Fabrication of Flame-Retardant Ammonium Polyphosphate Modified Phytic Acid-Based Rigid Polyurethane Foam with Enhanced Mechanical Properties
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Preparation of RPUFs
2.3. Characterization
3. Results and Discussion
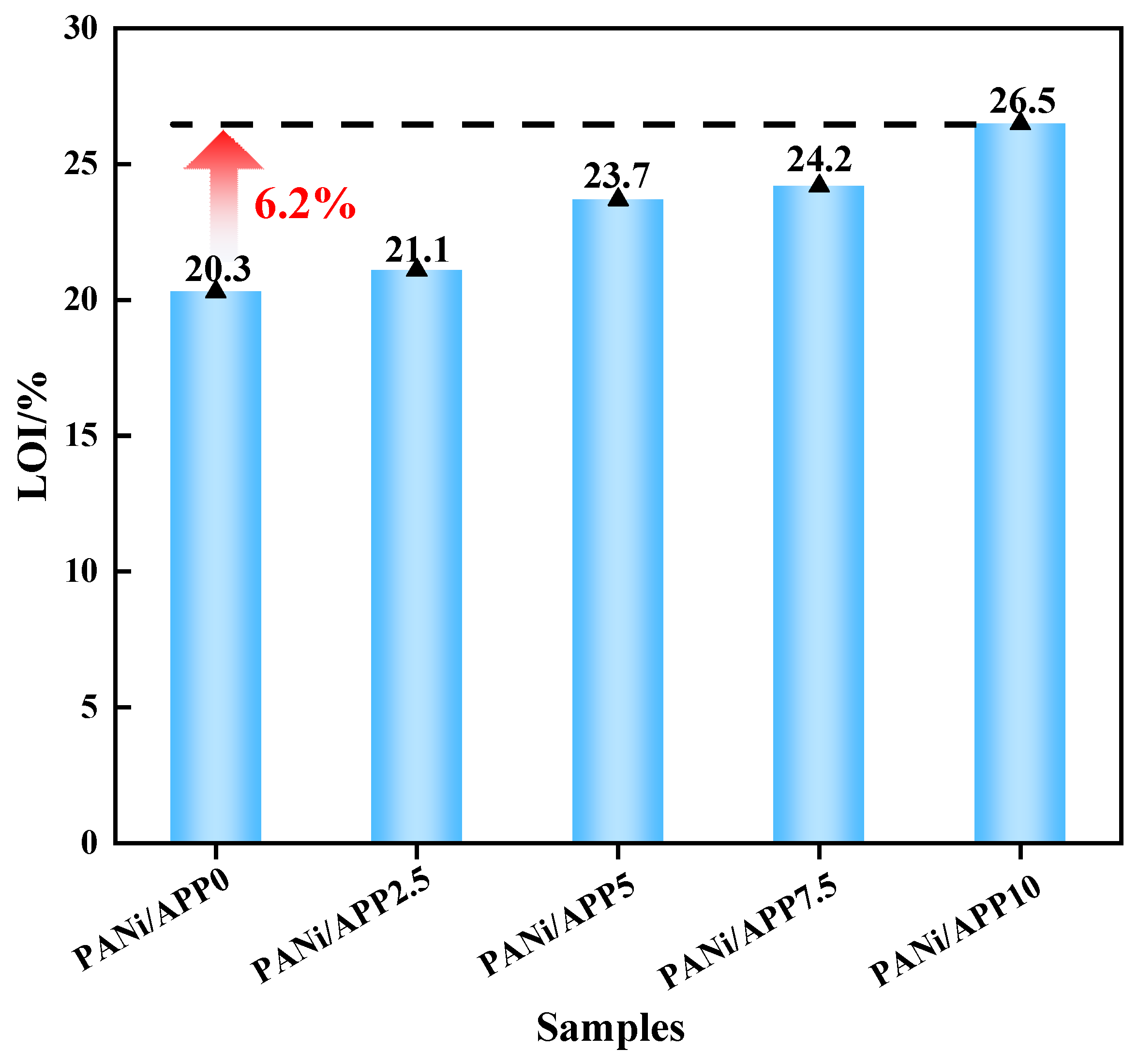
3.1. Flammability
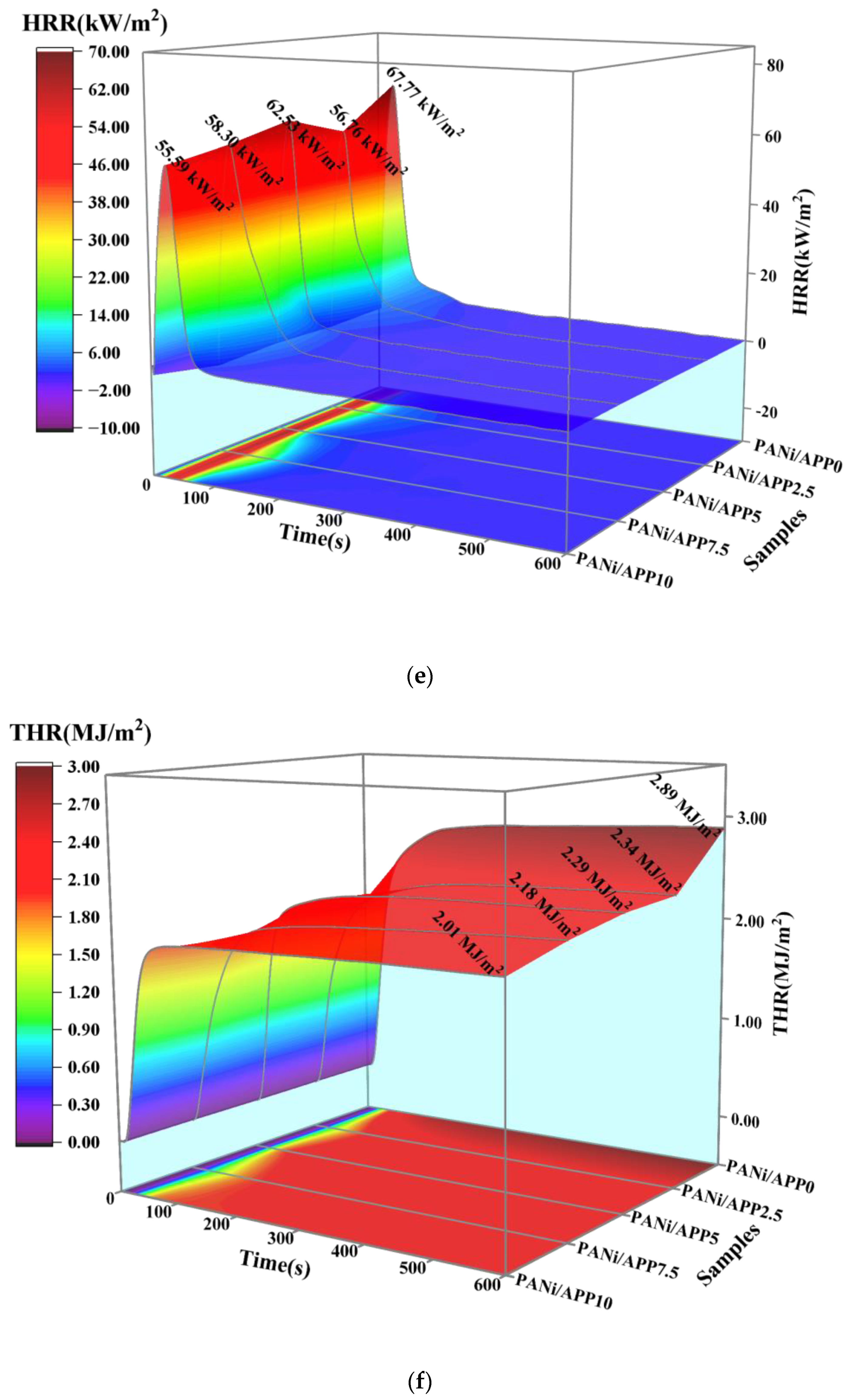
3.2. Combustibility
3.3. Smoke Toxicity
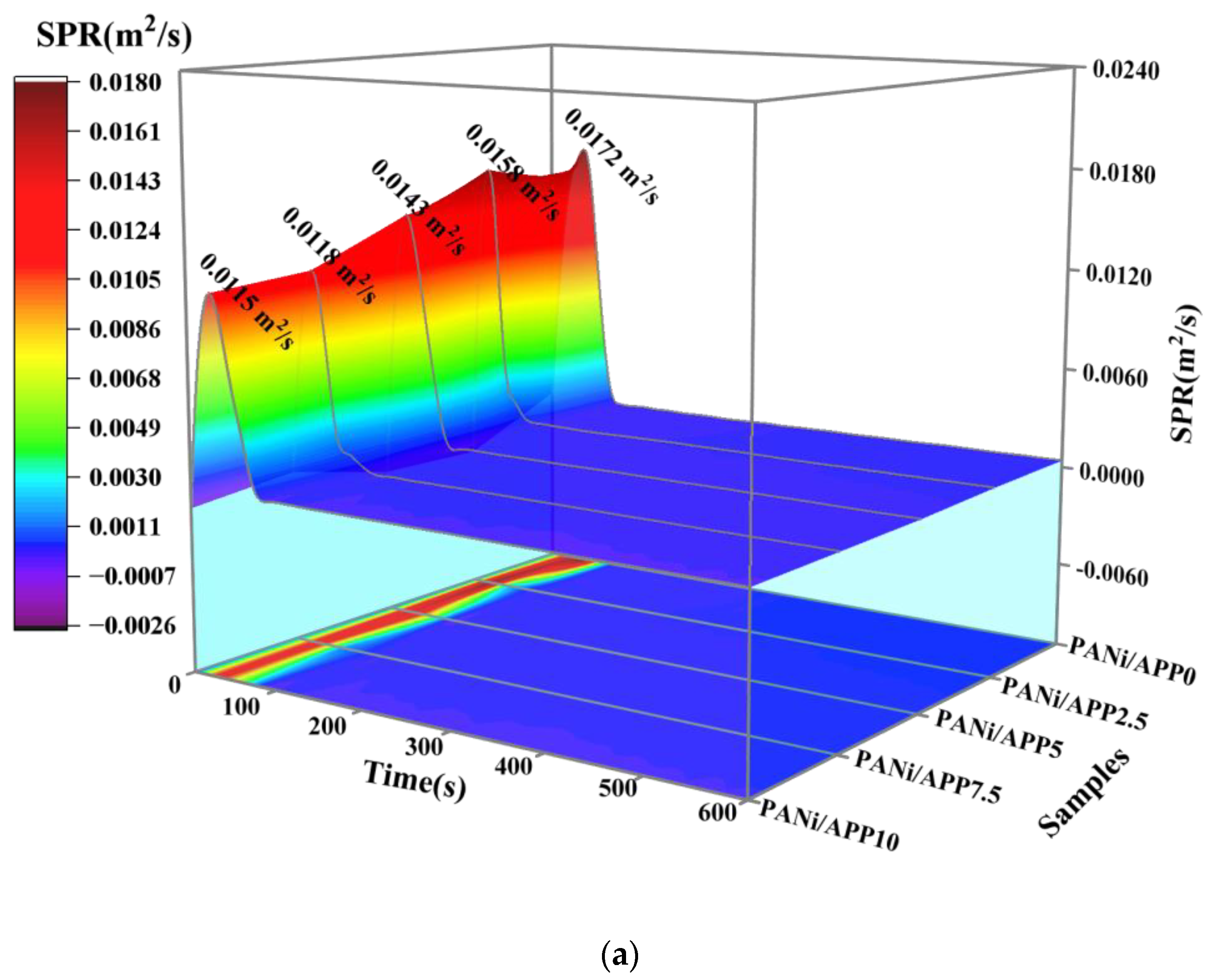
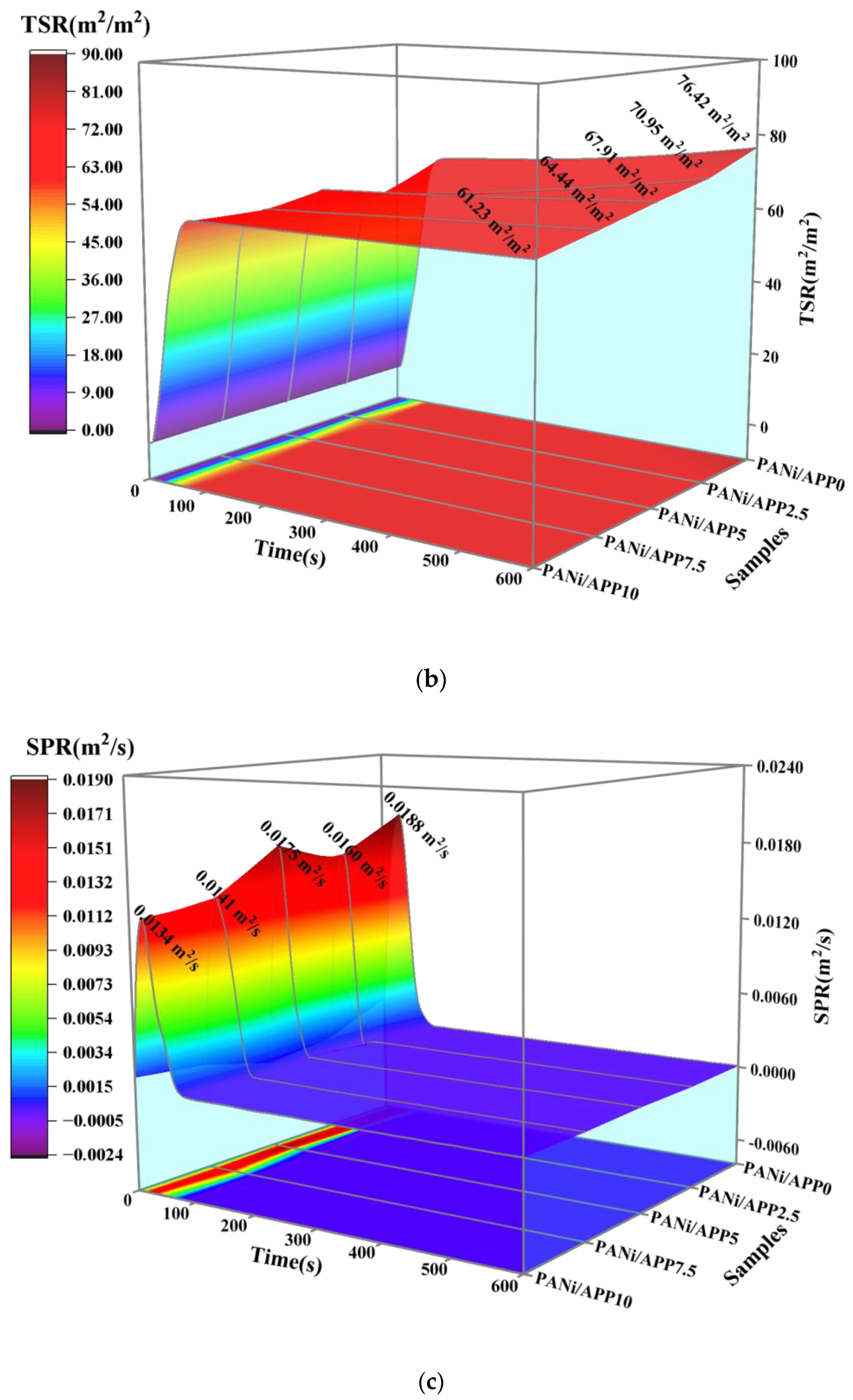
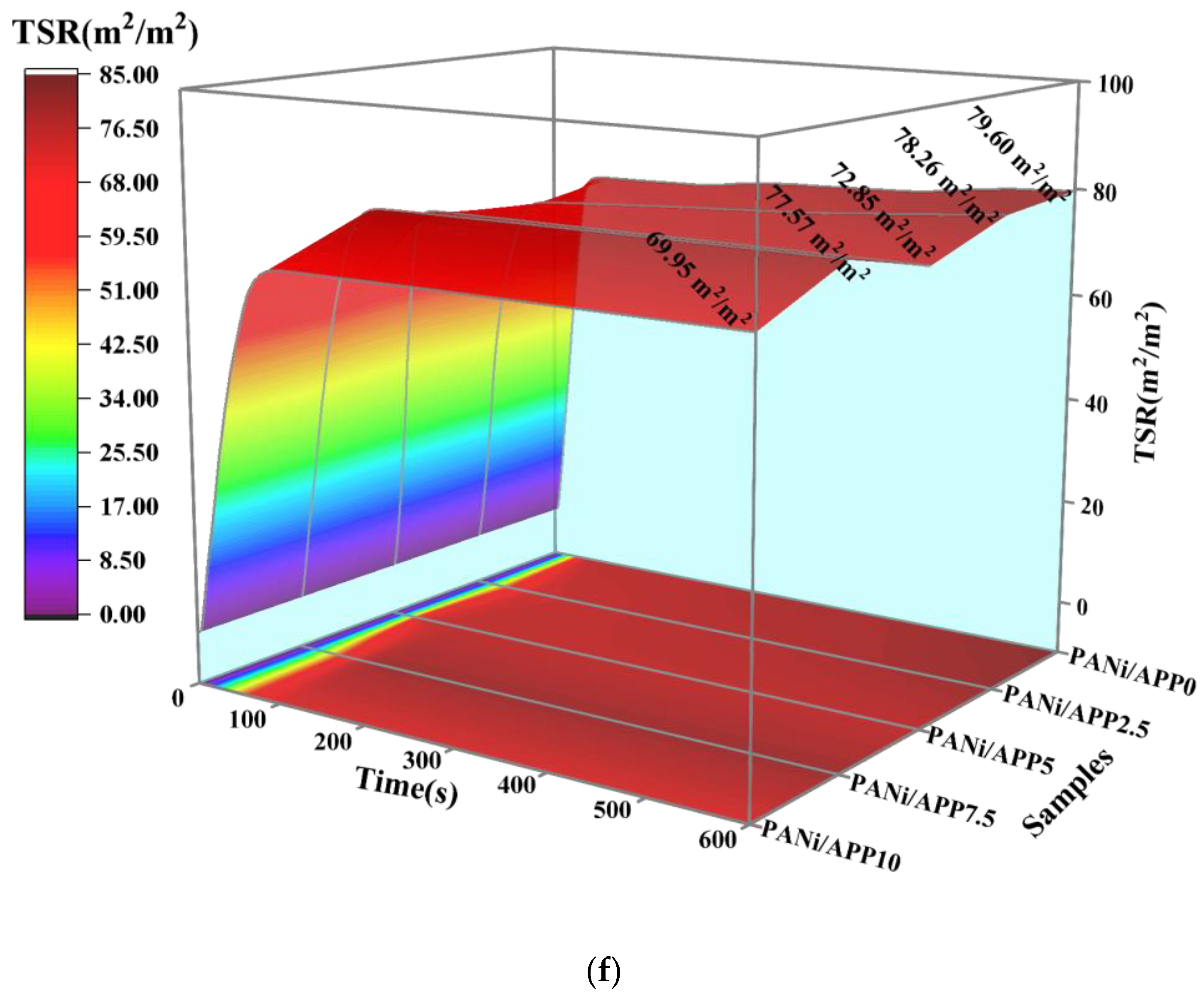
3.4. Fire Risk Assessment
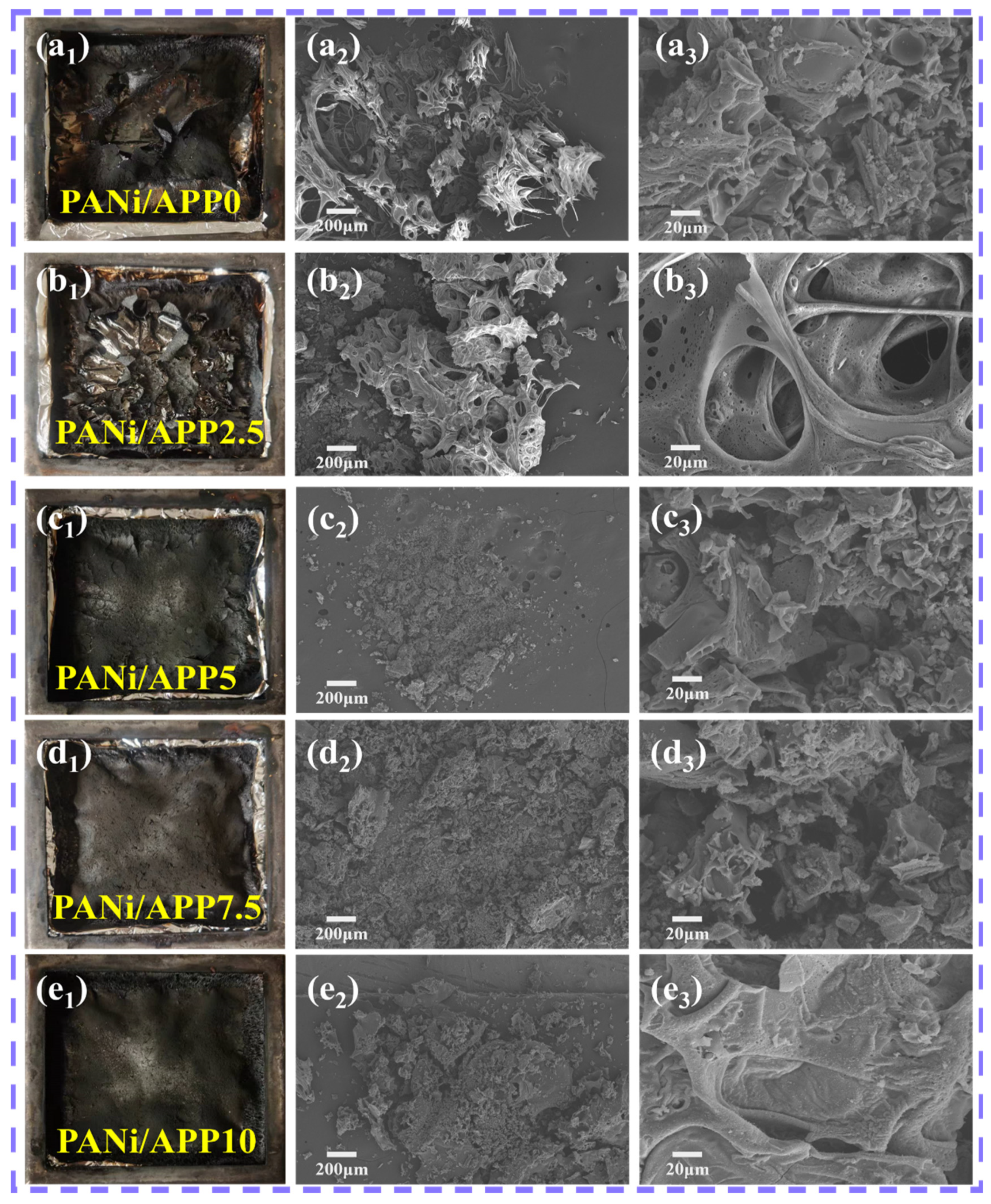
3.5. Carbon Layer Analysis
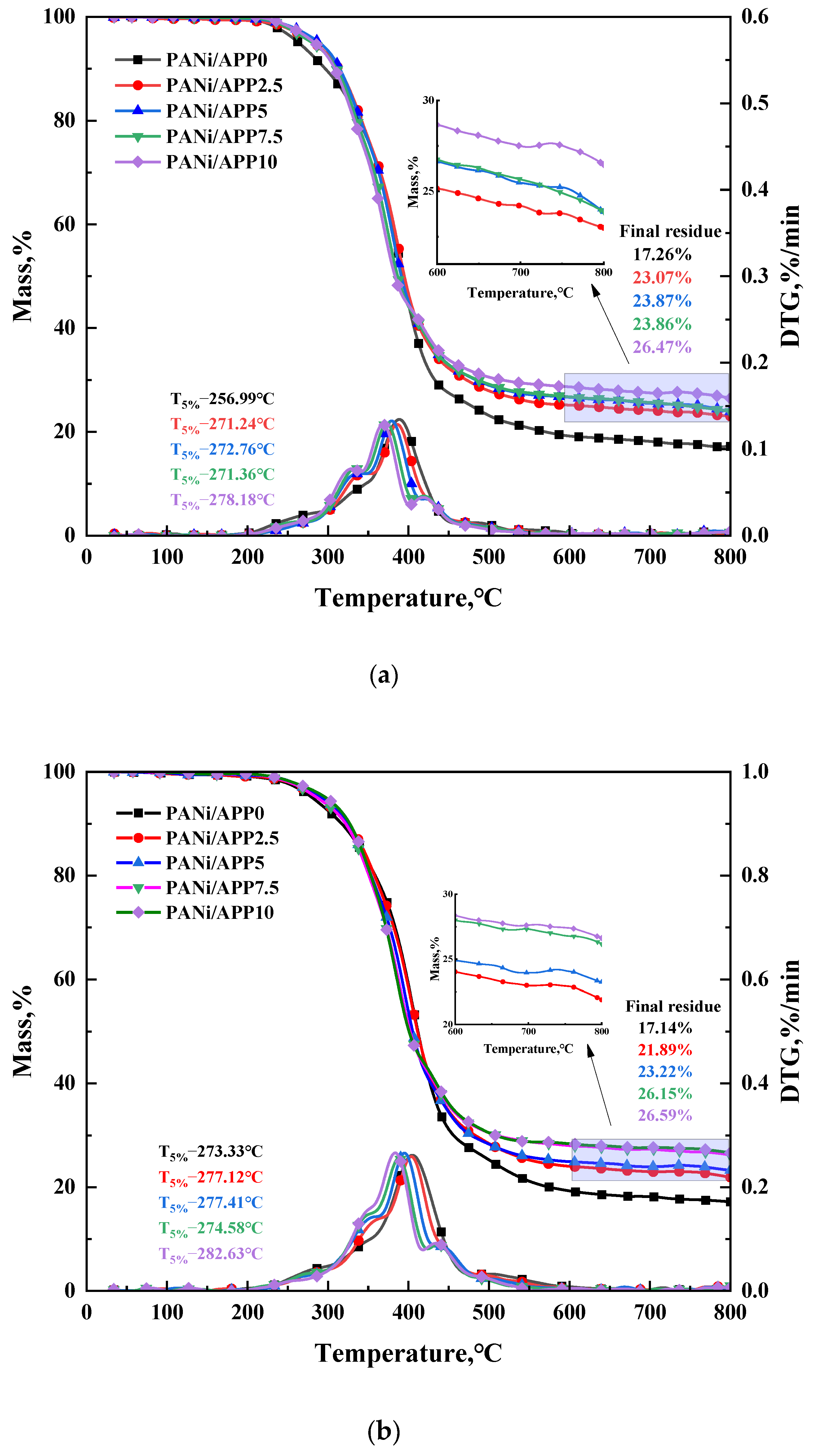
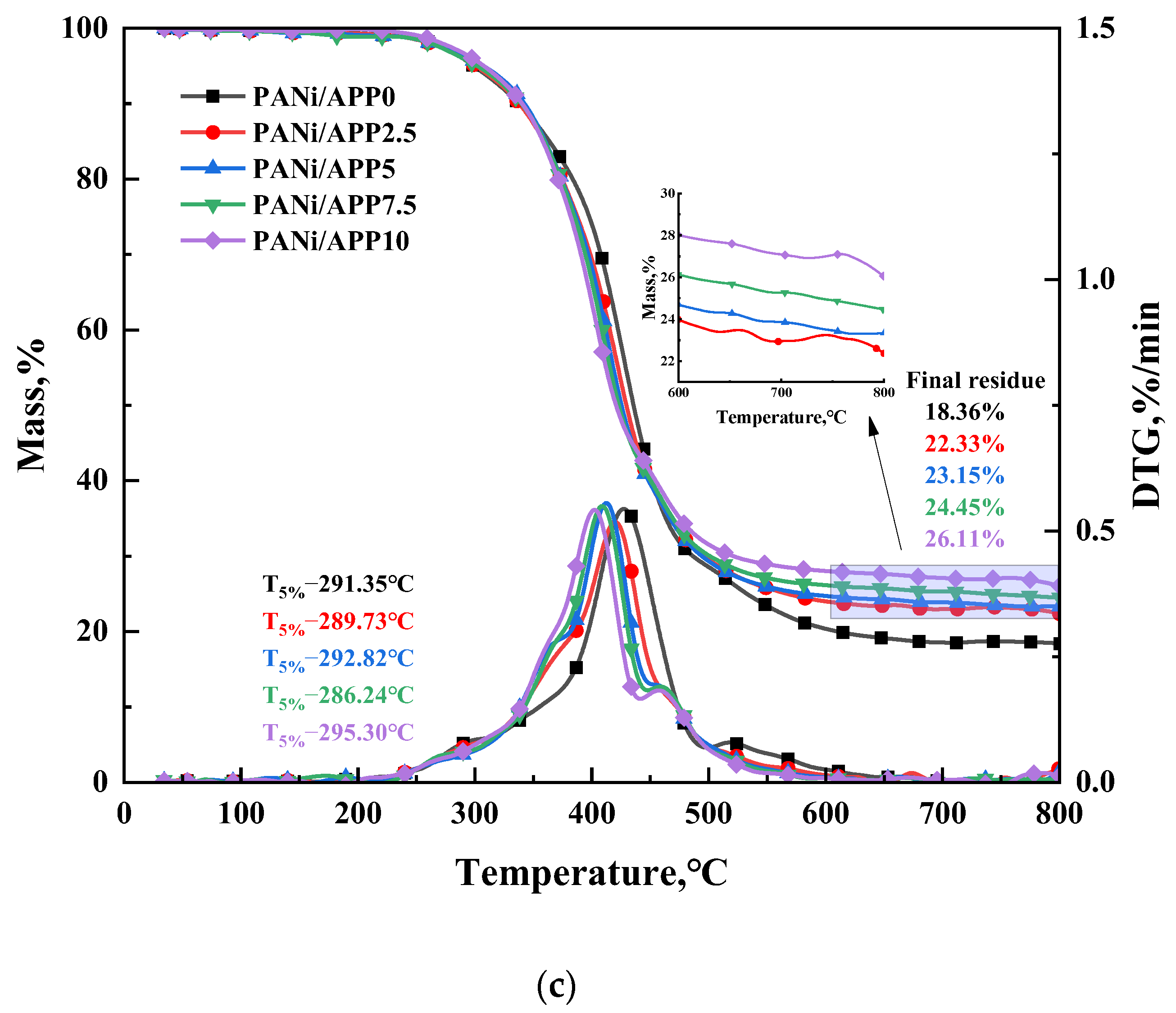
3.6. Thermal Stability
3.7. Activation Energy Analysis
3.8. Flame-Retardant Mechanism
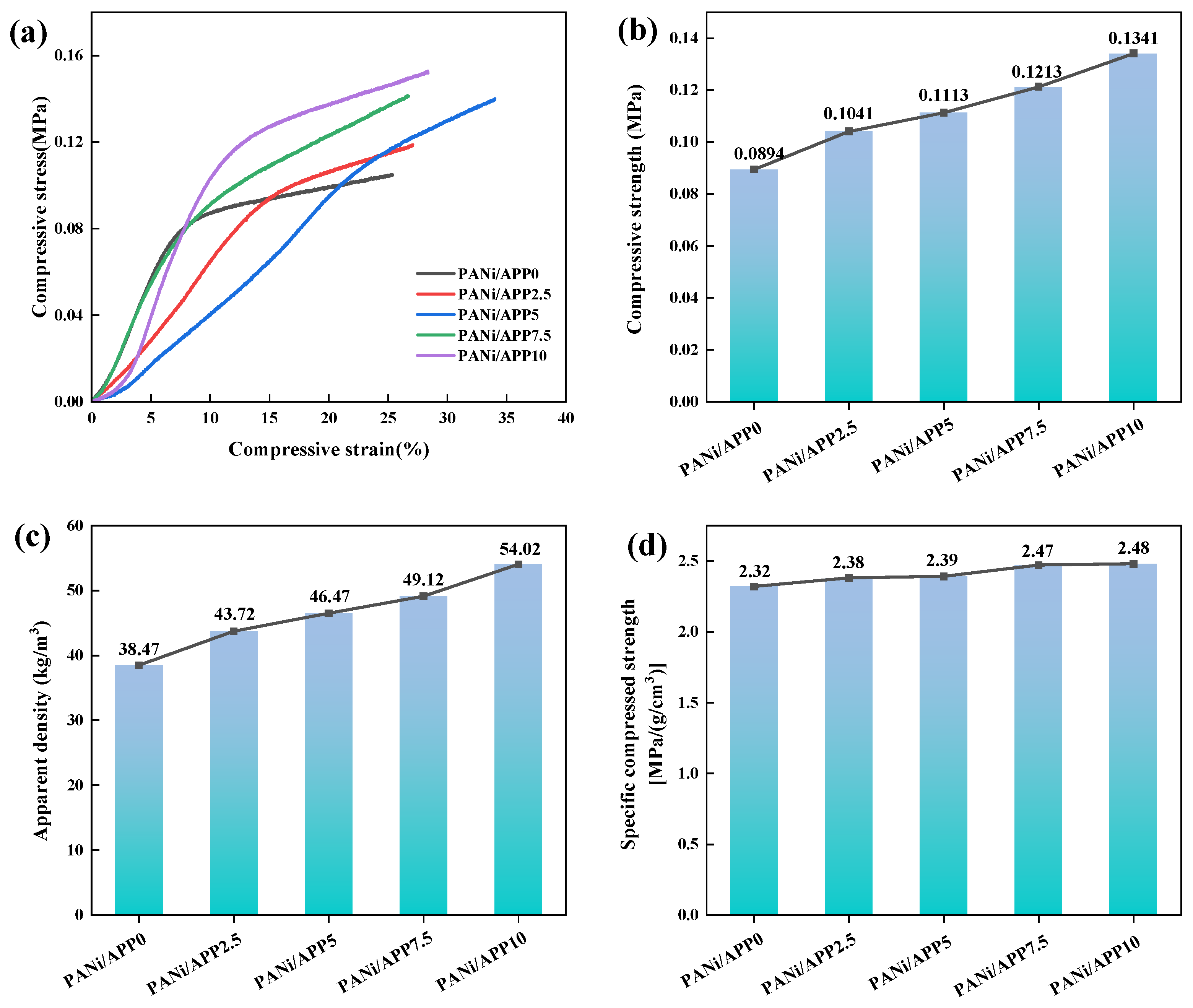
3.9. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pietro, M. Flexural behaviour of GFRP sandwich panels with eco-friendly PET foam core for the rehabilitation of building floors. Structures 2024, 60, 105818. [Google Scholar]
- Kirpluks, M.; Kalnbunde, D.; Benes, H.; Cabulis, U. Natural oil based highly functional polyols as feedstock for rigid polyurethane foam thermal insulation. Ind. Crops Prod. 2018, 122, 627–636. [Google Scholar] [CrossRef]
- Czupryński, B.; Paciorek-sadowska, J.; Liszkowska, J. Studies on Effect of Tri (2-hydroxypropyl), Tri (2-hydroxybutyl) and Tri (hydroxythiodiethylene) Borates on Thermal and Heat Properties of Rigid Polyurethane-Polyisocyanurate Foams. Chin. J. Chem. 2006, 24, 1796–1799. [Google Scholar] [CrossRef]
- Chai, H.; Duan, Q.; Jiang, L.; Sun, J. Effect of inorganic additive flame retardant on fire hazard of polyurethane exterior insulation material. J. Therm. Anal. Calorim. 2019, 135, 2857–2868. [Google Scholar] [CrossRef]
- Zhu, M.H.; Ma, Z.W.; Liu, L.; Zhang, J.Z.; Huo, S.Q.; Song, P.A. Recent advances in fire- retardant rigid polyurethane foam. J. Mater. Sci. Technol. 2022, 112, 315–328. [Google Scholar] [CrossRef]
- Michałowski, S.; Prociak, A. Flexible polyurethane foams modified with new bio-polyol based on rapeseed oil. J. Renew. Mater. 2015, 3, 14–18. [Google Scholar] [CrossRef]
- Ma, Z.W.; Liu, X.C.; Xu, X.D.; Liu, L.; Yu, B.; Maluk, C.; Huang, G.B.; Wang, H.; Song, P.G. Bioinspired, highly adhesive, nanostructured polymeric coatings for superhydrophobic fire-extinguishing thermal insulation foam. ACS Nano 2021, 15, 11667–11680. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Wang, L.; Xiao, A. Recent research progress on the flame-retardant mechanism of halogen-free flame retardant polypropylene. Polym. Plast. Technol. Eng. 2009, 48, 297–302. [Google Scholar] [CrossRef]
- Liao, S.F.; Deng, C.; Huang, S.C.; Cao, J.Y.; Wang, Y.Z. An efficient halogen-free flame retardant for polyethylene: Piperazinemodified ammonium poly-phosphates with different structures. Chin. J. Polym. Sci. 2016, 34, 1339–1353. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, R.Q.; Zhou, G.D.; Fang, Z.P.; Li, X.N. Flammability characterization and effects of magnesium oxide in halogen-free flame-retardant EVA blends. Chin. J. Polym. Sci. 2015, 33, 1683e1690. [Google Scholar] [CrossRef]
- Yang, H.T.; Yu, B.; Xu, X.D.; Bourbigot, S.; Wang, H.; Song, P.A. Lignin-derived bio-based flame retardants toward high-performance sustainable polymeric materials. Green Chem. 2020, 22, 2129–2161. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.M.; Yuan, D.H.; Wang, Z.; Xie, H.; Su, Z.Q. Chicken feather protein for the thermal stability and combustion performance of rigid polyurethane foam. Int. Polym. Process. 2023, 38, 593–605. [Google Scholar] [CrossRef]
- Xia, Y.R.; Chai, W.H.; Liu, Y.H.; Su, X.Y.; Liao, C.C.; Gao, M.H.; Li, Y.G.; Zheng, Z.H. Facile fabrication of starch-based, synergistic intumescent and halogen-free flame retardant strategy with expandable graphite in enhancing the fire safety of polypropylene. Ind. Crops Prod. 2022, 184, 115002. [Google Scholar] [CrossRef]
- Wei, A.; Ou, M.F.; Wang, S.X.; Zou, Y.J.; Xiang, C.L.; Xu, F.; Sun, L.X. Preparation of a Highly Flame-Retardant Urea–Formaldehyde Resin and Flame Retardance Mechanism. Polymers 2024, 16, 1761. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Zhang, W.P.; Ahmed, S.; Ali, M.; Xu, S.A. Effects of intumescent flame retardant system consisting of tris (2-hydroxyethyl) isocyanurate and ammonium polyphosphate on the flame retardant properties of high-density polyethylene composites. Compos. Part A Appl. Sci. Manuf. 2018, 112, 444–451. [Google Scholar] [CrossRef]
- Liu, X.Y.; Sui, Y.L.; Guo, P.Y.; Chen, R.; Mu, J.X. A flame retardant containing biomass-based polydopamine for high-performance rigid polyurethane foam. New J. Chem. 2022, 46, 11985–11993. [Google Scholar] [CrossRef]
- Yu, X.; Su, X.W.; Liu, Y.S.; Yu, D.; Ren, Y.L.; Liu, X.H. Biomass intumescent flame retardant polyacrylonitrile composite: Flame retardancy, smoke suppression and recycling. Compos. Part A Appl. Sci. Manuf. 2023, 173, 107647. [Google Scholar] [CrossRef]
- Wang, Y.D.; Ma, L.; Wang, H.; Cheng, C.Z.; Yin, X.Z.; Zhu, Z.M. Fabrication of a flame retardant, strong mechanical toughness and antimicrobial polylactic acid by chitosan Schiff base/ammonium polyphosphate. Polym. Degrad. Stab. 2023, 216, 110492. [Google Scholar] [CrossRef]
- Wang, Y.D.; Liu, L.Y.; Ma, L.; Yuan, J.; Wang, L.X.; Wang, H.; Xiao, F.; Zhu, Z.M. Transparent, flame retardant, mechanically strengthened and low dielectric EP composites enabled by a reactive bio-based P/N flame retardant. Polym. Degrad. Stab. 2022, 204, 110106. [Google Scholar] [CrossRef]
- He, X.D.; Zhang, W.C.; Yi, D.Q.; Yang, R.J. Flame retardancy of ammonium polyphosphate–montmorillonite nanocompounds on epoxy resin. J. Fire Sci. 2016, 34, 212–225. [Google Scholar] [CrossRef]
- Chen, M.; Tang, M.Q.; Qi, F.; Chen, X.L.; He, W.D. Microencapsulated ammonium polyphosphate and its application in the flame retardant polypropylene composites. J. Fire Sci. 2015, 33, 374–389. [Google Scholar] [CrossRef]
- Chen, X.L.; Jiang, Y.F.; Jiao, C.M. Smoke suppression properties of ferrite yellow on flame retardant thermoplastic polyurethane based on ammonium polyphosphate. J. Hazard. Mater. 2014, 266, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.Q.; Sun, S.M.; Yuan, D.H.; Wen, Y.Q.; Su, Z.P.; Wang, Z.; Xie, H. Fabrication of soybean oil-based polyol modified polyurethane foam from ammonium polyphosphate and its thermal stability and flame retardant properties. Int. Polym. Proc. 2024, 39, 32–46. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, Z.H.; Han, X.X.; Wang, B.B.; Yang, S.J.; Chen, D.P.; Yang, Y.D.; Liu, X.Y.; Tang, G. Effect of ammonium polyphosphate/cobalt phytate system on flame retardancy and smoke & toxicity suppression of rigid polyurethane foam composites. J. Polym. Res. 2021, 28, 1–16. [Google Scholar]
- Li, Q.M.; Wang, J.Y.; Chen, L.M.; Shi, H.; Hao, J.W. Ammonium polyphosphate modified with β-cyclodextrin crosslinking rigid polyurethane foam: Enhancing thermal stability and suppressing flame spread. Polym. Degrad. Stab. 2019, 161, 166–174. [Google Scholar] [CrossRef]
- Liao, M.; Chen, H.J.; Deng, L.M.; Wei, X.J.; Zou, Z.X.; Wang, H.; Chen, S.H.; Zhu, Z.M. Tannic acid-polyethyleneimine modified ammonium polyphosphate: For efficient flame retardant and UV resistant of polylactic acid. React. Funct. Polym. 2023, 192, 105735. [Google Scholar] [CrossRef]
- Fox, C.H.; Eberl, M. Phytic acid (IP6), novel broad spectrum anti-neoplastic agent: A systematic review. Complement. Ther. Med. 2002, 10, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.Q.; Ding, S.; Wang, Z. Fabrication of nickel phytate modified bio-based polyol rigid polyurethane foam with enhanced compression-resistant and improved flame-retardant. Sci. Rep. 2024, 14, 16651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Liu, Y.Z.; Fu, X.; Wang, T.; Sun, G.H.; Zhang, X.; Han, S.H. Fabrication of rigid isocyanate-based polyimide foam achieved excellent use safety via synergy between expandable graphite and phosphorus-containing Polyol. Polymers 2023, 15, 1381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.Q.; Sun, S.M.; Wang, Y.M.; Wang, Z.; Xie, H. Fabrication of expandable graphite and soybean oil-based synergistic modified polyurethane foam with improved thermal stability and flame retardant properties. Int. Polym. Proc. 2023, 39, 162–175. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.W.; Sun, S.M.; Xu, C.; Wang, Z.; Xie, H. Improved thermal stability and flame retardant performance of rigid polyurethane foam modified by hydrolyzed keratin-modified polyol and aluminum hypophosphite. Polym. Int. 2024, 73, 393–409. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Guida, M.Y.; Bouaik, H.; El Mouden, L.; Moubarik, A.; Aboulkas, A.; El Harfi, K.; Hannioui, A. Utilization of starink approach and avrami theory to evaluate the kinetic parameters of the pyrolysis of olive mill solid waste and olive mill wastewater. J. Adv. Chem. Eng. 2017, 7, 1–8. [Google Scholar]
- Kissinger, H.H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Coats, A.; Redfern, J. Kinetic parameters from the thermogravimetric data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.Q.; Sun, S.M.; Wang, Z.; Xie, H. Preparation of bio-based soybean oil polyol modified 3630 polyol-based rigid polyurethane foam and its improved thermal stability, flame retardant, and smoke suppression performances. Polym. Adv. Technol. 2024, 35, e6343. [Google Scholar] [CrossRef]
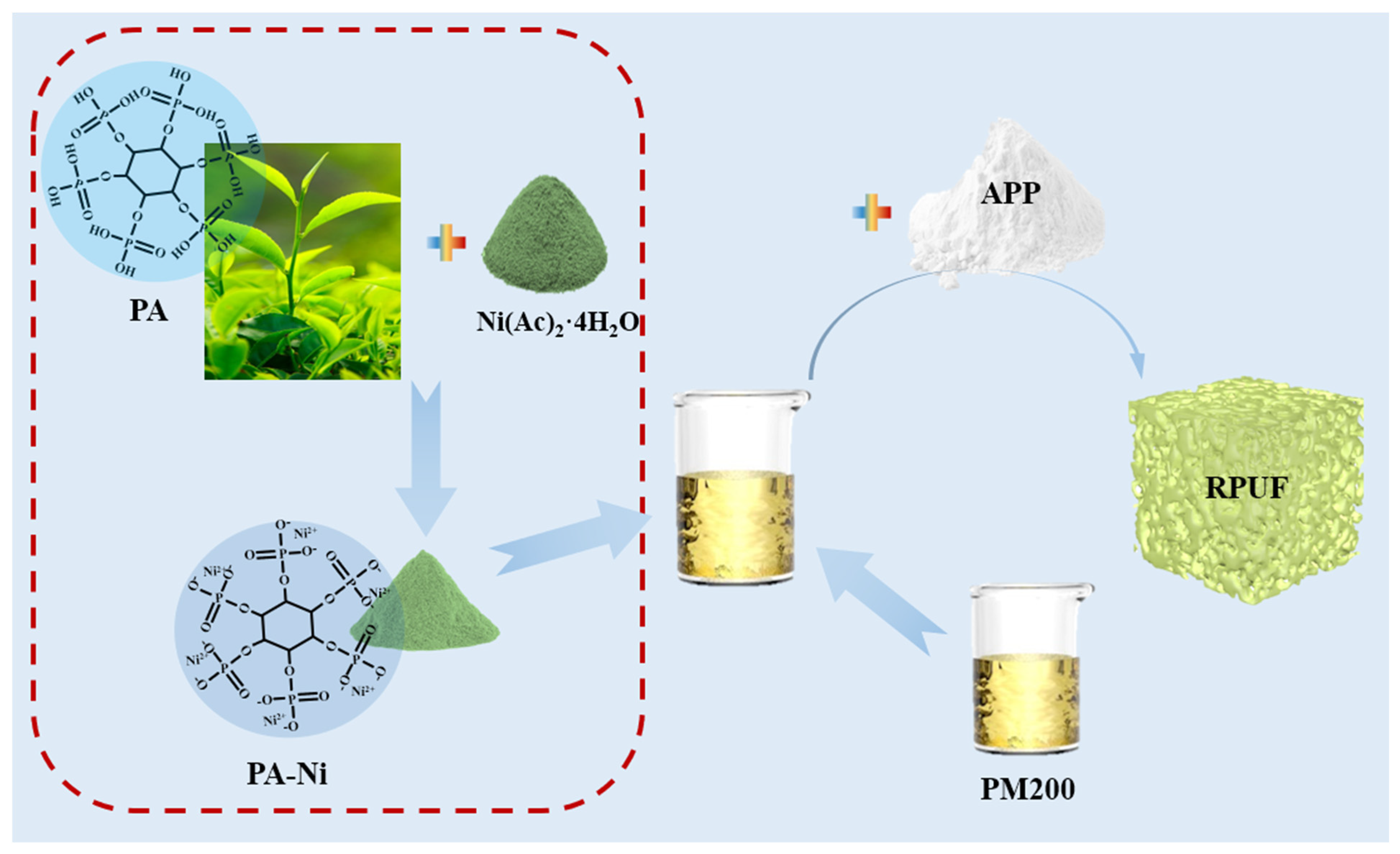





| Sample | 3630N (g) | PM200 (g) | T9 (g) | L-580 (g) | A33 (g) | Deionized Water (g) | PANi (wt%) | APP (wt%) |
|---|---|---|---|---|---|---|---|---|
| PANi/APP0 | 40 | 60 | 0.2 | 1 | 1.8 | 1 | 3 | 0 |
| PANi/APP2.5 | 40 | 60 | 0.2 | 1 | 1.8 | 1 | 3 | 2.5 |
| PANi/APP5 | 40 | 60 | 0.2 | 1 | 1.8 | 1 | 3 | 5 |
| PANi/APP7.5 | 40 | 60 | 0.2 | 1 | 1.8 | 1 | 3 | 7.5 |
| PANi/APP10 | 40 | 60 | 0.2 | 1 | 1.8 | 1 | 3 | 10 |
| Radiation Intensity (kW/m2) | Sample | ToxPI (kg/s) | TSPI (m2/s) | FGI (kW/m2·s) | THRI (MJ/m2) |
|---|---|---|---|---|---|
| 25 | PANi/APP0 | 0.43 | 2.61 | 1.46 | 2.61 |
| PANi/APP2.5 | 0.28 | 2.58 | 1.83 | 2.39 | |
| PANi/APP5 | 0.36 | 2.59 | 1.32 | 2.38 | |
| PANi/APP7.5 | 0.38 | 2.53 | 1.41 | 2.40 | |
| PANi/APP10 | 0.21 | 2.48 | 1.25 | 2.36 | |
| 35 | PANi/APP0 | 0.52 | 2.58 | 1.86 | 2.53 |
| PANi/APP2.5 | 0.50 | 2.61 | 1.55 | 2.41 | |
| PANi/APP5 | 0.57 | 2.53 | 1.96 | 2.41 | |
| PANi/APP7.5 | 0.52 | 2.71 | 1.53 | 2.42 | |
| PANi/APP10 | 0.40 | 2.51 | 1.49 | 2.35 | |
| 50 | PANi/APP0 | 0.62 | 2.64 | 2.55 | 2.50 |
| PANi/APP2.5 | 0.63 | 2.70 | 2.10 | 2.48 | |
| PANi/APP5 | 0.62 | 2.62 | 2.23 | 2.42 | |
| PANi/APP7.5 | 0.66 | 2.87 | 2.08 | 2.59 | |
| PANi/APP10 | 0.53 | 2.62 | 1.85 | 2.38 |
| Heating Rate (°C/min) | Sample | Weight Loss Temperature Range (°C) | Percent Weightlessness (%) | Initial Decomposition Temperature (°C) | IDPT (°C) |
|---|---|---|---|---|---|
| 10 | RPUF-Ni/APP0 | 164.70–457.82 | 73.232 | 164.70 | 647.75 |
| 457.82–800.00 | 9.39 | ||||
| RPUF-Ni/APP2.5 | 185.68–452.53 | 67.54 | 185.68 | 772.10 | |
| 452.53–800.00 | 8.73 | ||||
| RPUF-Ni/APP5 | 186.77–416.96 | 60.24 | 186.77 | 791.77 | |
| 416.96–800.00 | 15.42 | ||||
| RPUF-Ni/APP7.5 | 192.08–407.48 | 57.81 | 192.08 | 790.36 | |
| 407.48–800.00 | 18.19 | ||||
| RPUF-Ni/APP10 | 198.06–401.28 | 55.86 | 198.06 | 854.14 | |
| 401.28–800.00 | 19.80 | ||||
| 20 | RPUF-Ni/APP0 | 166.89–471.32 | 71.13 | 166.89 | 657.84 |
| 471.32–800.00 | 10.87 | ||||
| RPUF-Ni/APP2.5 | 203.37–477.70 | 68.68 | 203.37 | 752.22 | |
| 477.70–800.00 | 8.94 | ||||
| RPUF-Ni/APP5 | 205.96–431.55 | 59.90 | 205.96 | 779.23 | |
| 431.55–800.00 | 16.24 | ||||
| RPUF-Ni/APP7.5 | 207.17–424.26 | 56.51 | 207.17 | 849.07 | |
| 424.26–800.00 | 16.87 | ||||
| RPUF-Ni/APP10 | 209.46–418.06 | 55.38 | 209.46 | 860.83 | |
| 418.06–800.00 | 17.86 | ||||
| 40 | RPUF-Ni/APP0 | 185.56–497.58 | 70.32 | 185.56 | 694.79 |
| 497.58–800.00 | 10.56 | ||||
| RPUF-Ni/APP2.5 | 187.68–495.39 | 69.33 | 187.68 | 769.58 | |
| 495.39–800.00 | 7.952 | ||||
| RPUF-Ni/APP5 | 201.95–448.34 | 58.60 | 201.95 | 789.74 | |
| 448.34–800.00 | 17.20 | ||||
| RPUF-Ni/APP7.5 | 206.76–446.33 | 57.95 | 206.76 | 814.56 | |
| 446.33–800.00 | 16.58 | ||||
| RPUF-Ni/APP10 | 210.98–441.04 | 56.48 | 210.98 | 851.97 | |
| 441.01–800.00 | 17.38 |
| α | PANi/APP0 | PANi/APP2.5 | PANi/APP5 | PANi/APP7.5 | PANi/APP10 |
|---|---|---|---|---|---|
| (%) | kJ/mol | kJ/mol | kJ/mol | kJ/mol | kJ/mol |
| 5 | 95.45 | 139.34 | 121.47 | 156.92 | 148.61 |
| 10 | 91.83 | 150.49 | 106.68 | 129.63 | 149.57 |
| 20 | 100.65 | 135.77 | 123.44 | 115.90 | 112.03 |
| 30 | 102.48 | 125.35 | 121.76 | 112.87 | 114.42 |
| 40 | 112.44 | 133.67 | 133.49 | 122.22 | 122.25 |
| 50 | 120.52 | 138.49 | 142.36 | 128.90 | 132.67 |
| 60 | 122.54 | 143.25 | 144.46 | 134.30 | 131.01 |
| 70 | 124.18 | 140.60 | 143.04 | 135.96 | 127.86 |
| 80 | 125.77 | 143.50 | 143.44 | 143.03 | 156.62 |
| 90 | 137.10 | 142.73 | 144.83 | 147.46 | 206.24 |
| E | 113.30 | 139.32 | 132.50 | 132.72 | 140.13 |
| α | PANi/APP0 | PANi/APP2.5 | PANi/APP5 | PANi/APP7.5 | PANi/APP10 |
|---|---|---|---|---|---|
| (%) | kJ/mol | kJ/mol | kJ/mol | kJ/mol | kJ/mol |
| 5 | 91.57 | 137.60 | 126.19 | 159.15 | 147.40 |
| 10 | 87.30 | 148.78 | 116.52 | 139.99 | 147.84 |
| 20 | 95.89 | 132.84 | 119.88 | 121.42 | 115.34 |
| 30 | 97.39 | 121.65 | 117.81 | 121.65 | 110.15 |
| 40 | 107.59 | 130.11 | 129.87 | 118.08 | 118.22 |
| 50 | 115.90 | 134.96 | 139.02 | 132.34 | 131.92 |
| 60 | 117.86 | 139.72 | 141.09 | 130.48 | 131.96 |
| 70 | 119.41 | 136.86 | 139.41 | 132.01 | 126.42 |
| 80 | 120.80 | 139.58 | 154.79 | 148.92 | 153.45 |
| 90 | 131.87 | 138.09 | 150.84 | 151.69 | 205.04 |
| E | 108.56 | 136.02 | 133.54 | 135.57 | 138.77 |
| Sample | PANi/APP0 (kJ/mol) | PANi/APP2.5 (kJ/mol) | PANi/APP5 (kJ/mol) | PANi/APP7.5 (kJ/mol) | PANi/APP10 (kJ/mol) |
|---|---|---|---|---|---|
| K = −E/R | −14.78 | −17.44 | −16.59 | −15.28 | −17.62 |
| E | 122.85 | 144.99 | 137.95 | 127.07 | 146.48 |
| Heating Rate β (°C/min) | Sample | Temperature Range (°C) | E (kJ/mol) | Heating Rate β (°C/min) |
|---|---|---|---|---|
| 10 | PANi/APP0 | 164.70–457.82 | 47.91 | 49.10 |
| 457.82–800.00 | 1.19 | |||
| PANi/APP2.5 | 185.68–452.53 | 58.59 | 66.76 | |
| 452.53–800.00 | 7.87 | |||
| PANi/APP5 | 186.77–416.96 | 68.18 | 69.03 | |
| 416.96–800.00 | 0.85 | |||
| PANi/APP7.5 | 198.08–407.48 | 64.58 | 68.01 | |
| 407.48–800.00 | 3.43 | |||
| PANi/APP10 | 192.06–401.28 | 73.98 | 76.87 | |
| 401.28–800.00 | 2.89 | |||
| 20 | PANi/APP0 | 166.89–471.32 | 49.15 | 52.87 |
| 471.32–800.00 | 3.72 | |||
| PANi/APP2.5 | 203.37–477.70 | 51.92 | 61.33 | |
| 477.70–800.00 | 9.41 | |||
| PANi/APP5 | 205.96–431.55 | 57.34 | 67.56 | |
| 431.55–800.00 | 10.22 | |||
| PANi/APP7.5 | 197.17–424.26 | 57.38 | 61.44 | |
| 424.26–800.00 | 4.06 | |||
| PANi/APP10 | 209.46–418.06 | 65.23 | 75.18 | |
| 418.06–800.00 | 9.95 | |||
| 40 | PANi/APP0 | 185.56–497.58 | 51.50 | 68.44 |
| 497.58–800.00 | 16.94 | |||
| PANi/APP2.5 | 187.68–495.39 | 50.61 | 67.40 | |
| 495.39–800.00 | 16.79 | |||
| PANi/APP5 | 201.95–448.34 | 48.89 | 62.70 | |
| 448.34–800.00 | 13.81 | |||
| PANi/APP7.5 | 206.76–446.33 | 49.92 | 65.88 | |
| 446.33–800.00 | 15.96 | |||
| PANi/APP10 | 210.98–441.04 | 66.18 | 80.98 | |
| 441.01–800.00 | 14.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, Z.; Ding, S.; Wang, Z.; Xie, H. Fabrication of Flame-Retardant Ammonium Polyphosphate Modified Phytic Acid-Based Rigid Polyurethane Foam with Enhanced Mechanical Properties. Polymers 2024, 16, 2229. https://doi.org/10.3390/polym16152229
Zhang X, Wang Z, Ding S, Wang Z, Xie H. Fabrication of Flame-Retardant Ammonium Polyphosphate Modified Phytic Acid-Based Rigid Polyurethane Foam with Enhanced Mechanical Properties. Polymers. 2024; 16(15):2229. https://doi.org/10.3390/polym16152229
Chicago/Turabian StyleZhang, Xu, Zhaoqian Wang, Shuai Ding, Zhi Wang, and Hua Xie. 2024. "Fabrication of Flame-Retardant Ammonium Polyphosphate Modified Phytic Acid-Based Rigid Polyurethane Foam with Enhanced Mechanical Properties" Polymers 16, no. 15: 2229. https://doi.org/10.3390/polym16152229
APA StyleZhang, X., Wang, Z., Ding, S., Wang, Z., & Xie, H. (2024). Fabrication of Flame-Retardant Ammonium Polyphosphate Modified Phytic Acid-Based Rigid Polyurethane Foam with Enhanced Mechanical Properties. Polymers, 16(15), 2229. https://doi.org/10.3390/polym16152229





