Modulation of Interlayer Nanochannels via the Moderate Heat Treatment of Graphene Oxide Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
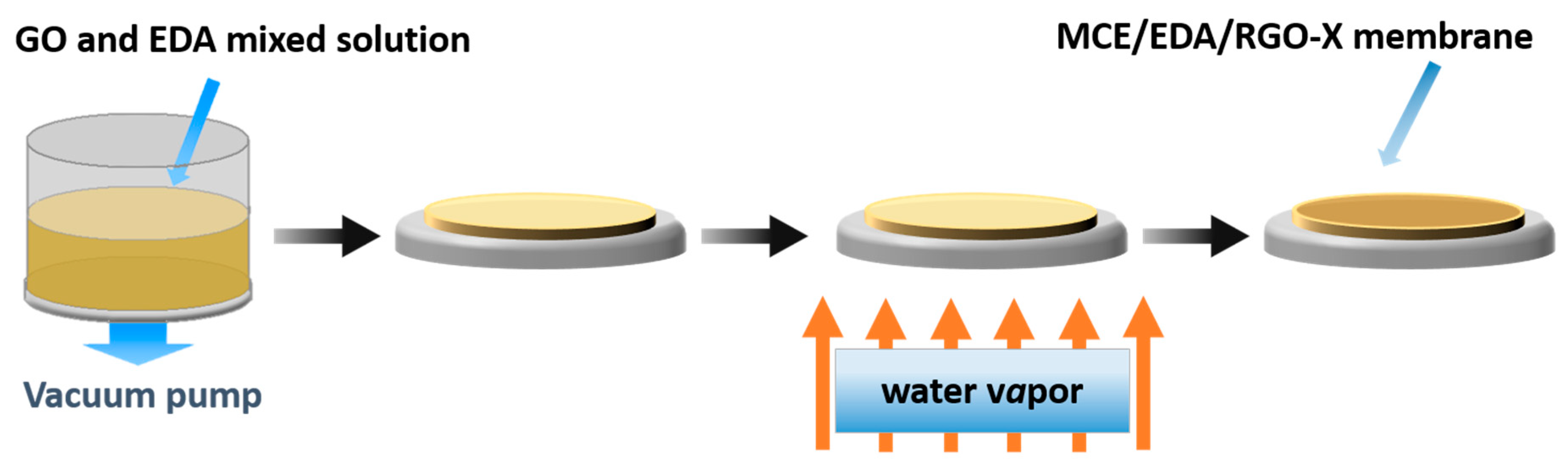
2.2. Preparation of MCE/EDA/RGO Composite Membranes
2.3. Characterization of MCE/EDA/RGO Membranes
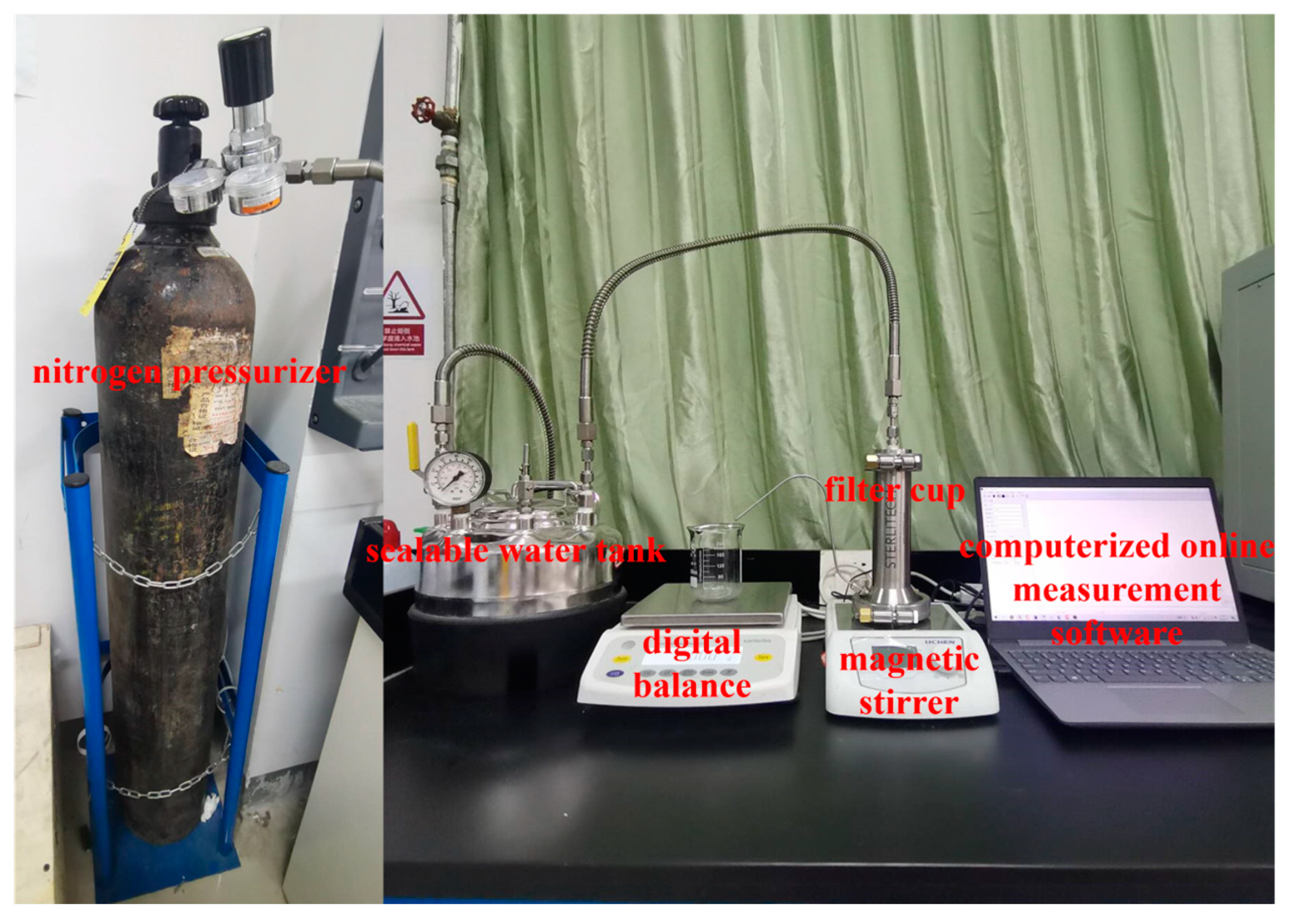
2.4. Evaluation of Membrane Performance
3. Results and Discussion
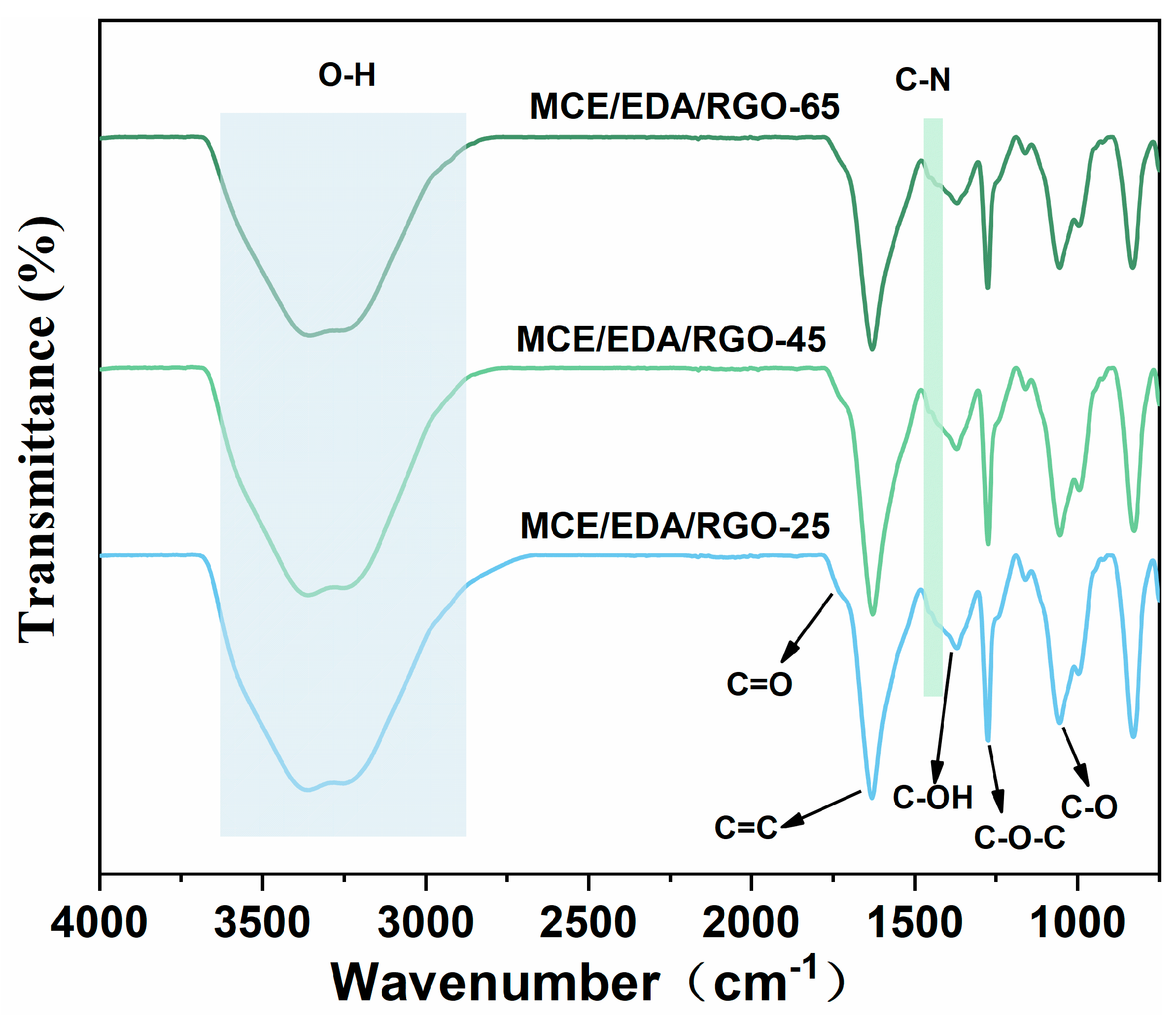
3.1. FTIR Characterization of MCE/EDA/RGO Membranes
3.2. XPS Characterization of MCE/EDA/RGO Membranes
3.3. Raman Testing of MCE/EDA/RGO Membranes
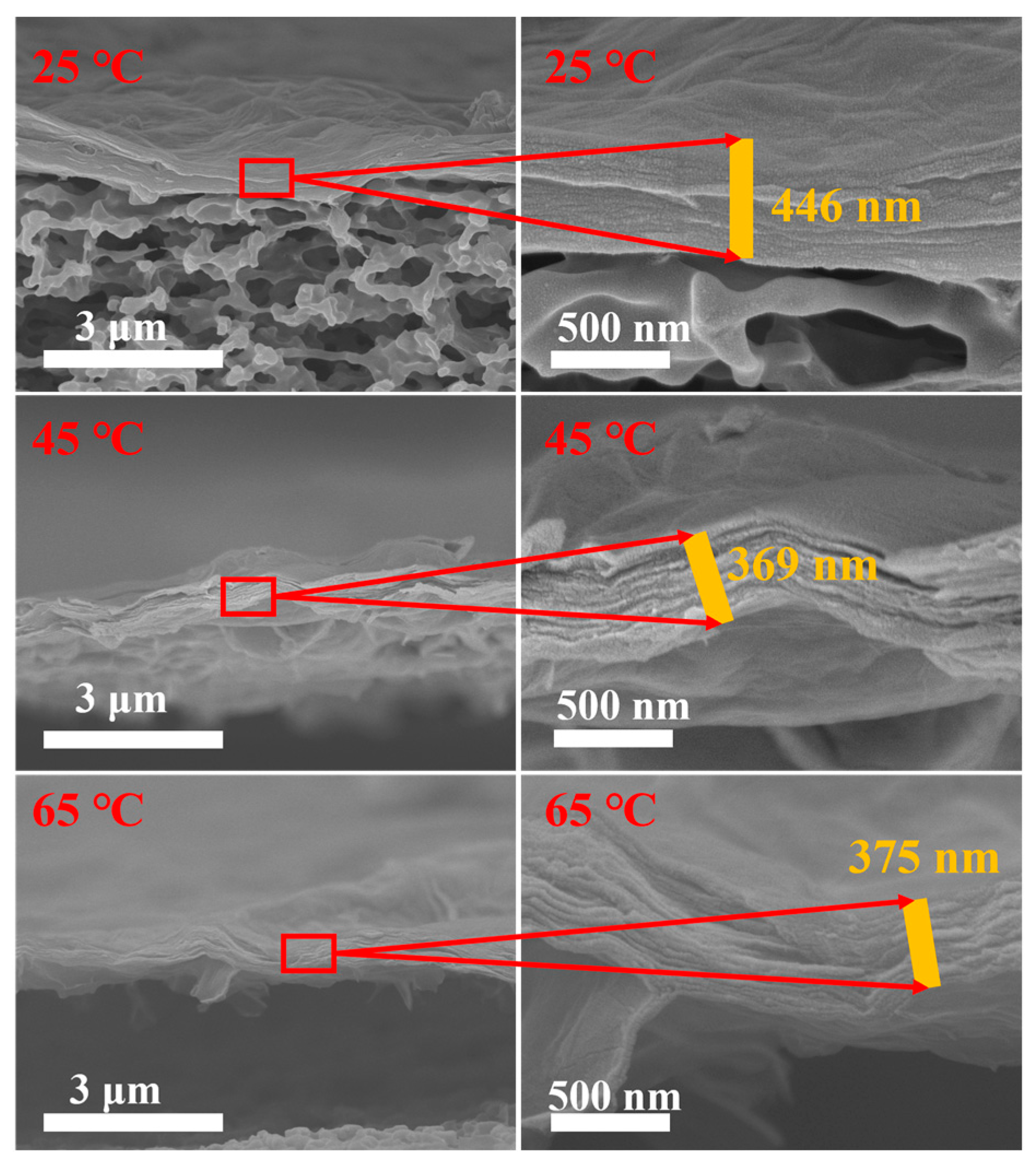
3.4. SEM Characterization of MCE/EDA/RGO Membranes
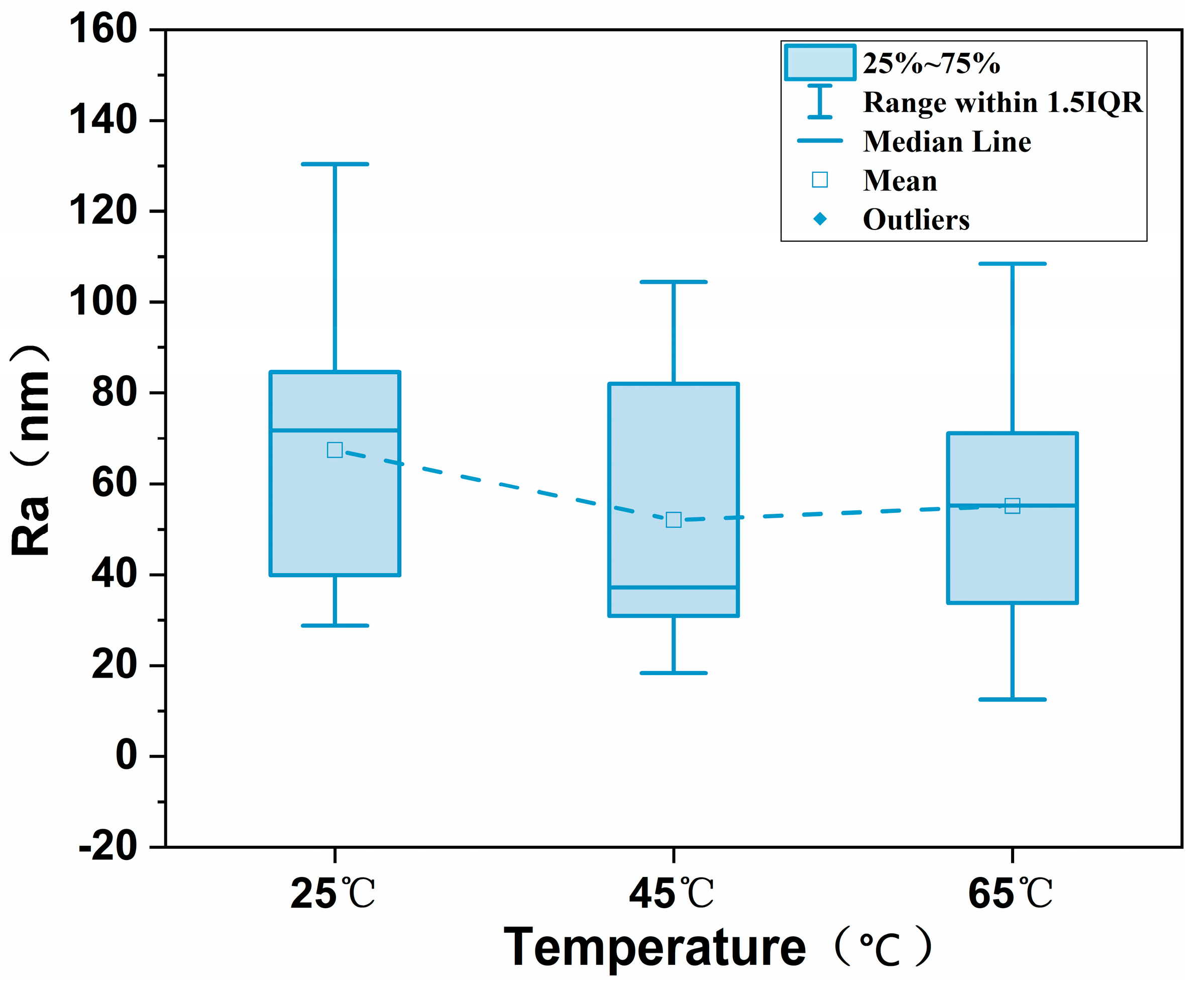
3.5. AFM Characterization of MCE/EDA/RGO Membranes
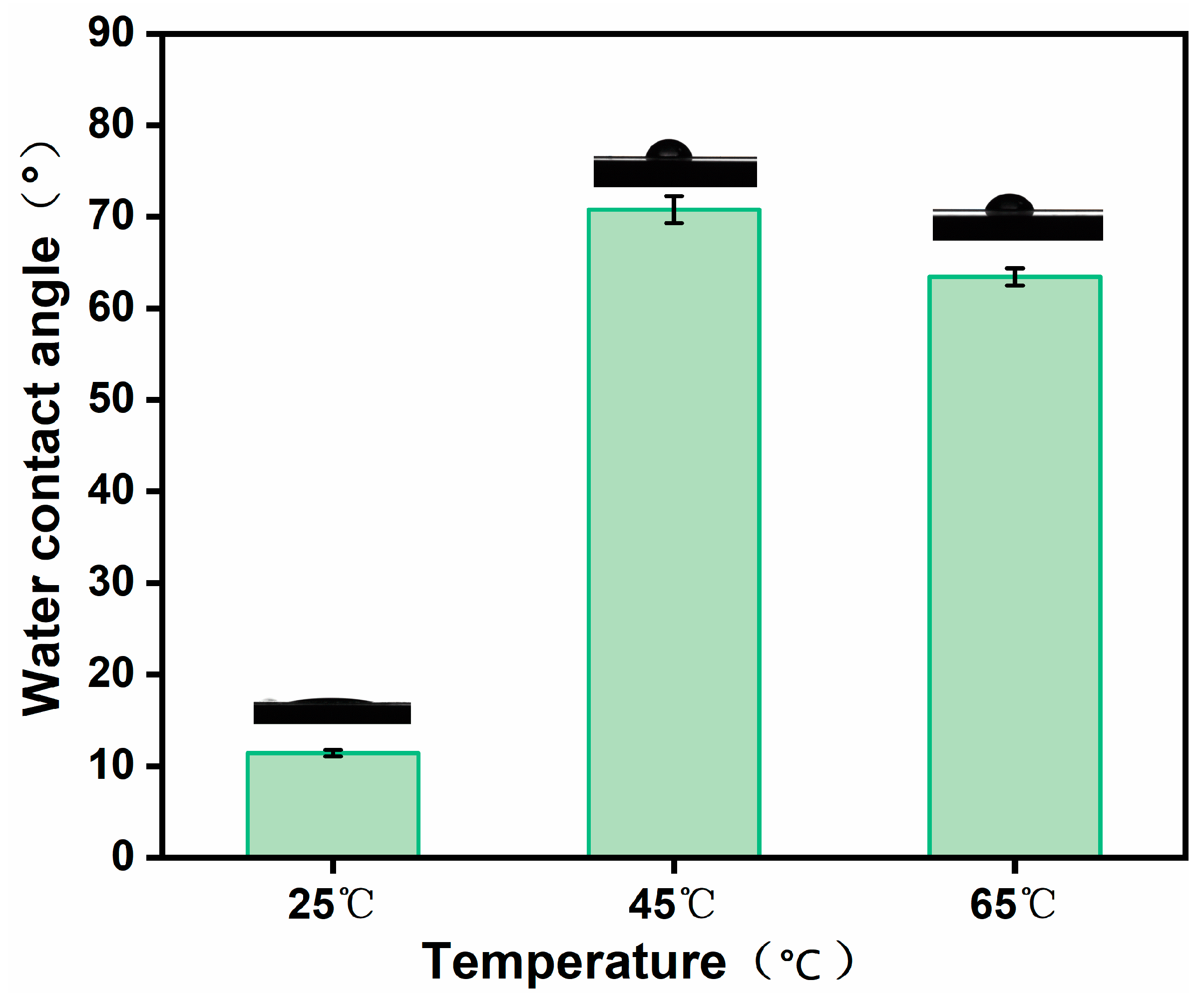
3.6. Water Contact Angle Testing of MCE/EDA/RGO Membranes
3.7. XRD Characterization of MCE/EDA/RGO Membranes
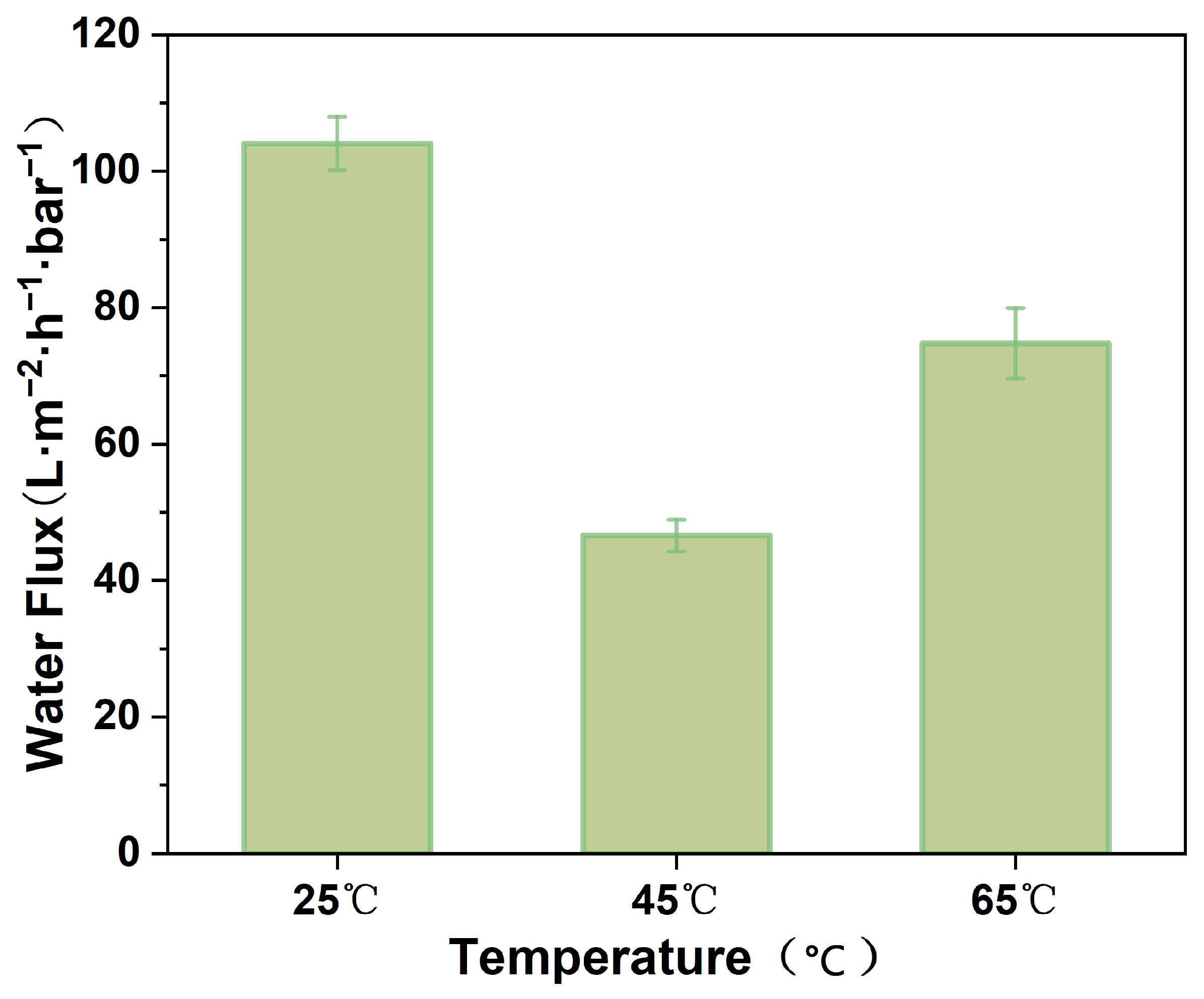
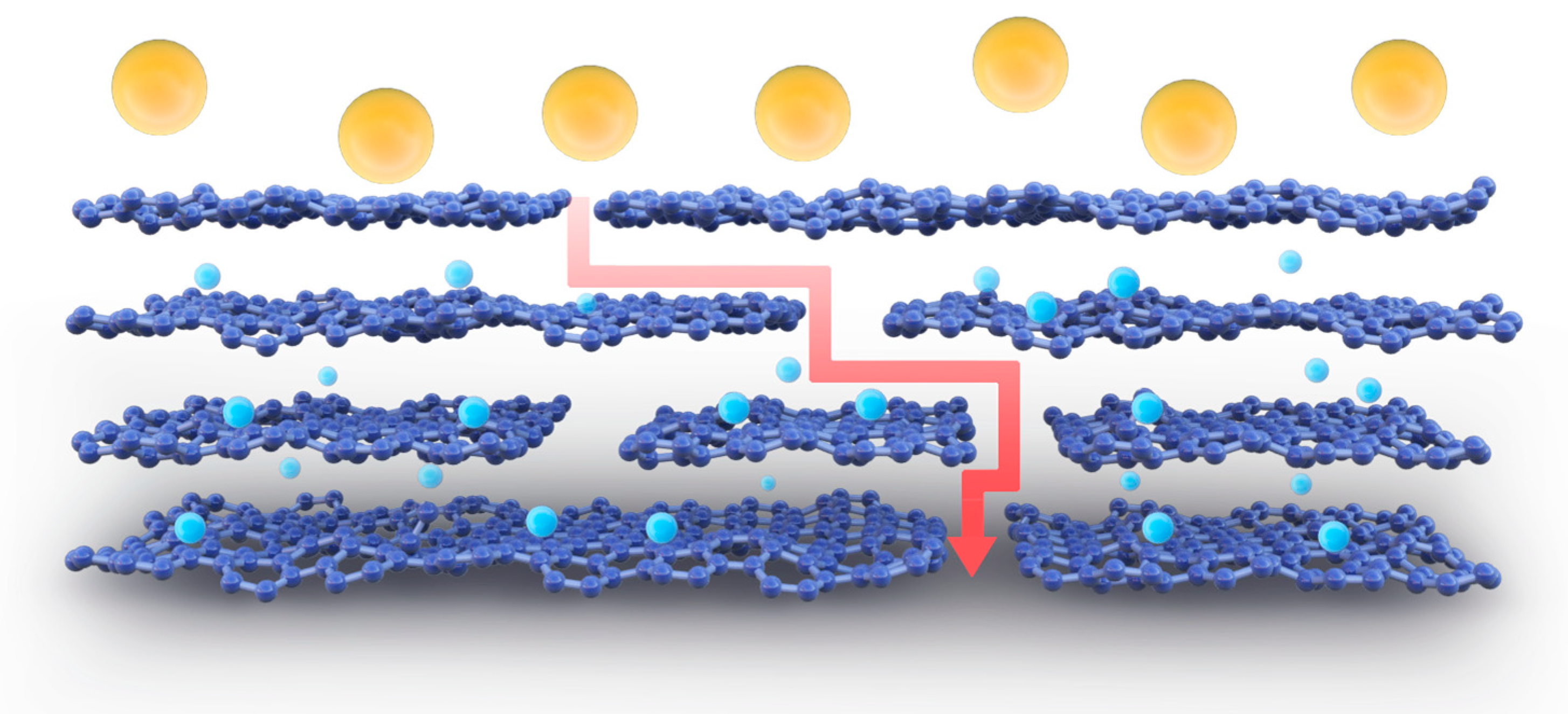
3.8. Permeability of MCE/EDA/RGO Membranes
3.9. Desalination Performance of MCE/EDA/RGO Membranes
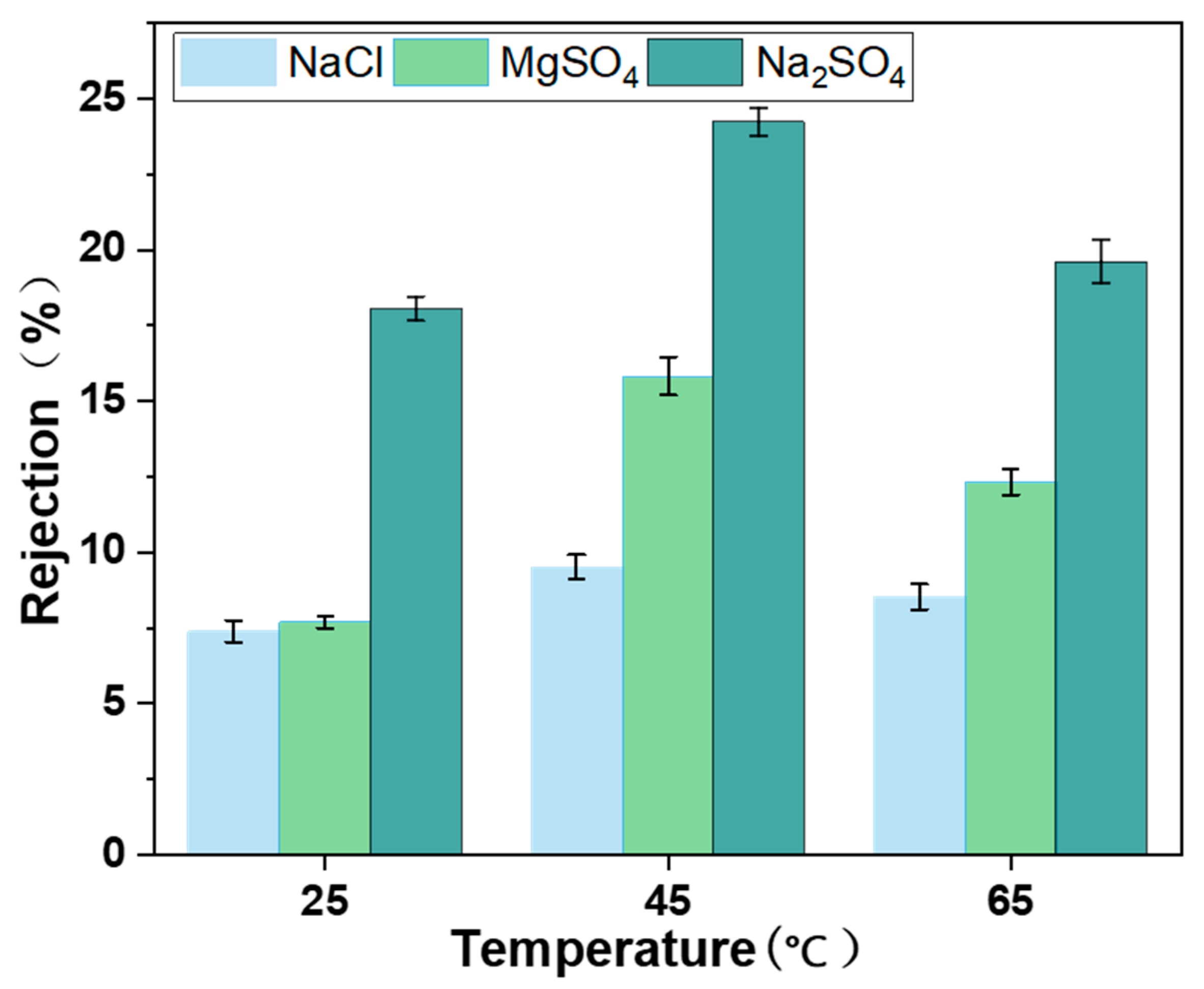
3.10. Decontamination Performance of MCE/EDA/RGO Membranes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, N.; Qi, W.; Huang, L.; Jiang, E.; Bao, J.; Zhang, X.; An, B.; He, G. Review on structural control and modification of graphene oxide-based membranes in water treatment: From separation performance to robust operation. Chin. J. Chem. Eng. 2019, 27, 1348–1360. [Google Scholar] [CrossRef]
- Cheng, X.; Lai, C.; Zhu, X.; Shao, S.; Xu, J.; Zhang, F.; Song, J.; Wu, D.; Liang, H.; Luo, X. Tailored ultra-low pressure nanofiltration membranes for advanced drinking water treatment. Desalination 2023, 548, 116264. [Google Scholar] [CrossRef]
- Miao, J.; Hu, Q.; Zhang, Z.; Hu, Y. Bioinspired cell membrane-based nanofiltration membranes with hierarchical pore channels for fast molecular separation. J. Membr. Sci. 2024, 697, 122578. [Google Scholar] [CrossRef]
- Figueira, M.; Rodríguez-Jiménez, D.; López, J.; Reig, M.; Cortina, J.L.; Valderrama, C. Evaluation of the nanofiltration of brines from seawater desalination plants as pre-treatment in a multimineral brine extraction process. Sep. Purif. Technol. 2023, 322, 124232. [Google Scholar] [CrossRef]
- Kang, Y.; Xia, Y.; Wang, H.; Zhang, X. 2D laminar membranes for selective water and ion transport. Adv. Funct. Mater. 2019, 29, 1902014. [Google Scholar] [CrossRef]
- Ma, J.; Ping, D.; Dong, X. Recent developments of graphene oxide-based membranes: A review. Membranes 2017, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mao, B.; Zhao, M.; Calatayud, D.G.; Qian, W.; Li, P.; Hu, Z.; Fu, H.; Zhao, X.; Yan, S. Ultrafast macroscopic assembly of high-strength graphene oxide membranes by implanting an interlaminar superhydrophilic aisle. ACS Nano 2022, 16, 3934–3942. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, W.; Weyland, M.; Yuan, S.; Xia, Y.; Liu, H.; Jian, M.; Yang, J.; Easton, C.D.; Selomulya, C. Thermally reduced nanoporous graphene oxide membrane for desalination. Environ. Sci. Technol. 2019, 53, 8314–8323. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, M.; Wang, B.; Yang, F.; Quan, X.; Tang, C.Y.; Dong, Y. Cross-linked graphene oxide framework membranes with robust nano-channels for enhanced sieving ability. Environ. Sci. Technol. 2020, 54, 15442–15453. [Google Scholar] [CrossRef]
- Gogoi, A.; Reddy, K.A.; Mondal, P.K. Influence of the presence of cations on the water and salt dynamics inside layered graphene oxide (GO) membranes. Nanoscale 2020, 12, 7273–7283. [Google Scholar] [CrossRef]
- Huang, H.-H.; Joshi, R.K.; De Silva, K.K.H.; Badam, R.; Yoshimura, M. Fabrication of reduced graphene oxide membranes for water desalination. J. Membr. Sci. 2019, 572, 12–19. [Google Scholar] [CrossRef]
- Fan, X.; Cai, C.; Gao, J.; Han, X.; Li, J. Hydrothermal reduced graphene oxide membranes for dyes removing. Sep. Purif. Technol. 2020, 241, 116730. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, X.; Thebo, K.H.; Cheng, H.-M.; Ren, W. Controlling reduction degree of graphene oxide membranes for improved water permeance. Sci. Bull. 2018, 63, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Ham, M.-H.; Park, H.B.; Kim, C.-M.; Song, J.-H.; Kim, I.S. Tunable semi-permeability of graphene-based membranes by adjusting reduction degree of laminar graphene oxide layer. J. Membr. Sci. 2018, 547, 73–79. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Song, B.; Fang, S.; Zhang, M.; Liu, H.-Y.; Huan, S.-Y.; Peng, P.; Niu, Q.-Y. Enhanced permeability of rGO/S-GO layered membranes with tunable inter-structure for effective rejection of salts and dyes. Sep. Purif. Technol. 2019, 220, 309–319. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Fan, X.; Wang, J.; Yuan, G.; Song, X.; Chen, J.; Li, Z. Study on the separation performance of the multi-channel reduced graphene oxide membranes. Appl. Surf. Sci. 2016, 384, 279–286. [Google Scholar] [CrossRef]
- Hung, W.-S.; Tsou, C.-H.; De Guzman, M.; An, Q.-F.; Liu, Y.-L.; Zhang, Y.-M.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Cross-linking with diamine monomers to prepare composite graphene oxide-framework membranes with varying d-spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, K.; Li, Z. Graphene oxide composite membranes cross-linked with urea for enhanced desalting properties. J. Membr. Sci. 2018, 563, 718–725. [Google Scholar] [CrossRef]
- Huang, H.-H.; De Silva, K.K.H.; Kumara, G.; Yoshimura, M. Structural evolution of hydrothermally derived reduced graphene oxide. Sci. Rep. 2018, 8, 6849. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Y. Covalently crosslinked graphene oxide membranes by esterification reactions for ions separation. J. Mater. Chem. A 2015, 3, 4405–4412. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-X.; Liu, Q.; You, L.; Lu, P.; Liu, T.; Sun, G.-D.; Wang, Y.; Chen, J.-F. Facile preparation of dopamine mediated graphene oxide composite membranes with enhanced stability for nanofiltration: Structure, performance and stability. Desalination 2022, 534, 115778. [Google Scholar] [CrossRef]
- Dong, H.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Clay nanosheets as charged filler materials for high-performance and fouling-resistant thin film nanocomposite membranes. J. Membr. Sci. 2015, 494, 92–103. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, F.; Yi, R.; He, Z.; Wang, Z.; Chen, J.; Liu, W.; Xu, J.; Chen, L. Effect of physical and chemical structures of graphene oxide on water permeation in graphene oxide membranes. Appl. Surf. Sci. 2020, 520, 146308. [Google Scholar] [CrossRef]
- Nair, R.; Wu, H.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A. Unimpeded permeation of water through helium-leak–tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef]
- Meng, N.; Zhao, W.; Shamsaei, E.; Wang, G.; Zeng, X.; Lin, X.; Xu, T.; Wang, H.; Zhang, X. A low-pressure GO nanofiltration membrane crosslinked via ethylenediamine. J. Membr. Sci. 2018, 548, 363–371. [Google Scholar] [CrossRef]
- Zhou, S.; Guan, K.; Fang, S.; Wang, Z.; Li, Z.; Xu, P.; Nakagawa, K.; Takagi, R.; Matsuyama, H. Nanochannel characteristics contributing to ion/ion selectivity in two-dimensional graphene oxide membranes. J. Membr. Sci. 2024, 689, 122185. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Yu, J.; He, Y.; Wang, Y.; Li, S.; Tian, S. Ethylenediamine-oxidized sodium alginate hydrogel cross-linked graphene oxide nanofiltration membrane with self-healing property for efficient dye separation. J. Membr. Sci. 2023, 670, 121366. [Google Scholar] [CrossRef]
- Kim, H.; Kang, S.-O.; Park, S.; Park, H.S. Adsorption isotherms and kinetics of cationic and anionic dyes on three-dimensional reduced graphene oxide macrostructure. J. Ind. Eng. Chem. 2015, 21, 1191–1196. [Google Scholar] [CrossRef]
- Soltanieh, M.; Mousavi, M. Application of charged membranes in water softening: Modeling and experiments in the presence of polyelectrolytes. J. Membr. Sci. 1999, 154, 53–60. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Two-dimensional-material membranes: A new family of high-performance separation membranes. Angew. Chem. Int. Ed. 2016, 55, 13384–13397. [Google Scholar] [CrossRef] [PubMed]
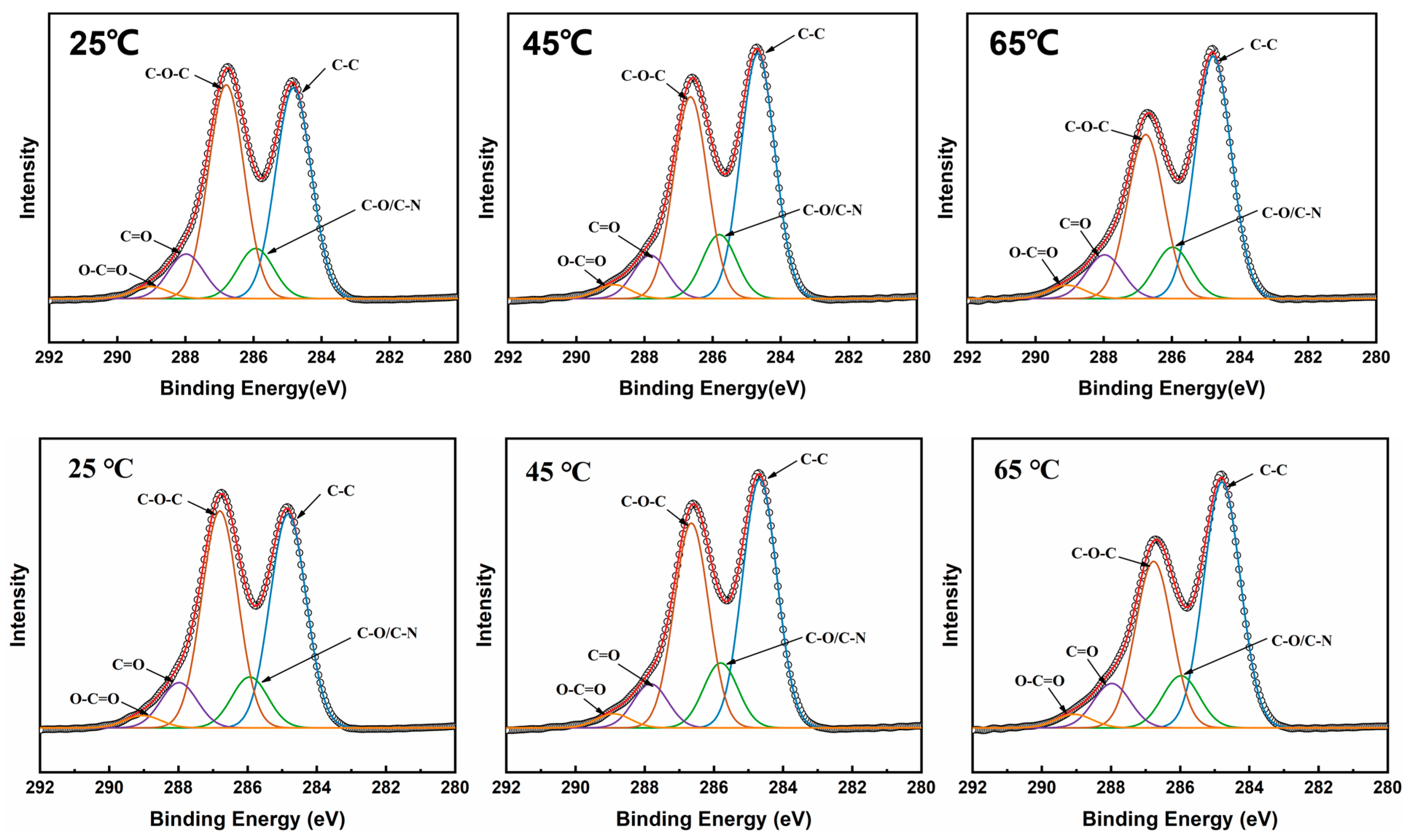






| Samples | O% | N% | C% | O/C |
|---|---|---|---|---|
| MCE/EDA/RGO-25 | 27.71% | 5.62% | 66.67% | 0.41 |
| MCE/EDA/RGO-45 | 26.34% | 5.81% | 67.85% | 0.38 |
| MCE/EDA/RGO-65 | 23.7% | 6.68% | 69.62% | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, N.; Sun, X.; Liu, J.; Mi, J.; Chen, X.; Rong, R. Modulation of Interlayer Nanochannels via the Moderate Heat Treatment of Graphene Oxide Membranes. Polymers 2024, 16, 2200. https://doi.org/10.3390/polym16152200
Meng N, Sun X, Liu J, Mi J, Chen X, Rong R. Modulation of Interlayer Nanochannels via the Moderate Heat Treatment of Graphene Oxide Membranes. Polymers. 2024; 16(15):2200. https://doi.org/10.3390/polym16152200
Chicago/Turabian StyleMeng, Na, Xin Sun, Jinxin Liu, Jialing Mi, Xuan Chen, and Rong Rong. 2024. "Modulation of Interlayer Nanochannels via the Moderate Heat Treatment of Graphene Oxide Membranes" Polymers 16, no. 15: 2200. https://doi.org/10.3390/polym16152200




