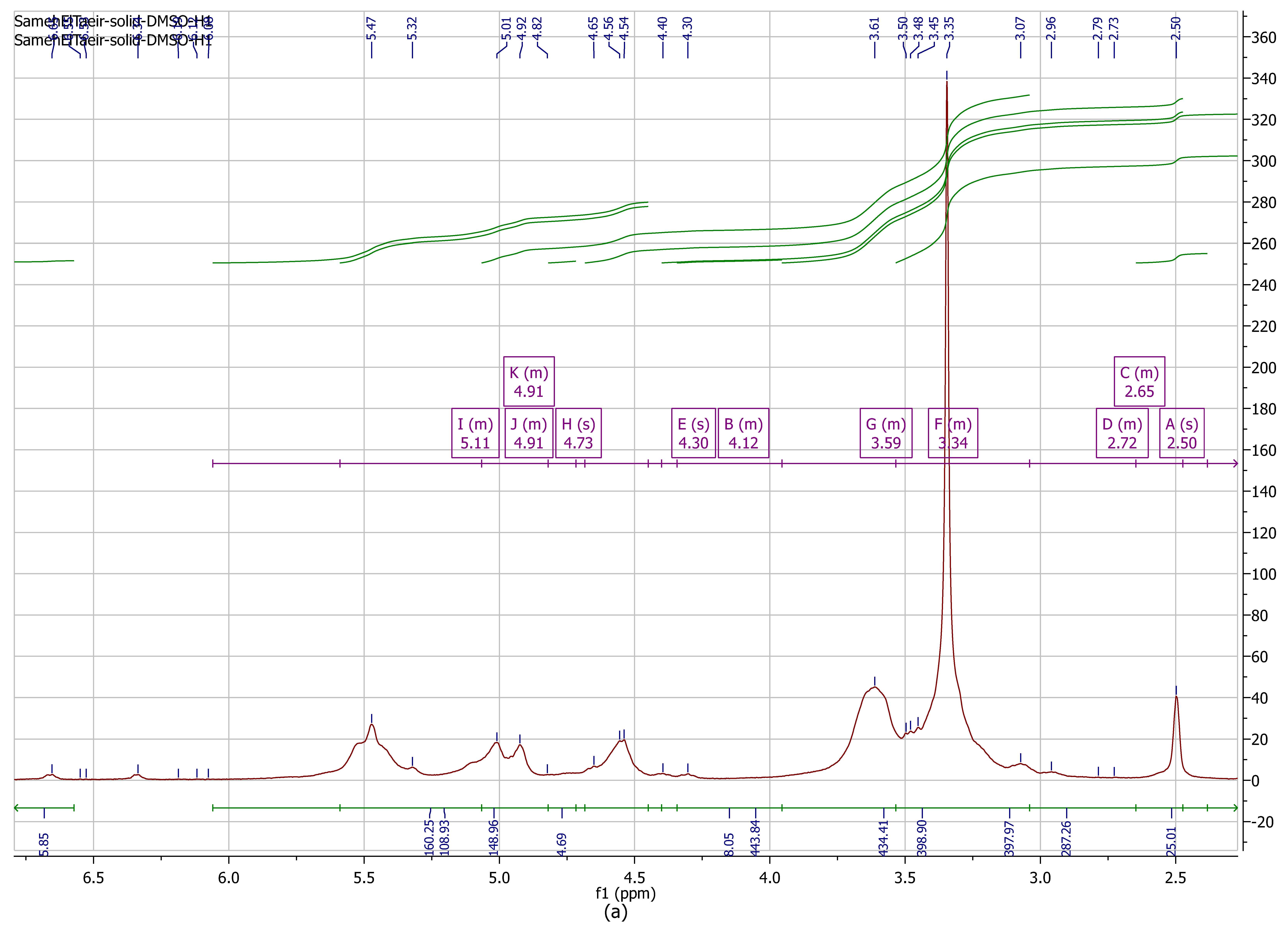
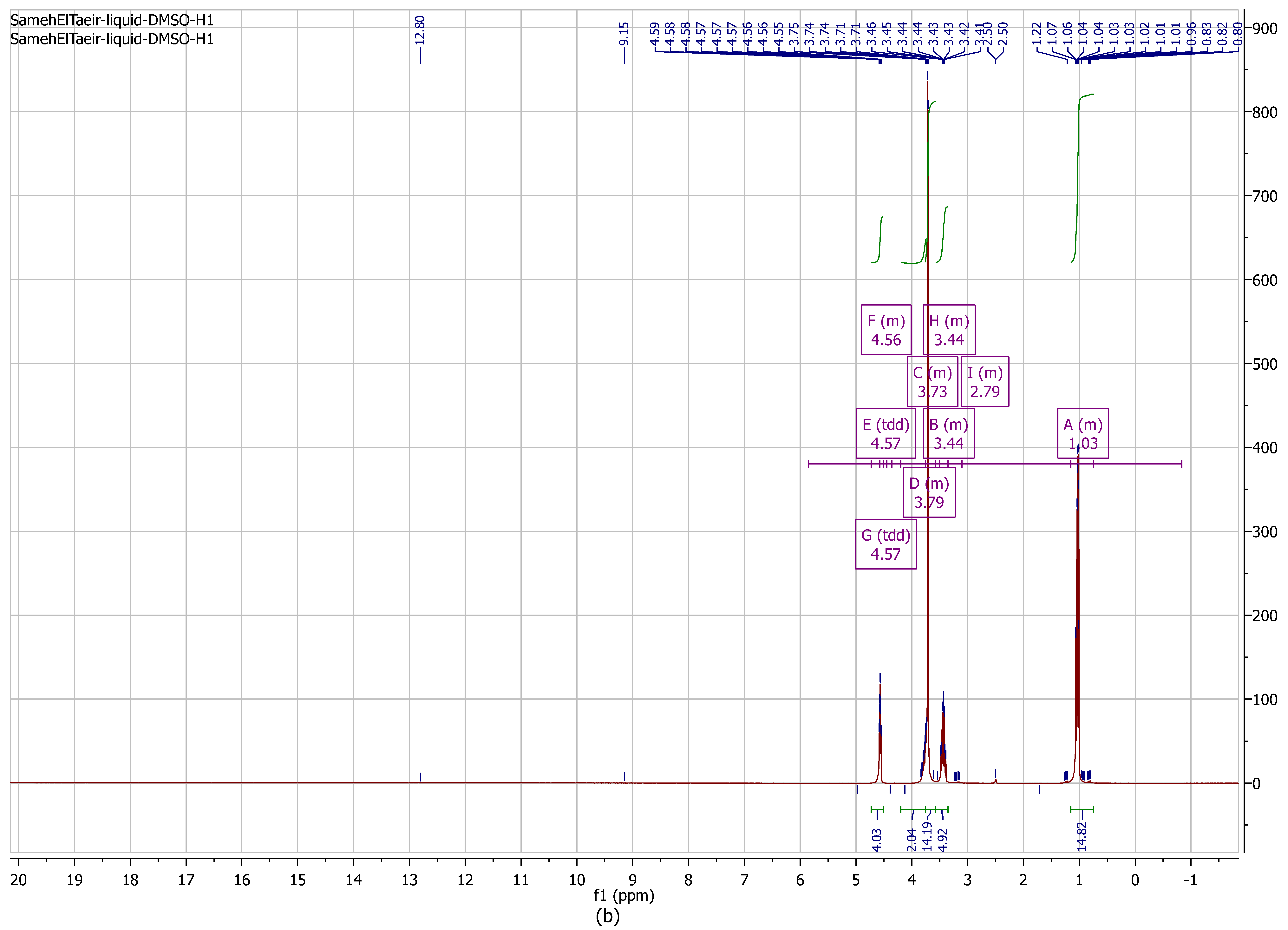
3.2.1. Proton Nuclear Magnetic Resonance (1H NMR) Analysis
3.2.2. FT-IR Analysis of Alginic Acids and Extracted from A. chroococcum MRHN (PP593706)
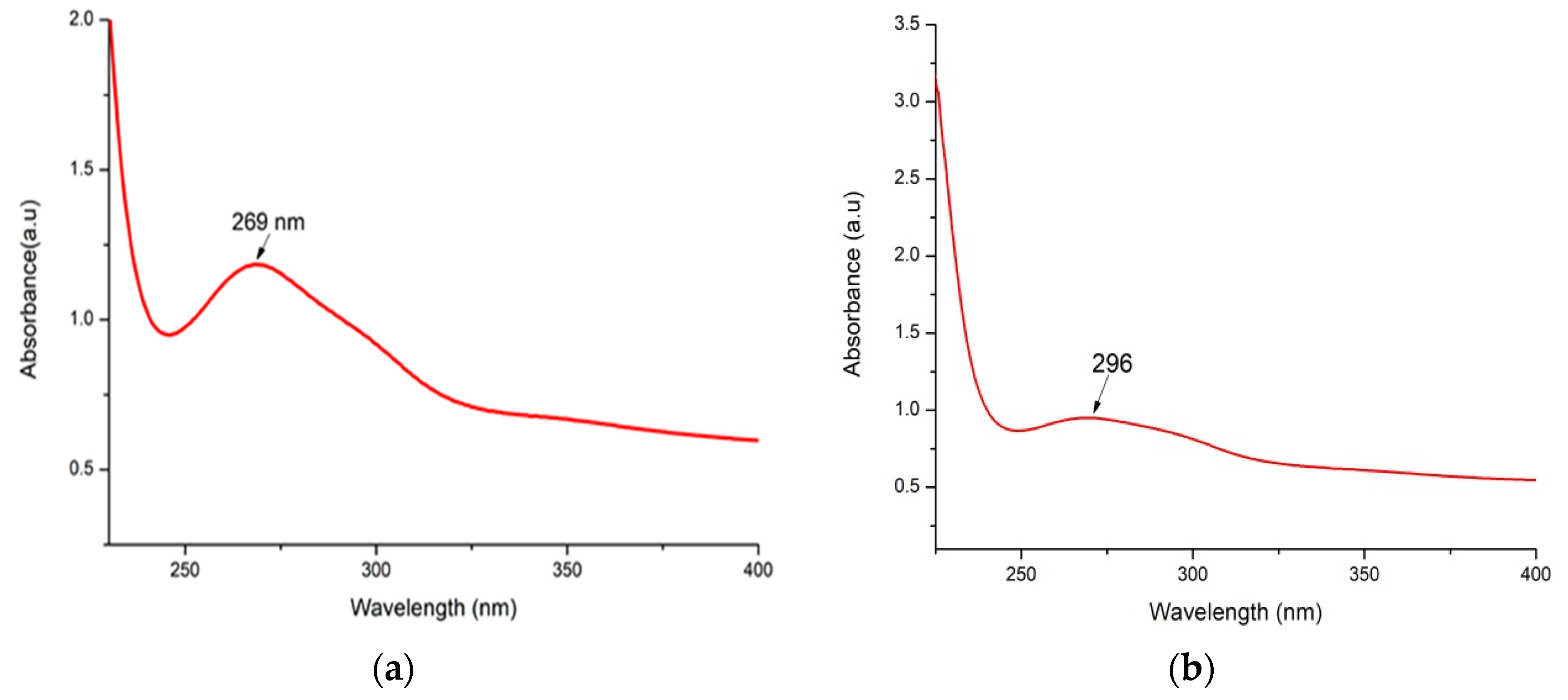
3.3. UV–Vis Spectroscopy Assessment
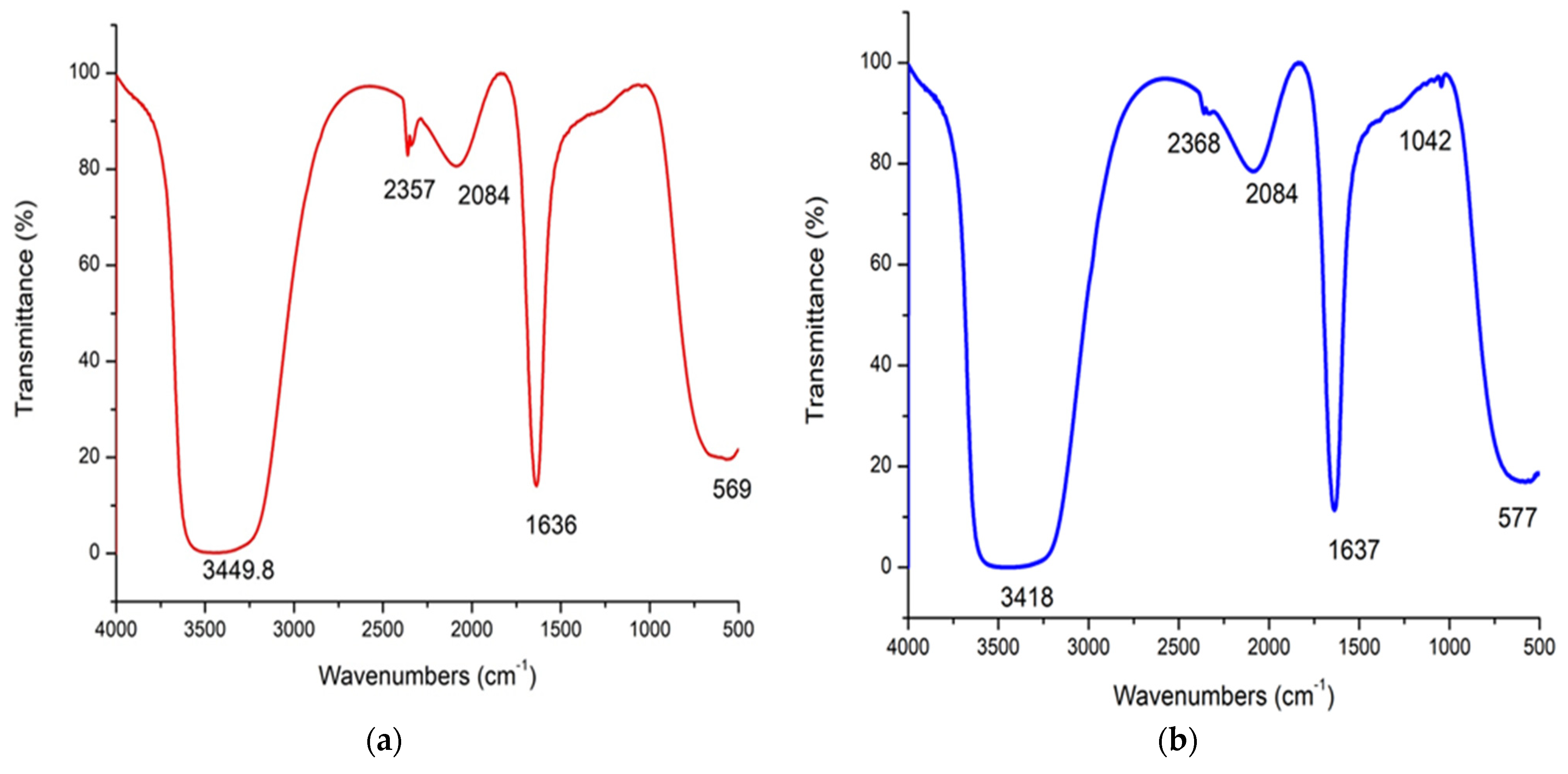
3.4. FT-IR Analysis of As/Se-NPs (a) and Alg-Se-NCMs
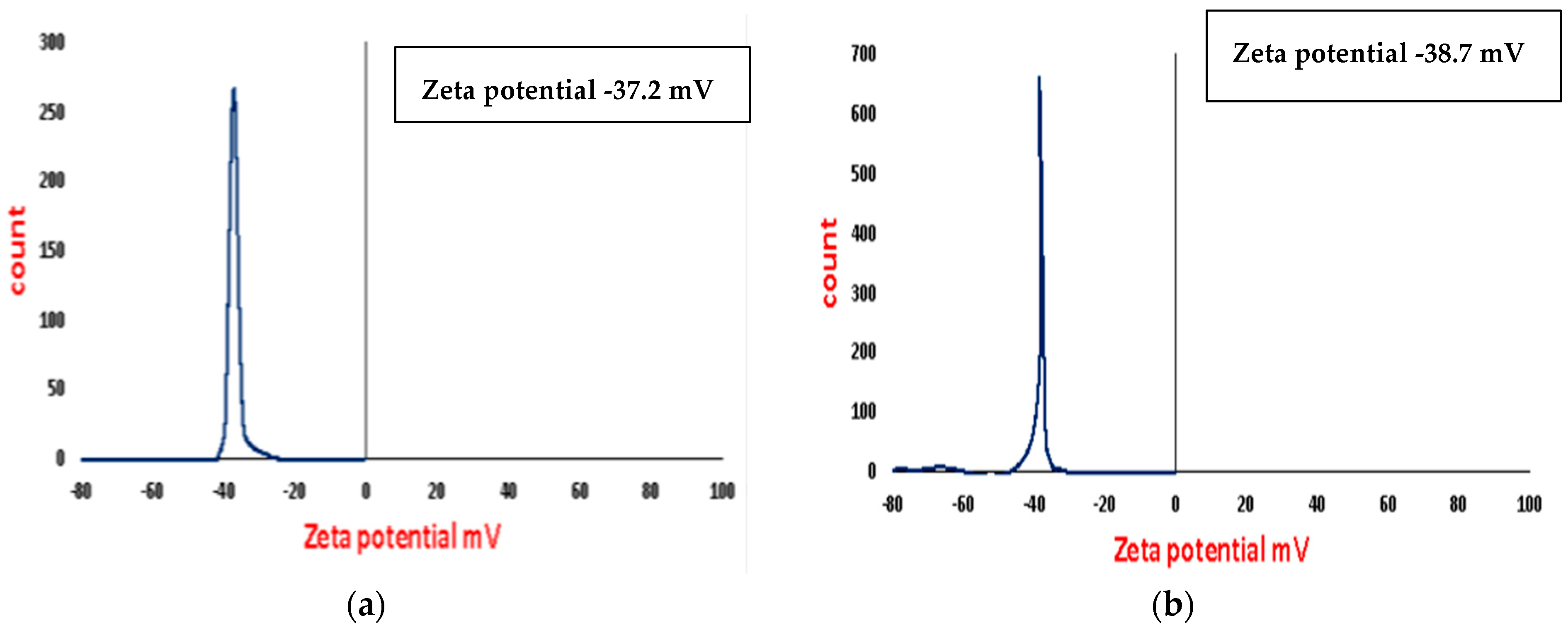
3.5. Zeta Potential
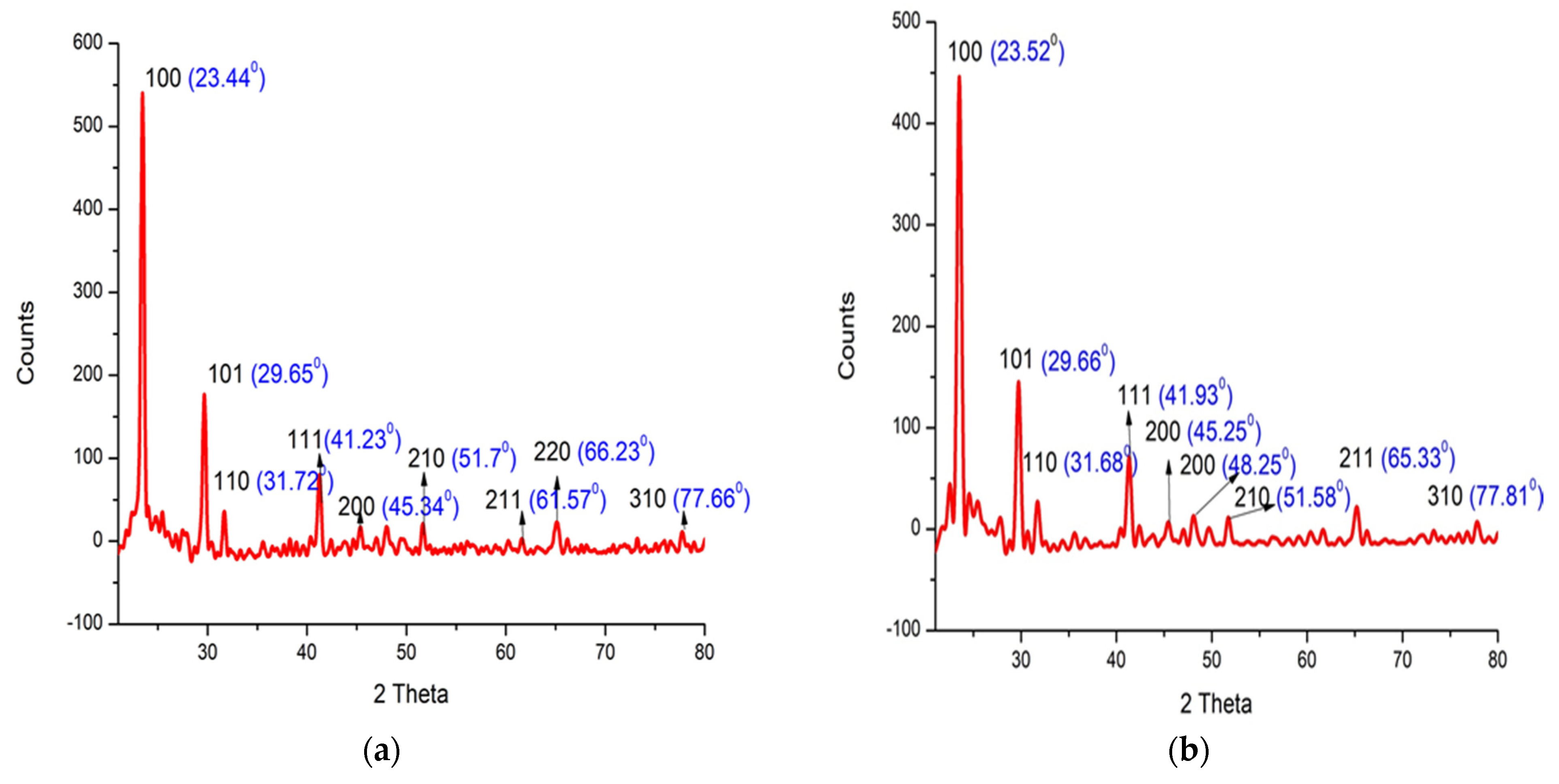
3.6. X-ray Diffraction (XRD)
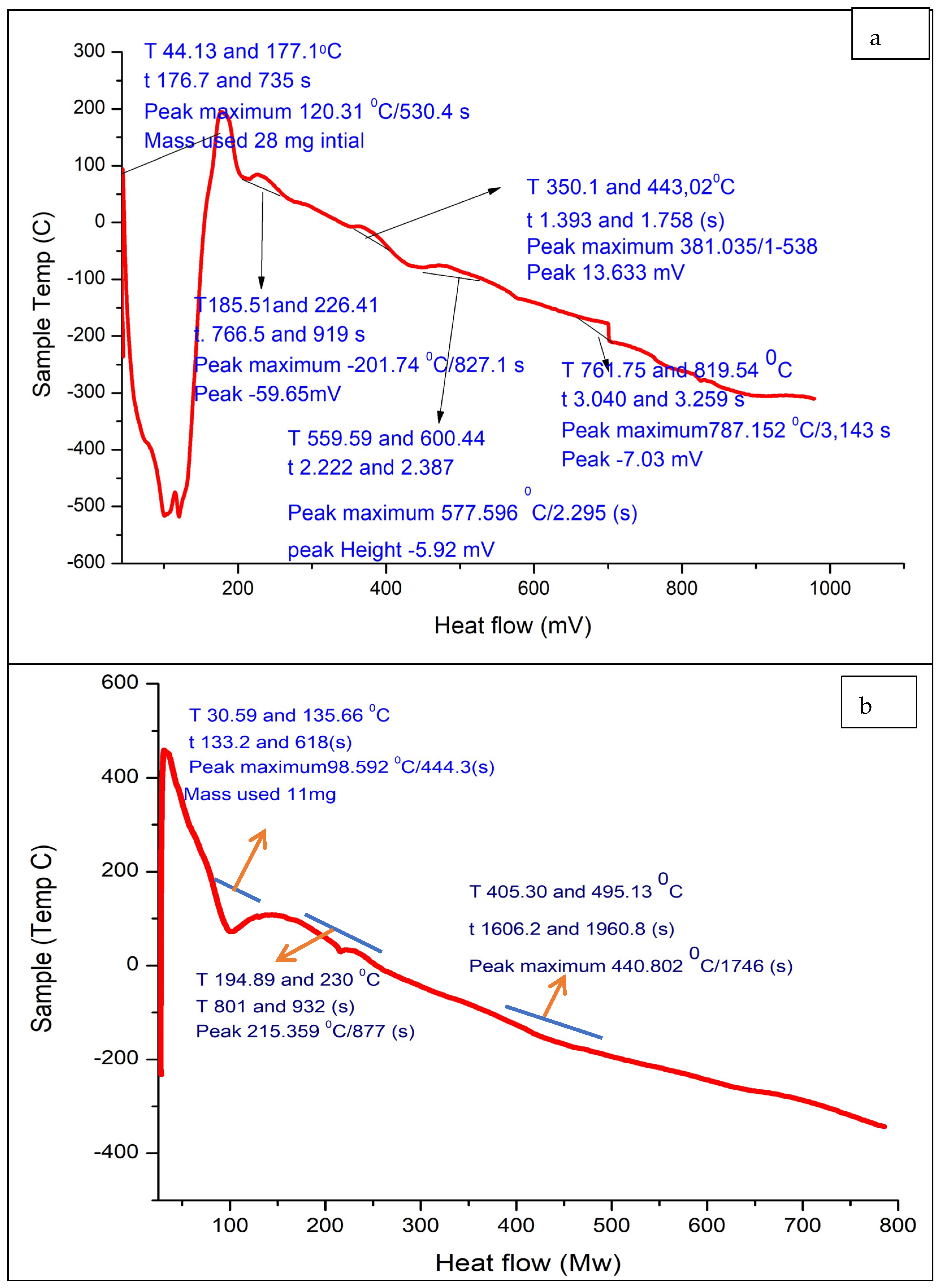
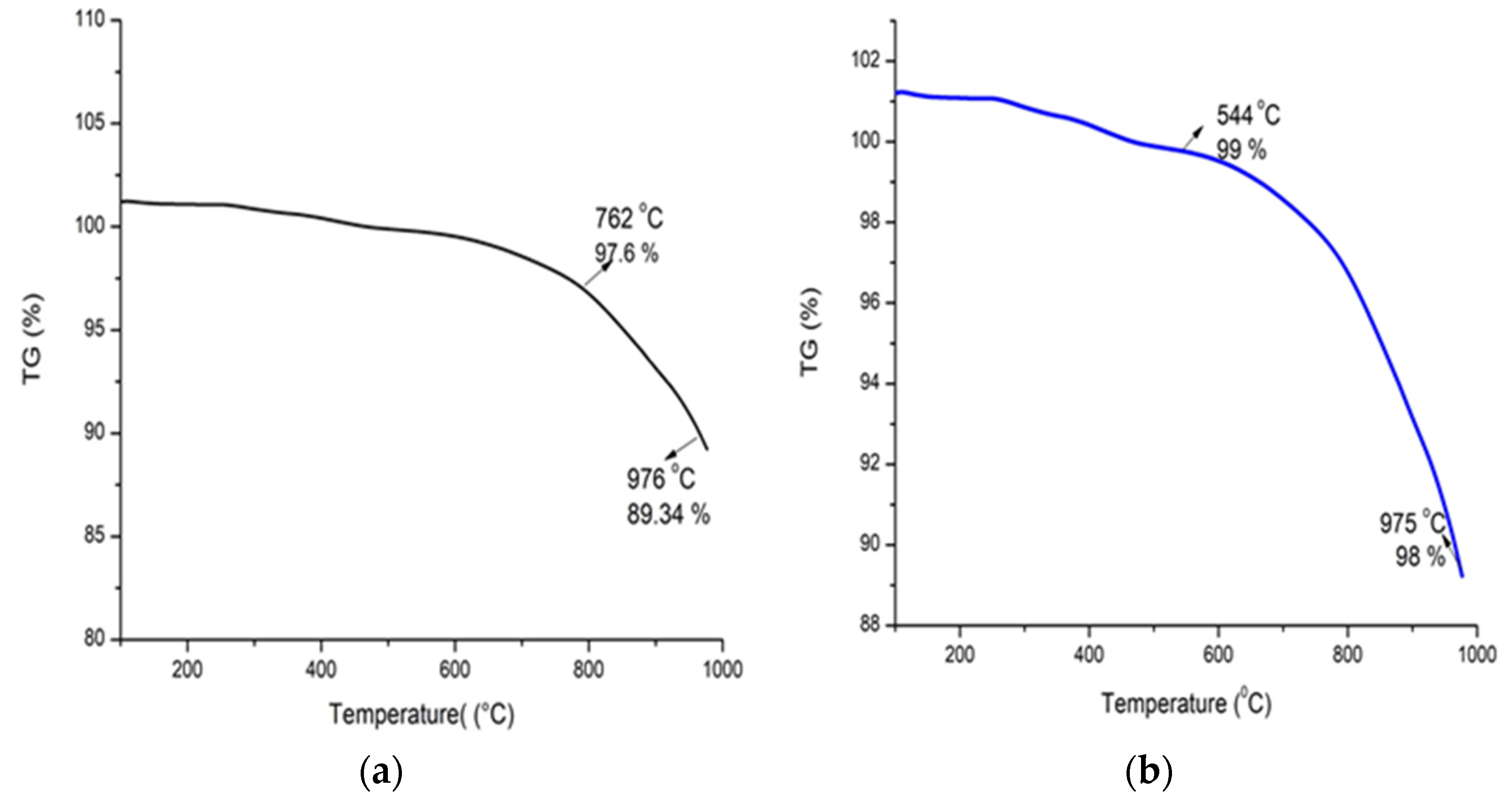
3.7. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) of Alg-Se-NCMs
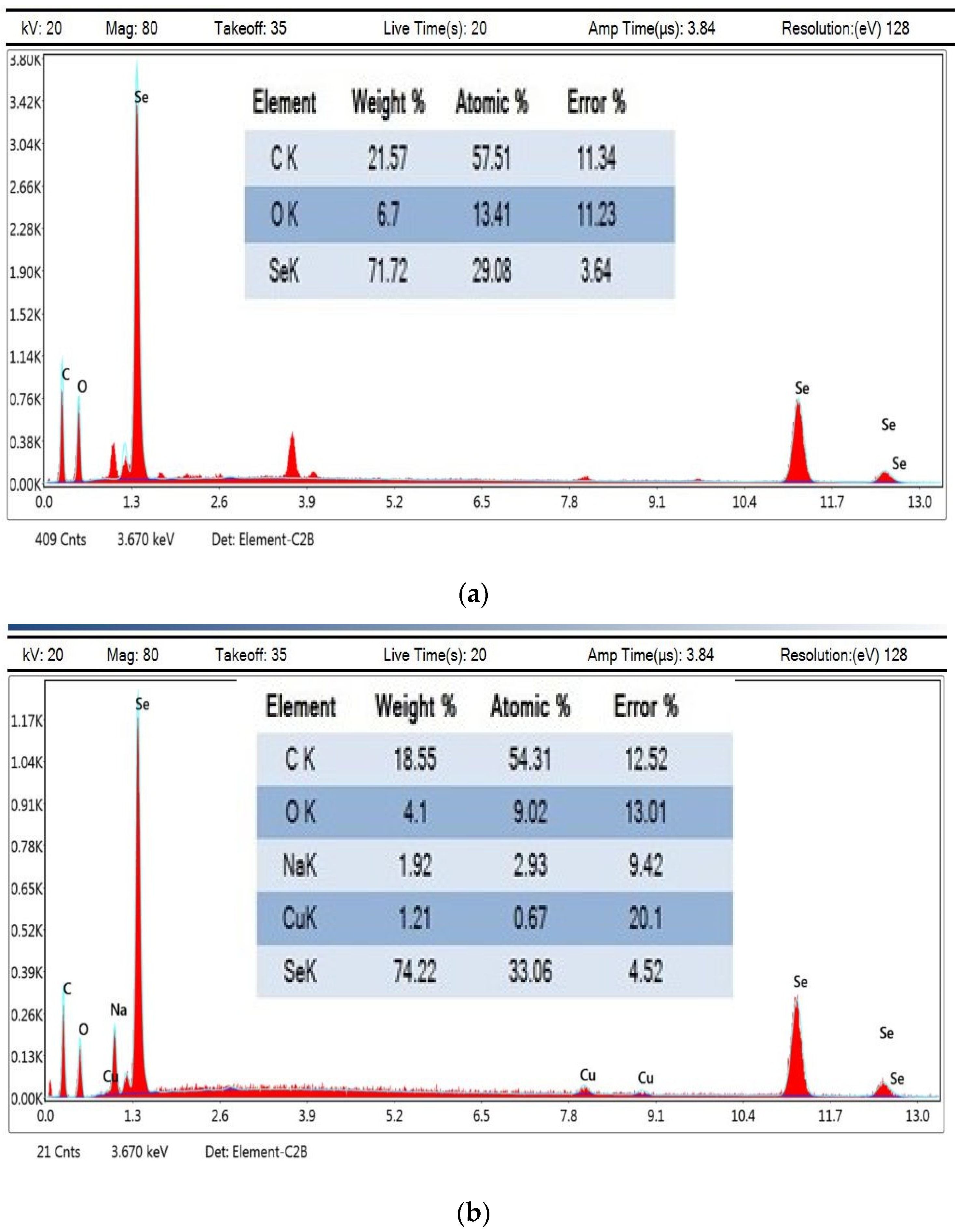
3.8. Energy-Dispersive X-ray Measurements
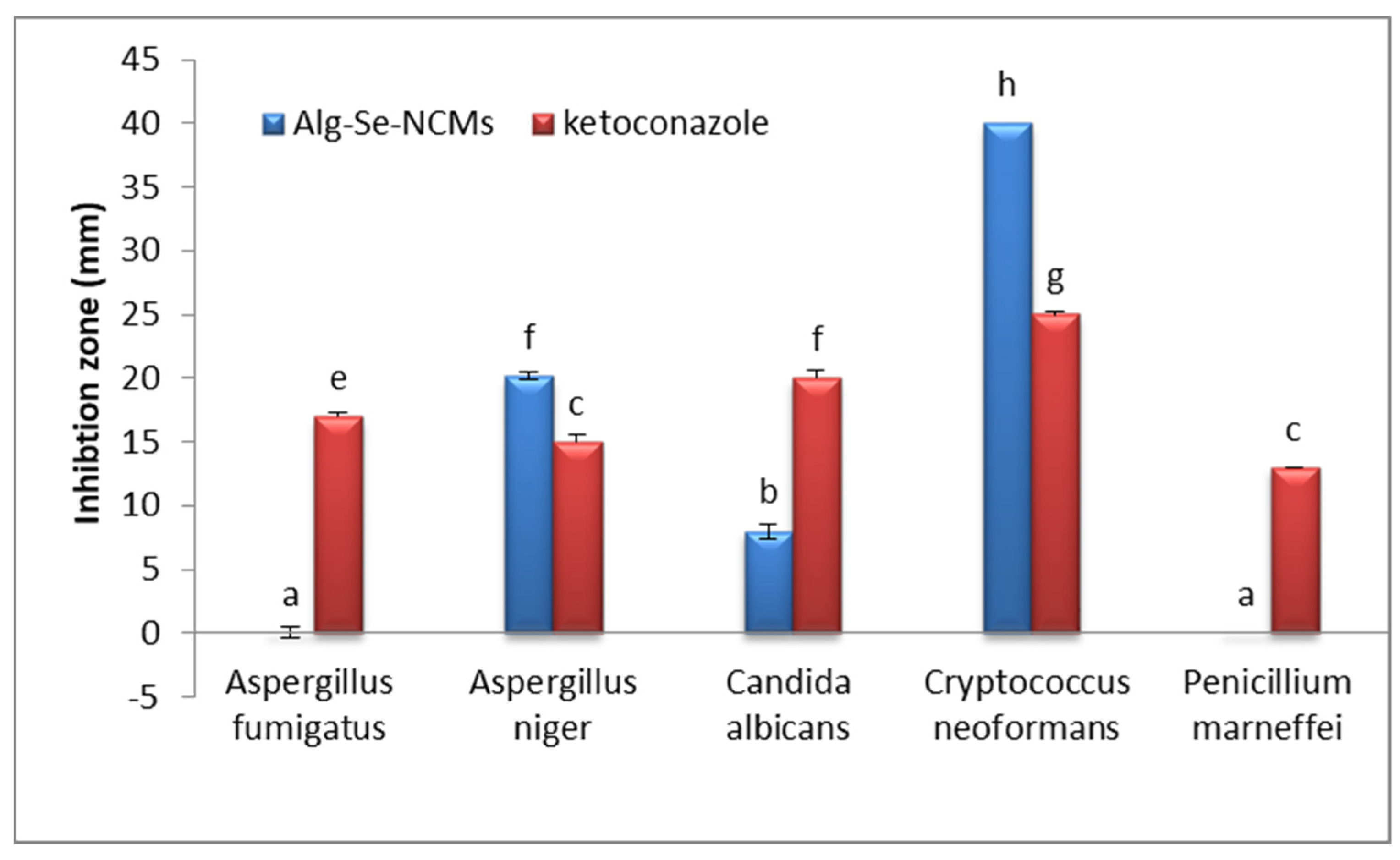
3.9. Antifungal Activities
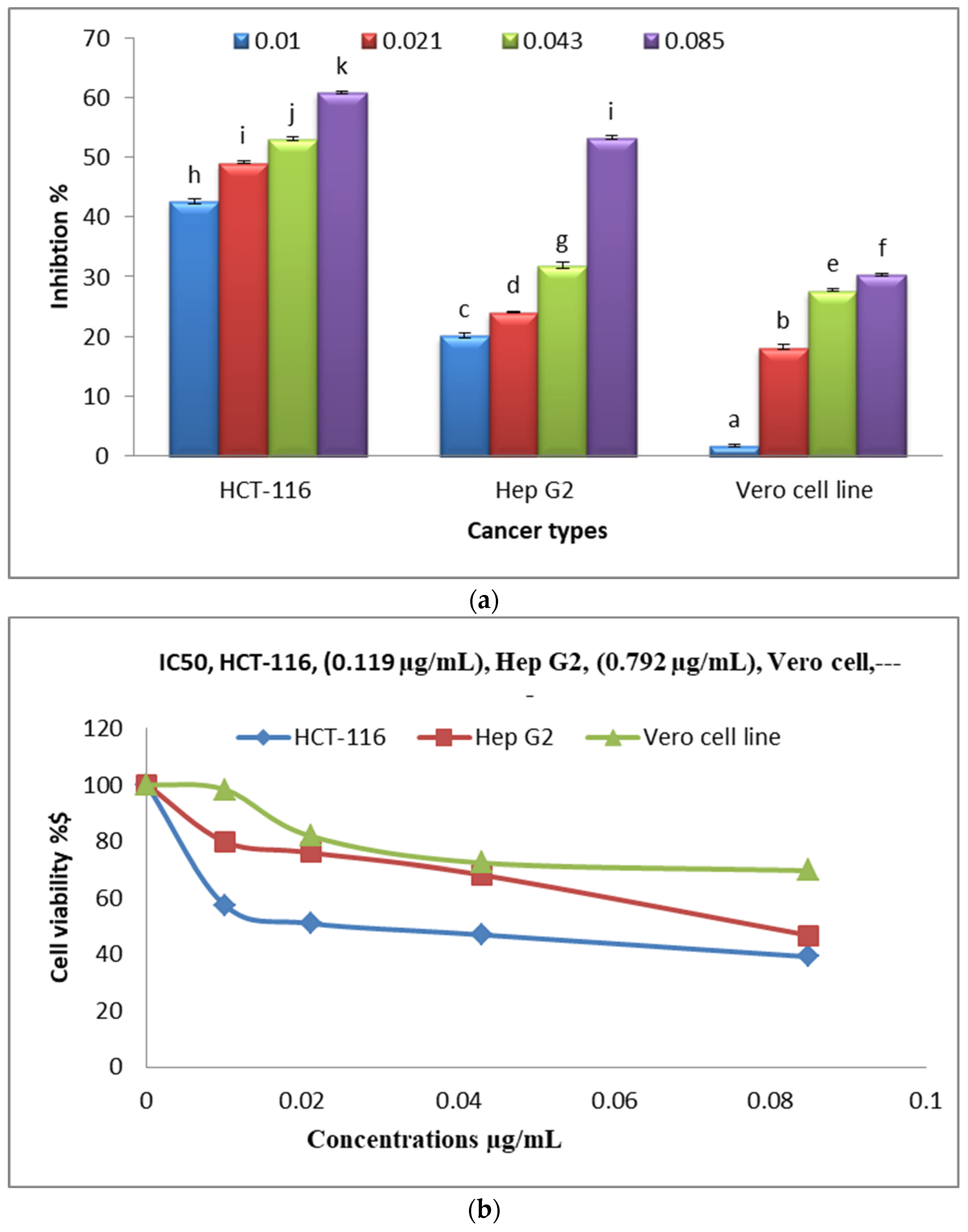
3.10. Anticancer Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2017. 2017. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html (accessed on 14 July 2024).
- Zhang, W.; Zhang, Z.; Zhang, Y. The Application of Carbon Nanotubes in Target Drug Delivery Systems for Cancer Therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Reinius, M.A.; Hatcher, H.M.; Ajithkumar, T.V. Anticancer Chemotherapy in Teenagers and Young Adults: Managing Long Term Side Effects. BMJ 2016, 354, i4567. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart Nanocarrier-Based Drug Delivery Systems for Cancer Therapy and Toxicity Studies: A Review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Asadpoor, M.; Ithakisiou, G.-N.; van Putten, J.P.M.; Pieters, R.J.; Folkerts, G.; Braber, S. Antimicrobial Activities of Alginate and Chitosan Oligosaccharides against Staphylococcus aureus and Group B Streptococcus. Front. Microbiol. 2021, 12, 700605. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Chen, M.; Ma, L.; Wang, X.; Chen, Y.-G.; Liu, S.-L. Role of CD44high/CD133high HCT-116 Cells in the Tumorigenesis of Colon Cancer. Oncotarget 2016, 7, 7657–7666. [Google Scholar] [CrossRef] [PubMed]
- Arzumanian, V.A.; Kiseleva, O.I.; Poverennaya, E.V. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int. J. Mol. Sci. 2021, 22, 13135. [Google Scholar] [CrossRef] [PubMed]
- Deepa, A.; Nair, B.; Sivakumar, T.; Joseph, A. Uncommon Opportunistic Fungal Infections of Oral. J. Oral Maxillofac. Pathol. 2014, 18, 235. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Xess, I.; Swetha, J.; Tanveer, N.; Asati, D.; Ramam, M.; Singh, M. Primary Cutaneous Aspergillosis due to Aspergillus niger in an Immunocompetent Patient. Indian J. Med. Microbiol. 2009, 27, 367–370. [Google Scholar] [CrossRef]
- Kanaujia, R.; Singh, S.; Rudramurthy, S.M. Aspergillosis: An Update on Clinical Spectrum, Diagnostic Schemes, and Management. Curr. Fungal Infect. Rep. 2023, 17, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Merad, Y.; Derrar, H.; Belmokhtar, Z.; Belkacemi, M. Aspergillus Genus and Its Various Human Superficial and Cutaneous Features. Pathogens 2021, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; Lee, P.H.A.; Yamasaki, K.; Gallo, R.L. Anti-Fungal Activity of Cathelicidins and Their Potential Role in Candida albicans Skin Infection. J. Investig. Dermatol. 2005, 125, 108–115. [Google Scholar] [CrossRef]
- Halverstam, C.; Cohen, S.R. Cutaneous Candidiasis and Chronic Mucocutaneous Candidiasis. In Treatment of Skin Disease, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 171–174. [Google Scholar] [CrossRef]
- Morales, E.G.; Guidi, M.; Peterka, T.; Rabufetti, A.; Blum, R.; Mainetti, C. Primary Cutaneous Cryptococcosis due to Cryptococcus neoformans in an Immunocompetent Host Treated with Itraconazole and Drainage: Case Report and Review of the Literature. Case Rep. Dermatol. 2021, 13, 89–97. [Google Scholar] [CrossRef]
- Wong, S.Y.N.; Wong, K.F. Penicillium marneffei Infection in AIDS. Pathol. Res. Int. 2011, 2011, 764293. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium Alginate/Poly(Vinyl Alcohol)/Nano ZnO Composite Nanofibers for Antibacterial Wound Dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef]
- Spadari, C.d.C.; Lopes, L.B.; Ishida, K. Potential Use of Alginate-Based Carriers as Antifungal Delivery System. Front. Microbiol. 2017, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Alginate-Based Micro- and Nanosystems for Targeted Cancer Therapy. Mar. Drugs 2022, 20, 598. [Google Scholar] [CrossRef] [PubMed]
- Kubyshkin, A.; Chegodar, D. Antimicrobial Effects of Silver Nanoparticles Stabilized in Solution by Sodium Alginate. Biochem. Mol. Biol. J. 2016, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Clementi, F. Alginate Production by Azotobacter vinelandii. Crit. Rev. Biotechnol. 1997, 17, 327–361. [Google Scholar] [CrossRef] [PubMed]
- Sabra, W.; Zeng, A.P.; Deckwer, W.D. Bacterial Alginate: Physiology, Product Quality and Process Aspects. Appl. Microbiol. Biotechnol. 2001, 56, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef] [PubMed]
- Shengyu, C.; Yinhua, L.; Yuanhong, L.; Jinbo, Z.; Can, F.; Hao, X.; Changjiang, Z. Selenium Alleviates Heart Remodeling through Sirt1/AKT/GSK-3β Pathway. Int. Immunopharmacol. 2022, 111, 109158. [Google Scholar] [CrossRef] [PubMed]
- Shimada, B.K.; Alfulaij, N.; Seale, L.A. The Impact of Selenium Deficiency on Cardiovascular Function. Int. J. Mol. Sci. 2021, 22, 10713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Wu, P.; Chu, Y.; Gui, S.; Zheng, Y.; Chen, X. Dietary Selenium Alleviated Mouse Liver Oxidative Stress and NAFLD Induced by Obesity by Regulating the KEAP1/NRF2 Pathway. Antioxidants 2022, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Zhang, J.; Song, M.; Shao, B.; Han, Y.; Yang, X.; Li, Y. The Protective Effect of Selenium on T-2-Induced Nephrotoxicity Is Related to the Inhibition of ROS-Mediated Apoptosis in Mice Kidney. Biol. Trace Elem. Res. 2021, 200, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1297658. [Google Scholar] [CrossRef]
- Torres, D.J.; Alfulaij, N.; Berry, M.J. Stress and the Brain: An Emerging Role for Selenium. Front. Neurosci. 2021, 15, 666601. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.R.; McKenzie, R.C.; Beckett, G.J. Selenium in the Immune System. J. Nutr. 2003, 133, 1457S1459S. [Google Scholar] [CrossRef] [PubMed]
- Shalihat, A.; Hasanah, A.N.; Mutakin; Lesmana, R.; Budiman, A.; Gozali, D. The Role of Selenium in Cell Survival and Its Correlation with Protective Effects against Cardiovascular Disease: A Literature Review. Biomed. Pharmacother. 2021, 134, 111125. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xu, H. Selenium-Containing Nanomaterials for Cancer Treatment. Cell Rep. Phys. Sci. 2020, 1, 100111. [Google Scholar] [CrossRef]
- Liao, G.; Tang, J.; Wang, D.; Zuo, H.; Zhang, Q.; Liu, Y.; Xiong, H. Selenium Nanoparticles (SeNPs) Have Potent Antitumor Activity against Prostate Cancer Cells through the Upregulation of MiR-16. World J. Surg. Oncol. 2020, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Khaledizade, E.; Tafvizi, F.; Jafari, P. Anti-Breast Cancer Activity of Biosynthesized Selenium Nanoparticles Using Bacillus coagulans Supernatant. J. Trace Elem. Med. Biol. 2024, 82, 127357. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wei, W.; Wang, J.; Chen, T. Theranostic Applications of Selenium Nanomedicines against Lung Cancer. J. Nanobiotechnol. 2023, 21, 96. [Google Scholar] [CrossRef]
- Gao, X.; Yao, Y.; Chen, X.; Lin, X.; Yang, X.; Ho, C.-T.; Li, B.; Chen, Z. Lentinan-Functionalized Selenium Nanoparticles Induce Apoptosis and Cell Cycle Arrest in Human Colon Carcinoma HCT-116 Cells. Front. Nutr. 2022, 9, 987807. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.K.; Kumar, S.; Tomar, M.S.; Singh, R.K.; Acharya, A.; Kumar, S.; Ram, B. Selenium Nanoparticles Induce Suppressed Function of Tumor Associated Macrophages and Inhibit Dalton’s Lymphoma Proliferation. Biochem. Biophys. Rep. 2017, 12, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Cai, L.; Yang, Q.; Luo, Z.; Liang, L.; Liang, Y.; Wu, B.; Ding, L.; Zhang, D.; Xu, X.; et al. Anti-Leukemia Activities of Selenium Nanoparticles Embedded in Nanotube Consisted of Triple-Helix β-d-Glucan. Carbohydr. Polym. 2020, 240, 116329. [Google Scholar] [CrossRef] [PubMed]
- Shakibaie, M.; Salari Mohazab, N.; Ayatollahi Mousavi, S.A. Antifungal Activity of Selenium Nanoparticles Synthesized by Bacillus Species Msh-1 against Aspergillus fumigatus and Candida albicans. Jundishapur J. Microbiol. 2015, 8, e26381. [Google Scholar] [CrossRef]
- Salem, M.L.; Abd-Elraoof, W.A.; Tayel, A.A.; AlZuaibr, F.M.; Abonama, O.M. Antifungal Application of Biosynthesized Selenium Nanoparticles with Pomegranate Peels and Nanochitosan as Edible Coatings for Citrus Green Mold Protection. J. Nanobioil. 2022, 20, 182. [Google Scholar] [CrossRef] [PubMed]
- Squarzanti, D.F.; Zavattaro, E.; Pizzimenti, S.; Amoruso, A.; Savoia, P.; Azzimonti, B. Non-Melanoma Skin Cancer: News from microbiota research. Crit. Rev. Microbiol. 2020, 46, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Huët, M.A.L.; Lee, C.Z.; Rahman, S. A review on association of fungi with the development and progression of carcinogenesis in the human body. Curr. Res. Microb. Sci. 2022, 3, 100090. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Kong, C.; Li, H.; Huang, L.; Qu, X.; Qin, N.; Qin, H. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2457–2468. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Josheghani, S.; Bakhshi, B. Formulation of selenium nanoparticles encapsulated by alginate-chitosan for controlled delivery of Vibrio cholerae LPS: A novel delivery system candidate for nanovaccine. Int. J. Biol. Macromol. 2022, 208, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Naveenkumar, S.; Alagumanikumaran, N.; Kaviyarasu, K.; Muthukumaran, A. Influence of Encapsulated Sodium Alginates and Pectin on Selenium Nanoparticles and Efficient Cardioprotective Effect in C2C12 Cell Line. J. Nanoparticle Res. 2024, 26, 52. [Google Scholar] [CrossRef]
- Cavalu, S.; Prokisch, J.; Laslo, V.; Vicas, S. Preparation, structural characterisation and release study of novel hybrid microspheres entrapping nanoselenium, produced by green synthesis. IET Nanobiotechnol. 2017, 11, 426–432. [Google Scholar] [CrossRef]
- Stella, M.; Suhaimi, M. Selection of Suitable Growth Medium for Free-Living Diazotrophs Isolated from Compost. J. Trop. Agric. Food Sci. 2010, 38, 211–219. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.S.; Saad, M.W.; Badawy, G.A.; Hamza, H.A.; Haroun, A.A. Synthesis and Examination of Hydroxyapatite Nanocomposites Based on Alginate Extracted from Azotobacter chroococcum New Strain MWGH ShKB In Vitro. Biosci. Res. 2018, 15, 293–3306. [Google Scholar]
- Hamouda, R.A.; Abdel-Hamid, M.S.; Hagagy, N.; Nofal, A.M. The Potent Effect of Selenium Nanoparticles Insight into: The Antifungal Activity and Preservation of Postharvest Strawberries from Gray Mold Diseases. J. Sci. Food Agric. 2024. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Aarstad, O.A.; Stanisci, A.; Sætrom, G.I.; Tøndervik, A.; Sletta, H.; Aachmann, F.L.; Skjåk-Bræk, G. Biosynthesis and function of long guluronic acid-blocks in alginate produced by Azotobacter vinelandii. Biomacromolecules 2019, 20, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Huamani-Palomino, R.G.; Córdova, B.M.; Pichilingue L., E.R.; Venâncio, T.; Valderrama, A.C. Functionalization of an Alginate-Based Material by Oxidation and Reductive Amination. Polymers 2021, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Salomonsen, T.; Jensen, H.M.; Stenbæk, D.; Engelsen, S.B. Rapid Determination of Alginate Monomer Compostion Using Raman Spectroscopy and Chemometrics. R. Soc. Chem. 2008, 14, 543–551. [Google Scholar] [CrossRef]
- Salomonsen, T.; Jensen, H.M.; Larsen, F.H.; Steuernagel, S.; Engelsen, S.B. Alginate Monomer Composition Studied by Solution- and Solid-State NMR—A Comparative Chemometric Study. Food Hydrocoll. 2009, 23, 1579–1586. [Google Scholar] [CrossRef]
- Sindi, H.A.; Hamouda, R.A.; Alhazmi, N.M.; Abdel-Hamid, M.S. Functionalized gold nanoparticles coated with bacterial alginate and their antibacterial and anticancer activities. Green Process. Synth. 2024, 13, 20230170. [Google Scholar] [CrossRef]
- Ramamurthy, C.H.; Sampath, K.S.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green Synthesis and Characterization of Selenium Nanoparticles and its Augmented Cytotoxicity with Doxorubicin on Cancer Cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Sani-e-Zahra; Iqbal, M.S.; Abbas, K.; Qadir, M.I. Synthesis, Characterization and Evaluation of Biological Properties of Selenium Nanoparticles from Solanum lycopersicum. Arab. J. Chem. 2022, 15, 103901. [Google Scholar] [CrossRef]
- Anu, K.; Devanesan, S.; Prasanth, R.; AlSalhi, M.S.; Ajithkumar, S.; Singaravelu, G. Biogenesis of Selenium Nanoparticles and Their Anti-Leukemia Activity. J. King Saud Univ. Sci. 2020, 32, 2520–2526. [Google Scholar] [CrossRef]
- Ghazali, N.M.; Mazuki, N.F.; Samsudin, A.S. Characterization of Biopolymer Blend-Based on Alginate and Poly (Vinyl Alcohol) as an Application for Polymer Host in Polymer Electrolyte. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012047. [Google Scholar] [CrossRef]
- Huang, N.; Wang, J. A TGA-FTIR Study on the Effect of CaCO3 on the Thermal Degradation of EBA Copolymer. J. Anal. Appl. Pyrolysis 2009, 84, 124–130. [Google Scholar] [CrossRef]
- Ivanova, E.; Mihaylov, M.; Thibaultstarzyk, F.; Daturi, M.; Hadjiivanov, K. New Type of Rhodium Gem-Dicarbonyls Formed in Rh-ZSM-5: An FTIR Spectroscopy Study. J. Catal. 2005, 236, 168–171. [Google Scholar] [CrossRef]
- Tareb, R.; Bernardeau, M.; Amiel, C.; Vernoux, J.-P. Usefulness of FTIR Spectroscopy to Distinguish Rough and Smooth Variants of Lactobacillus farciminis CNCM-I-3699. FEMS Microbiol. Lett. 2017, 364, fnw298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamouda, R.A.; Almaghrabi, F.Q.; Alharbi, O.M.; Al-Harbi, A.D.M.; Alsulami, R.M.; Alhumairi, A.M. Antifungal Activities of Biogenic Silver Nanoparticles Mediated by Marine Algae: In Vitro and In Vivo Insights of Coating Tomato Fruit to Protect against Penicillium italicum Blue Mold. Mar. Drugs 2024, 22, 225. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, V.; Anitha, R.; Thenmozhi, S.; Marchewka, M.K.; Mohan, S. Potential Energy Profile, Structural, Vibrational and Reactivity Descriptors of Trans-2-Methoxycinnamic Acid by FTIR, FT-Raman and Quantum Chemical Studies. J. Mol. Struct. 2016, 1113, 42–54. [Google Scholar] [CrossRef]
- Alghanmi, R.M.; Hamouda, R.A.; Al-Moubaraki, A.H.; Allouzi, A.A.; Abuelmagd, M.A. Biofabrication of Silver Nanoparticles Using Uncaria tomentosa L.: Insight into Characterization, Antibacterial Activities Combined with Antibiotics, and Effect on Triticum aestivum Germination. Green Process. Synth. 2024, 13, 20230207. [Google Scholar] [CrossRef]
- Suriyakalaa, U.; Antony, J.J.; Suganya, S.; Siva, D.; Sukirtha, R.; Kamalakkannan, S.; Pichiah, P.B.T.; Achiraman, S. Hepatocurative Activity of Biosynthesized Silver Nanoparticles Fabricated Using Andrographis Paniculata. Colloids Surf. B Biointerfaces 2013, 102, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Naveenkumar, S.; Venkateshan, N.; Kaviyarasu, K.; Christyraj, J.R.S.S.; Muthukumaran, A. Optimum Performance of a Novel Biocompatible Scaffold Comprising Alginate-Pectin-Selenium Nanoparticles for Cardiac Tissue Engineering Using C2C12 Cells. J. Mol. Struct. 2023, 1294, 136457. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Mosallam, F.M.; Ghorab, M.M.; Hanora, A.; Gobara, M.; Baraka, A.; Elsayed, M.A.; Pal, K.; Fathy, R.M.; Elkodous, M.A.; et al. Factorial Design-Optimized and Gamma Irradiation-Assisted Fabrication of Selenium Nanoparticles by Chitosan and Pleurotus ostreatus Fermented Fenugreek for a Vigorous In Vitro Effect against Carcinoma Cells. Int. J. Biol. Macromol. 2020, 156, 1584–1599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sevonkaev, I.; Goia, D.V. Synthesis of Selenium Particles with Various Morphologies. J. Colloid Interface Sci. 2014, 416, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Yu, D.; Wang, F.; Wang, Z. Controlled Growth of Hexagonal Trigonal Selenium Microtubes. Cryst. Growth Des. 2006, 6, 2159–2162. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; El-Sayed, S.M.; Youssef, A.M. Designated Functional Microcapsules Loaded with Green Synthesis Selenium Nanorods and Probiotics for Enhancing Stirred Yogurt. Sci. Rep. 2022, 12, 14751. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.S.; Alkhatib, S.N.; Ahmed, F.M.; Hamouda, R.A. Chitosan Nanoparticles Loaded with Capparis cartilaginea Decne Extract: Insights into Characterization and Antigenotoxicity In Vivo. Pharmaceutics 2023, 15, 2551. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Mahmood, S.; Enezei, H.H.; Hussain, S.A.; Hamad, H.A.; Aldoghachi, A.F.; Hagar, A.; Doolaanea, A.A.; Ibrahim, W.N. Formulation, Characterization and Biological Activity Screening of Sodium Alginate-Gum Arabic Nanoparticles Loaded with Curcumin. Molecules 2020, 25, 2244. [Google Scholar] [CrossRef] [PubMed]
- Pakolpakçıl, A.; Draczynski, Z. Green Approach to Develop Bee Pollen-Loaded Alginate Based Nanofibrous Mat. Materials 2021, 14, 2775. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal Behavior of Alginic Acid and Its Sodium Salt. Eclética Química 2004, 29, 57–64. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Fauzia, A.K.Q.; Shahabuddin, F.S.; Al-Shaikh, T.M.; Makharita, R.R. Antibacterial Activity of Ulva/Nanocellulose and Ulva/Ag/Cellulose Nanocomposites and Both Blended with Fluoride against Bacteria Causing Dental Decay. Polymers 2023, 15, 1047. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Collado, J.L.; García-San-Martín, N.; Molina-Mateo, J.; Torregrosa Cabanilles, C.; Donderis Quiles, V.; Serrano-Aroca, A.; Sabater i Serra, R. Electroactive Calcium-Alginate/Polycaprolactone/Reduced Graphene Oxide Nanohybrid Hydrogels for Skeletal Muscle Tissue Engineering. Colloids Surf. B Biointerfaces 2022, 214, 112455. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Shah, C.P.; Singh, K.; Kumar, M.; Bajaj, P.N. An organic acid-induced synthesis and characterization of selenium nanoparticles. J. Nanotechnol. 2011, 2011, 651971. [Google Scholar] [CrossRef]
- Langi, B.; Shah, C.; Singh, K.; Chaskar, A.; Kumar, M.; Bajaj, P.N. Ionic liquid-induced synthesis of selenium nanoparticles. Mater. Res. Bull. 2010, 45, 668–671. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhu, P.; Zhao, J.; Zhang, C.-J.; Wilkinson, A.J.; Cui, L. Thermal Degradation Properties of Biobased Iron Alginate Film. J. Anal. Appl. Pyrolysis 2016, 119, 87–96. [Google Scholar] [CrossRef]
- Vishakha, V.; Abdel-Mohsen, A.M.; Michalicka, J.; White, P.B.; Lepcio, P.; Katherine, L.; Jančář, J. Carboxymethyl Starch as a Reducing and Capping Agent in the Hydrothermal Synthesis of Selenium Nanostructures for Use with Three-Dimensional-Printed Hydrogel Carriers. R. Soc. Open Sci. 2023, 10, 230829. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Zhang, M.; Shen, H. Structure of Alginate Polysaccharide Selenium-Nanoparticles and the Mechanism of Promoting Selenium Accumulation in Rice. J. Plant Nutr. Fertil. 2023, 29, 158–171. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Elhadary, A.M.A.; Abuelmagd, M.A. Extruded Polysaccharide/Protein Matrix from Arthrospira Platensis Cultures Mediated Silver Nanoparticles Biosynthesis and Capping. Appl. Nanosci. 2020, 10, 3839–3855. [Google Scholar] [CrossRef]
- Alagesan, V.; Venugopal, S. Green Synthesis of Selenium Nanoparticle Using Leaves Extract of Withania somnifera and Its Biological Applications and Photocatalytic Activities. BioNanoScience 2018, 9, 105–116. [Google Scholar] [CrossRef]
- Serov, D.A.; Khabatova, V.V.; Vodeneev, V.; Li, R.; Gudkov, S.V. A Review of the Antibacterial, Fungicidal and Antiviral Properties of Selenium Nanoparticles. Materials 2023, 16, 5363. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Le, X.C.; Tran, T.N.M.; Nguyen, N.T.T.; Pham, B.N.; Vu, D. Nano Selenium–Alginate Edible Coating Extends Hydroponic Strawberry Shelf Life and Provides Selenium Fortification as a Micro-Nutrient. Food Biosci. 2023, 53, 102597. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Bach, H. Mechanisms of Antifungal Properties of Metal Nanoparticles. Nanomaterials 2022, 12, 4470. [Google Scholar] [CrossRef] [PubMed]
- Tarek, A.; Elnasr, S.; Ibrahim, O.M.; Alhumaimess, M.S.; Alsohaimi, I.H.; El-Ossaily, Y.A.; Hussein, M.F.; Nassar, A.M.; Hassan, H.M.A.; El-Aassar, M.R.; et al. Chitosan/Selenium@Olive Oil Nanocomplex Targeted Therapy for Multiple Cancers. J. Polym. Environ. 2023, 32, 658–671. [Google Scholar] [CrossRef]
- El-Zayat, M.; Eraqi, M.M.; Alrefai, H.; El-Khateeb, A.Y.; Ibrahim, M.A.; Aljohani, H.M.; Aljohani, M.M.; Elshaer, M.M. The Antimicrobial, Antioxidant, and Anticancer Activity of Greenly Synthesized Selenium and Zinc Composite Nanoparticles Using Ephedra aphylla Extract. Biomolecules 2021, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Khaled, A.M.; Othman, M.S.; Obeidat, S.T.; Aleid, G.M.; Aboelnaga, S.M.; Fehaid, A.; Hathout, H.M.R.; Bakkar, A.A.; Abdel, A.E.; El-Garawani, I.M.; et al. Green-Synthesized Silver and Selenium Nanoparticles Using Berberine: A Comparative Assessment of In Vitro Anticancer Potential on Human Hepatocellular Carcinoma Cell Line (HepG2). Cells 2024, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Estevez, H.; Garcia-Lidon, J.C.; Luque-Garcia, J.L.; Camara, C. Effects of Chitosan-Stabilized Selenium Nanoparticles on Cell Proliferation, Apoptosis and Cell Cycle Pattern in HepG2 Cells: Comparison with Other Selenospecies. Colloids Surf. B Biointerfaces 2014, 122, 184–193. [Google Scholar] [CrossRef]
- Abdelhamid, A.E.; Ahmed, E.H.; Awad, H.M.; Ayoub, H. Synthesis and Cytotoxic Activities of Selenium Nanoparticles Incorporated Nano-Chitosan. Polym. Bull. 2023, 81, 1421–1437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindi, H.A.; Hamouda, R.A.; Abdel-Hamid, M.S.; Alhazmi, N.M. Alginate Extracted from Azotobacter chroococcum Loaded in Selenium Nanoparticles: Insight on Characterization, Antifungal and Anticancer Activities. Polymers 2024, 16, 2065. https://doi.org/10.3390/polym16142065
Sindi HA, Hamouda RA, Abdel-Hamid MS, Alhazmi NM. Alginate Extracted from Azotobacter chroococcum Loaded in Selenium Nanoparticles: Insight on Characterization, Antifungal and Anticancer Activities. Polymers. 2024; 16(14):2065. https://doi.org/10.3390/polym16142065
Chicago/Turabian StyleSindi, Hebah A., Ragaa A. Hamouda, Marwa S. Abdel-Hamid, and Nuha M. Alhazmi. 2024. "Alginate Extracted from Azotobacter chroococcum Loaded in Selenium Nanoparticles: Insight on Characterization, Antifungal and Anticancer Activities" Polymers 16, no. 14: 2065. https://doi.org/10.3390/polym16142065
APA StyleSindi, H. A., Hamouda, R. A., Abdel-Hamid, M. S., & Alhazmi, N. M. (2024). Alginate Extracted from Azotobacter chroococcum Loaded in Selenium Nanoparticles: Insight on Characterization, Antifungal and Anticancer Activities. Polymers, 16(14), 2065. https://doi.org/10.3390/polym16142065








