Sodium-Alginate-Doped Lignin Nanoparticles for PBAT Composite Films to Dually Enhance Tensile Strength and Elongation Performance with Functionality
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Purified Lignin
2.3. Preparation of Lignin Nanoparticles (LNPs)
2.4. Preparation of PBAT/LNP Composite Films
2.5. Characterizations
2.5.1. Morphology
2.5.2. Zeta Potential and Particle Size Analysis
2.5.3. Contact Angle Measurement
2.5.4. Properties of PBAT/LNP Composite Films
Functional Groups
Mechanical Properties
Moisture Barrier Properties
UV Resistance
Thermal Stability
3. Results and Discussion
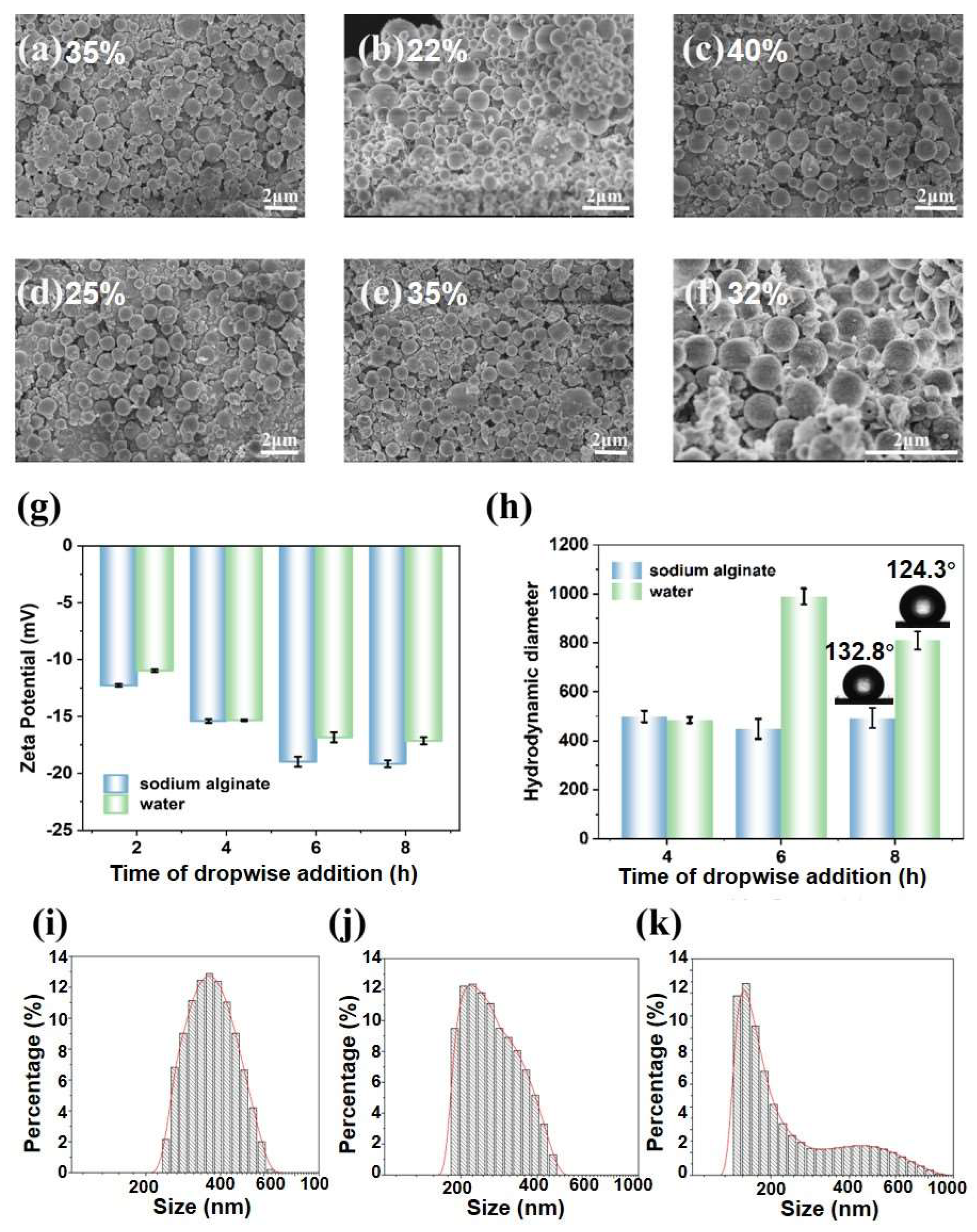
3.1. Characterization of SLNPs
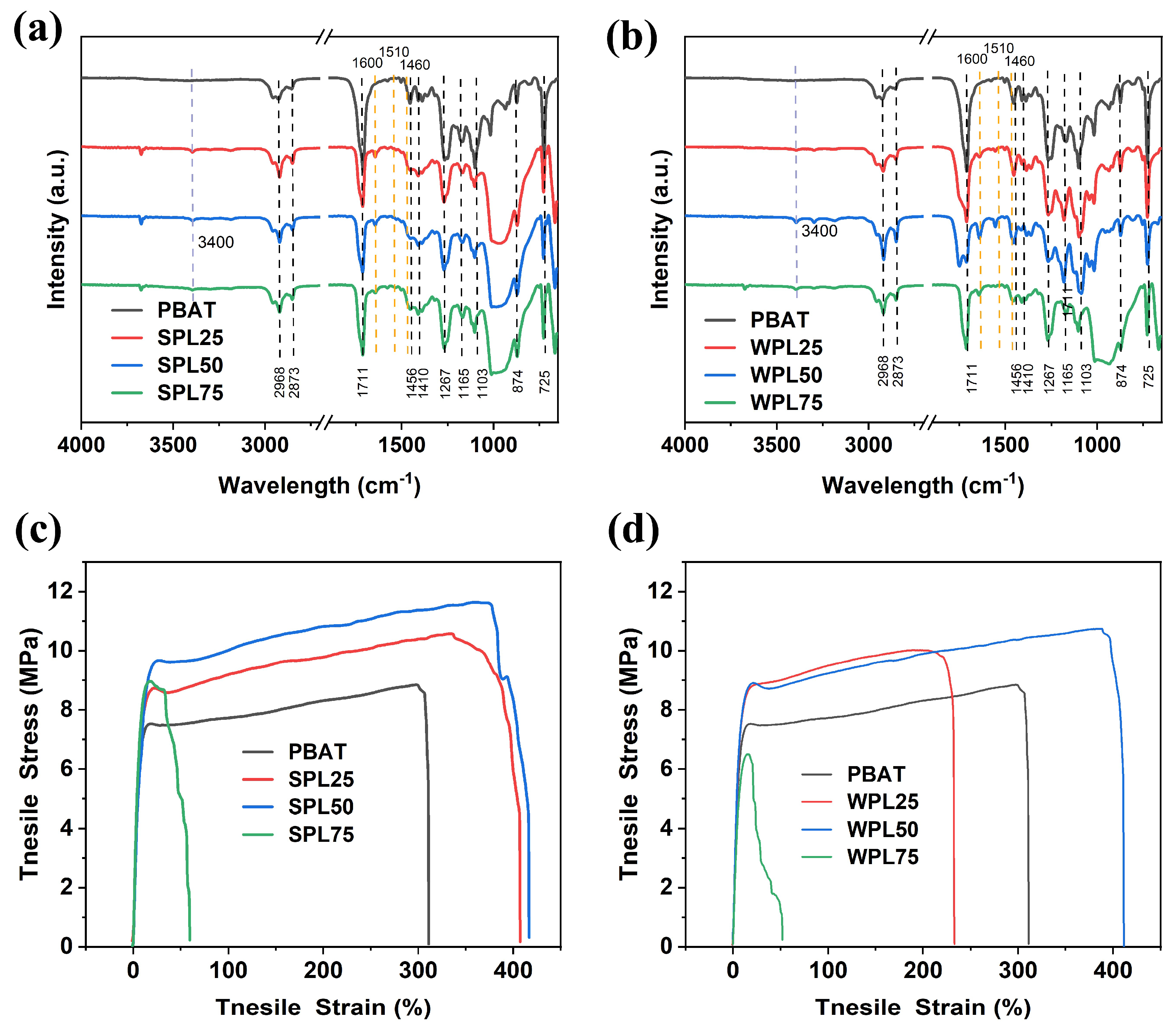
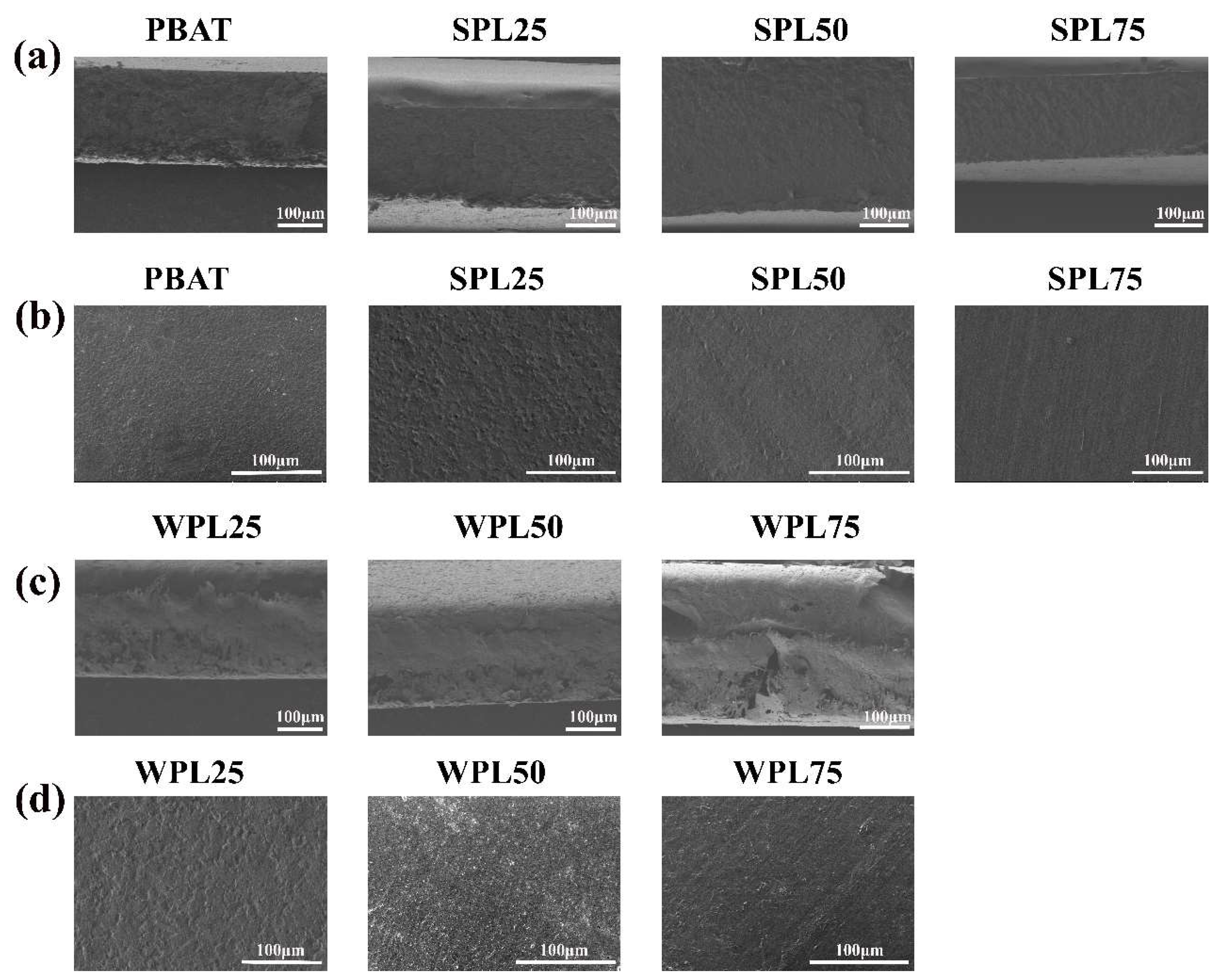
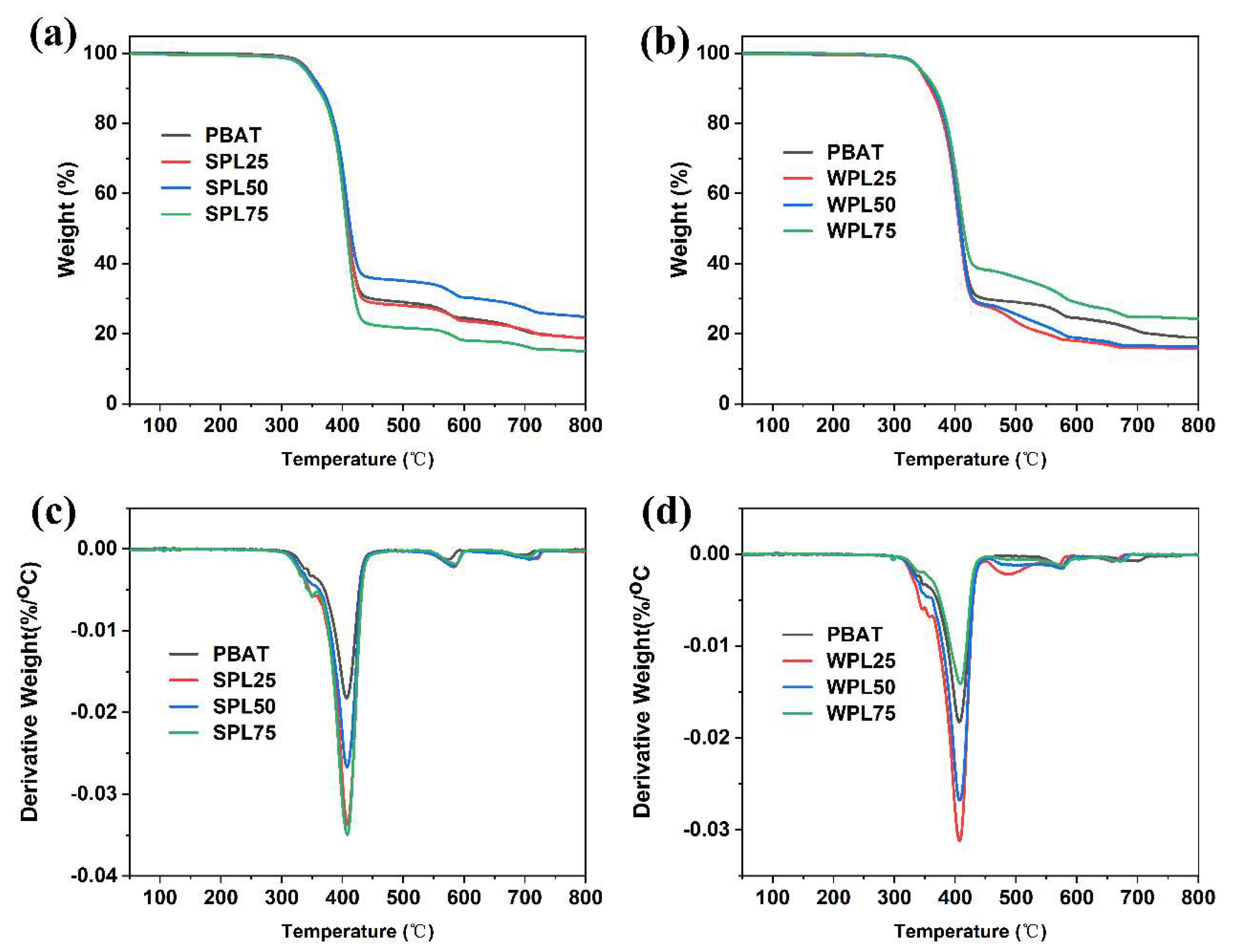
3.2. Characterization of PBAT/LNP Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Filiciotto, L.; Rothenberg, G. Biodegradable Plastics: Standards, Policies, and Impacts. ChemSusChem 2021, 14, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Arend, M. Malaysian Investment Development Authority. Available online: https://www.mida.gov.my/ (accessed on 15 July 2024).
- Jiao, J.; Zeng, X.; Huang, X. An Overview on Synthesis, Properties and Applications of Poly. (Butylene-Adipate-Co-Terephthalate)—PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Morro, A.; Catalina, F.; Sanchez-León, E.; Abrusci, C. Photodegradation and Biodegradation Under Thermophile Conditions of Mulching Films Based on Poly (Butylene Adipate-Co-Terephthalate) and Its Blend with Poly (Lactic Acid). J. Polym. Environ. 2019, 27, 352–363. [Google Scholar] [CrossRef]
- Xie, J.; Yan, Y.; Fan, S.; Min, X.; Wang, L.; You, X.; Jia, X.; Waterhouse, G.I.; Wang, J.; Xu, J. Prediction Model of Photodegradation for PBAT/PLA Mulch Films: Strategy to Fast Evaluate Service Life. Environ. Sci. Technol. 2022, 56, 9041–9051. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Renders, A.T.; van den Bosch, S.; Koelewijn, S.F.; Beckham, G.; Sels, B.F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gu, Y.; Feng, S.; Yang, W.; Dai, H.; Xiao, H.; Han, J. Lignin-Containing Biodegradable UV-Blocking Films: A Review. Green Chem. 2023, 25, 9020–9044. [Google Scholar] [CrossRef]
- Jędrzejczak, P.; Collins, M.N.; Jesionowski, T.; Klapiszewski, Ł. The Role of Lignin and Lignin-Based Materials in Sustainable Construction—A Comprehensive Review. Int. J. Biol. Macromol. 2021, 187, 624–650. [Google Scholar] [CrossRef]
- Xiong, S.J.; Pang, B.; Zhou, S.J.; Li, M.K.; Yang, S.; Wang, Y.Y.; Shi, Q.; Wang, S.F.; Yuan, T.Q.; Sun, R.C. Economically Competitive Biodegradable PBAT/Lignin Composites: Effect of Lignin Methylation and Compatibilizer. ACS Sustain. Chem. Eng. 2020, 8, 5338–5346. [Google Scholar] [CrossRef]
- Kim, J.; Bang, J.; Park, S.; Jung, M.; Jung, S.; Yun, H.; Kim, J.H.; Choi, I.G.; Kwak, H.W. Enhanced Barrier Properties of Biodegradable PBAT/Acetylated Lignin Films. Sustain. Mater. Technol. 2023, 37, e00686. [Google Scholar] [CrossRef]
- Han, M.; Yang, W. Improving the Antiradical Activity and UV Resistance of PBAT/Nanolignin Composite Films through Amination of Various Amino Acids. Ind. Crops Prod. 2023, 200, 116779. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, X.; Fu, Q.; Xu, P.; Liu, T.; Wang, Z.; Yang, W.; Ma, P. One Step to Simultaneously Improve the Antibacterial Activity and Compatibility with PBAT of Nanolignin Via Surface Modification. ACS Sustain. Chem. Eng. 2023, 11, 14773–14781. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhong, X.; Zhang, D.; Deng, Y.; Yang, D.; Zhu, S. Lignin Reverse Micelles for UV-Absorbing and High Mechanical Performance Thermoplastics. Ind. Eng. Chem. Res. 2015, 54, 12025–12030. [Google Scholar] [CrossRef]
- Schneider, W.D.H.; Dillon, A.J.P.; Camassola, M. Lignin Nanoparticles Enter the Scene: A Promising Versatile Green Tool for Multiple Applications. Biotechnol. Adv. 2021, 47, 107685. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Poonia, M.; Yoo, C.G.; Ragauskas, A.J. Recent Advances in Synthesis and Application of Lignin Nanoparticles. Lignin Util. Strateg. 2021, 1377, 273–293. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.L.; Hult, E.L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A Simple Process for Lignin Nanoparticle Preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Ma, M.; Dai, L.; Xu, J.; Liu, Z.; Ni, Y. A Simple and Effective Approach to Fabricate Lignin Nanoparticles with Tunable Sizes Based on Lignin Fractionation. Green Chem. 2020, 22, 2011–2017. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Yang, D.; Fang, Z.; Liu, W.; Xiang, T.; Qiu, X. Monodispersed Lignin Colloidal Spheres with Tailorable Sizes for Bio-Photonic Materials. Small 2022, 18, 2200671. [Google Scholar] [CrossRef] [PubMed]
- Aneem, T.; Wong, S.; Afrin, H.; Nurunnabi, M.; Li, X.; Arafat, M. Investigation of Coagulation Process of Wet-Spun Sodium Alginate Polymannuronate Fibers with Varied Functionality Using Organic Coagulants and Cross-Linkers. Mater. Today Chem. 2021, 22, 100580. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2016. Available online: www.astm.org (accessed on 1 January 2024).
- Ma, L.; Zhu, Y.; Huang, Y.; Zhang, L.; Wang, Z. Strong water-resistant, UV-Blocking Cellulose/Glucomannan/Lignin Composite Films Inspired by Natural LCC Bonds. Carbohydr. Polym. 2022, 281, 119083. [Google Scholar] [CrossRef]
- Xu, X.Q.; Xu, R.Y.; Wang, Z.Y.; Xu, G. Influence of Adsorption of Alginate on the Zeta Potential and Colloidal Stability of Fe3O4 Nanoparticles. In Proceedings of the Abstracts of the 25th Annual Conference of the Chinese Chemical Society (Part II), Changchun, China, 11–15 July 2006; p. 739. [Google Scholar]
- Menendez-Manjon, A.; Chichkov, B.N.; Barcikowski, S. Influence of Water Temperature on the Hydrodynamic Diameter of Gold Nanoparticles from Laser Ablation. J. Phys. Chem. C 2010, 114, 2499–2504. [Google Scholar] [CrossRef]
- Cai, Y.; Lv, J.; Feng, J. Spectral Characterization of Four Kinds of Biodegradable Plastics: Poly (Lactic Acid), Poly (Butylenes Adipate-Co-Terephthalate), Poly (Hydroxybutyrate-Co-Hydroxyvalerate) and Poly (Butylenes Succinate) with FTIR and Raman Spectroscopy. J. Polym. Environ. 2013, 21, 108–114. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’Ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions Between Gelatin and Sodium Alginate: UV and FTIR Studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Tran, N.T.; Nguyen, T.T.T.; Ha, D.; Nguyen, T.H.; Nguyen, N.N.; Baek, K.; Nguyen, N.T.; Tran, C.K.; Tran, T.T.V.; Le, H.V.; et al. Highly Functional Materials Based on Nano-Lignin, Lignin, and Lignin/Silica Hybrid Capped Silver Nanoparticles with Antibacterial Activities. Biomacromolecules 2021, 22, 5327–5338. [Google Scholar] [CrossRef] [PubMed]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV Light Blocker—A Review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Xiong, W.; Qiu, X.; Yang, D.; Zhong, R.; Qian, Y.; Li, Y.; Wang, H. A Simple One-Pot Method to Prepare UV-Absorbent Lignin/Silica Hybrids Based on Alkali Lignin from Pulping Black Liquor and Sodium Metasilicate. Chem. Eng. J. 2017, 326, 803–810. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Fu, S.; Chen, Y. High-Value Utilization of Kraft Lignin: Color Reduction and Evaluation as Sunscreen Ingredient. Int. J. Biol. Macromol. 2019, 133, 86–92. [Google Scholar] [CrossRef]


| Samples | Composition | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| SLP25 | PBAT, 0.25% SLNP | 10.6 ± 0.6 | 394 ± 35.4 |
| SLP50 | PBAT, 0.5% SLNP | 11.6 ± 0.7 | 404 ± 42.2 |
| SLP75 | PBAT, 0.75% SLNP | 9.5 ± 0.3 | 44 ± 28.4 |
| WLP25 | PBAT, 0.25% WLNP | 10 ± 0.5 | 220 ± 37.6 |
| WLP50 | PBAT, 0.5% WLNP | 10.7 ± 0.4 | 398 ± 39.8 |
| WLP75 | PBAT, 0.75% WLNP | 6.5 ± 0.3 | 16 ± 9.2 |
| PBAT | PBAT | 8.8 ± 0.6 | 299 ± 23.8 |
| Samples Composition | TS | TS Increment | Elongation at Break | Elongation at Break Increment | References |
|---|---|---|---|---|---|
| PBAT, 2% LZM | Increased from 33.2 to 37.6 MPa. | +13% | Decreased from 1100 to ~1048% | −4.73% | [12] |
| PBAT, 0.5% LNP | Increased from 22.6 ± 1.2 to 24.6 ± 0.6 MPa | +9% | Increased from 1450 ± 80 to 1520 ± 20% | +4.82% | [27] |
| PBAT, 30% acylated lignin | Decreased from 29.8 ± 2.2 to 20.9 ± 1.5 MPa | −29.9% | Decreased from 879 ± 48 to 692 ± 67% | −21.3% | [10] |
| PBAT, 3% MP, and 60% lignin | Decreased from 23.7 ± 1.5 to 16.7 ± 0.55 MPa | −29.5% | Decreased sharply from 816.49 ± 52.95 to 49.26 ± 5.34% | −93.5% | [9] |
| PBAT, 0.5% SLNP | Increased from 8.8 ± 0.6 to 11.6 ± 0.7 MPa | +31.8% | Increased from 299 ± 23.8% to 404 ± 42.2% | +35.1% | In this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; He, Y.; Wu, J.; Ye, H.; You, T.; Xu, F. Sodium-Alginate-Doped Lignin Nanoparticles for PBAT Composite Films to Dually Enhance Tensile Strength and Elongation Performance with Functionality. Polymers 2024, 16, 2312. https://doi.org/10.3390/polym16162312
Guo Q, He Y, Wu J, Ye H, You T, Xu F. Sodium-Alginate-Doped Lignin Nanoparticles for PBAT Composite Films to Dually Enhance Tensile Strength and Elongation Performance with Functionality. Polymers. 2024; 16(16):2312. https://doi.org/10.3390/polym16162312
Chicago/Turabian StyleGuo, Qiyue, Yuan He, Jianyu Wu, Haichuan Ye, Tingting You, and Feng Xu. 2024. "Sodium-Alginate-Doped Lignin Nanoparticles for PBAT Composite Films to Dually Enhance Tensile Strength and Elongation Performance with Functionality" Polymers 16, no. 16: 2312. https://doi.org/10.3390/polym16162312
APA StyleGuo, Q., He, Y., Wu, J., Ye, H., You, T., & Xu, F. (2024). Sodium-Alginate-Doped Lignin Nanoparticles for PBAT Composite Films to Dually Enhance Tensile Strength and Elongation Performance with Functionality. Polymers, 16(16), 2312. https://doi.org/10.3390/polym16162312








