Recent Advancements in Acrylic Fabric Applications: A Comprehensive Review and Future Trends
Abstract
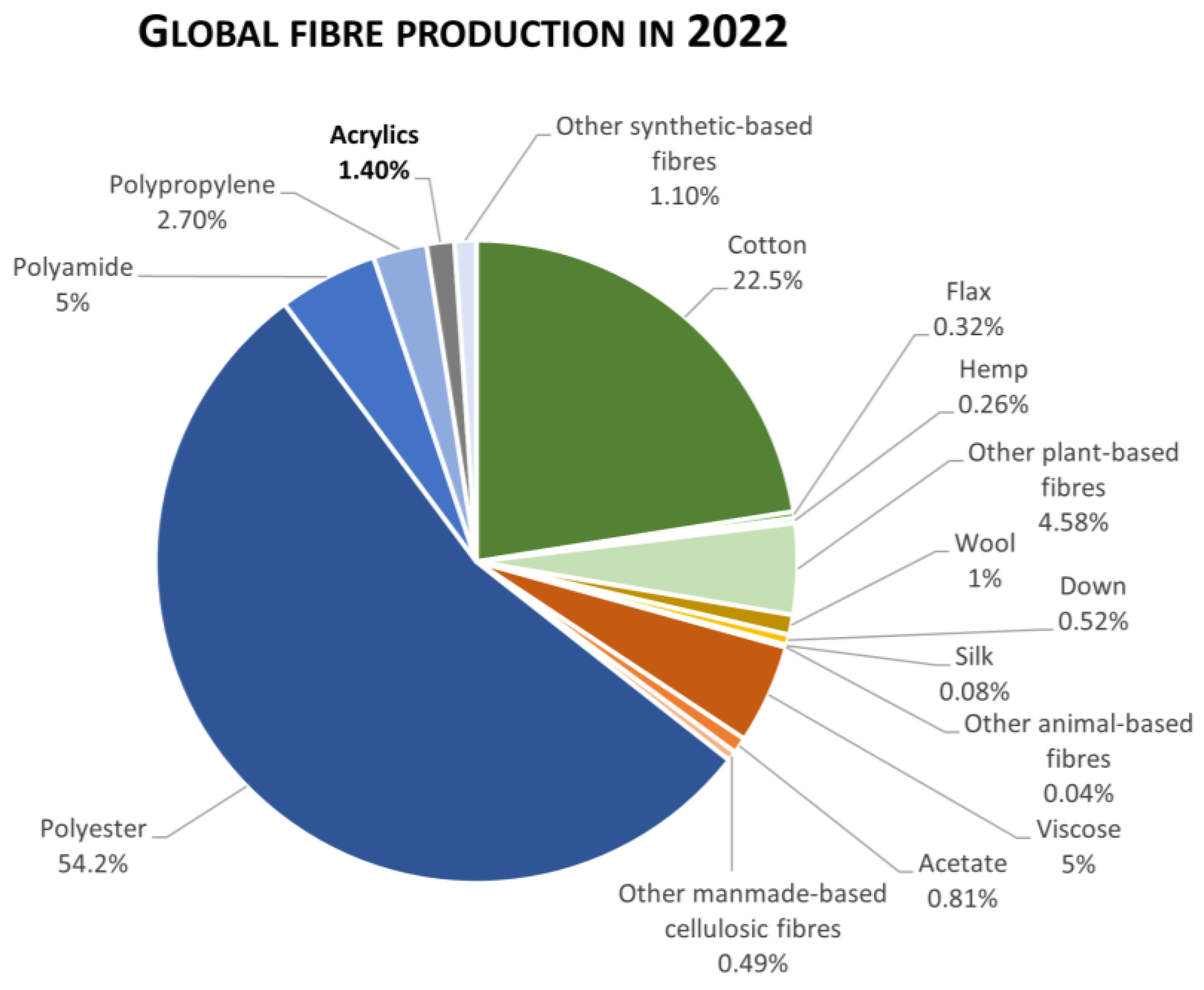
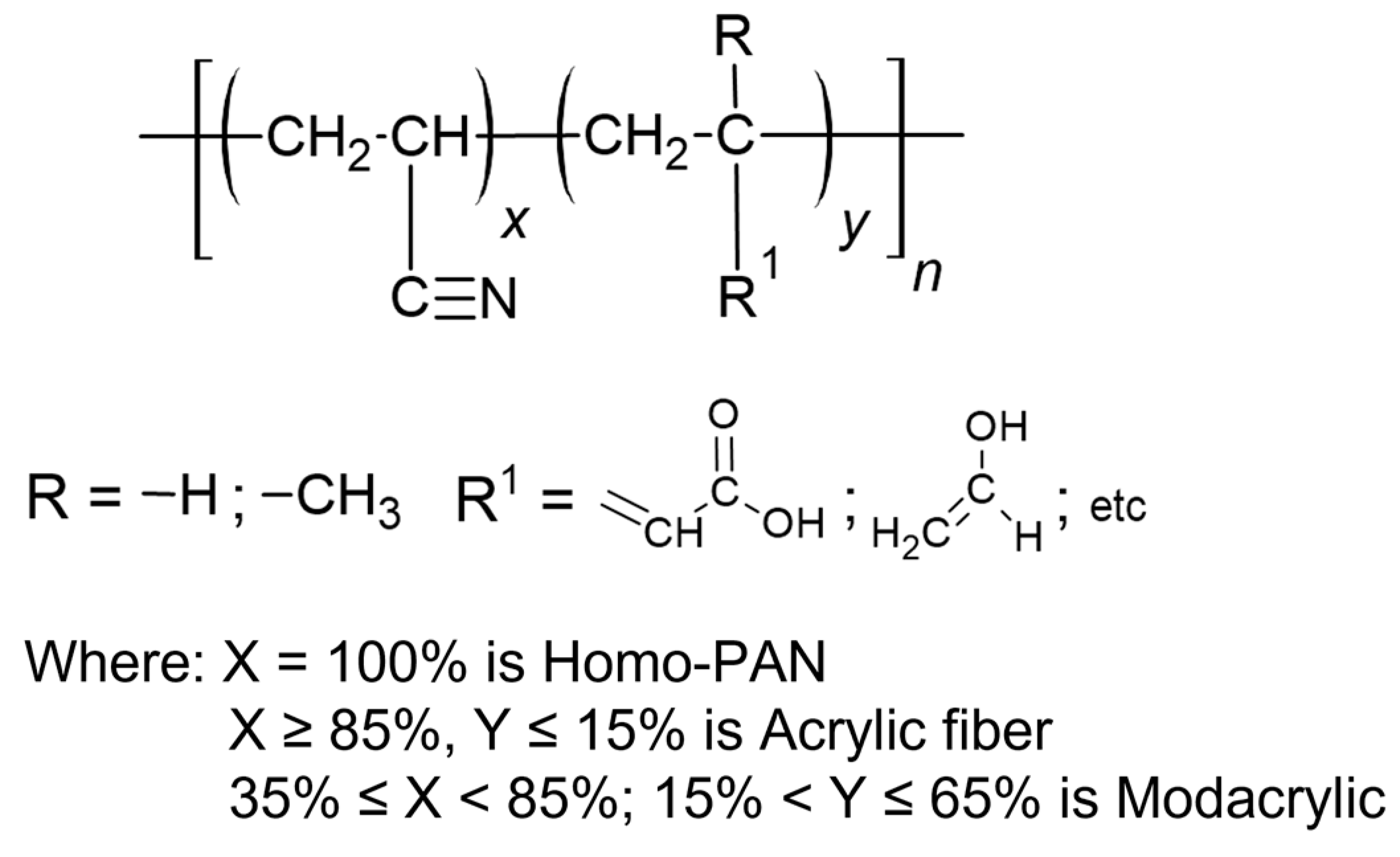
:1. Introduction
2. Functional Finishing of Acrylic Fibres
2.1. Antimicrobial Fibres
| Author | Material Used | Inhibition Zone Diameters (mm) | Ref. | |
|---|---|---|---|---|
| E. coli | S. aureus | |||
| Houng et al. | Silver/graphene oxide (Ag/GO) nanocomposite | - | 24.00 ± 0.34 | [37] |
| Okay et al. | Silver nanoparticles, acrylic acid, and acrylamide | 10.25 ± 0.35 | 11.00 ± 1.41 | [42] |
| Patel et al. | Zinc oxide and silver nanoparticles | 10.00 | - | [43] |
| Mofidfar et al. | Poly(acrylic acid) fibres with silver nanoparticles | - | 3.00 ± 0.3 | [44] |
| Chen et. al. | Tannic acid and silver nanoparticles | 11.00 ± 0.5 a | 11.60 ± 0.5 a | [45] |
| Sarwar et al. | Diclofenac Sodium Salt | 16 ± 0.46 | 15.5 ± 0.28 | [46] |
| Wahab et al. | Titania/AgNP composite nanoparticles | 3.23 | 4.1 | [47] |
2.2. Electronic Textiles
| Author | Material | Amount | Final Conductive (µS/cm) | Ref. |
|---|---|---|---|---|
| Ahn et al. | Carbon black nanoparticles | 12% | 890 | [66] |
| Rehan et al. | Silver nanoparticles | 0.05% | 324 ± 0.5 | [68] |
| Karbownik et al. | Polyaniline fibres | 1% | 100 | [69] |
| Mustafov et al. | Lignin with graphite | 20%, 1 wt.% | 19.2 × 103 | [70] |
| Xi et al. | Poly (3,4-ethylene dioxythiophene) (PEDOT) | 150 µL/10 mL | 20.51 × 106 | [71] |
| Deng et al. | Multi-walled carbon nanotubes (MWCNTs) | 6 wt.% | 51.6 × 106 | [72] |
| Mpukuta et al. | Silica nanoparticles | 1 wt.% | 8.11 × 103 | [73] |
2.3. Flame Resistance
2.4. Water Repellency and Waterproof Finishes
| Author | Materials/Method | Amount | Final Contact Angle | Water Vapour Transmittance (kg m−2 d−1) | Hydrostatic Pressure (kPa) | Ref. |
|---|---|---|---|---|---|---|
| Li et al. | Blocked isocyanate prepolymer (BIP) and fluorine-free waterborne hydroxyl acrylic resin (HAR) | 2 wt.% of BIP and 2% of HAR | 151° | 12.7 | 112.5 | [100] |
| Wang et al. | Polyurethane (PU) and silicon dioxide nanoparticles (SiO2) | 50 wt.% of SiO2 | 151.2° | 10.8 | 85.7 | [101] |
| Zhang et al. | Amino functional modified polysiloxane (AMP) and 4, 4′-methyl diphenylene diisocyanate (MDI) | 1 wt.% of AMP and 2 wt.% of MDI | 139.2° | 4.7 | 93.8 | [102] |
| Gu et al. | Polyvinylidene fluoride (PVDF) | 3 wt.% | 137° | 4.65 | 18.04 | [103] |
| Yu et al. | PU and SiO2 nanoparticles | 3 wt.% of SiO2 | 137.2° | 10.3 | - | [104] |
| Liu et al. | (3-aminopropyl) triethoxysilane (APS) and hexadecyltrimethoxysilane (HDTMS) | 10 wt.% of APS | 150.1° | 3.92 | - | [105] |
2.5. UV Protection
| Author | Material | Amount (%) | UPF | UV Protection Improvement (%) | Ref. |
|---|---|---|---|---|---|
| Hassan et al. | Silver nanoparticles | 3 | - | 94.8 | [107] |
| El Gabry et al. | Sodium polyacrylate/bentonite nanocomposite | - | 36 | 300 | [29] |
| Rehan et al. | Silver nanoparticles | 0.05 | 541 | 1400 | [68] |
| Jiang et al. | Titanium dioxide (TiO2) nanoparticles | 10 | 175 | - | [113] |
| Koozekonan et al. | Titanium dioxide nanoparticles | 15 | 133 | 1425 | [114] |
| Carbon nanotubes (CNT) | 10 | 48 | 450 | ||
| TiO2 with CNT | 15 | 685 | 7755 | ||
| Nasouri et al. | Multi-walled carbon nanotubes (MWCNTs) | 10 | 677 | - | [115] |
3. Recent Technologies in Acrylic Textile Finishing
3.1. Plasma Treatment
3.2. Sol-Gel
3.3. Bio-Based Finishings
3.4. Grafting
3.5. Carbonisation
3.6. Dyeing Process
4. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Exchange Textile Materials Market Report. Available online: https://textileexchange.org/knowledge-center/reports/materials-market-report-2023/ (accessed on 11 July 2024).
- Masson, J. (Ed.) Acrylic Fiber Technology and Applications; CRC Press: Boca Raton, FL, USA, 1995; ISBN 9780429189913. [Google Scholar]
- Gojic, A.; Bukhonka, N. Recycled Textile Fibers and Materials—Current State and Development Perspectives. In Proceedings of the ICPAE, Zrenjanin, Serbia, 24–26 August 2023; pp. 95–100. [Google Scholar]
- Kaitwade, N. Acrylic Fibre Market Outlook (2023 to 2033). Available online: https://www.futuremarketinsights.com/reports/acrylic-fibre-market (accessed on 10 May 2024).
- Muthu, S.S. Introduction to Sustainability and the Textile Supply Chain and Its Environmental Impact. In Assessing the Environmental Impact of Textiles and the Clothing Supply Chain; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–32. [Google Scholar]
- Seddik, K.M.; El-Gabry, L.K.; Ali, M.A. Improving Some Functional Utility Properties of Garment Using Cross-Linked Acrylic Fabrics. Res. J. Text. Appar. 2023, in press. [Google Scholar] [CrossRef]
- Mather, R.R. Synthetic Textile Fibres. In Textiles and Fashion; Elsevier: Amsterdam, The Netherlands, 2015; pp. 115–138. [Google Scholar]
- Danish, A.; Ozbakkaloglu, T. Fiber Classifications and Physical and Mechanical Properties of Different Fibers Used in Alkali-Activated Composites. In Advanced Fiber-Reinforced Alkali-Activated Composites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 23–58. [Google Scholar]
- Ghalia, M.A.; Abdelrasoul, A. Compressive and Fracture Toughness of Natural and Synthetic Fiber-Reinforced Polymer. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–140. [Google Scholar]
- Gupta, B.S.; Afshari, M. Polyacrylonitrile Fibers. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 545–593. [Google Scholar]
- Rebenfeld, L. Chapter VI—Fibers and Fibrous Materials. In Absorbent Technology; Chatterjee, P.K., Gupta, B.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 13, pp. 199–232. ISBN 0920-4083. [Google Scholar]
- Nogaj, A.; Süling, C.; Schweizer, M. Fibers, 8. Polyacrylonitrile Fibers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Niaounakis, M. The Problem of Marine Plastic Debris. In Management of Marine Plastic Debris; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–55. [Google Scholar]
- Rudin, A.; Choi, P. Introductory Concepts and Definitions. In The Elements of Polymer Science & Engineering; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1–62. ISBN 978-0-12-382178-2. [Google Scholar]
- Adegbola, T.A.; Agboola, O.; Fayomi, O.S.I. Review of Polyacrylonitrile Blends and Application in Manufacturing Technology: Recycling and Environmental Impact. Results Eng. 2020, 7, 100144. [Google Scholar] [CrossRef]
- Hesse, M.; Meier, H.; Zeeh, B. (Eds.) Spectroscopic Methods in Organic Chemistry; Georg Thieme Verlag: Stuttgart, Germany, 2008; ISBN 9783131060426. [Google Scholar]
- Mahltig, B. High-Performance Fibres—A Review of Properties and IR-Spectra. Tekstilec 2021, 64, 96–118. [Google Scholar] [CrossRef]
- Moody, V.; Needles, H.L. Major Fibers and Their Properties. In Tufted Carpet; Elsevier: Amsterdam, The Netherland, 2004; pp. 35–59. [Google Scholar]
- El-Sayed, H.; Vineis, C.; Varesano, A.; Mowafi, S.; Andrea Carletto, R.; Tonetti, C.; Abou Taleb, M. A Critique on Multi-Jet Electrospinning: State of the Art and Future Outlook. Nanotechnol. Rev. 2019, 8, 236–245. [Google Scholar] [CrossRef]
- Rohani Shirvan, A.; Nouri, A.; Sutti, A. A Perspective on the Wet Spinning Process and Its Advancements in Biomedical Sciences. Eur. Polym. J. 2022, 181, 111681. [Google Scholar] [CrossRef]
- Temesgen, S.; Rennert, M.; Tesfaye, T.; Nase, M. Review on Spinning of Biopolymer Fibers from Starch. Polymers 2021, 13, 1121. [Google Scholar] [CrossRef] [PubMed]
- Sayyar, S.; Moskowitz, J.; Fox, B.; Wiggins, J.; Wallace, G. Wet-spinning and Carbonization of Graphene/PAN-based Fibers: Toward Improving the Properties of Carbon Fibers. J. Appl. Polym. Sci. 2019, 136, 47932. [Google Scholar] [CrossRef]
- Ozipek, B.; Karakas, H. Wet Spinning of Synthetic Polymer Fibers. In Advances in Filament Yarn Spinning of Textiles and Polymers; Elsevier: Amsterdam, The Netherland, 2014; pp. 174–186. [Google Scholar]
- Choi, D.; Kil, H.-S.; Lee, S. Fabrication of Low-Cost Carbon Fibers Using Economical Precursors and Advanced Processing Technologies. Carbon N. Y. 2019, 142, 610–649. [Google Scholar] [CrossRef]
- Shang, L.; Yu, Y.; Liu, Y.; Chen, Z.; Kong, T.; Zhao, Y. Spinning and Applications of Bioinspired Fiber Systems. ACS Nano 2019, 13, 2749–2772. [Google Scholar] [CrossRef]
- Hu, J.; Jahid, M.A.; Harish Kumar, N.; Harun, V. Fundamentals of the Fibrous Materials. In Handbook of Fibrous Materials; Wiley: Hoboken, NJ, USA, 2020; pp. 1–36. [Google Scholar]
- Kim, T.; Kim, D.; Park, Y. Recent Progress in Regenerated Fibers for “Green” Textile Products. J. Clean. Prod. 2022, 376, 134226. [Google Scholar] [CrossRef]
- Imura, Y.; Hogan, R.M.C.; Jaffe, M. Dry Spinning of Synthetic Polymer Fibers. In Advances in Filament Yarn Spinning of Textiles and Polymers; Elsevier: Amsterdam, The Netherland, 2014; pp. 187–202. [Google Scholar]
- El Gabry, L.K.; Abou El-Kheir, A.A.; El-Sayad, H.S.; El-Kashouty, M.A. Ecofriendly Modification of Acrylic Fabrics for Enhanced Transfer Printability. Fibers Polym. 2021, 22, 421–429. [Google Scholar] [CrossRef]
- Commission, E. Recycling of Waste ACrylic Textiles (REACT) Project; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial Textile: Recent Developments and Functional Perspective. Polym. Bull. 2022, 79, 5747–5771. [Google Scholar] [CrossRef] [PubMed]
- Selvasudha, N.; Sweety, J.P.; Dhanalekshmi, U.M.; Devi, N.S.D. Smart Antimicrobial Textiles for Healthcare Professionals and Individuals. In Antimicrobial Textiles from Natural Resources; Elsevier: Amsterdam, The Netherland, 2021; pp. 455–484. [Google Scholar]
- McQueen, R.H.; Ehnes, B.L. Antimicrobial Textiles and Infection Prevention—Clothes and Inanimate Environment. In Infection Prevention; Springer International Publishing: Cham, Switzerland, 2022; pp. 139–149. [Google Scholar]
- Aguda, O.N.; Lateef, A. Recent Advances in Functionalization of Nanotextiles: A Strategy to Combat Harmful Microorganisms and Emerging Pathogens in the 21st Century. Heliyon 2022, 8, e09761. [Google Scholar] [CrossRef] [PubMed]
- Atef El-Sayed, A.; El Gabry, L.K.; Allam, O.G. Application of Prepared Waterborne Polyurethane Extended with Chitosan to Impart Antibacterial Properties to Acrylic Fabrics. J. Mater. Sci. Mater. Med. 2010, 21, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Fromm, K.M. Silver Coordination Compounds with Antimicrobial Properties. Appl. Organomet. Chem. 2013, 27, 683–687. [Google Scholar] [CrossRef]
- Huong, Q.T.T.; Nam, N.T.H.; Hai, N.D.; Dat, N.M.; Linh, N.T.T.; Tinh, N.T.; Chau, N.M.; Phuc, N.V.H.; Le Hoai Nhi, T.; Phong, M.T.; et al. Surface Modification and Antibacterial Activity Enhancement of Acrylic Fabric by Coating Silver/Graphene Oxide Nanocomposite. J. Polym. Res. 2023, 30, 109. [Google Scholar] [CrossRef]
- Yu, D. Characterization and Inhibitory Effect of Antibacterial PAN-Based Hollow Fiber Loaded with Silver Nitrate. J. Memb. Sci. 2003, 225, 115–123. [Google Scholar] [CrossRef]
- Allehyani, E.S.; Almulaiky, Y.Q.; Al-Harbi, S.A.; El-Shishtawy, R.M. Polydopamine-AgNPs Coated Acrylic Fabric for Antimicrobial and Antioxidant Textiles. J. Coat. Technol. Res. 2022, 20, 1133–1143. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Zhang, L.; Zhong, S.; Yu, D. Electrospinning of PAN/Ag NPs Nanofiber Membrane with Antibacterial Properties. J. Mater. Res. 2019, 34, 1669–1677. [Google Scholar] [CrossRef]
- Lee, J.; Broughton, R.M.; Liang, J.; Worley, S.D.; Huang, T.S. Antimicrobial Acrylic Fiber. Res. J. Text. Appar. 2006, 10, 61–66. [Google Scholar] [CrossRef]
- Okay, Z.; Erdoğan, M.K.; Karaca, B.; Karakişla, M.; Saçak, M. Investigation of Antibacterial Properties of Polyacrylonitrile Fibers Modified by New Functional Groups and Silver Nanoparticles. Turk. J. Chem. 2022, 46, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Konar, M.; Sahoo, H.; Hota, G. Surface Functionalization of Electrospun PAN Nanofibers with ZnO–Ag Heterostructure Nanoparticles: Synthesis and Antibacterial Study. Nanotechnology 2019, 30, 205704. [Google Scholar] [CrossRef] [PubMed]
- Mofidfar, M.; Kim, E.S.; Larkin, E.L.; Long, L.; Jennings, W.D.; Ahadian, S.; Ghannoum, M.A.; Wnek, G.E. Antimicrobial Activity of Silver Containing Crosslinked Poly(Acrylic Acid) Fibers. Micromachines 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chai, M.; Mai, Z.; Liao, M.; Xie, X.; Lu, Z.; Zhang, W.; Zhao, H.; Dong, X.; Fu, X.; et al. Electrospinning Polyacrylonitrile (PAN) Based Nanofiberous Membranes Synergic with Plant Antibacterial Agent and Silver Nanoparticles (AgNPs) for Potential Wound Dressing. Mater. Today Commun. 2022, 31, 103336. [Google Scholar] [CrossRef]
- Sarwar, M.N.; Ullah, A.; Haider, M.K.; Hussain, N.; Ullah, S.; Hashmi, M.; Khan, M.Q.; Kim, I.S. Evaluating Antibacterial Efficacy and Biocompatibility of PAN Nanofibers Loaded with Diclofenac Sodium Salt. Polymers 2021, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- Wahab, J.A.; Mamun, S. Al Polyacrylonitrile Nanofiber Mats Containing Titania/AgNP Composite Nanoparticles for Antibacterial Applications. Mater. Res. Express 2020, 7, 015416. [Google Scholar] [CrossRef]
- Stegmaier, T. Recent Advances in Textile Manufacturing Technology. In The Global Textile and Clothing Industry; Elsevier: Amsterdam, The Netherland, 2012; pp. 113–130. [Google Scholar]
- Kim, S.-C.; Son, J.S. Double-Layered Fiber for Lightweight Flexible Clothing Providing Shielding from Low-Dose Natural Radiation. Sci. Rep. 2021, 11, 3676. [Google Scholar] [CrossRef] [PubMed]
- Šafářová, V.; Militký, J. Multifunctional Metal Composite Textile Shields against Electromagnetic Radiation—Effect of Various Parameters on Electromagnetic Shielding Effectiveness. Polym. Compos. 2017, 38, 309–323. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Yin Yin Nyein, H.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous Sweat Extraction and Analysis Applied to Cystic Fibrosis and Glucose Monitoring Using a Fully Integrated Wearable Platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef]
- Steinberg, C.; Philippon, F.; Sanchez, M.; Fortier-Poisson, P.; O’Hara, G.; Molin, F.; Sarrazin, J.-F.; Nault, I.; Blier, L.; Roy, K.; et al. A Novel Wearable Device for Continuous Ambulatory ECG Recording: Proof of Concept and Assessment of Signal Quality. Biosensors 2019, 9, 17. [Google Scholar] [CrossRef]
- Gao, W.; Brooks, G.A.; Klonoff, D.C. Wearable Physiological Systems and Technologies for Metabolic Monitoring. J. Appl. Physiol. 2018, 124, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Kario, K. Management of Hypertension in the Digital Era. Hypertension 2020, 76, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Hou, L.; Lu, Y. An Integrated Wearable Strain, Temperature and Humidity Sensor for Multifunctional Monitoring. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106504. [Google Scholar] [CrossRef]
- Ilén, E.; Elsehrawy, F.; Palovuori, E.; Halme, J. Washable Textile Embedded Solar Cells for Self-Powered Wearables. Res. J. Text. Appar. 2024, 28, 133–151. [Google Scholar] [CrossRef]
- Fernández-Caramés, T.; Fraga-Lamas, P. Towards The Internet-of-Smart-Clothing: A Review on IoT Wearables and Garments for Creating Intelligent Connected E-Textiles. Electronics 2018, 7, 405. [Google Scholar] [CrossRef]
- Sundriyal, P.; Bhattacharya, S. Textile-Based Supercapacitors for Flexible and Wearable Electronic Applications. Sci. Rep. 2020, 10, 13259. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.M.; Ahuja, K. E-Textiles: A Revolutionary Technology. Int. J. Syst. Assur. Eng. Manag. 2023, 14, 2031–2047. [Google Scholar] [CrossRef]
- Maity, S.; Singha, K.; Pandit, P. Advanced Applications of Green Materials in Wearable E-Textiles. In Applications of Advanced Green Materials; Elsevier: Amsterdam, The Netherland, 2021; pp. 239–263. [Google Scholar]
- Ruckdashel, R.R.; Khadse, N.; Park, J.H. Smart E-Textiles: Overview of Components and Outlook. Sensors 2022, 22, 6055. [Google Scholar] [CrossRef] [PubMed]
- Singha, K.; Kumar, J.; Pandit, P. Recent Advancements in Wearable & Smart Textiles: An Overview. Mater. Today Proc. 2019, 16, 1518–1523. [Google Scholar] [CrossRef]
- Ismar, E.; Kurşun Bahadir, S.; Kalaoglu, F.; Koncar, V. Futuristic Clothes: Electronic Textiles and Wearable Technologies. Glob. Chall. 2020, 4, 1900092. [Google Scholar] [CrossRef]
- Younes, B. Smart E-Textiles: A Review of Their Aspects and Applications. J. Ind. Text. 2023, 53, 15280837231215493. [Google Scholar] [CrossRef]
- Baseri, S.; Zadhoush, A.; Morshed, M.; Amirnasr, M.; Azarnasab, M. Synthesis and Optimization of Copper Sulfide-coated Electrically Conducting Poly(Acrylonitrile) Fibers. J. Appl. Polym. Sci. 2007, 104, 2579–2586. [Google Scholar] [CrossRef]
- Ahn, D.; Choi, H.-J.; Kim, H.; Yeo, S.Y. Properties of Conductive Polyacrylonitrile Fibers Prepared by Using Benzoxazine Modified Carbon Black. Polymers 2020, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Kayabaşı, G.; Özen, Ö.; Yılmaz, D. A Novel Yarn Spinning Method for Fabricating Conductive and Nanofiber-Coated Hybrid Yarns. J. Ind. Text. 2022, 51, 953–976. [Google Scholar] [CrossRef]
- Rehan, M.; Nada, A.A.; Khattab, T.A.; Abdelwahed, N.A.M.; El-Kheir, A.A.A. Development of Multifunctional Polyacrylonitrile/Silver Nanocomposite Films: Antimicrobial Activity, Catalytic Activity, Electrical Conductivity, UV Protection and SERS-Active Sensor. J. Mater. Res. Technol. 2020, 9, 9380–9394. [Google Scholar] [CrossRef]
- Karbownik, I.; Rac-Rumijowska, O.; Fiedot-Toboła, M.; Rybicki, T.; Teterycz, H. The Preparation and Characterization of Polyacrylonitrile-Polyaniline (PAN/PANI) Fibers. Materials 2019, 12, 664. [Google Scholar] [CrossRef] [PubMed]
- Demiroğlu Mustafov, S.; Mohanty, A.K.; Misra, M.; Seydibeyoğlu, M.Ö. Fabrication of Conductive Lignin/PAN Carbon Nanofibers with Enhanced Graphene for the Modified Electrodes. Carbon N. Y. 2019, 147, 262–275. [Google Scholar] [CrossRef]
- Zhang, X.; Shiu, B.; Li, T.-T.; Liu, X.; Ren, H.-T.; Wang, Y.; Lou, C.-W.; Lin, J.-H. Photo-Thermoelectric Nanofiber Film Based on the Synergy of Conjugated Polymer and Light Traps for the Solar-Energy Harvesting of Textile Solar Panel. Sol. Energy Mater. Sol. Cells 2021, 232, 111353. [Google Scholar] [CrossRef]
- Deng, B.; Fang, L.; Fang, K.; Han, X.; Liang, Y. Scalable Preparation of MWCNTs/PAN Conductive Composite Fibers with Tai Chi Structure for Thermotherapy Textiles. Compos. Sci. Technol. 2023, 232, 109866. [Google Scholar] [CrossRef]
- Mukongo Mpukuta, O.; Dincer, K.; Okan Erdal, M. Investigation of Electrical Conductivity of PAN Nanofibers Containing Silica Nanoparticles Produced by Electrospinning Method. Mater. Today Proc. 2019, 18, 1927–1935. [Google Scholar] [CrossRef]
- Zhang, J.; Silcock, G.W.H.; Shields, T.J. Study of the Combustion and Fire Retardancy of Polyacrylonitrile and Its Copolymers by Using Cone Calorimetry. J. Fire Sci. 1995, 13, 141–161. [Google Scholar] [CrossRef]
- Tretsiakova-McNally, S.; Arun, M.; Guerrieri, M.; Joseph, P. An Overview of Some Reactive Routes to Flame-Retardant Fibre-Forming Polymers: Polypropylene and Polyacrylonitrile. Organics 2023, 4, 386–402. [Google Scholar] [CrossRef]
- Horrocks, A.R. Fundamentals: Flammability, Ignition, and Fire Spread in Polymers. In Analysis of Flame Retardancy in Polymer Science; Elsevier: Amsterdam, The Netherland, 2022; pp. 1–72. [Google Scholar]
- Mazumder, N.; Islam, M.T. Flame Retardant Finish for Textile Fibers. In Innovative and Emerging Technologies for Textile Dyeing and Finishing; Wiley: Hoboken, NJ, USA, 2021; pp. 373–405. [Google Scholar]
- Visakh, P.M.; Arao, Y. (Eds.) Flame Retardants; Engineering Materials; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-03466-9. [Google Scholar]
- Mahltig, B.; Grethe, T. High-Performance and Functional Fiber Materials—A Review of Properties, Scanning Electron Microscopy SEM and Electron Dispersive Spectroscopy EDS. Textiles 2022, 2, 209–251. [Google Scholar] [CrossRef]
- Kaneka Chemical Resistance The Advantages of Kanecaron®. Available online: https://www.modacrylic.com/en/about/advantage05 (accessed on 11 July 2024).
- Yusuf, M. A Review on Flame Retardant Textile Finishing: Current and Future Trends. Curr. Smart Mater. 2019, 3, 99–108. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, X.; Liu, P.; Jiang, M.; Xu, J. Flame Retardant Modification of Acrylic Fiber with Hydrazine Hydrate and Sodium Ions. J. Appl. Polym. Sci. 2015, 132, 41996. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, X.; Jiang, M.; Liu, P.; Xu, J. Study of Flame-Resistant Acrylic Fibers Reinforced by Poly(Vinyl Alcohol). J. Appl. Polym. Sci. 2016, 133, 43006. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J. Flame Retardant Multilayered Coatings on Acrylic Fabrics Prepared by One-Step Deposition of Chitosan/Montmorillonite Complexes. Fibers 2018, 6, 36. [Google Scholar] [CrossRef]
- Kim, J.; You, N.-H.; Ku, B.-C. Highly Efficient Halogen-Free Flame Retardants of Thermally-Oxidized Polyacrylonitrile Copolymers Containing Bio-Derived Caffeic Acid Derivatives. Polym. Chem. 2020, 11, 6658–6669. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, Y.; Gu, Y.; Zeng, Q. Flame Retardant Polyacrylonitrile Fabrics Prepared by Organic-Inorganic Hybrid Silica Coating via Sol-Gel Technique. Prog. Org. Coat. 2017, 112, 225–233. [Google Scholar] [CrossRef]
- Yan, X.; Zhou, W.; Zhao, X.; Xu, J.; Liu, P. Preparation, Flame Retardancy and Thermal Degradation Behaviors of Polyacrylonitrile Fibers Modified with Diethylenetriamine and Zinc Ions. J. Therm. Anal. Calorim. 2016, 124, 719–728. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, Q.; Li, L.; Li, Y.; Zhu, M. Flame Retardance Enhancement of Polyacrylonitrile with Dimethyl Vinylphosphonate. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Liu, X.; Huo, T.; Qin, Y. Preparation of Durable Flame Retardant PAN Fabrics Based on Amidoximation and Phosphorylation. Appl. Surf. Sci. 2018, 428, 395–403. [Google Scholar] [CrossRef]
- Ren, Y.; Jiang, L.; Tian, T.; Liu, X.; Han, Z. Durable Flame Retardant Polyacrylonitrile Fabric via UV-Induced Grafting Polymerization and Surface Chemical Modification. RSC Adv. 2018, 8, 41389–41396. [Google Scholar] [CrossRef]
- Zuo, C.; Guo, Y.; Jiang, L.; Yu, D.; Chen, X.; Ren, Y.; Liu, X. Fabrication of Durable Flame Retardant PAN Fibers through Bio-Based Ammonium Phytate Surface Modification and Highly Efficient Thermal Oxidation. Eur. Polym. J. 2023, 196, 112304. [Google Scholar] [CrossRef]
- John, M.J. Flammability Performance of Biocomposites. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 43–58. [Google Scholar]
- Carosio, F.; Alongi, J. Influence of Layer by Layer Coatings Containing Octapropylammonium Polyhedral Oligomeric Silsesquioxane and Ammonium Polyphosphate on the Thermal Stability and Flammability of Acrylic Fabrics. J. Anal. Appl. Pyrolysis 2016, 119, 114–123. [Google Scholar] [CrossRef]
- Schellenberger, S.; Hill, P.J.; Levenstam, O.; Gillgard, P.; Cousins, I.T.; Taylor, M.; Blackburn, R.S. Highly Fluorinated Chemicals in Functional Textiles Can Be Replaced by Re-Evaluating Liquid Repellency and End-User Requirements. J. Clean. Prod. 2019, 217, 134–143. [Google Scholar] [CrossRef]
- Kwon, J.; Jung, H.; Jung, H.; Lee, J. Micro/Nanostructured Coating for Cotton Textiles That Repel Oil, Water, and Chemical Warfare Agents. Polymers 2020, 12, 1826. [Google Scholar] [CrossRef]
- Loghin, C.; Ciobanu, L.; Ionesi, D.; Loghin, E.; Cristian, I. Introduction to Waterproof and Water Repellent Textiles. In Waterproof and Water Repellent Textiles and Clothing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–24. [Google Scholar]
- Whittaker, M.H.; Heine, L. Toxicological and Environmental Issues Associated with Waterproofing and Water Repellent Formulations. In Waterproof and Water Repellent Textiles and Clothing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–120. [Google Scholar]
- Mohsin, M.; Farooq, A.; Abbas, N.; Noreen, U.; Sarwar, N.; Khan, A. Environment Friendly Finishing for the Development of Oil and Water Repellent Cotton Fabric. J. Nat. Fibers 2016, 13, 261–267. [Google Scholar] [CrossRef]
- Ceria, A.; Hauser, P.J. Atmospheric Plasma Treatment to Improve Durability of a Water and Oil Repellent Finishing for Acrylic Fabrics. Surf. Coat. Technol. 2010, 204, 1535–1541. [Google Scholar] [CrossRef]
- Li, P.; Feng, Q.; Chen, L.; Zhao, J.; Lei, F.; Yu, H.; Yi, N.; Gan, F.; Han, S.; Wang, L.; et al. Environmentally Friendly, Durably Waterproof, and Highly Breathable Fibrous Fabrics Prepared by One-Step Fluorine-Free Waterborne Coating. ACS Appl. Mater. Interfaces 2022, 14, 8613–8622. [Google Scholar] [CrossRef]
- Wang, J.; Wei, C.; Yuan, Y.; Shi, Q.; Zhong, D.; Wang, X. UV-Assisted Stabilization of Superhydrophobic Nanofibrous Membranes for Waterproof, Breathable and Thermal Insulation Applications. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132634. [Google Scholar] [CrossRef]
- Zhang, L.; Sheng, J.; Yao, Y.; Yan, Z.; Zhai, Y.; Tang, Z.; Li, H. Fluorine-Free Hydrophobic Modification and Waterproof Breathable Properties of Electrospun Polyacrylonitrile Nanofibrous Membranes. Polymers 2022, 14, 5295. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Quan, Z.; Wang, L.; Zhang, H.; Wang, N.; Qin, X.; Wang, R.; Yu, J. Fiber-Microsphere Binary Structured Composite Fibrous Membranes for Waterproof and Breathable Applications. Fibers Polym. 2022, 23, 1500–1509. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, F.; Liu, Y.; Zheng, Y.; Xin, B.; Jiang, Z.; Peng, X.; Jin, S. Waterproof and Breathable Polyacrylonitrile/(Polyurethane/Fluorinated-Silica) Composite Nanofiber Membrane via Side-by-Side Electrospinning. J. Mater. Res. 2020, 35, 1173–1181. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, C.; Wang, Y.; Wang, H.; Li, N. In-Situ Grown SiO2 on Amino-Silane Modified Polyacrylonitrile Nanofibrous Membranes and Its Waterproof-Breathable and Light-Shielding Properties. Appl. Surf. Sci. 2024, 642, 158536. [Google Scholar] [CrossRef]
- Bashari, A.; Shakeri, M.; Shirvan, A.R. UV-Protective Textiles. In The Impact and Prospects of Green Chemistry for Textile Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 327–365. [Google Scholar]
- Hassan, M.M.; Koyama, K. Multifunctional Acrylic Fibers Prepared via In-Situ Formed Silver Nanoparticles: Physicochemical, UV Radiation Protection, and Antistatic Properties. Dye. Pigment. 2018, 159, 517–526. [Google Scholar] [CrossRef]
- Kocić, A.; Bizjak, M.; Popović, D.; Poparić, G.B.; Stanković, S.B. UV Protection Afforded by Textile Fabrics Made of Natural and Regenerated Cellulose Fibres. J. Clean. Prod. 2019, 228, 1229–1237. [Google Scholar] [CrossRef]
- Yang, H.; Zhu, S.; Pan, N. Studying the Mechanisms of Titanium Dioxide as Ultraviolet-Blocking Additive for Films and Fabrics by an Improved Scheme. J. Appl. Polym. Sci. 2004, 92, 3201–3210. [Google Scholar] [CrossRef]
- Nazari, A.; Montazer, M. Durable Multifunctional Properties on Acrylic Fabric Using Nano TiO2 and Polysiloxane. Fibers Polym. 2014, 15, 698–706. [Google Scholar] [CrossRef]
- Syafiuddin, A. Toward a Comprehensive Understanding of Textiles Functionalized with Silver Nanoparticles. J. Chin. Chem. Soc. 2019, 66, 793–814. [Google Scholar] [CrossRef]
- Singh, A.; Sheikh, J. Synthesis of a Novel Cationic Dye to Impart Mosquito-Repellent and UV Protection to an Acrylic Fabric. ACS Omega 2023, 8, 10214–10224. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dong, K.; An, J.; Liang, F.; Yi, J.; Peng, X.; Ning, C.; Ye, C.; Wang, Z.L. UV-Protective, Self-Cleaning, and Antibacterial Nanofiber-Based Triboelectric Nanogenerators for Self-Powered Human Motion Monitoring. ACS Appl. Mater. Interfaces 2021, 13, 11205–11214. [Google Scholar] [CrossRef] [PubMed]
- Koozekonan, A.G.; Esmaeilpour, M.R.M.; Kalantary, S.; Karimi, A.; Azam, K.; Moshiran, V.A.; Golbabaei, F. Fabrication and Characterization of PAN/CNT, PAN/TiO2, and PAN/CNT/TiO2 Nanofibers for UV Protection Properties. J. Text. Inst. 2021, 112, 946–954. [Google Scholar] [CrossRef]
- Nasouri, K.; Shoushtari, A.M.; Mirzaei, J.; Merati, A.A. Synthesis of Carbon Nanotubes Composite Nanofibers for Ultrahigh Performance UV Protection and Microwave Absorption Applications. Diam. Relat. Mater. 2020, 107, 107896. [Google Scholar] [CrossRef]
- Naebe, M.; Haque, A.N.M.A.; Haji, A. Plasma-Assisted Antimicrobial Finishing of Textiles: A Review. Engineering 2022, 12, 145–163. [Google Scholar] [CrossRef]
- Saleem, M.; Naz, M.Y.; Shoukat, B.; Shukrullah, S.; Hussain, Z. Functionality and Applications of Non-Thermal Plasma Activated Textiles: A Review. Mater. Today Proc. 2021, 47, S74–S82. [Google Scholar] [CrossRef]
- Zille, A.; Oliveira, F.R.; Souto, A.P. Plasma Treatment in Textile Industry. Plasma Process. Polym. 2015, 12, 98–131. [Google Scholar] [CrossRef]
- Jelil, R.A. A Review of Low-Temperature Plasma Treatment of Textile Materials. J. Mater. Sci. 2015, 50, 5913–5943. [Google Scholar] [CrossRef]
- Pane, S.; Tedesco, R.; Greger, R. Acrylic Fabrics Treated with Plasma for Outdoor Applications. J. Ind. Text. 2001, 31, 135–145. [Google Scholar] [CrossRef]
- Haji, A.; Naebe, M. Cleaner Dyeing of Textiles Using Plasma Treatment and Natural Dyes: A Review. J. Clean. Prod. 2020, 265, 121866. [Google Scholar] [CrossRef]
- Kil, H.-S.; Lee, S. High Flame Retardancy of Oxidized Polyacrylonitrile Fibers Prepared by Effective Plasma-Assisted Thermal Stabilization and Electron-Beam Irradiation. Compos. Part B Eng. 2019, 178, 107458. [Google Scholar] [CrossRef]
- Sfameni, S.; Hadhri, M.; Rando, G.; Drommi, D.; Rosace, G.; Trovato, V.; Plutino, M.R. Inorganic Finishing for Textile Fabrics: Recent Advances in Wear-Resistant, UV Protection and Antimicrobial Treatments. Inorganics 2023, 11, 19. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in Sol-Gel Technology for the Coatings of Fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef]
- Ismail, W.N.W. Sol–Gel Technology for Innovative Fabric Finishing—A Review. J. Sol-Gel Sci. Technol. 2016, 78, 698–707. [Google Scholar] [CrossRef]
- Ren, Y.; Huo, T.; Qin, Y.; Liu, X. Preparation of Flame Retardant Polyacrylonitrile Fabric Based on Sol-Gel and Layer-by-Layer Assembly. Materials 2018, 11, 483. [Google Scholar] [CrossRef]
- Ying, Z.-Y.; Shao, Z.-D.; Wang, L.; Cheng, X.; Zheng, Y.-M. Sol–Gel SiO2 on Electrospun Polyacrylonitrile Nanofiber for Efficient Oil-in-Water Emulsion Separation. J. Mater. Sci. 2020, 55, 16129–16142. [Google Scholar] [CrossRef]
- De Smet, D.; Goethals, F.; Demedts, B.; Uyttendaele, W.; Vanneste, M. Bio-Based Textile Coatings and Composites. In Biobased Products and Industries; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–402. [Google Scholar]
- Hahn, T.; Bossog, L.; Hager, T.; Wunderlich, W.; Breier, R.; Stegmaier, T.; Zibek, S. Chitosan Application in Textile Processing and Fabric Coating. In Chitin and Chitosan; Wiley: Hoboken, NJ, USA, 2019; pp. 395–428. [Google Scholar]
- Lou, T.; Yan, X.; Wang, X. Chitosan Coated Polyacrylonitrile Nanofibrous Mat for Dye Adsorption. Int. J. Biol. Macromol. 2019, 135, 919–925. [Google Scholar] [CrossRef]
- Guo, Y.; Zuo, C.; Liu, Y.; Chen, X.; Ren, Y.; Liu, X. Construction of a Fully Bio-Based Intumescent Flame Retardant for Improving the Flame Retardancy of Polyacrylonitrile. Polym. Degrad. Stab. 2023, 214, 110385. [Google Scholar] [CrossRef]
- Ren, Y.; Qin, Y.; Liu, X.; Huo, T.; Jiang, L.; Tian, T. Flame-retardant Polyacrylonitrile Fabric Prepared by Ultraviolet-induced Grafting with Glycidyl Methacrylate Followed by Ammoniation and Phosphorylation. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Nazri, N.A.M.; Lau, W.J.; Ismail, A.F.; Matsuura, T.; Veerasamy, D.; Hilal, N. Performance of PAN-Based Membranes with Graft Copolymers Bearing Hydrophilic PVA and PAN Segments in Direct Ultrafiltration of Natural Rubber Effluent. Desalination 2015, 358, 49–60. [Google Scholar] [CrossRef]
- Ren, Y.; Tian, T.; Jiang, L.; Guo, Y. Fabrication of Chitosan-Based Intumescent Flame Retardant Coating for Improving Flame Retardancy of Polyacrylonitrile Fabric. Molecules 2019, 24, 3749. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Ren, Y.; Zhang, G.; Liu, X.; Qu, H. Biomaterial Arginine Encountering with UV Grafting Technology to Prepare Flame Retardant Coating for Polyacrylonitrile Fabric. Prog. Org. Coat. 2022, 163, 106599. [Google Scholar] [CrossRef]
- Basuoni, A.S.; Sabaa, M.W.; Mohamed, R.R.; El-Sayed, H.Z. An Eco-Friendly Strategy for Grafting Keratin onto Polyacrylonitrile Fabrics Using Bi-Functional Amino Acids as Coupling Agents. J. Adhes. Sci. Technol. 2024, 38, 861–876. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Y.; Huang, Y.; Chen, W.; Dong, X. Surface Modification Method of Polyacrylonitrile (PAN) Fibers by L-Cysteine Coupling Protein. Fibers Polym. 2019, 20, 2581–2586. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Yang, J.; Ji, M.; Yu, J.; Wang, M.; Chai, X.; Yang, B.; Zhu, C.; Xu, J. Preparation, Stabilization and Carbonization of a Novel Polyacrylonitrile-Based Carbon Fiber Precursor. Polymers 2019, 11, 1150. [Google Scholar] [CrossRef]
- Morris, E.A.; Weisenberger, M.C.; Abdallah, M.G.; Vautard, F.; Grappe, H.; Ozcan, S.; Paulauskas, F.L.; Eberle, C.; Jackson, D.; Mecham, S.J.; et al. High Performance Carbon Fibers from Very High Molecular Weight Polyacrylonitrile Precursors. Carbon N. Y. 2016, 101, 245–252. [Google Scholar] [CrossRef]
- Cipriani, E.; Zanetti, M.; Bracco, P.; Brunella, V.; Luda, M.P.; Costa, L. Crosslinking and Carbonization Processes in PAN Films and Nanofibers. Polym. Degrad. Stab. 2016, 123, 178–188. [Google Scholar] [CrossRef]
- Xu, W.; Xin, B.; Yang, X. Carbonization of Electrospun Polyacrylonitrile (PAN)/Cellulose Nanofibril (CNF) Hybrid Membranes and Its Mechanism. Cellulose 2020, 27, 3789–3804. [Google Scholar] [CrossRef]
- Sabantina, L.; Böttjer, R.; Wehlage, D.; Grothe, T.; Klöcker, M.; García-Mateos, F.J.; Rodríguez-Mirasol, J.; Cordero, T.; Ehrmann, A. Morphological Study of Stabilization and Carbonization of Polyacrylonitrile/TiO2 Nanofiber Mats. J. Eng. Fibers Fabr. 2019, 14, 155892501986224. [Google Scholar] [CrossRef]
- Langhals, H. Color Chemistry. Synthesis, Properties and Applications of Organic Dyes and Pigments. 3rd Revised Edition. By Heinrich Zollinger. Angew. Chem. Int. Ed. 2004, 43, 5291–5292. [Google Scholar] [CrossRef]
- Burkinshaw, S.M. Acrylic BT. In Chemical Principles of Synthetic Fibre Dyeing; Burkinshaw, S.M., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 157–193. ISBN 978-94-011-0593-4. [Google Scholar]
- Hou, A.; Chen, H.; Xu, Y.; Yang, X.; Zhang, Y.; Xie, K.; Gao, A. Rapid and Environmental-Friendly Continuous Gel-Dyeing of Polyacrylonitrile Fiber with Cationic Dyes. J. Clean. Prod. 2020, 274, 122935. [Google Scholar] [CrossRef]
- Esmaeilian, N.; Malek, R.M.A.; Arami, M.; Mazaheri, F.M.; Dabir, B. Environmentally Friendly Sugar-Based Cationic Surfactant as a New Auxiliary in Polyacrylonitrile Dyeing. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 103–109. [Google Scholar] [CrossRef]
- Popescu, V.; Buciscanu, I.I.; Pruneanu, M.; Maier, S.S.; Danila, A.; Maier, V.; Pîslaru, M.; Rotaru, V.; Cristian, I.N.; Popescu, A.; et al. Sustainable Functionalization of PAN to Improve Tinctorial Capacity. Polymers 2021, 13, 3665. [Google Scholar] [CrossRef]
- Ma, M.; Sun, G. Antimicrobial Cationic Dyes. Part 3: Simultaneous Dyeing and Antimicrobial Finishing of Acrylic Fabrics. Dye. Pigment. 2005, 66, 33–41. [Google Scholar] [CrossRef]
- Abedi, D.; Mortazavi, S.M.; Mehrizi, M.K.; Feiz, M. Antimicrobial Properties of Acrylic Fabrics Dyed with Direct Dye and a Copper Salt. Text. Res. J. 2008, 78, 311–319. [Google Scholar] [CrossRef]
- Khajeh Mehrizi, M.; Mortazavi, S.M.; Abedi, D. The Antimicrobial Characteristic Study of Acrylic Fiber Treated with Metal Salts and Direct Dyes. Fibers Polym. 2009, 10, 601–605. [Google Scholar] [CrossRef]
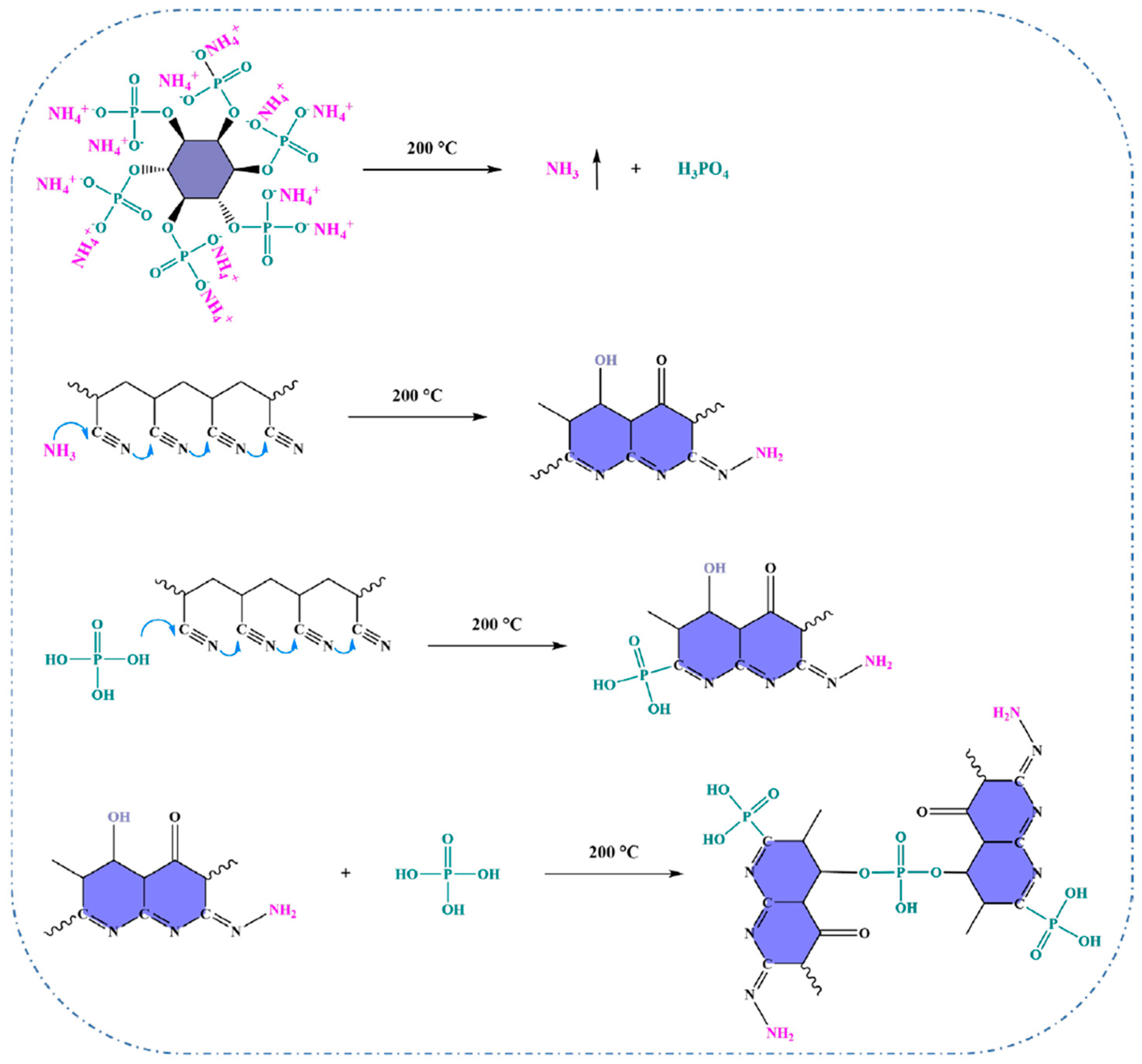
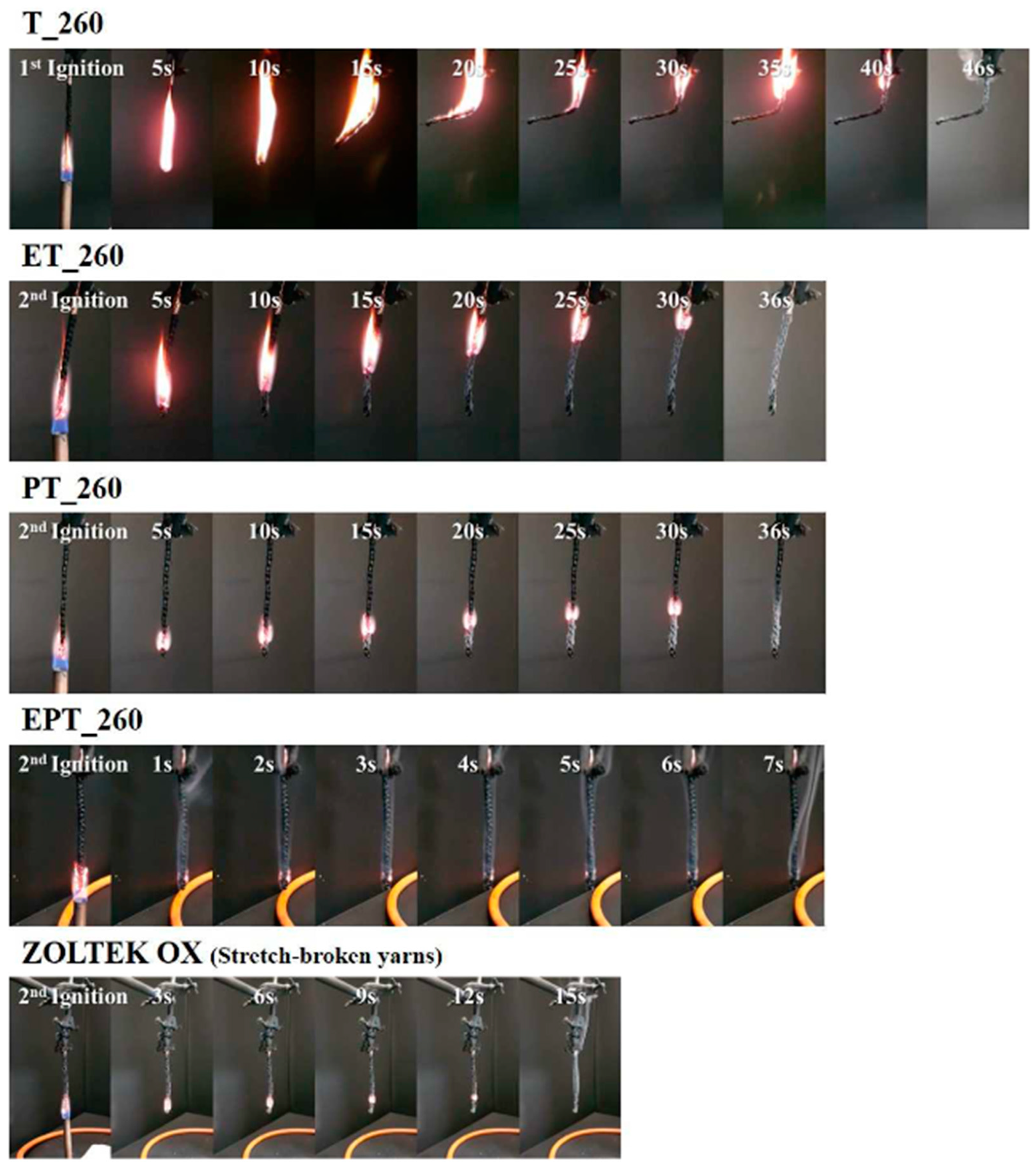

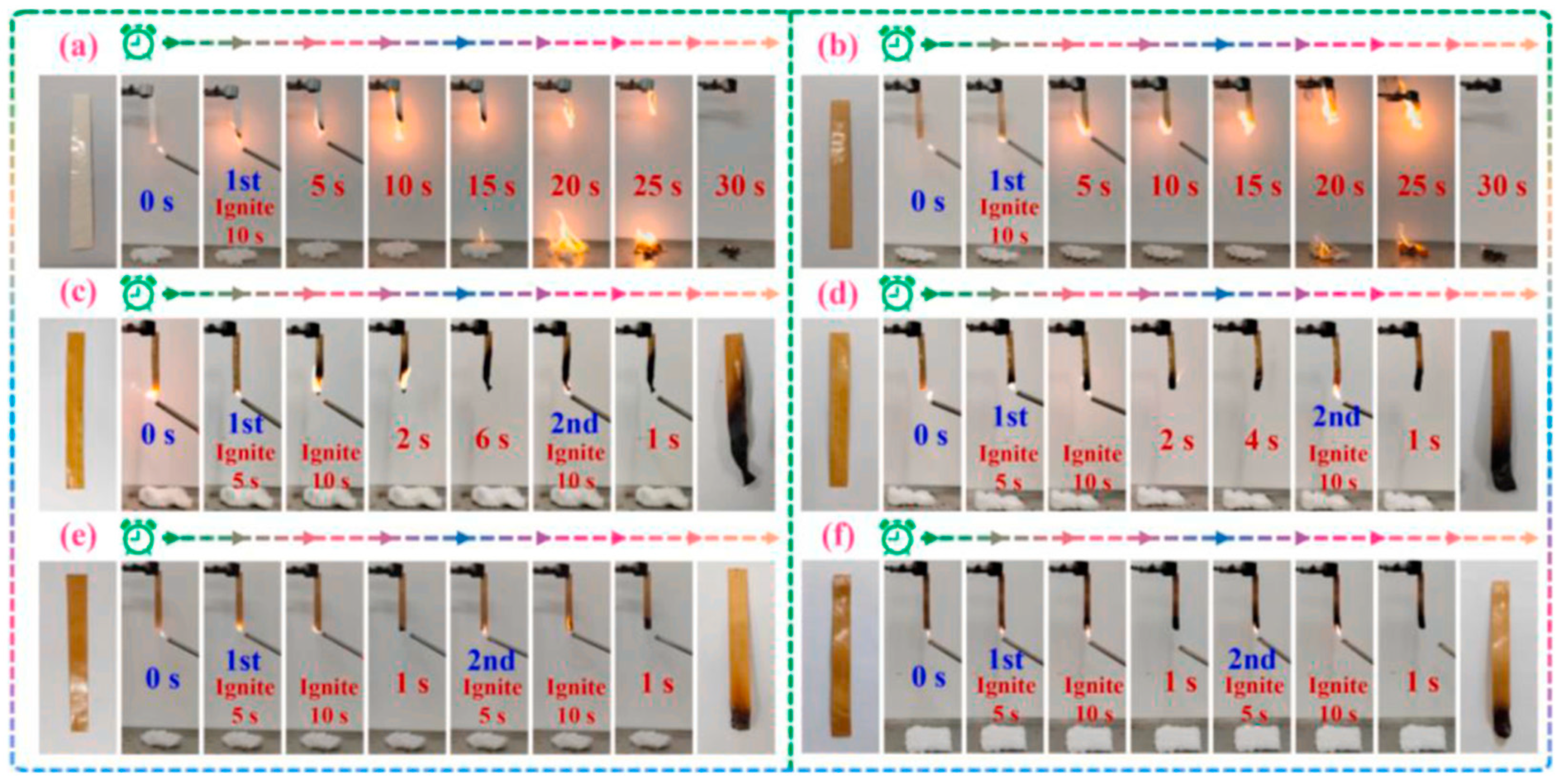
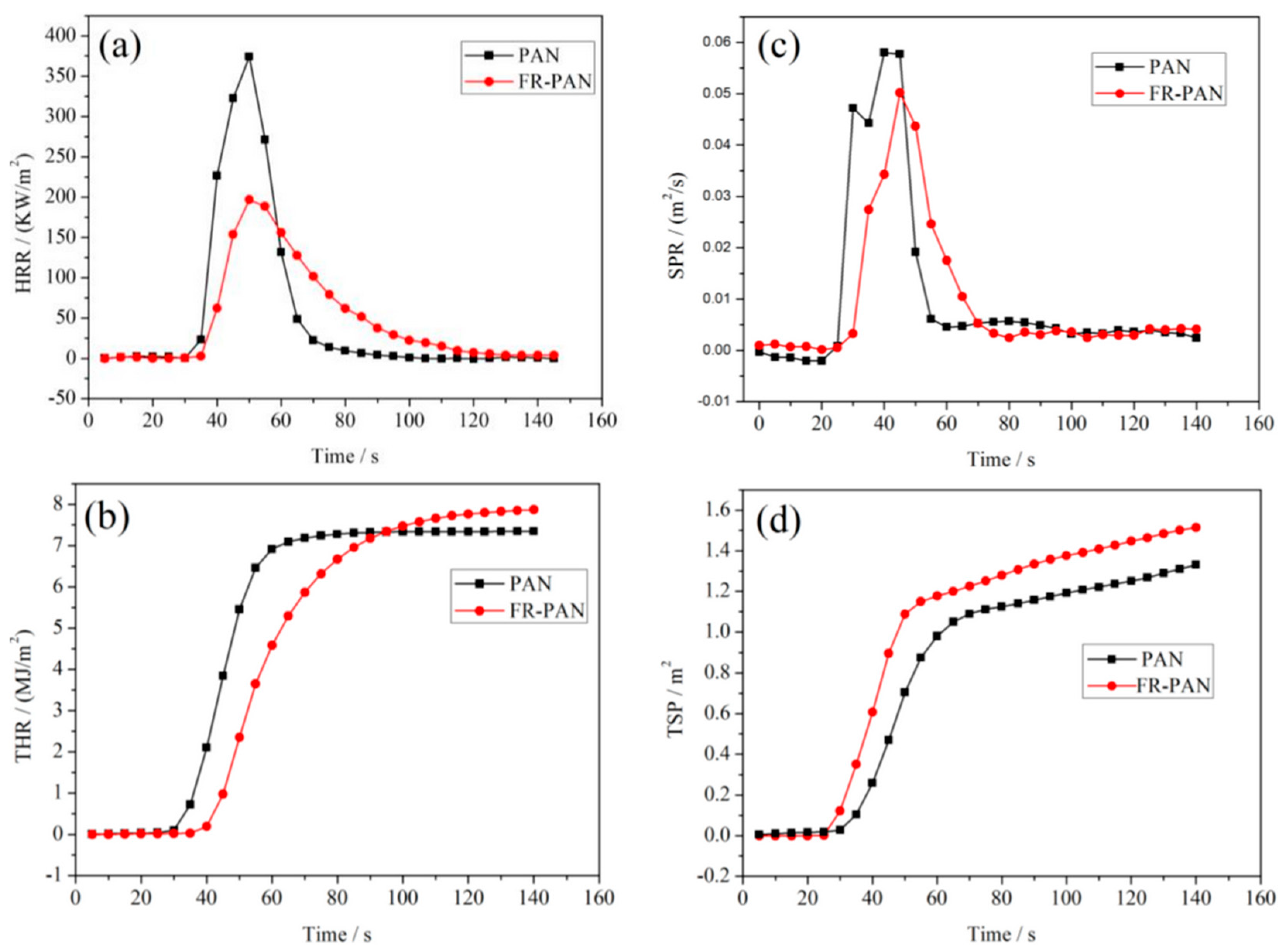
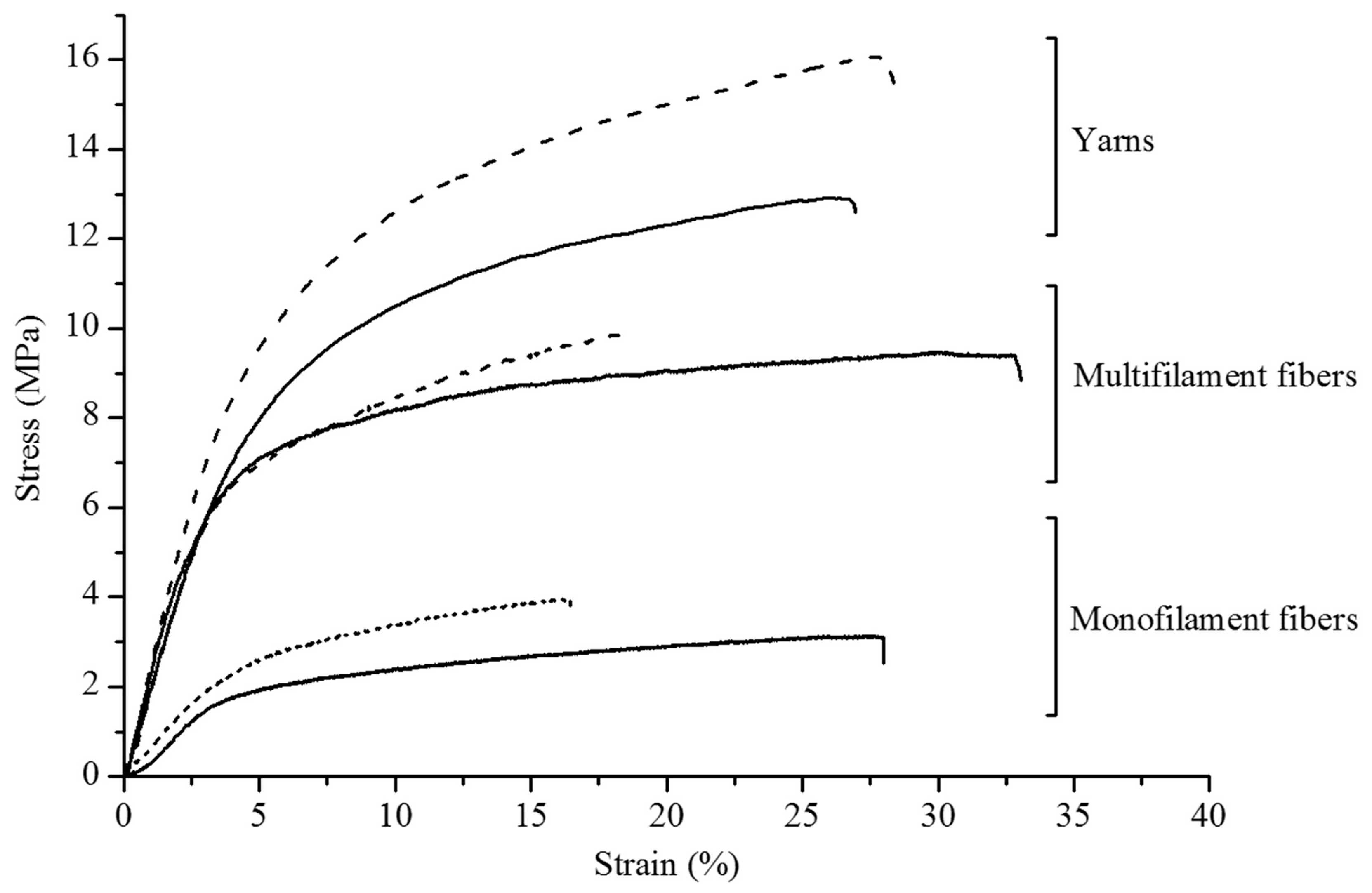

| Author | Method | Char Residue in Air (%) | Char Residue in N2 (%) | LOI Improvement (%) | Ref. |
|---|---|---|---|---|---|
| Zhou et al. | Immersion in HHA and NaOH | 46.3 a | - | 163 | [82] |
| Zhou et al. | Fibre reinforcement with PVA and immersion in HHA and NaOH | - | - | 53.2 | [83] |
| Carosio et al. | Coating with chitosan and montmorillonite | 12.0 b | 53.0 b | 10 | [84] |
| Kim et al. | Copolymerisation of PAN with methyl caffeate | - | 46.7 c | 24.8 | [85] |
| Ren et al. | Sol-gel between PAN fibres and TEOS and urea | 23.4 b | 55.9 b | 89.4 | [86] |
| Yan et al. | Immersion in diethylenetriamine and zinc sulphate | - | 37.5 a | 161.1 | [87] |
| Dong et al. | Copolymerisation of PAN with dimethyl vinylphosphonate (DMVP) | 44.9 a | 67.1 a | 61.3 | [88] |
| Zhang et al. | Amidoximation using hydroxylamine hydrochloride (HA) followed by phosphorylation with phosphoric acid (PA) | - | 55.7 b | 88.4 | [89] |
| Ren et al. | UV grafting of PAN fibres with glycidyl methacrylate (GMA) | 61.6 b | - | 90% | [90] |
| Zou et al. | Soaked in ammonium phytate solution followed by thermal oxidation | 9.29 b | 72.35 b | 36.6 | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, R.P.; Rosace, G.; Trovato, V. Recent Advancements in Acrylic Fabric Applications: A Comprehensive Review and Future Trends. Polymers 2024, 16, 2111. https://doi.org/10.3390/polym16152111
Rosa RP, Rosace G, Trovato V. Recent Advancements in Acrylic Fabric Applications: A Comprehensive Review and Future Trends. Polymers. 2024; 16(15):2111. https://doi.org/10.3390/polym16152111
Chicago/Turabian StyleRosa, Raphael Palucci, Giuseppe Rosace, and Valentina Trovato. 2024. "Recent Advancements in Acrylic Fabric Applications: A Comprehensive Review and Future Trends" Polymers 16, no. 15: 2111. https://doi.org/10.3390/polym16152111






