A Green Approach to Oil Spill Mitigation: New Hybrid Materials for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Lignin Esterification
2.3. Xanthan Esterification
2.4. Adsorptive Materials Obtainment
2.5. Adsorption of Degraded Motor Oil
2.6. Adsorption Kinetics
2.7. Isotherms Analysis
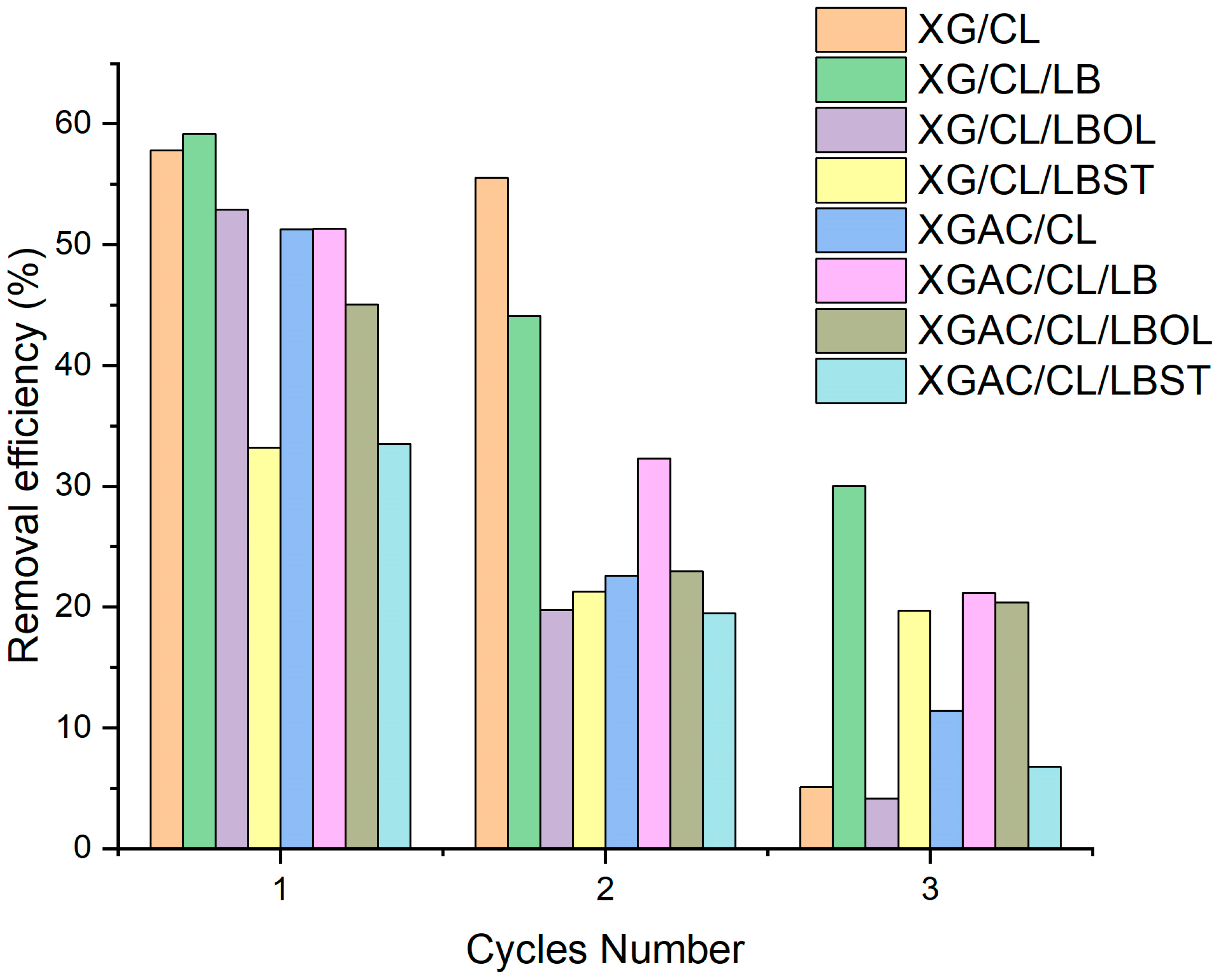
2.8. Regeneration and Reusability Evaluation
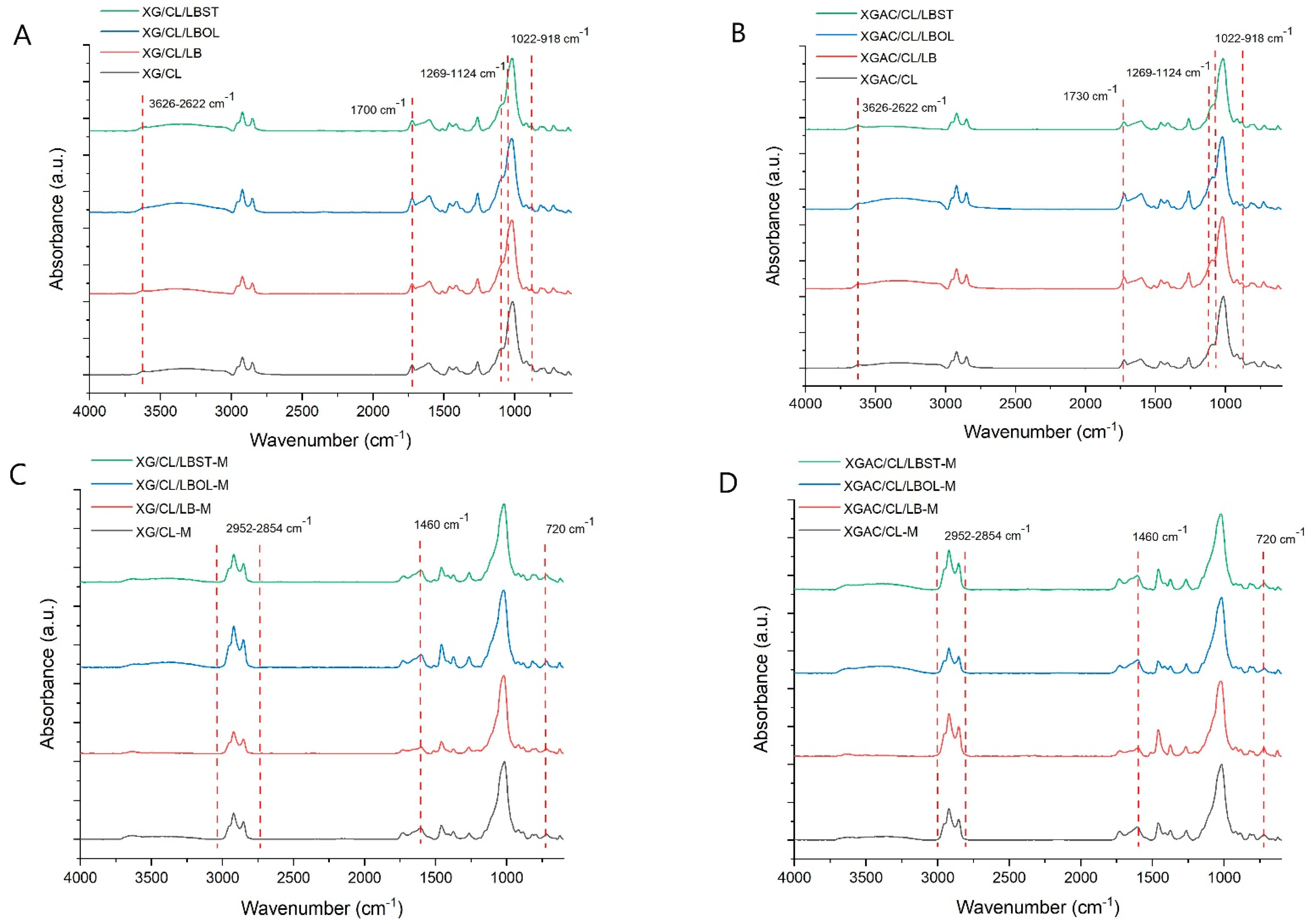
2.9. The Characterization of Materials
3. Results and Discussion
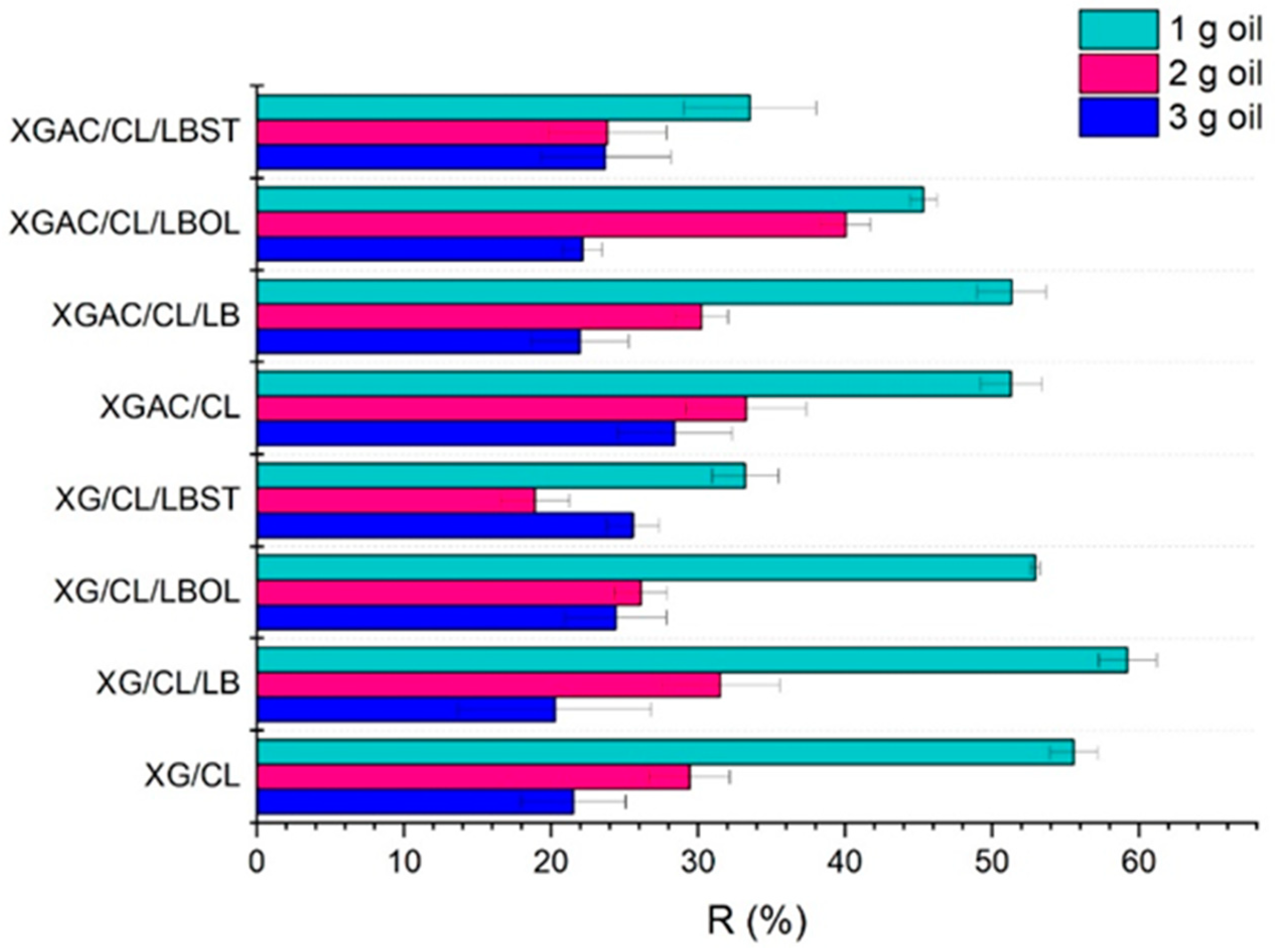
3.1. The Adsorption Capacity of Degraded Motor Oil
3.2. The Impact of Chemical Modification of XG and LB upon the Adsorption Capacity
3.3. The Effect of CL on Adsorption Capacity of Materials
3.4. The Impact of the Structural Properties of Materials on Their Adsorption Capacity
3.4.1. The Effect of Materials’ Density and Porosity on the Adsorption Capacity
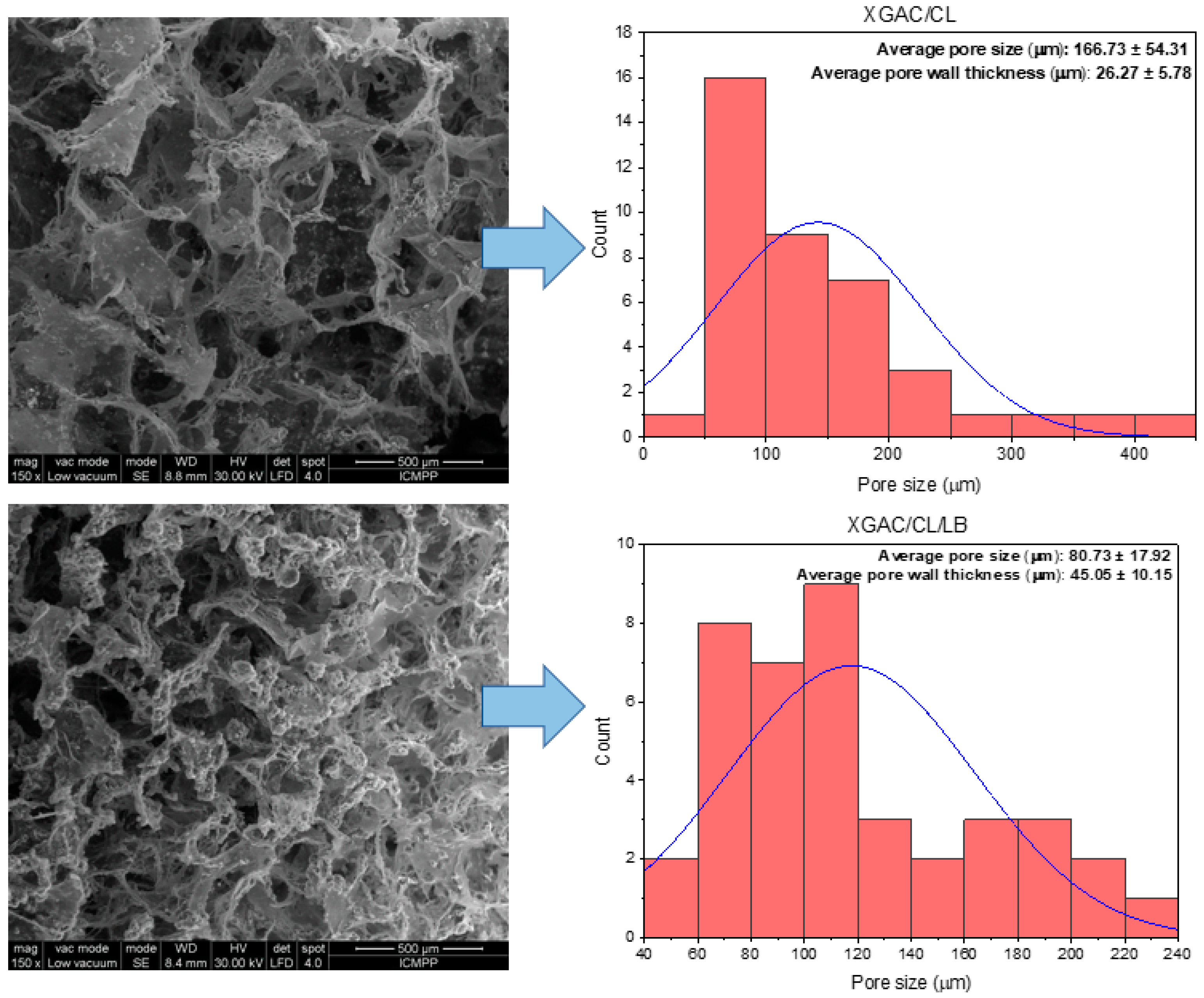
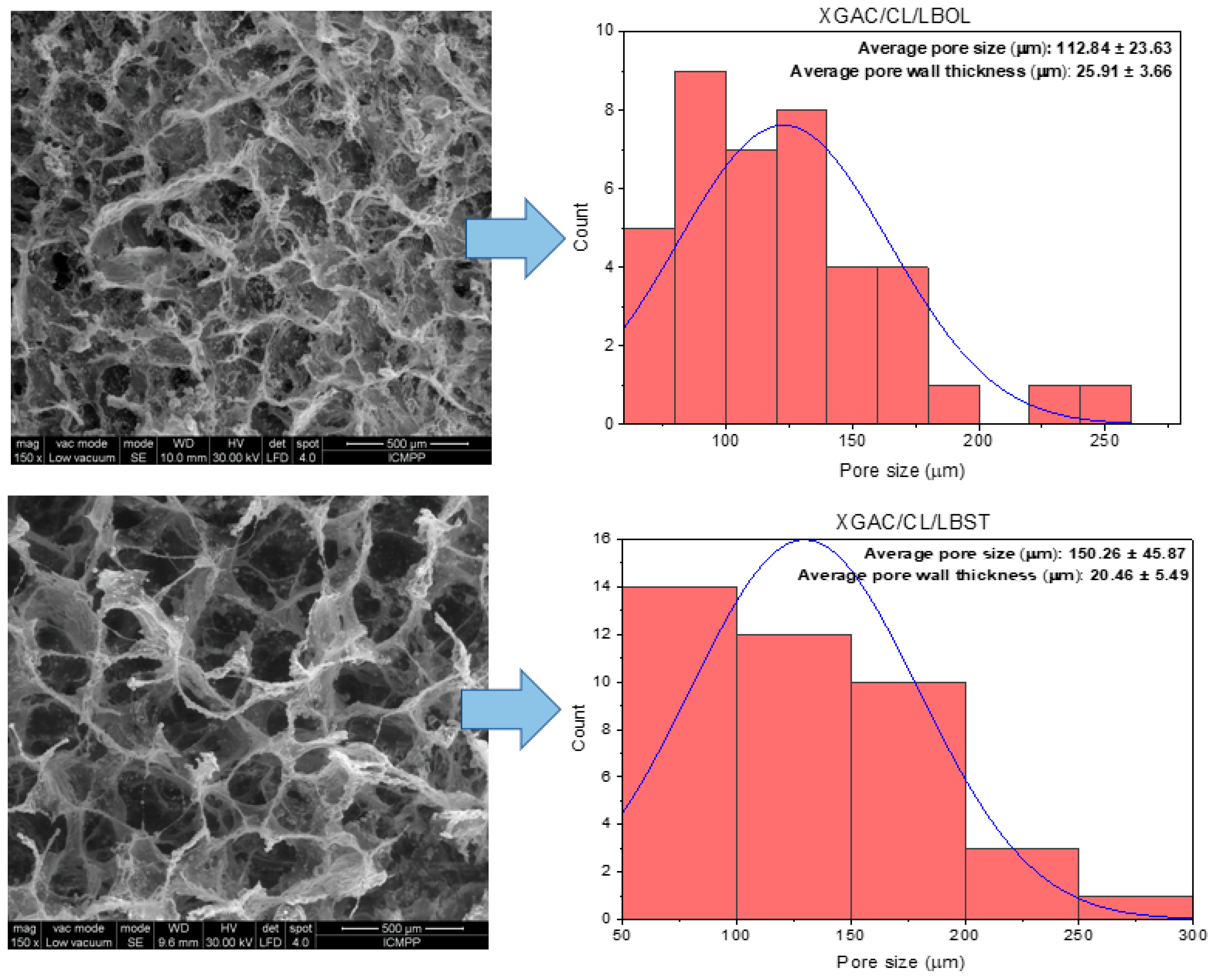
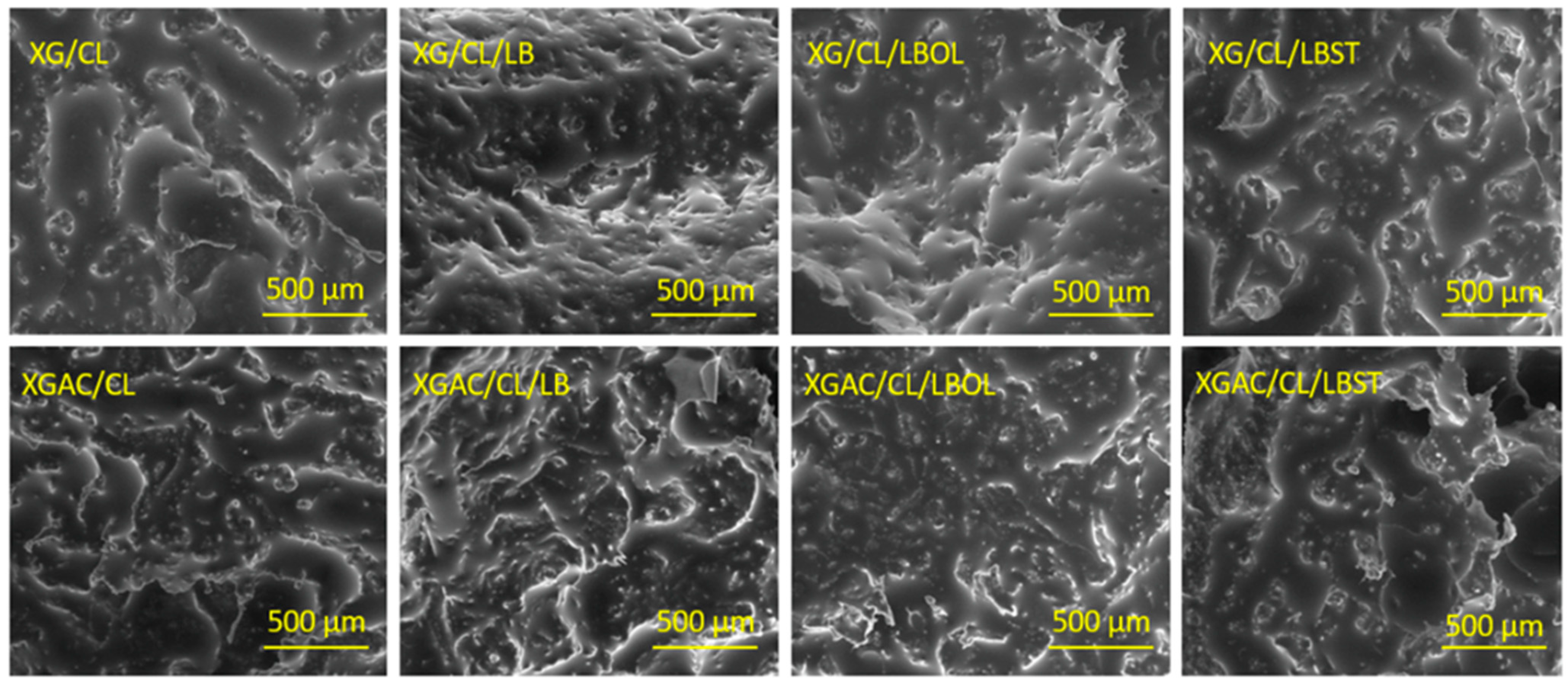
3.4.2. The Impact of Materials’ Morphology upon Their Adsorption Capacity
3.4.3. The Effect of Materials’ Surface Area on Their Adsorption Capacity
3.4.4. The Impact of the Materials’ Compression Resistance upon Their Adsorption Capacity
3.4.5. The Effect of Additives Zeta Potential upon Materials Adsorptive Properties
3.5. The Influence of Experimental Parameters upon Materials Adsorptive Capacity
3.5.1. The Effect of Contact Time upon Adsorption Process (Kinetic Study)
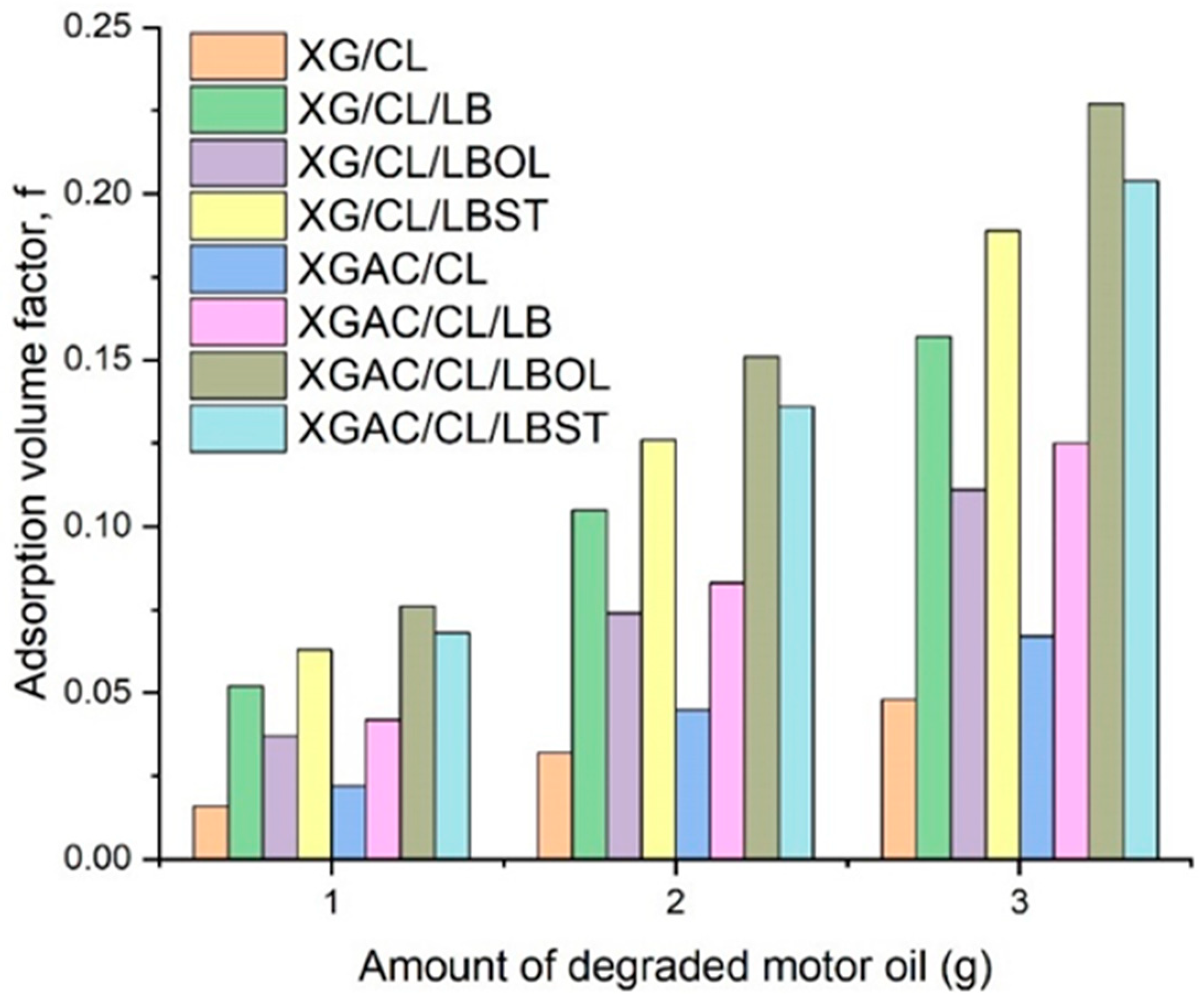
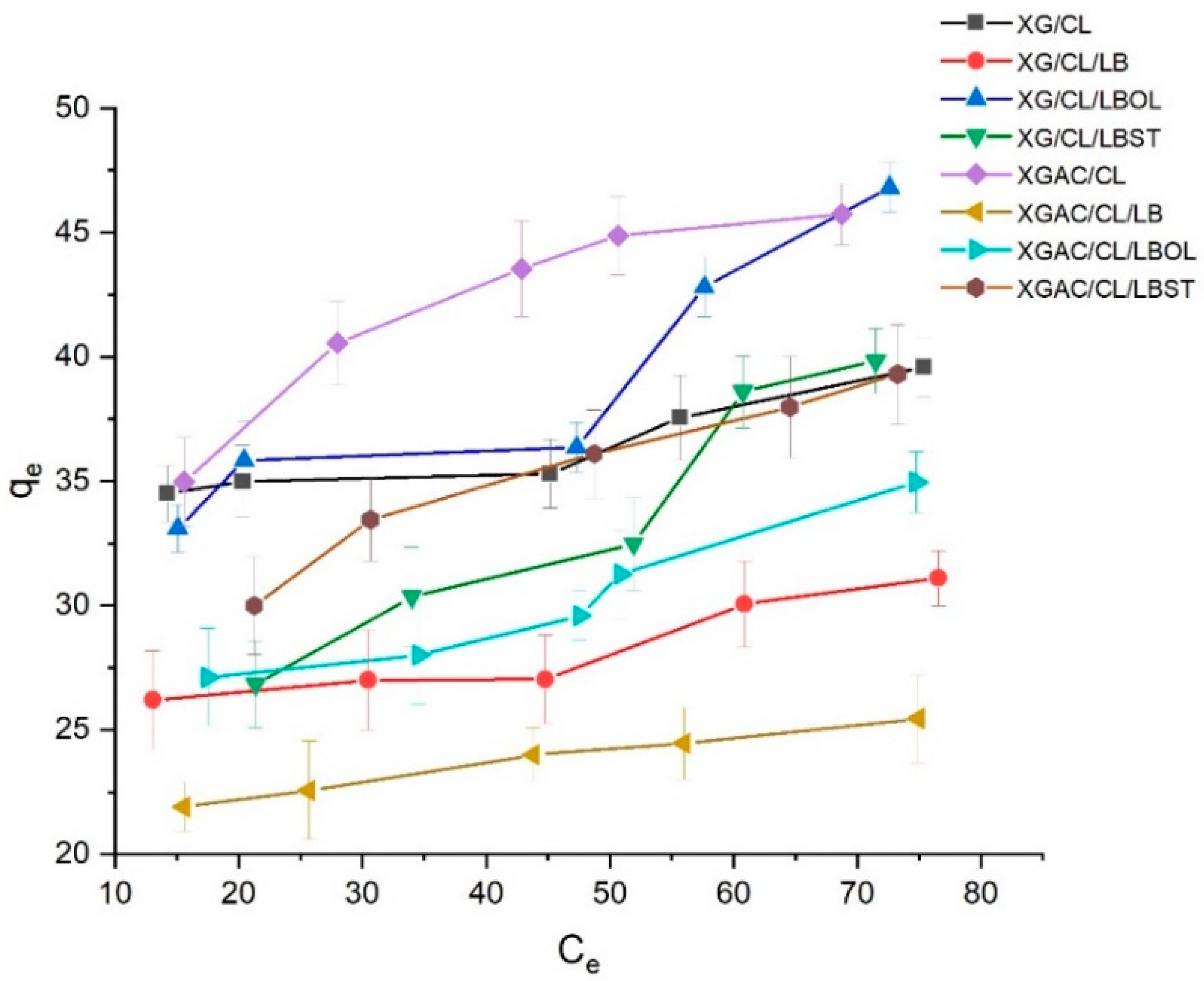
3.5.2. The Influence of Initial Degraded Motor Oil Amount upon Adsorption Process (Adsorption Isotherms Study)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Omar, B.M.; Abdelgalil, S.A.; Fakhry, H.; Tamer, M.T.; Mervat, A.E.-S. Wheat husk-based sorbent as an economical solution for removal of oil spills from sea water. Sci. Rep. 2023, 13, 2575. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.M.; Eweida, B.Y.; Tamer, T.M.; Soliman, H.M.A.; Ali, S.M.; Zaatot, A.A.; Mohy-Eldin, M.S. Removal of oil spills by novel developed amphiphilic chitosan-g-citronellal schiff base polymer. Sci. Rep. 2021, 11, 19879. [Google Scholar] [CrossRef] [PubMed]
- Jernelöv, A. The threats from oil spills: Now, then, and in the future. Ambio 2010, 39, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Jin, J.; Du, S.; Ren, Y.; Li, Q. Research on performance evaluation of polymeric surfactant cleaning gel-breaking fluid (GBF) and its enhanced oil recovery (EOR) effect. Polymers 2024, 16, 397. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, Y.M.; Al-Sabagh, A.M.; Younis, S.A.; Mostafa, M.H.K.; Abdel-Salam, M.O. Preparation of magnetic carbon nanotube nanocomposite for enhancing the separation of dissolved hydrocarbon from petroleum wastewater. J. Environ. Chem. Eng. 2017, 5, 2240–2250. [Google Scholar] [CrossRef]
- Nasiri, M.; Jafari, I. Produced water from oil-gas plants: A short review on challenges and opportunities. Period. Polytech. Chem. Eng. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Abdel-Salam, M.O.; Younis, S.A.; Moustafa, Y.M.; Al-Sabagh, A.M.; Mostafa, M.H.K. Microwave—Assisted production of hydrophilic carbon-based magnetic nanocomposites from saw-dust for elevating oil from oil field waste water. J. Clean. Prod. 2020, 249, 119355. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Oil removal from water using biomaterials. Bioresour. Technol. 2010, 101, 6594–6600. [Google Scholar] [CrossRef] [PubMed]
- Kamgar, A.; Hassanajili, S.; Unbehaun, H. Oil spill remediation from water surface using induction of magnetorheological behavior in oil by functionalized sawdust. Chem. Eng. Res. Des. 2020, 160, 119–128. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ang, H.-M.; Wang, S. Removal of emulsified food and mineral oils from wastewater using surfactant modified barley straw. Bioresour. Technol. 2009, 100, 5744–5749. [Google Scholar] [CrossRef]
- Wahi, R.; Chuah, L.A.; Yaw Choong, T.S.; Ngaini, Z.; Nourouzi, M.M. Oil removal from aqueous state by natural fibrous sorbent: An overview. Sep. Purif. Technol. 2013, 113, 51–63. [Google Scholar] [CrossRef]
- Ribeiro, T.H.; Rubio, J.; Smith, R.W. A dried hydrophobic aquaphyte as an oil filter for oil/water emulsions. Spill Sci. Technol. Bull. 2003, 8, 483–489. [Google Scholar] [CrossRef]
- Spiridon, I.; Bele, A.; Apostol, I.; Dinu, M.V.; Anghel, N. Enhancing natural polymers-based materials using montmorillonite: Preparation, characterization, and environmental applications. J. Polym. Environ. 2024, 32, 2014–2030. [Google Scholar] [CrossRef]
- Zamani, A.M.; Moslemi, A.; Hassani, K. Experimental investigation into effects of the natural polymer and nanoclay particles on the EOR performance of chemical flooding in carbonate reservoirs. Pet. Sci. 2024, 21, 951–961. [Google Scholar] [CrossRef]
- Ayazi, Z.; Khoshhesab, Z.M.; Azhar, F.F.; Mohajeri, Z. Modeling and optimization of adsorption removal of reactive Orange 13 on the alginate–montmorillonite–polyaniline nanocomposite via response surface methodology. J. Chin. Chem. Soc. 2017, 64, 627–639. [Google Scholar] [CrossRef]
- Apostol, I.; Anghel, N.; Dinu, M.V.; Ziarelli, F.; Mija, A.; Spiridon, I. An eco-friendly strategy for preparing lignin esters as filler in materials for removal of argan oil and sunflower oil. React. Funct. Polym. 2023, 190, 105620. [Google Scholar] [CrossRef]
- Apostol, I.; Anghel, N.; Doroftei, F.; Bele, A.; Spiridon, I. Xanthan or esterified xanthan/cobalt ferrite-lignin hybrid materials for methyl blue and basic fuchsine dyes removal: Equilibrium, kinetic and thermodynamic studies. Mater. Today Chem. 2023, 27, 101299. [Google Scholar] [CrossRef]
- Raschip, I.E.; Dinu, M.V.; Fifere, N.; Darie-Nita, R.; Pamfil, D.; Popirda, A.; Logigan, C. Thermal, mechanical and water sorption properties of xanthan-based composite cryogels. Cellulose Chem. Technol. 2020, 54, 915–924. [Google Scholar] [CrossRef]
- Spiridon, I.; Andrei, I.-M.; Anghel, N.; Dinu, M.V.; Ciubotaru, B.-I. Development and characterization of novel cellulose composites obtained in 1-ethyl-3-methylimidazolium chloride used as drug delivery systems. Polymers 2021, 13, 2176. [Google Scholar] [CrossRef]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Designing smart triple-network cationic cryogels with outstanding efficiency and selectivity for deep cleaning of phosphate. Chem. Eng. J. 2021, 426, 131411. [Google Scholar] [CrossRef]
- De Rivas, B.L.; Vivancos, J.-L.; Ordieres-Meré, J.; Capuz-Rizo, S.F. Determination of the total acid number (TAN) of used mineral oils in aviation engines by FTIR using regression models. Chemometr. Intell. Lab. Syst. 2017, 160, 32–39. [Google Scholar] [CrossRef]
- Tiwari, S.; Gupta, V.; Pandey, P.C.; Singh, H.; Mishra, P.K. Adsorption of emulsified oil from spent metalworking fluid using agro-waste of Cajanus cajan. Int. J. Environ. Technol. Manag. 2011, 14, 115–131. [Google Scholar] [CrossRef]
- Bandura, L.; Franus, M.; Józefaciuk, G.; Franus, W. Synthetic zeolites from fly ash as effective mineral sorbents for land-based petroleum spills cleanup. Fuel 2015, 147, 100–107. [Google Scholar] [CrossRef]
- Darvish Pour-Mogahi, S.; Ansari-Asl, Z.; Darabpour, E. Polycaprolactone/ZIF-8 nanocomposites fabricated for oil sorption and antibacterial applications. Inorg. Chem. Commun. 2021, 133, 108945. [Google Scholar] [CrossRef]
- Shi, M.; Thang, C.; Young, X.; Zhou, J.; Jia, F.; Han, Y.; Li, Z. Superhydrophobic silica aerogels reinforced with polyacrylonitrile fibers for adsorbing oil from water and oil mixtures. RSC Adv. 2017, 7, 4039–4045. [Google Scholar] [CrossRef]
- Kovačević, A.; Marković, D.; Radoičić, M.; Šaponjić, Z.; Radetić, M. Sustainable non-woven sorbents based on jute post-industrial waste for cleaning of oil spills. J. Clean. Prod. 2022, 386, 135811. [Google Scholar] [CrossRef]
- Sathasivam, K.; Mas Haris, M.R.H. Adsorption kinetics and capacity of fatty acid-modified banana trunk fibers for oil in water. Water Air Soil Pollut. 2010, 213, 413–423. [Google Scholar] [CrossRef]
- Łukawski, D.; Lisiecki, F.; Dudkowiak, A. Coating cellulosic materials with graphene for selective absorption of oils and organic solvents from water. Fibers Polym. 2018, 19, 524–530. [Google Scholar] [CrossRef]
- Asadpour, R.; Yavari, S.; Kamyab, H.; Ashokkumar, V.; Chelliapan, S.; Yuzir, A. Study of oil sorption behaviour of esterified oil palm empty fruit bunch (OPEFB) fibre and its kinetics and isotherm studies. Environ. Technol. Innov. 2021, 22, 101397. [Google Scholar] [CrossRef]
- Zare, Y.; Garmabi, H. Modeling of interfacial bonding between two nanofillers (montmorillonite and CaCO3) and a polymer matrix (PP) in a ternary polymer nanocomposite. Appl. Surf. Sci. 2014, 321, 219–225. [Google Scholar] [CrossRef]
- Al-Mulla, E.A.J. A new biopolymer-based polycaprolactone/starch modified clay nanocomposite. Cellul. Chem. Technol. 2014, 48, 515–520. [Google Scholar]
- Zhao, Z.; Ren, J.; Liu, W.; Yan, W.; Zhu, K.; Kong, Y.; Jiang, X.; Shen, X. Facile synthesis of polymer-reinforced silica aerogel microspheres as robust, hydrophobic and recyclable sorbents for oil removal from water. Polymers 2023, 15, 3526. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.; Wang, S.; Bai, L.; Deng, Y.; Tao, J. Effect of pore size distribution and amination on adsorption capacities of polymeric adsorbents. Molecules 2021, 26, 5267. [Google Scholar] [CrossRef]
- Chen, Y.K.; Sun, Y.; Wang, K.Q.; Kuang, W.Y.; Yan, S.R.; Wang, Z.H.; Lee, H.S. Utilization of bio-waste eggshell powder as a potential filler material for cement: Analyses of zeta potential, hydration and sustainability. Constr. Build Mater. 2022, 325, 126220. [Google Scholar] [CrossRef]
- Pellenz, L.; de Oliveira, C.R.S.; da Silva Júnior, A.H.; da Silva, L.J.S.; da Silva, L.; de Souza, A.A.U.; de Arruda Guelli Ulson de Souza, S.M.; Borba, F.H.; da Silva, A. A comprehensive guide for characterization of adsorbent materials. Sep. Purif. Technol. 2023, 305, 122435. [Google Scholar] [CrossRef]
- Huang, S.; Jiang, G.; Guo, C.; Feng, Q.; Yang, J.; Dong, T.; He, Y.; Yang, L. Experimental study of adsorption/desorption and enhanced recovery of shale oil and gas by zwitterionic surfactants. Chem. Eng. J. 2024, 487, 150628. [Google Scholar] [CrossRef]
- Estrada, D.; Murga, R.; Rubilar, O.; Amalraj, J.; Gutierrez, L.; Uribe, L. On the use of styrene-based nanoparticles to mitigate the effect of montmorillonite in copper sulfide recovery by flotation. Polymers 2024, 16, 1682. [Google Scholar] [CrossRef]
- Kamble, S.; Agrawal, S.; Cherumukkil, S.; Sharma, V.; Jasra, R.V.; Munshi, P. Revisiting zeta potential, the key feature of interfacial phenomena, with applications and recent advancements. Chem. Select. 2022, 7, e202103084. [Google Scholar] [CrossRef]
- Soliman, E.M.; Ahmed, S.A.; Fadl, A.A. Adsorptive removal of oil spill from sea water surface using magnetic wood sawdust as a novel nano-composite synthesized via microwave approach. J. Environ. Health Sci. Eng. 2020, 18, 79–90. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Investigation of acetylated kapok fibers on the sorption of oil in water. J. Environ. Sci. 2013, 25, 246–253. [Google Scholar] [CrossRef]
- Sidik, S.M.; Jalil, A.A.; Triwahyono, S.; Adam, S.H.; Satar, M.A.H.; Hameed, B.H. Modified oil palm leaves adsorbent with enhanced hydrophobicity for crude oil removal. Chem. Eng. J. 2012, 203, 9–18. [Google Scholar] [CrossRef]
- Nguyen, K.D. Preparation and characterization of chitin hydrogel composited with halloysite clay solution via phase inversion. Cellulose Chem. Technol. 2022, 56, 1071–1080. [Google Scholar] [CrossRef]
- Spiridon, I.; Apostol, I.; Anghel, N.C.; Zaltariov, M.F. Equilibrium, kinetic, and thermodynamic studies of new materials based on xanthan gum and cobalt ferrite for dye adsorption. Appl. Organomet. Chem. 2022, 36, e6670. [Google Scholar] [CrossRef]
- Anghel, N.; Dinu, M.V.; Doroftei, F.; Spiridon, I. Xanthan matrix as drug delivery system. Rev. Chim. 2021, 72, 90. [Google Scholar] [CrossRef]
- Jozanikohan, G.; Abarghooei, M.N. The Fourier transform infrared spectroscopy (FTIR) analysis for the clay mineralogy studies in a clastic reservoir. J. Petrol Explor. Prod. Technol. 2022, 12, 2093–2106. [Google Scholar] [CrossRef]
- Esteves, B.; Velez Marques, A.; Domingos, I.; Pereira, H. Chemical changes of heat-treated pine and eucalypt wood monitored by FTIR. Maderas Cienc. Tecnol. 2013, 15, 245–258. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, Y.; Ren, H.; Hao, X.; Song, X.; Liu, Z. A novel co-treatment scheme for waste motor oil and low rank coal slime: Waste dispose waste. Fuel 2021, 292, 120275. [Google Scholar] [CrossRef]
- Belchinskaya, L.; Zhuzhukin, K.V.; Ishchenko, T.; Platonov, A. Impregnation of wood with waste engine oil to increase water- and bio-resistance. Forests 2021, 12, 1762. [Google Scholar] [CrossRef]
- Amrhar, O.; El Gana, L.; Mobarak, M. Calculation of adsorption isotherms by statistical physics models: A review. Environ. Chem. Lett. 2021, 19, 4519–4547. [Google Scholar] [CrossRef]
- Guo, X.; Liu, Y.; Wang, J. Sorption of sulfamethazine onto different types of microplastics: A combined experimental and molecular dynamics simulation study. Mar. Pollut. Bull. 2019, 145, 547–554. [Google Scholar] [CrossRef]
- Majd, M.M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, Y.; Li, F.; Liu, Y.; Wang, X.; Hu, X. Preparation and application of magnetic superhydrophobic polydivinylbenzene nanofibers for oil adsorption in wastewater. Environ. Sci. Pollut. Res. 2018, 25, 22911–22919. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar] [CrossRef]
- Akanji, O.; Iwarere, S.A.; Sani, B.S.; Mukhtar, B.; Jibril, B.; Daramola, M.O. Polystyrene-reduced graphene oxide composite as sorbent for oil removal from an Oil-Water mixture. Chem. Eng. Sci. 2024, 298, 120383. [Google Scholar] [CrossRef]


| Material | Composition | Mass Ratio |
|---|---|---|
| XG/CL | XG and CL | 1:0.5 |
| XG/CL/LB | XG, CL and LB | 1:0.5:1 |
| XG/CL/LBOL | XG, CL and LBOL | 1:0.5:1 |
| XG/CL/LBST | XG, CL and LBST | 1:0.5:1 |
| XGAC/CL | XGAC and CL | 1:0.5 |
| XGAC/CL/LB | XGAC, CL and LB | 1:0.5:1 |
| XGAC/CL/LBOL | XGAC, CL and LBOL | 1:0.5:1 |
| XGAC/CL/LBST | XGAC, CL and LBST | 1:0.5:1 |
| a qe ± SD, g/g | |||
|---|---|---|---|
| Material | Degraded 5w40 Motor Oil | ||
| 1 g | 2 g | 3 g | |
| XG/CL | 34.49 ± 1.60 | 35.29 ± 2.73 | 39.58 ± 3.57 |
| XG/CL/LB | 26.19 ± 1.94 | 27.03 ± 4.01 | 31.09 ± 6.57 |
| XG/CL/LBOL | 33.10 ± 0.34 | 36.35 ± 1.79 | 46.80 ± 3.43 |
| XG/CL/LBST | 26.82 ± 2.26 | 32.47 ± 2.35 | 36.70 ± 1.78 |
| XGAC/CL | 34.97 ± 2.08 | 43.53 ± 4.11 | 45.73 ± 3.90 |
| XGAC/CL/LB | 21.89 ± 2.32 | 23.99 ± 1.78 | 25.44 ± 3.30 |
| XGAC/CL/LBOL | 27.11 ± 0.90 | 27.89 ± 1.72 | 34.96 ± 1.37 |
| XGAC/CL/LBST | 29.99 ± 4.49 | 36.10 ± 3.99 | 39.31 ± 4.42 |
| Material | a Density, g/cm3 | a Porosity, % | b Pore Area, % |
|---|---|---|---|
| XG/CL | 0.034 ± 0.011 | 90.21 ± 3.12 | 55.20 |
| XG/CL/LB | 0.028 ± 0.004 | 54.06 ± 1.23 | 40.94 |
| XG/CL/LBOL | 0.021 ± 0.010 | 98.25 ± 2.47 | 62.18 |
| XG/CL/LBST | 0.048 ± 0.002 | 84.97 ± 2.34 | 37.60 |
| XGAC/CL | 0.028 ± 0.0024 | 71.91 ± 0.93 | 50.55 |
| XGAC/CL/LB | 0.035 ± 0.0032 | 77.15 ± 1.45 | 49.66 |
| XGAC/CL/LBOL | 0.064 ± 0.0013 | 96.56 ± 3.11 | 43.77 |
| XGAC/CL/LBST | 0.068 ± 0.0027 | 72.94 ± 2.97 | 59.76 |
| BET Data | |||
|---|---|---|---|
| Material | a Sorption Capacity, % d.b. | a Surface Area, m2/g | a Monolayer, g/g |
| XG/CL | 25.59 ± 2.00 | 169.25 ± 1.17 | 0.048 ± 0.020 |
| XG/CL/LB | 20.56 ± 1.69 | 143.62 ± 3.55 | 0.040 ± 0.040 |
| XG/CL/LBOL | 23.91 ± 3.77 | 170.44 ± 2.54 | 0.048 ± 0.010 |
| XG/CL/LBST | 18.35 ± 3.38 | 129.14 ± 5.90 | 0.036 ± 0.040 |
| XGAC/CL | 24.37 ± 1.45 | 172.80 ± 6.27 | 0.049 ± 0.030 |
| XGAC/CL/LB | 16.96 ± 1.98 | 163.55 ± 2.76 | 0.046 ± 0.021 |
| XGAC/CL/LBOL | 20.93 ± 2.03 | 154.22 ± 9.85 | 0.043 ± 0.024 |
| XGAC/CL/LBST | 19.63 ± 3.33 | 143.48 ± 0.63 | 0.040 ± 0.017 |
| Material | Compressive Nominal Stress, kPa | Strain, % | Compressive Elastic Modulus, kPa | R2 |
|---|---|---|---|---|
| XGAC/CL | 211.88 ± 10.30 | 88.46 ± 0.27 | 10.29 ± 0.47 | 0.999 |
| XGAC/CL/LB | 194.95 ± 9.47 | 64.93 ± 0.50 | 7.34 ± 0.39 | 0.992 |
| XGAC/CL/LBOL | 242.70 ± 8.52 | 78.43 ± 0.42 | 5.11 ± 0.63 | 0.994 |
| XGAC/CL/LBST | 173.49 ± 11.33 | 100.11 ± 0.09 | 1.24 ± 0.07 | 0.981 |
| Sample | Zeta Potential, mV |
|---|---|
| CL | 16.85 ± 3.01 |
| LB | −31.06 ± 0.70 |
| LBOL | −21.67 ± 0.90 |
| LBST | −14.19 ± 0.06 |
| Material | PFO Model | PSO Model | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 g Oil | 2 g Oil | 3 g Oil | 1 g Oil | 2 g Oil | 3 g Oil | |||||||
| k1, min−1 | R2 | k1, min−1 | R2 | k1, min−1 | R2 | k2 × 10−4, g/min × g | R2 | k2 × 10−4, g/min × g | R2 | k2 × 10−4, g/min × g | R2 | |
| XG/CL | −0.04 | 0.8425 | −0.04 | 0.8491 | −0.04 | 0.8477 | 1.80 | 0.9985 | 1.18 | 0.9977 | 1.89 | 0.9993 |
| XG/CL/LB | −0.03 | 0.9231 | −0.03 | 0.8501 | −0.04 | 0.7097 | 4.26 | 0.9991 | 3.92 | 0.9971 | 3.06 | 0.9998 |
| XG/CL/LBOL | −0.03 | 0.4641 | −0.04 | 0.7338 | −0.04 | 0.8787 | 3.09 | 0.9985 | 2.48 | 0.9989 | 0.60 | 0.9889 |
| XG/CL/LBST | −0.04 | 0.8386 | −0.03 | 0.9398 | −0.04 | 0.8350 | 7.10 | 0.9999 | 1.66 | 0.9981 | 0.85 | 0.9560 |
| XGAC/CL | −0.04 | 0.8496 | −0.05 | 0.8491 | −0.03 | 0.9511 | 1.26 | 0.9980 | 9.95 | 0.9977 | 0.44 | 0.9889 |
| XGAC/CL/LB | −0.04 | 0.8396 | −0.04 | 0.8394 | −0.04 | 0.8441 | 4.84 | 0.9995 | 4.48 | 0.9998 | 2.44 | 0.9983 |
| XGAC/CL/LBOL | −0.03 | 0.4609 | −0.04 | 0.8356 | −0.04 | 0.8443 | 6.65 | 0.9995 | 4.13 | 0.9996 | 1.27 | 0.9955 |
| XGAC/CL/LBST | −0.03 | 0.4633 | −0.04 | 0.8449 | −0.03 | 0.8951 | 7.07 | 0.9997 | 1.76 | 0.9986 | 1.05 | 0.9909 |
| Materials | Henry | Langmuir | Freundlich | Temkin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KH, L/g | R2 | qmax, g/g | KL, L/g | RL | R2 | n | KF, L/g | R2 | AT, L/g | BT, J/mol | R2 | |
| XG/CL | 0.083 | 0.8923 | 38.70 | 0.54 | 0.16 | 0.6253 | 13.90 | 28.09 | 0.8395 | 24.63 | 2.65 | 0.9337 |
| XG/CL/LB | 0.077 | 0.9207 | 30.14 | 0.48 | 0.17 | 0.6035 | 11.82 | 20.72 | 0.8411 | 31.99 | 2.41 | 0.5402 |
| XG/CL/LBOL | 0.232 | 0.9656 | 45.52 | 0.17 | 0.37 | 0.7177 | 5.21 | 19.15 | 0.8120 | 4.67 | 7.50 | 0.8164 |
| XG/CL/LBST | 0.253 | 0.9484 | 44.74 | 0.07 | 0.59 | 0.8988 | 3.30 | 10.42 | 0.9707 | 0.67 | 9.83 | 0.9222 |
| XGAC/CL | 0.203 | 0.8601 | 50.40 | 0.15 | 0.41 | 0.9088 | 5.37 | 21.12 | 0.9673 | 7.25 | 7.44 | 0.9662 |
| XGAC/CL/LB | 0.060 | 0.9829 | 26.10 | 0.33 | 0.23 | 0.9075 | 10.57 | 16.86 | 0.9949 | 20.43 | 2.23 | 0.9780 |
| XGAC/CL/LBOL | 0.136 | 0.9305 | 35.31 | 0.18 | 0.36 | 0.6610 | 6.23 | 16.83 | 0.9094 | 12.45 | 4.92 | 0.7988 |
| XGAC/CL/LBST | 0.180 | 0.9540 | 44.25 | 0.10 | 0.50 | 0.9335 | 4.56 | 15.34 | 0.9913 | 29.53 | 7.51 | 0.9924 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostol, I.; Dinu, M.V.; Anghel, N.; Spiridon, I. A Green Approach to Oil Spill Mitigation: New Hybrid Materials for Wastewater Treatment. Polymers 2024, 16, 2225. https://doi.org/10.3390/polym16152225
Apostol I, Dinu MV, Anghel N, Spiridon I. A Green Approach to Oil Spill Mitigation: New Hybrid Materials for Wastewater Treatment. Polymers. 2024; 16(15):2225. https://doi.org/10.3390/polym16152225
Chicago/Turabian StyleApostol, Irina, Maria Valentina Dinu, Narcis Anghel, and Iuliana Spiridon. 2024. "A Green Approach to Oil Spill Mitigation: New Hybrid Materials for Wastewater Treatment" Polymers 16, no. 15: 2225. https://doi.org/10.3390/polym16152225
APA StyleApostol, I., Dinu, M. V., Anghel, N., & Spiridon, I. (2024). A Green Approach to Oil Spill Mitigation: New Hybrid Materials for Wastewater Treatment. Polymers, 16(15), 2225. https://doi.org/10.3390/polym16152225







