Thermal Stability of Highly Filled Cellulosic Biocomposites Based on Ethylene–Vinyl Acetate Copolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Preparation of Biocomposites
2.2. Methods
- (1)
- Determination of oxidation induction temperature (dynamic OIT)
- (2)
- Determination of oxidation induction time (isothermal OIT)
- (3)
- Thermogravimetric analysis (TGA)
- (4)
- Fourier-transform infrared spectroscopy (FTIR)
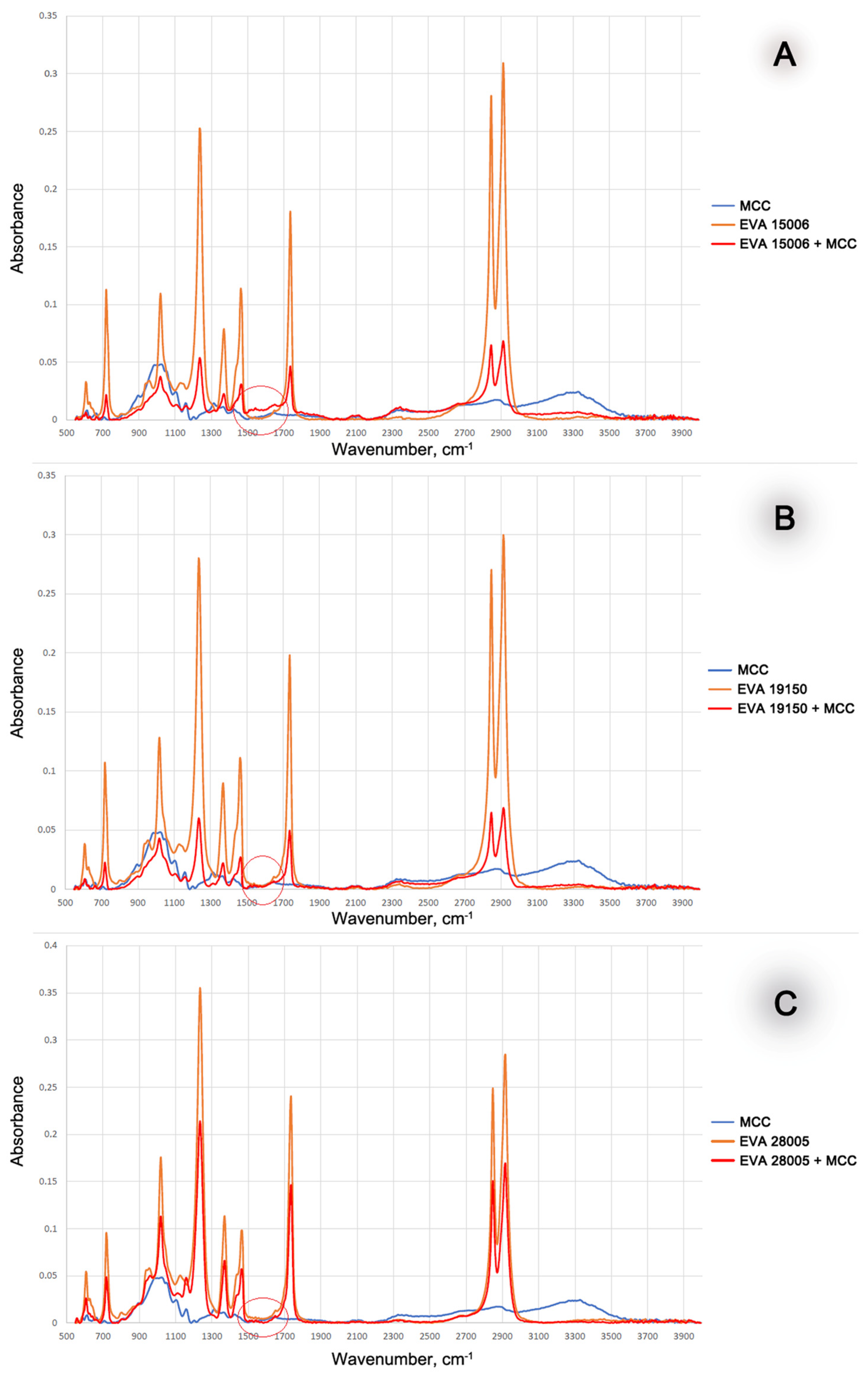
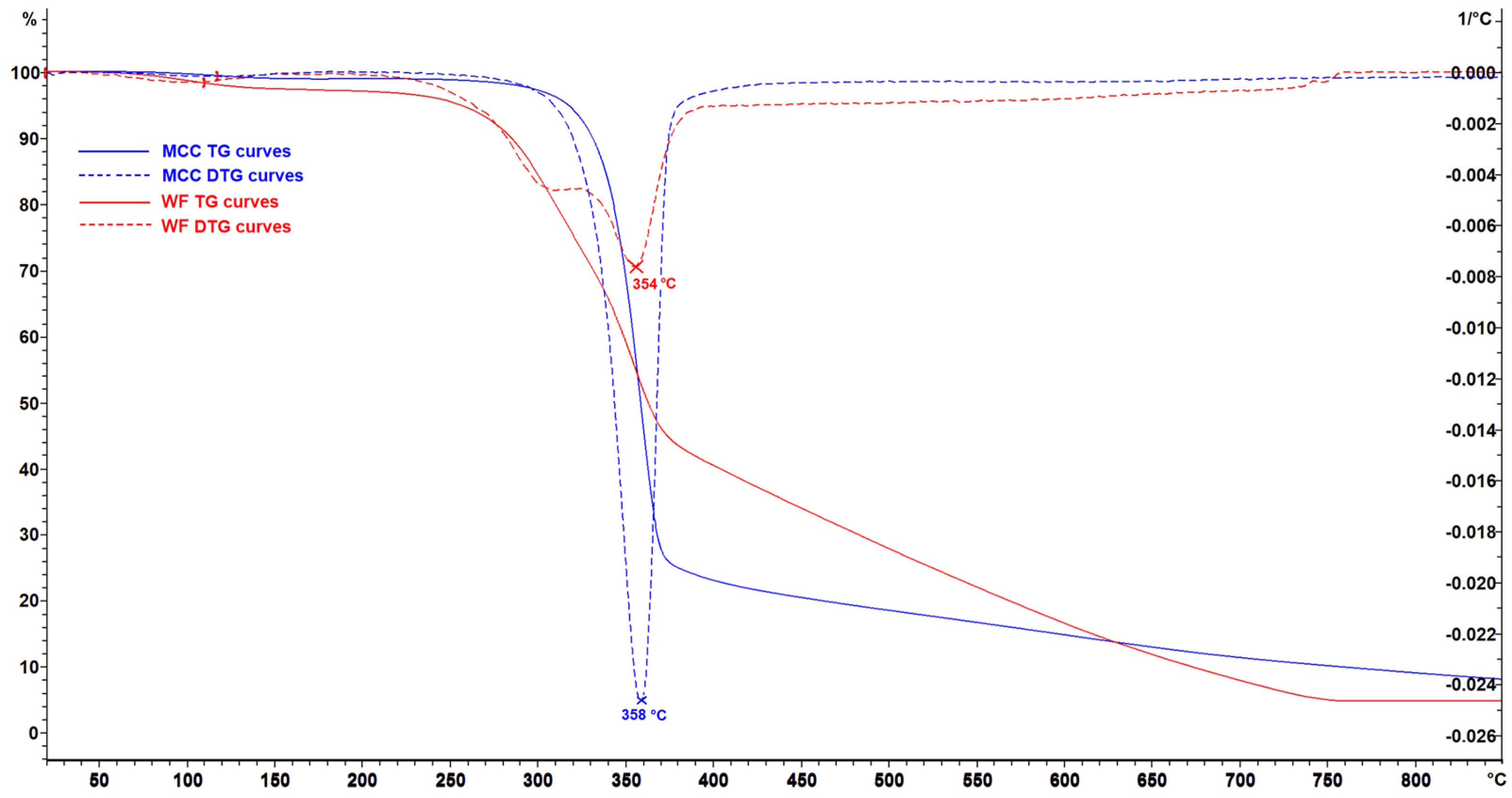
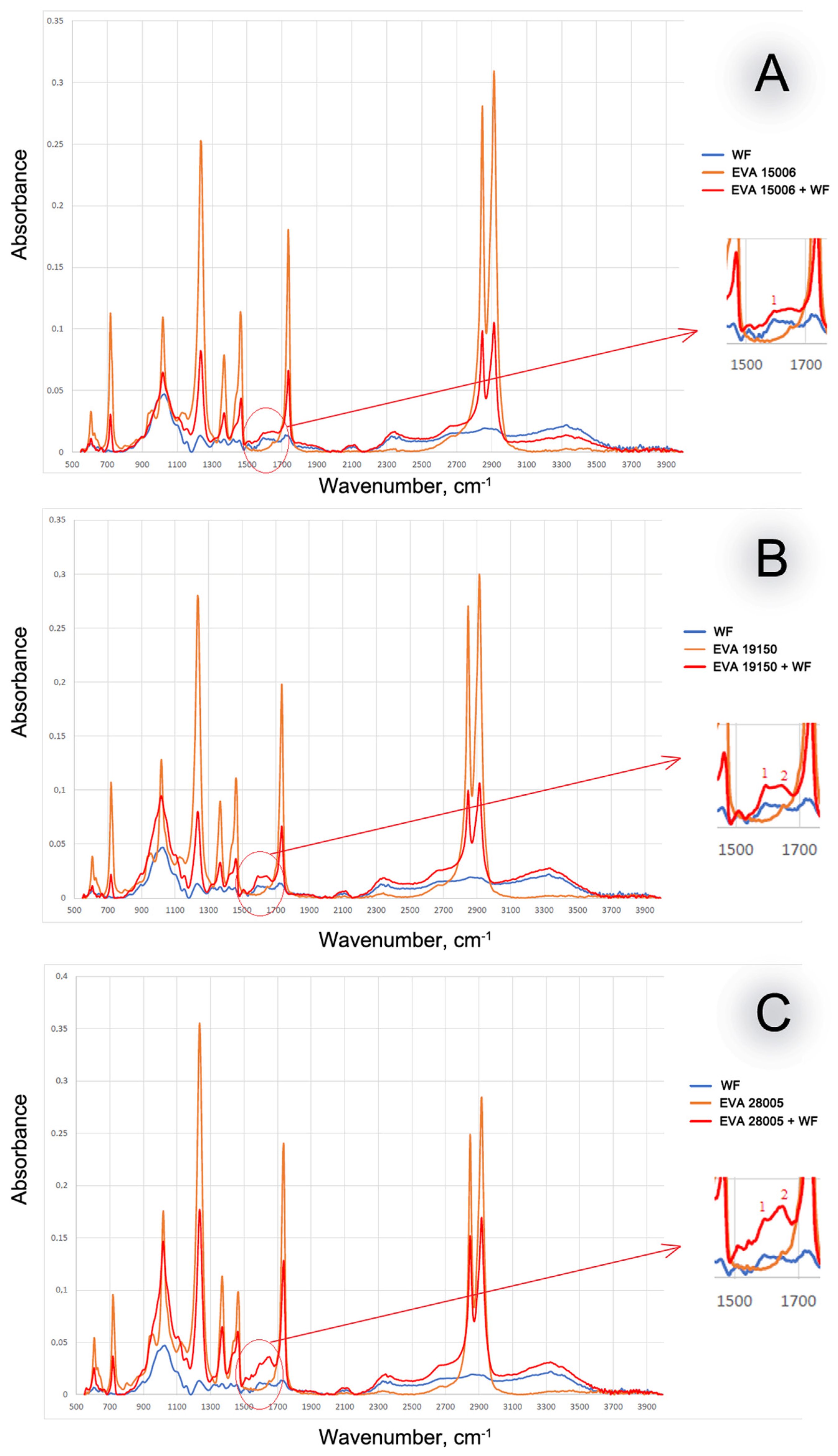
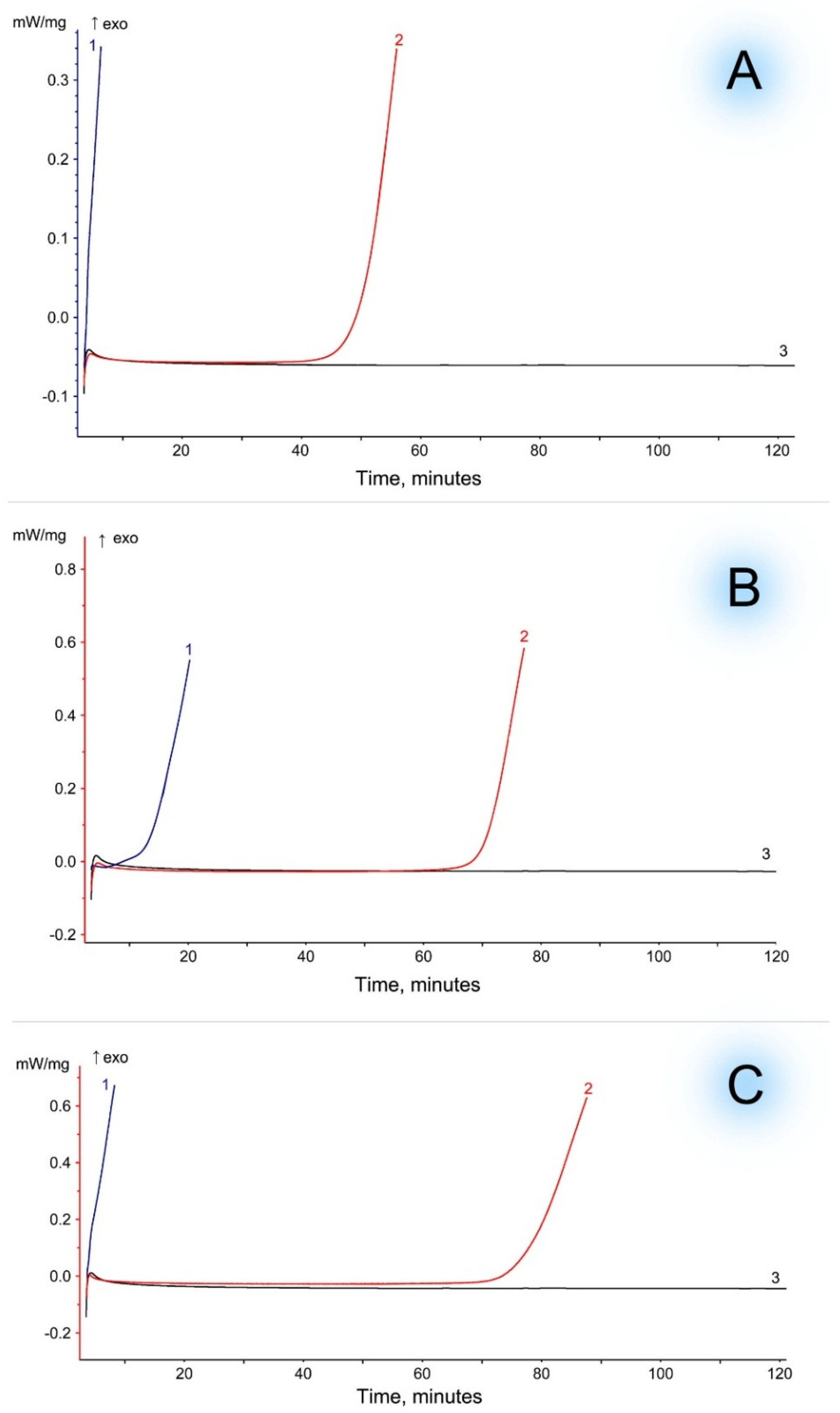
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, M.V.G.; Turella, T.; Santana, R.M.C.; Zattera, A.J. Comparative study between poly(ethylene-co-vinyl acetate)—EVA expanded composites filled with banana fiber and wood flour. Mater. Res. 2014, 17, 1535–1544. [Google Scholar] [CrossRef]
- Sartore, L.; D’Amore, A.; Di Landro, L. Ethylene vinyl acetate blends with cellulosic fillers and reinforcements. Polym. Compos. 2015, 36, 980–986. [Google Scholar] [CrossRef]
- Sessini, V.; Arrieta, M.P.; Raquez, J.-M.; Dubois, P.; Kenny, J.M.; Peponi, L. Thermal and composting degradation of EVA/Thermoplastic starch blends and their nanocomposites. Polym. Degrad. Stab. 2019, 159, 184–198. [Google Scholar] [CrossRef]
- Lightsey, G.R. Organic Fillers for Thermoplastics. In Polymer Applications of Renewable-Resource Materials; Springer: Boston, MA, USA, 1983; pp. 193–211. [Google Scholar] [CrossRef]
- Buchachenko, A.L.; Emanuel, N.M. Chemical Physics of Polymer Degradation and Stabilization; CRC Press: Boca Raton, FL, USA, 1987. [Google Scholar]
- Maghsoud, Z.; Rafiei, M.; Famili, M.H.N. Effect of processing method on migration of antioxidant from HDPE packaging into a fatty food simulant in terms of crystallinity. Packag. Technol. Sci. 2018, 31, 141–149. [Google Scholar] [CrossRef]
- Kang, K.; Chang, Y.; Choi, J.C.; Park, S.; Han, J. Migration Study of Butylated Hydroxytoluene and Irganox 1010 from Polypropylene Treated with Severe Processing Conditions. J. Food Sci. 2018, 83, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Poletto, M. Natural oils as coupling agents in recycled polypropylene wood flour composites: Mechanical, thermal and morphological properties. Polym. Polym. Compos. 2020, 28, 443–450. [Google Scholar] [CrossRef]
- Vorobyova, E.V.; Prykhod’ko, E.L. Stabilization of polyethylene by natural fillers and their extracts. Chem. Plant Raw Mater. 2019, 2, 213–223. [Google Scholar] [CrossRef]
- Cerruti, P.; Malinconico, M.; Rychly, J.; Matisova-Rychla, L.; Carfagna, C. Effect of natural antioxidants on the stability of polypropylene films. Polym. Degrad. Stab. 2009, 94, 2095–2100. [Google Scholar] [CrossRef]
- Koontz, J.L.; Marcy, J.E.; O’Keefe, S.F.; Duncan, S.E.; Long, T.E.; Moffitt, R.D. Polymer processing and characterization of LLDPE films loaded with α-tocopherol, quercetin, and their cyclodextrin inclusion complexes. J. Appl. Polym. Sci. 2010, 117, 2299–2309. [Google Scholar] [CrossRef]
- Samper, M.D.; Fages, E.; Fenollar, O.; Boronat, T.; Balart, R. The potential of flavonoids as natural antioxidants and UV light stabilizers for polypropylene. J. Appl. Polym. Sci. 2013, 129, 1707–1716. [Google Scholar] [CrossRef]
- Shelenkov, P.G.; Pantyukhov, P.V.; Popov, A.A. Mechanical Properties of Superconcentrates Based on Ethylene-Vinyl Acetate Copolymer and Microcrystalline Cellulose. Mater. Sci. Forum. 2020, 992, 306–310. [Google Scholar] [CrossRef]
- Shelenkov, P.G.; Pantyukhov, P.V.; Popov, A.A. Highly filled biocomposites based on ethylene-vinyl acetate copolymer and wood flour. IOP Conf. Ser. Mater. Sci. Eng. 2018, 369, 012043. [Google Scholar] [CrossRef]
- Shelenkov, P.G.; Pantyukhov, P.V.; Poletto, M.; Popov, A.A. Influence of Vinyl Acetate Content and Melt Flow Index of Ethylene-Vinyl Acetate Copolymer on Physico-Mechanical and Physico-Chemical Properties of Highly Filled Biocomposites. Polymers 2023, 15, 2639. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, N.I. Chemistry of Wood and Cellulose; Academy of Sciences of the USSR: Leningrad, Russia, 1962. [Google Scholar]
- ISO 11357-6:2018; Differential Scanning Calorimetry (DSC). ISO: Geneva, Switzerland, 2018.
- ISO 11358-1:2022; Plastics—Thermogravimetry (TG) of Polymers. ISO: Geneva, Switzerland, 2022.
- Shurvell, H.F. Spectra- Structure Correlations in the Mid- and Far-Infrared. In Handbook of Vibrational Spectroscopy; Chalmers, J.M., Ed.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Wenwei, Z.; Xiaoguang, Z.; Li, Y.; Yuefang, Z.; Jiazhen, S. Determination of the vinyl acetate content in ethylene-vinyl acetate copolymers by thermogravimetric analysis. Polymer 1994, 35, 3348–3350. [Google Scholar] [CrossRef]
- Pantyukhov, P.; Monakhova, T.; Popov, A.; Zykova, A. Chemical interaction of polyethylene matrix with vegetable fillers in biocomposites. AIP Conf. Proc. 2016, 1736, 020122. [Google Scholar] [CrossRef]
- Pantyukhov, P.V.; Khvatov, A.V.; Monakhova, T.V.; Popov, A.A.; Kolesnikova, N.N. The Degradation of Materials Based on Low-Density Polyethylene and Natural Fillers. Int. Polym. Sci. Technol. 2013, 40, 55–58. [Google Scholar] [CrossRef]
- Iyer, K.A.; Zhang, L.; Torkelson, J.M. Direct Use of Natural Antioxidant-rich Agro-wastes as Thermal Stabilizer for Polymer: Processing and Recycling, ACS Sustain. Chem. Eng. 2016, 4, 881–889. [Google Scholar] [CrossRef]



| EVA Grade | Content of Vinyl Acetate Groups | Melt Flow Index (MFI) at 190 °C/2.16 kg [g/10 min] | |
|---|---|---|---|
| [wt.%] | [mol.%] | ||
| 28005 | 28 | 11 | 5 |
| 28025 | 28 | 11 | 25 |
| 28150 | 28 | 11 | 150 |
| 15006 | 15 | 5 | 6 |
| 19150 | 19 | 7 | 150 |
| Filler | Cellulose [wt.%] | Lignin [wt.%] | Pentosanes [wt.%] | Polyuronic Acid [wt.%] | Reference |
|---|---|---|---|---|---|
| Wood flour (WF) | 46 | 20 | 29 | 5 | [16] |
| Microcrystalline cellulose (MCC) | 100 | - | - | - | - |
| Composition | Tonset, °C | Tpeak, °C | Tend, °C |
|---|---|---|---|
| WF | 253 | 354 | 756 |
| MCC | 311 | 358 | 374 |
| EVA 15006 | 330 | 374/481 | 517 |
| EVA 15006 + WF | 266 | 373/492 | 515 |
| EVA 15006 + MCC | 317 | 359/489 | 513 |
| EVA 19150 | 313 | 372/484 | 513 |
| EVA 19150 + WF | 261 | 374/492 | 515 |
| EVA 19150 + MCC | 306 | 358/486 | 514 |
| EVA 28005 | 329 | 367/487 | 513 |
| EVA 28005 + WF | 259 | 371/488 | 835 |
| EVA 28005 + MCC | 308 | 356/490 | 514 |
| Composition | Tangent of the Inclination Angle in the Range of 220–240 °C |
|---|---|
| Wood flour (WF) | 0.029 ± 0.001 |
| EVA (15 wt.% of VA) + WF | 0.010 ± 0.001 |
| EVA (19 wt.% of VA) + WF | 0.009 ± 0.001 |
| EVA (28 wt.% of VA) + WF | 0.008 ± 0.001 |
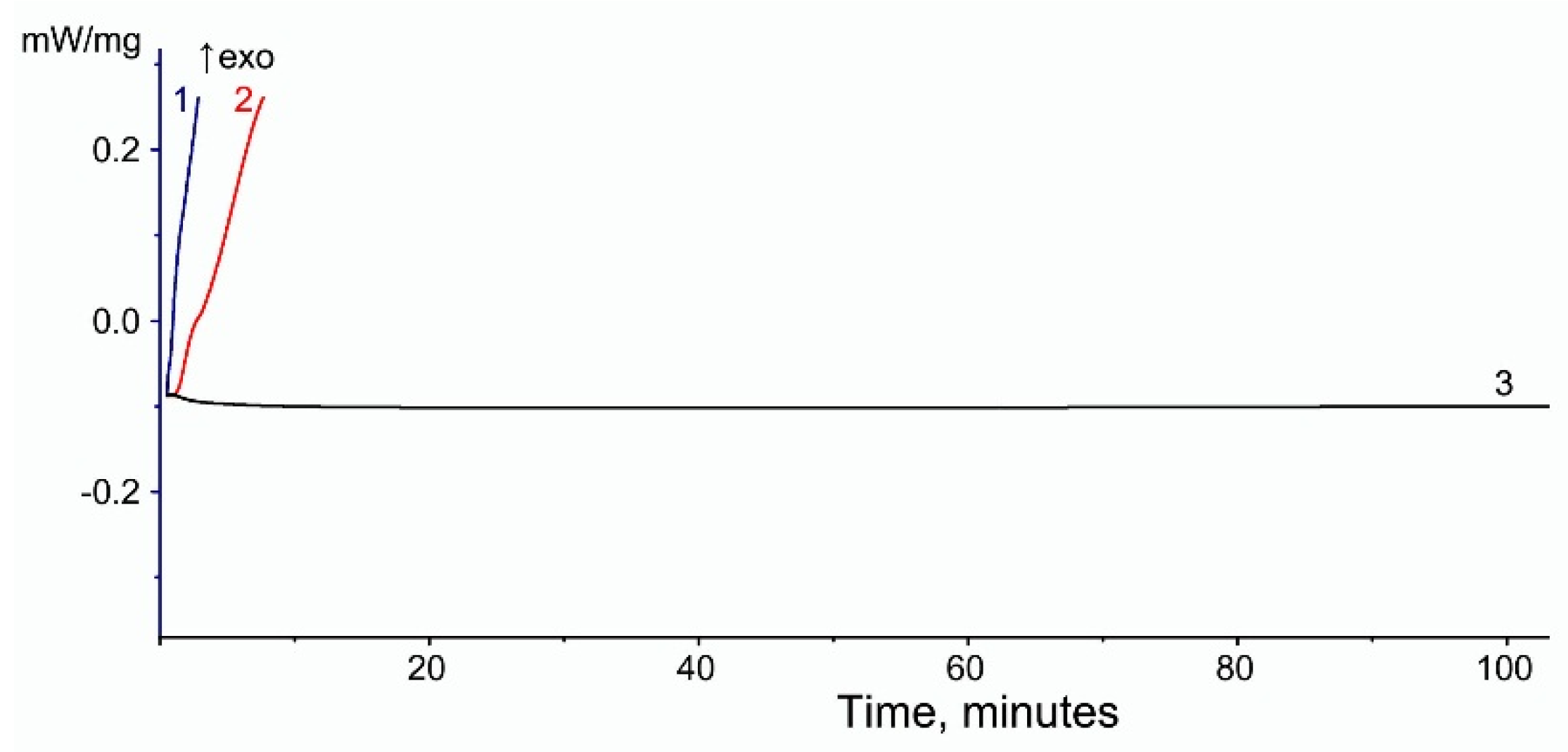
| Content of VA in EVA, [wt.%] | Filler | Onset Time of Thermal-Oxidative Degradation [min] | ||
|---|---|---|---|---|
| EVA | Filler | EVA + Filler | ||
| 15 | WF | 0.2 | >120 | 46.2 |
| 19 | 9.8 | 66.8 | ||
| 28 | 0.2 | 73.4 | ||
| 15 | MCC | 0.2 | >120 | 0.2 |
| 19 | 9.8 | 9.8 | ||
| 28 | 0.2 | 0.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelenkov, P.G.; Pantyukhov, P.V.; Aleshinskaya, S.V.; Maltsev, A.A.; Abushakhmanova, Z.R.; Popov, A.A.; Saavedra-Arias, J.J.; Poletto, M. Thermal Stability of Highly Filled Cellulosic Biocomposites Based on Ethylene–Vinyl Acetate Copolymer. Polymers 2024, 16, 2103. https://doi.org/10.3390/polym16152103
Shelenkov PG, Pantyukhov PV, Aleshinskaya SV, Maltsev AA, Abushakhmanova ZR, Popov AA, Saavedra-Arias JJ, Poletto M. Thermal Stability of Highly Filled Cellulosic Biocomposites Based on Ethylene–Vinyl Acetate Copolymer. Polymers. 2024; 16(15):2103. https://doi.org/10.3390/polym16152103
Chicago/Turabian StyleShelenkov, Pavel Gennadievich, Petr Vasilievich Pantyukhov, Svetlana Vladimirovna Aleshinskaya, Alexander Andreevich Maltsev, Zubarzhat Rafisovna Abushakhmanova, Anatoly Anatolievich Popov, Jose Javier Saavedra-Arias, and Matheus Poletto. 2024. "Thermal Stability of Highly Filled Cellulosic Biocomposites Based on Ethylene–Vinyl Acetate Copolymer" Polymers 16, no. 15: 2103. https://doi.org/10.3390/polym16152103
APA StyleShelenkov, P. G., Pantyukhov, P. V., Aleshinskaya, S. V., Maltsev, A. A., Abushakhmanova, Z. R., Popov, A. A., Saavedra-Arias, J. J., & Poletto, M. (2024). Thermal Stability of Highly Filled Cellulosic Biocomposites Based on Ethylene–Vinyl Acetate Copolymer. Polymers, 16(15), 2103. https://doi.org/10.3390/polym16152103









