Research Progress on Lignin Depolymerization Strategies: A Review
Abstract
:1. Introduction
2. Lignocellulosic Structure
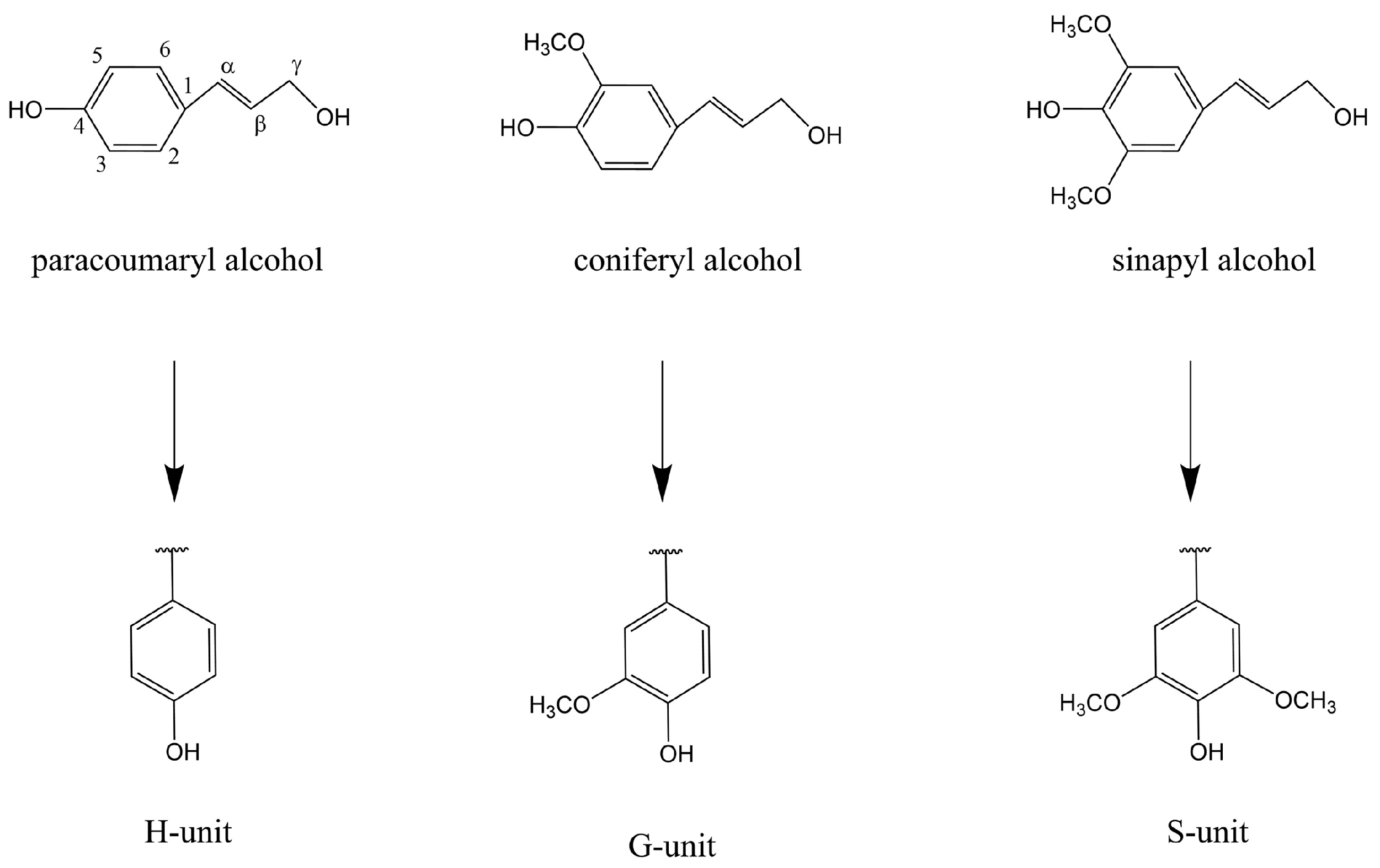
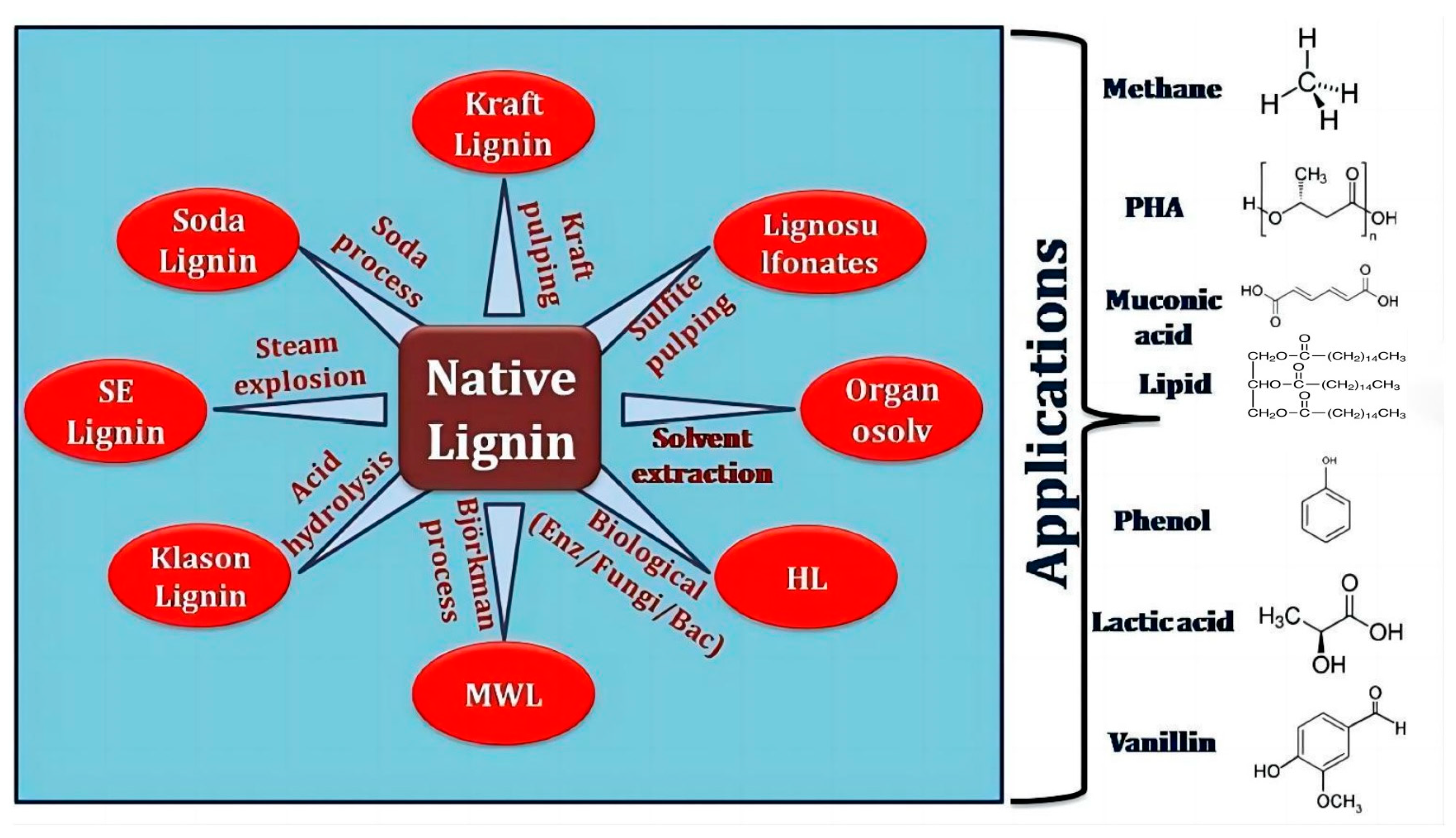
Lignin Structure, Properties, and Classification
3. Thermochemical Depolymerization of Lignin
3.1. Pyrolysis
3.2. Microwave-Assisted Lignin Depolymerization
4. Chemocatalytic Depolymerization of Lignin
4.1. Acid or Base Catalysts
4.2. Metal Catalysts
4.3. Ionic Liquids
4.4. Deep Eutectic Solvents
5. Photocatalytic Lignin Depolymerization
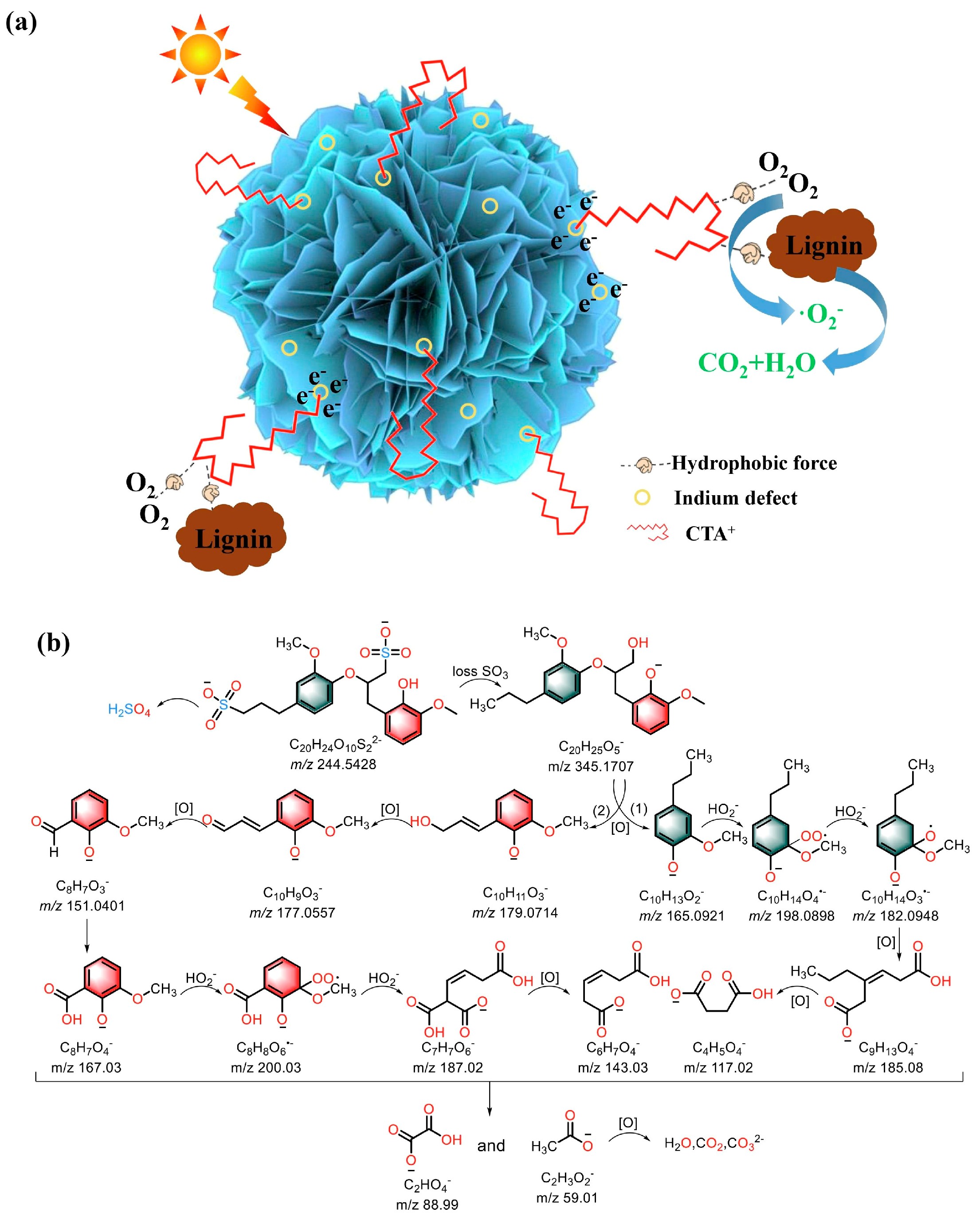
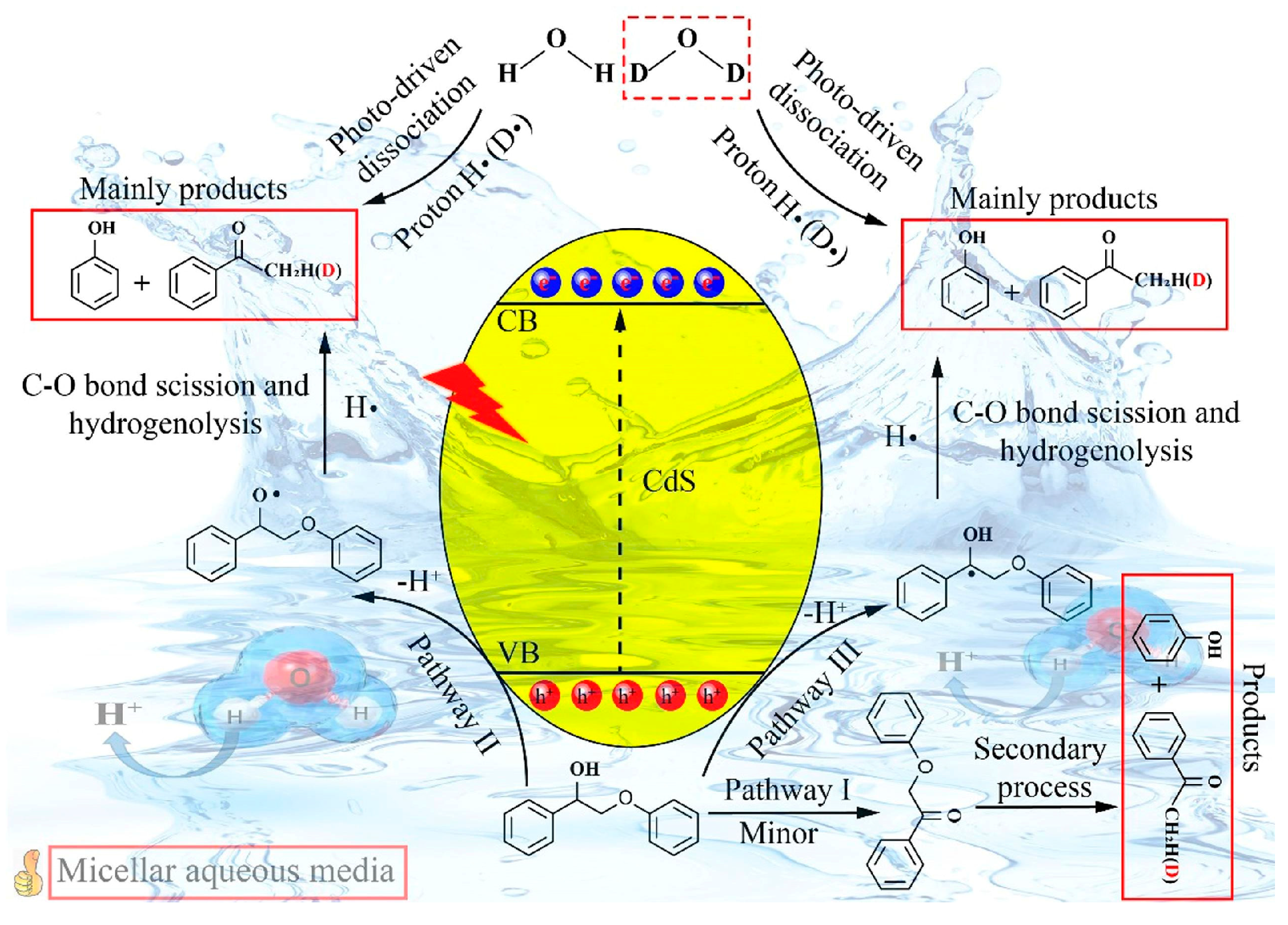
5.1. Sole Photocatalysis
5.2. Other Strategies of Assisted Photocatalytic Lignin Depolymerization
6. Electrocatalytic Lignin Depolymerization
6.1. Product Distribution of Lignin Electrochemical Depolymerization
6.2. Electrode Materials
6.3. Combined Strategy for Electrocatalytic Lignin Depolymerization
7. Biological Depolymerization of Lignin
7.1. Fungi
7.2. Bacteria
7.3. Enzymes
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, L.; Sun, Z.F.; Zhang, C.C.; Nan, J.; Ren, N.Q.; Lee, D.J.; Chen, C. Advances in Pretreatment of Lignocellulosic Biomass for Bioenergy Production: Challenges and Perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, J.; Huang, Z.; Liu, T.; Li, H. Photothermal Technique-Enabled Ambient Production of Microalgae Biodiesel: Mechanism and Life Cycle Assessment. Bioresour. Technol. 2023, 369, 128390. [Google Scholar] [CrossRef] [PubMed]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Tan, J.; Huang, J.; Yuan, J.; Chen, J.; Pei, Z.; Li, H.; Yang, S. Novel Supramolecular Deep Eutectic Solvent-Enabled in-Situ Lignin Protection for Full Valorization of All Components of Wheat Straw. Bioresour. Technol. 2023, 388, 129722. [Google Scholar] [CrossRef]
- Cao, Y.; He, M.; Dutta, S.; Luo, G.; Zhang, S.; Tsang, D.C.W. Hydrothermal Carbonization and Liquefaction for Sustainable Production of Hydrochar and Aromatics. Renew. Sustain. Energy Rev. 2021, 152, 111722. [Google Scholar] [CrossRef]
- Sun, R. Lignin Source and Structural Characterization. ChemSusChem 2020, 13, 4385–4393. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Mahanwar, P.A.; Gadekar, P.T. Lignin-Polyurethane Based Biodegradable Foam. Open J. Polym. Chem. 2018, 8, 1. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Chen, L.; Mustapha, A.T.; Okonkwo, C.E.; Zhou, C.; Liu, X. Comprehensive Depolymerization of Lignin from Lignocellulosic Biomass: A Review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1866–1887. [Google Scholar] [CrossRef]
- Menezes Fabricia, F.; Nascimento, V.M.; George, G.R.G.; Rocha, J.M.; Strauss, M.; Junqueira, T.L.; Driemeier, C. Depolymerization of Enzymatic Hydrolysis Lignin: Review of Technologies and Opportunities for Research. Fuel 2023, 342, 127796. [Google Scholar] [CrossRef]
- Leng, E.; Guo, Y.; Chen, J.; Liu, S.; Jiaqiang, E.; Xue, Y. A Comprehensive Review on Lignin Pyrolysis: Mechanism, Modeling and the Effects of Inherent Metals in Biomass. Fuel 2022, 309, 122102. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Zhang, H.; Xiao, R. Thermal Conversion of Lignin to Phenols: Relevance between Chemical Structure and Pyrolysis Behaviors. Fuel 2016, 182, 864–870. [Google Scholar] [CrossRef]
- Camas, K.L.; Ullah, A. Depolymerization of Lignin into High-Value Products. Biocatal. Agric. Biotechnol. 2022, 40, 102306. [Google Scholar]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Radhika, N.L.; Sachdeva, S.; Kumar, M. Lignin Depolymerization and Biotransformation to Industrially Important Chemicals/Biofuels. Fuel 2022, 312, 122935. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin Utilization: A Review of Lignin Depolymerization from Various Aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Wendisch, V.F.; Kim, Y.; Lee, J.-H. Chemicals from Lignin: Recent Depolymerization Techniques and Upgrading Extended Pathways. Curr. Opin. Green Sustain. Chem. 2018, 14, 33–39. [Google Scholar] [CrossRef]
- Datta, R.; Kelkar, A.; Baraniya, D.; Molaei, A.; Moulick, A.; Meena, R.S.; Formanek, P. Enzymatic Degradation of Lignin in Soil: A Review. Sustainability 2017, 9, 1163. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, D.; Singh, P.M.; Ragauskas, A.J. The New Forestry Biofuels Sector. Biofuels Bioprod. Bioref. 2008, 2, 58–73. [Google Scholar] [CrossRef]
- Tong, Z.; Meng, J.; Liu, S.; Liu, Y.; Zeng, S.; Wang, L.; Xia, Q.; Yu, H. Room Temperature Dissolving Cellulose with a Metal Salt Hydrate-Based Deep Eutectic Solvent. Carbohydr. Polym. 2021, 272, 118473. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from Lignin: An Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2010, 34, 29–41. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the Oxidative Valorization of Lignin to High-Value Chemicals: A Critical Review of Opportunities and Challenges. ChemSusChem 2022, 15, 1232. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Abdelaziz, O.Y.; Hulteberg, C.P.; Riisager, A. New Synthetic Approaches to Biofuels from Lignocellulosic Biomass. Curr. Opin. Green Sustain. Chem. 2020, 21, 16–21. [Google Scholar] [CrossRef]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Ind. Crops Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Brink, D.P.; Prothmann, J.; Ravi, K.; Sun, M.; García-Hidalgo, J.; Sandahl, M.; Hulteberg, C.P.; Turner, C.; Lidén, G.; et al. Biological Valorization of Low Molecular Weight Lignin. Biotechnol. Adv. 2016, 34, 1318–1346. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.M.; Rajendran, K.; Taherzadeh, M.J.; Horváth, I.S. Experimental and Economical Evaluation of Bioconversion of Forest Residues to Biogas Using Organosolv Pretreatment. Bioresour. Technol. 2015, 178, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. The Isolation, Characterization and Effect of Lignin Isolated from Steam Pretreated Douglas-Fir on the Enzymatic Hydrolysis of Cellulose. Bioresour. Technol. 2011, 102, 4507–4517. [Google Scholar] [CrossRef]
- Wright, M.M.; Daugaard, D.E.; Satrio, J.A.; Brown, A.C. Techno-Economic Analysis of Biomass Fast Pyrolysis to Transportation Fuels. Fuel 2010, 89, S2–S10. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stylianos, D.; Stefanidis, C.M.; Michailof, A.A. Lappas, and Elisabeth Sjöholm. Pyrolysis of Lignin with 2dgc Quantification of Lignin Oil: Effect of Lignin Type, Process Temperature and Zsm-5 In Situ Upgrading. J. Anal. Appl. Pyrolysis 2015, 115, 410–418. [Google Scholar] [CrossRef]
- Beneroso, D.; Monti, T.; Kostas, E.T.; Robinson, J. Microwave Pyrolysis of Biomass for Bio-Oil Production: Scalable Processing Concepts. Chem. Eng. J. 2017, 316, 481–498. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Wang, X.; Shao, J.; Chen, H. Recent Developments in Lignocellulosic Biomass Catalytic Fast Pyrolysis: Strategies for the Optimization of Bio-Oil Quality and Yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Zhu, G.; Qiu, X.; Zhao, Y.; Qian, Y.; Pang, Y.; Ouyang, X. Depolymerization of Lignin by Microwave-Assisted Methylation of Benzylic Alcohols. Bioresour. Technol. 2016, 218, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, P.; Liu, N.; Shen, D. Lignin Depolymerization to Aromatic Monomers and Oligomers in Isopropanol Assisted by Microwave Heating. Polym. Degrad. Stab. 2017, 135, 54–60. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the Lignocellulosic Biomass Pyrolysis for Biofuel Production toward Environmental Sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Monteil-Rivera, F.; Paquet, L. Solvent-Free Catalyst-Free Microwave-Assisted Acylation of Lignin. Ind. Crops Prod. 2015, 65, 446–453. [Google Scholar] [CrossRef]
- Ziwei, W.; Hao, S.; Yizhen, C.; Ben, L.; Yaowei, X.; Wanxia, W.; Kaiyue, W.; Mengheng, L.; Li, G.; Lei, W. Thermal, Photonic, and Electrocatalysis in Lignin Depolymerization Research. RSC Adv. 2023, 13, 32627–32640. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Liu, Y.; Ruan, X.; Tsang, D.C.W.; Hunt, A.J.; Ok, Y.S.; Song, H.; Zhang, S. Lignin Valorization for the Production of Renewable Chemicals: State-of-the-Art Review and Future Prospects. Bioresour. Technol. 2018, 269, 465–475. [Google Scholar] [CrossRef]
- Dhar, P.; Vinu, R. Understanding Lignin Depolymerization to Phenols Via Microwave-Assisted Solvolysis Process. J. Environ. Chem. Eng. 2017, 5, 4759–4768. [Google Scholar] [CrossRef]
- Lam, S.S.; Mahari, W.A.W.; Jusoh, A.; Chong, C.T.; Lee, C.L.; Chase, H.A. Pyrolysis Using Microwave Absorbents as Reaction Bed: An Improved Approach to Transform Used Frying Oil into Biofuel Product with Desirable Properties. J. Clean. Prod. 2017, 147, 263–272. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.; YuhYek, P.N.; Mahari, W.A.W.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q.; et al. Valorization of Biomass Waste to Engineered Activated Biochar by Microwave Pyrolysis: Progress, Challenges, and Future Directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Duan, D.; Wang, Y.; Dai, L.; Ruan, R.; Zhao, Y.; Fan, L.; Tayier, M.; Liu, Y. Ex-Situ Catalytic Co-Pyrolysis of Lignin and Polypropylene to Upgrade Bio-Oil Quality by Microwave Heating. Bioresour. Technol. 2017, 241, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Soria, J.; Gauthier, D.; Mazza, G.; Flamant, G. Modeling of Beech Wood Pellet Pyrolysis under Concentrated Solar Radiation. Renew. Energy 2016, 99, 721–729. [Google Scholar] [CrossRef]
- Muley, P.D.; Mobley, J.K.; Tong, X.; Novak, B.; Stevens, J.; Moldovan, D.; Shi, J.; Boldor, D. Rapid Microwave-Assisted Biomass Delignification and Lignin Depolymerization in Deep Eutectic Solvents. Energy Convers. Manag. 2019, 196, 1080–1088. [Google Scholar] [CrossRef]
- Zhu, G.; Jin, D.; Zhao, L.; Ouyang, X.; Chen, C.; Qiu, X. Microwave-Assisted Selective Cleavage of C C Bond for Lignin Depolymerization. Fuel Process. Technol. 2017, 161, 155–161. [Google Scholar] [CrossRef]
- Nastasiienko, N.; Kulik, T.; Palianytsia, B.; Larsson, M.; Kartel, M. Microwave-Assisted Catalytic Pyrolysis of Ferulic Acid, as a Lignin Model Compound. J. Therm. Anal. Calorim. 2023, 148, 5485–5492. [Google Scholar] [CrossRef]
- Wang, H.; Li, D.; Hong, M.; Dou, J. Syntheses, Structural Characterization and In vitro Cytotoxic Activity Of triorganotin(Iv) Complexes Based on 1,7-Dihydroxycarbonyl-1,7-Dicarba-Closo-Dodecaborane Ligand. J. Organomet. Chem. 2013, 740, 1–9. [Google Scholar] [CrossRef]
- Wang, H.; Tucker, M.P.; Ji, Y. Recent Development in Chemical Depolymerization of Lignin: A Review. J. Appl. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Huang, X.; Ouyang, X.; Hendriks, B.M.S.; Gonzalez, O.M.M.; Zhu, J.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Selective Production of Mono-Aromatics from Lignocellulose over Pd/C Catalyst: The Influence of Acid Co-Catalysts. Faraday Discuss. 2017, 202, 141–156. [Google Scholar] [CrossRef]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C. Hydrolytic Depolymerization of Hydrolysis Lignin: Effects of Catalysts and Solvents. Bioresour. Technol. 2015, 190, 416–419. [Google Scholar] [CrossRef]
- Forsythe, W.G.; Garrett, M.D.; Hardacre, C.; Nieuwenhuyzen, M.; Sheldrake, G.N. An Efficient and Flexible Synthesis of Model Lignin Oligomers. Green Chem. 2013, 15, 3031. [Google Scholar] [CrossRef]
- Deuss, P.J.; Scott, M.; Tran, F.; Westwood, N.J.; De Vries, J.G.; Barta, K. Aromatic Monomers by in Situ Conversion of Reactive Intermediates in the Acid-Catalyzed Depolymerization of Lignin. J. Am. Chem. Soc. 2015, 137, 7456–7467. [Google Scholar] [CrossRef]
- Jasiukaitytė, E.; Kunaver, M.; Crestini, C. Lignin Behaviour During Wood Liquefaction—Characterization by Quantitative 31p, 13c Nmr and Size-Exclusion Chromatography. Catal. Today 2010, 156, 23–30. [Google Scholar] [CrossRef]
- Forchheim, D.; Gasson, J.R.; Hornung, U.; Kruse, A.; Barth, T. Modeling the Lignin Degradation Kinetics in a Ethanol/Formic Acid Solvolysis Approach. Part 2. Validation and Transfer to Variable Conditions. Ind. Eng. Chem. Res. 2012, 51, 15053–15063. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Xu, J. Hydrogenolysis of Lignosulfonate into Phenols over Heterogeneous Nickel Catalysts. Chem. Commun. 2012, 48, 7019–7021. [Google Scholar] [CrossRef]
- Oregui-Bengoechea, M.; Gandarias, I.; Arias, P.L.; Barth, T. Solvent and Catalyst Effect in the Formic Acid Aided Lignin-to-Liquids. Bioresour. Technol. 2018, 270, 529–536. [Google Scholar] [CrossRef]
- Bjelić, A.; Grilc, M.; Huš, M.; Likozar, B. Hydrogenation and Hydrodeoxygenation of Aromatic Lignin Monomers over Cu/C, Ni/C, Pd/C, Pt/C, Rh/C and Ru/C Catalysts: Mechanisms, Reaction Micro-Kinetic Modelling and Quantitative Structure-Activity Relationships. Chem. Eng. J. 2019, 359, 305–320. [Google Scholar] [CrossRef]
- Barta, K.; Warner, G.R.; Beach, E.S.; Anastas, P.T. Depolymerization of Organosolv Lignin to Aromatic Compounds over Cu-Doped Porous Metal Oxides. Green Chem. 2014, 16, 191–196. [Google Scholar] [CrossRef]
- Zhang, J.; Asakura, H.; Van Rijn, J.; Yang, J.; Duchesne, P.; Zhang, B.; Chen, X.; Zhang, P.; Saeys, M.; Yan, N. Highly Efficient, Niau-Catalyzed Hydrogenolysis of Lignin into Phenolic Chemicals. Green Chem. 2014, 16, 2432–2437. [Google Scholar] [CrossRef]
- Rawat, S.; Kumar, A.; Bhaskar, T. Ionic Liquids for Separation of Lignin and Transformation into Value-Added Chemicals. Curr. Opin. Green Sustain. Chem. 2022, 34, 100582. [Google Scholar] [CrossRef]
- García, A.; Torres-González, L.C.; Padmasree, K.P.; Benavides-Garcia, M.G.; Sánchez, E.M. Conductivity and Viscosity Properties of Associated Ionic Liquids Phosphonium Orthoborates. J. Mol. Liq. 2013, 178, 57–62. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Li, D.; Jiang, J.; Li, K.; Zhang, K.; An, Q.; Zhai, S.; Wei, L. 1-Ethyl-3-Methylimidazolium Acetate Ionic Liquid as Simple and Efficient Catalytic System for the Oxidative Depolymerization of Alkali Lignin. Int. J. Biol. Macromol. 2021, 183, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Zakzeski, J.; Jongerius, A.L.; Weckhuysen, B.M. Transition Metal Catalyzed Oxidation of Alcell Lignin, Soda Lignin, and Lignin Model Compounds in Ionic Liquids. Green Chem. 2010, 12, 1225–1236. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. The Chemistry of Ammonium-Based Ionic Liquids in Depolymerization Process of Lignin. J. Mol. Liq. 2017, 248, 227–234. [Google Scholar] [CrossRef]
- Stärk, K.; Taccardi, N.; Bösmann, A.; Wasserscheid, P. Oxidative Depolymerization of Lignin in Ionic Liquids. ChemSusChem 2010, 3, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, H.; Meng, Q.; Zhang, Z.; Yang, G.; Han, B. Ionic Liquid [Omim][Oac] Directly Inducing Oxidation Cleavage of the Β-O-4 Bond of Lignin Model Compounds. Chem. Commun. 2017, 53, 8850–8853. [Google Scholar] [CrossRef]
- Chen, Z.; Ragauskas, A.; Wan, C. Lignin Extraction and Upgrading Using Deep Eutectic Solvents. Ind. Crops Prod. 2020, 147, 112241. [Google Scholar] [CrossRef]
- Tan, J.; Yu, D.; Yuan, J.; Wu, H.; Luo, H.; Zhang, H.; Li, X.; Li, H.; Yang, S. Efficient Delignification of Wheat Straw for Microbial Lipid Production Enabled by a Novel Ternary Deep Eutectic Solvent Containing Ethylene Glycol. Fuel 2023, 347, 128485. [Google Scholar] [CrossRef]
- Di Marino, D.; Aniko, V.; Stocco, A.; Kriescher, S.; Wessling, M. Emulsion Electro-Oxidation of Kraft Lignin. Green Chem. 2017, 19, 4778–4784. [Google Scholar] [CrossRef]
- Di Marino, D.; Stöckmann, D.; Kriescher, S.; Stiefel, S.; Wessling, M. Electrochemical Depolymerisation of Lignin in a Deep Eutectic Solvent. Green Chem. 2016, 18, 6021–6028. [Google Scholar] [CrossRef]
- Xiang, Z.; Han, W.; Deng, J.; Zhu, W.; Zhang, Y.; Wang, H. Photocatalytic Conversion of Lignin into Chemicals and Fuels. ChemSusChem 2020, 13, 4199–4213. [Google Scholar] [CrossRef]
- He, G.; Hu, J.; Sun, C.; Zhao, Y.; Yan, H. Advances in Photocatalytic Lignin Depolymerization: Photocatalytic Materials and Mechanisms. J. Wood Chem. Technol. 2024, 44, 65–87. [Google Scholar] [CrossRef]
- Granone, L.I.; Sieland, F.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Photocatalytic Conversion of Biomass into Valuable Products: A Meaningful Approach? Green Chem. 2018, 20, 1169–1192. [Google Scholar] [CrossRef]
- Chen, H.; Wan, K.; Zheng, F.; Zhang, Z.; Zhang, Y.; Long, D. Mechanism Insight into Photocatalytic Conversion of Lignin for Valuable Chemicals and Fuels Production: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2021, 147, 111217. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding Tio2photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Xiao, X.; Han, Y.; Liu, C.; Li, Y.; Sun, G.; Wang, X. Visible-Light-Activated Tio2 Photocatalysis Regionally Modified by Sio2 for Lignin Depolymerization. Mater. Today Energy 2022, 30, 101190. [Google Scholar] [CrossRef]
- Xu, J.; Li, M.; Yang, L.; Qiu, J.; Chen, Q.; Zhang, X.; Feng, Y.; Yao, J. Synergy of Ni Dopant and Oxygen Vacancies in Zno for Efficient Photocatalytic Depolymerization of Sodium Lignosulfonate. Chem. Eng. J. 2020, 394, 125050. [Google Scholar] [CrossRef]
- Dhar, P.; Teja, V.; Vinu, R. Sonophotocatalytic Degradation of Lignin: Production of Valuable Chemicals and Kinetic Analysis. J. Environ. Chem. Eng. 2020, 8, 104286. [Google Scholar] [CrossRef]
- Chen, R.; Huang, Y.; Rao, C.; Su, H.; Pang, Y.; Lou, H.; Yang, D.; Qiu, X. Enhanced Photocatalytic Degradation of Lignin by In2s3 with Hydrophobic Surface and Metal Defects. Appl. Surf. Sci. 2022, 600, 154110. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Lu, J.; Zeng, S.; Wang, M.; Luo, N.; Xu, S.; Wang, F. Photocatalytic Cleavage of C–C Bond in Lignin Models under Visible Light on Mesoporous Graphitic Carbon Nitride through π–π Stacking Interaction. ACS Catal. 2018, 8, 4761–4771. [Google Scholar] [CrossRef]
- Ku, C.; Guo, H.; Li, K.; Wu, Q.; Yan, L. One-Step Fabrication of Mesoporous Sulfur-Doped Carbon Nitride for Highly Selective Photocatalytic Transformation of Native Lignin to Monophenolic Compounds. Chin. Chem. Lett. 2023, 34, 107298. [Google Scholar] [CrossRef]
- Luo, H.; Liu, X.; Yu, D.; Yuan, J.; Tan, J.; Li, H. Research Progress on Lignocellulosic Biomass Degradation Catalyzed by Enzymatic Nanomaterials. Chem. Asian J. 2022, 17, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lin, F.; Wang, J.; Wang, Y. Photocatalyst Cds for Efficient Cleavage of Lignin C O Bonds in Micellar Aqueous Medium. Chem. Phys. Lett. 2022, 805, 139981. [Google Scholar] [CrossRef]
- Dai, D.; Qiu, J.; Xia, G.; Zhang, L.; Ma, H.; Yang, L.; Yao, J. Interspersing Cds Nanodots into Iodine Vacancy-Rich Bioi Sphere for Photocatalytic Lignin Valorization. Int. J. Biol. Macromol. 2023, 227, 1317–1324. [Google Scholar] [CrossRef]
- Cui, M.; Liang, C.; Zhao, W.; Liu, X.; Dong, L.; Wang, D.; Fu, S.; Jiang, Z.; Wang, F.; Wei, X. Fabrication of Z-Scheme Cds/H5PMo10V2O40/G-C3N4 for the Photocatalytic Depolymerization of Lignin into Aromatic Monomers. Fuel Process. Technol. 2022, 238, 107481. [Google Scholar] [CrossRef]
- Choi, Y.; Mehrotra, R.; Lee, S.-H.; Nguyen, T.V.T.; Lee, I.; Kim, J.; Yang, H.-Y.; Oh, H.; Kim, H.; Lee, J.-W.; et al. Bias-Free Solar Hydrogen Production at 19.8 Ma Cm−2 Using Perovskite Photocathode and Lignocellulosic Biomass. Nat. Commun. 2022, 13, 5709. [Google Scholar] [CrossRef]
- Butti, S.K.; Velvizhi, G.; Sulonen, M.L.K.; Haavisto, J.M.; Koroglu, E.O.; Cetinkaya, A.Y.; Singh, S.; Arya, D.; Modestra, J.A.; Krishna, K.V.; et al. Microbial Electrochemical Technologies with the Perspective of Harnessing Bioenergy: Maneuvering Towards Upscaling. Renew. Sustain. Energy Rev. 2016, 53, 462–476. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Park, J.-H. Advancement in Technologies for the Depolymerization of Lignin. Fuel Process. Technol. 2018, 181, 115–132. [Google Scholar] [CrossRef]
- Du, X.; Zhang, H.; Sullivan, K.P.; Gogoi, P.; Deng, Y. Electrochemical Lignin Conversion. ChemSusChem 2020, 13, 4318–4343. [Google Scholar] [CrossRef]
- Chen, A.; Wen, Y.; Han, X.; Qi, J.; Liu, Z.-H.; Zhang, S.; Li, G. Electrochemical Decomposition of Wheat Straw Lignin into Guaiacyl-, Syringyl-, and Phenol-Type Compounds Using Pb/PbO2 Anode and Alloyed Steel Cathode in Alkaline Solution. Environ. Prog. Sustain. Energy 2019, 38, 13117. [Google Scholar] [CrossRef]
- Du, X.; Liu, W.; Zhang, Z.; Mulyadi, A.; Brittain, A.; Gong, J.; Deng, Y. Low-Energy Catalytic Electrolysis for Simultaneous Hydrogen Evolution and Lignin Depolymerization. ChemSusChem 2017, 10, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Zirbes, M.; Waldvogel, S.R. Electro-Conversion as Sustainable Method for the Fine Chemical Production from the Biopolymer Lignin. Curr. Opin. Green Sustain. Chem. 2018, 14, 19–25. [Google Scholar] [CrossRef]
- Garedew, M.; Lin, F.; Song, B.; DeWinter, T.M.; Jackson, J.E.; Saffron, C.M.; Lam, C.H.; Anastas, P.T. Greener Routes to Biomass Waste Valorization: Lignin Transformation through Electrocatalysis for Renewable Chemicals and Fuels Production. ChemSusChem 2020, 13, 4214–4237. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Tian, M.; Jiang, Z.-H.; Kjartanson, B.; Chen, A. Electrochemical Oxidation of Lignin at Lead Dioxide Nanoparticles Photoelectrodeposited on TiO2 Nanotube Arrays. Electrochim. Acta 2012, 60, 147–153. [Google Scholar] [CrossRef]
- Hao, X.; Quansheng, Y.; Dan, S.; Honghui, Y.; Jidong, L.; Jiangtao, F.; Wei, Y. Fabrication and Characterization of PbO2 Electrode Modified with [Fe(CN)6]3− and Its Application on Electrochemical Degradation of Alkali Lignin. J. Hazard. Mater. 2015, 286, 509–516. [Google Scholar] [CrossRef]
- Lan, C.; Fan, H.; Shang, Y.; Shen, D.; Li, G. Electrochemically Catalyzed Conversion of Cornstalk Lignin to Aromatic Compounds: An Integrated Process of Anodic Oxidation of a Pb/Pbo2electrode and Hydrogenation of a Nickel Cathode in Sodium Hydroxide Solution. Sustain. Energy Fuels 2020, 4, 1828–1836. [Google Scholar] [CrossRef]
- Zirbes, M.; Schmitt, D.; Beiser, N.; Pitton, D.; Hoffmann, T.; Waldvogel, S.R. Anodic Degradation of Lignin at Active Transition Metal-Based Alloys and Performance-Enhanced Anodes. ChemElectroChem 2018, 6, 155–161. [Google Scholar] [CrossRef]
- Ayub, R.; Raheel, A. High-Value Chemicals from Electrocatalytic Depolymerization of Lignin: Challenges and Opportunities. Int. J. Mol. Sci. 2022, 23, 3767. [Google Scholar] [CrossRef]
- Reichert, E.; Wintringer, R.; Volmer, D.A.; Hempelmann, R. Volmer, and Rolf Hempelmann. Electro-Catalytic Oxidative Cleavage of Lignin in a Protic Ionic Liquid. Phys. Chem. Chem. Phys. 2012, 14, 5214–5221. [Google Scholar] [CrossRef]
- Wang, J.; Cao, F.; Su, E.; Zhao, L.; Qin, W. Improvement of Animal Feed Additives of Ginkgo Leaves through Solid-State Fermentation Using Aspergillus Niger. Int. J. Biol. Sci. 2018, 14, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, A.K.; Naas, A.; Kracun, S.K.; Schückel, J.; Fangel, J.U.; Agger, J.W.; Willats, W.G.T.; Eijsink, V.G.H.; Pope, P.B.; Drake, H.L. A Polysaccharide Utilization Locus from an Uncultured Bacteroidetes Phylotype Suggests Ecological Adaptation and Substrate Versatility. Appl. Environ. Microbiol. 2015, 81, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Sharma-Shivappa, R.R.; Chinn, M.; Howell, N. Effect of Microbial Pretreatment on Enzymatic Hydrolysis and Fermentation of Cotton Stalks for Ethanol Production. Biomass Bioenergy 2009, 33, 88–96. [Google Scholar] [CrossRef]
- Zhu, Y.; Ouyang, X.; Zhao, Y.; Jiang, L.; Guo, H.; Qiu, X. Oxidative Depolymerization of Lignin Improved by Enzymolysis Pretreatment with Laccase. J. Energy Chem. 2018, 27, 801–805. [Google Scholar] [CrossRef]
- Civzele, A.; Stipniece-Jekimova, A.A.; Mezule, L. Fungal Ligninolytic Enzymes and Their Application in Biomass Lignin Pretreatment. J. Fungi 2023, 9, 780. [Google Scholar] [CrossRef]
- Sainsbury, P.D.; Mineyeva, Y.; Mycroft, Z.; Bugg, T.D.H. Chemical Intervention in Bacterial Lignin Degradation Pathways: Development of Selective Inhibitors for Intradiol and Extradiol Catechol Dioxygenases. Bioorganic Chem. 2015, 60, 102–109. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P. Production of Biofuels and Biochemicals by in Vitro Synthetic Biosystems: Opportunities and Challenges. Biotechnol. Adv. 2015, 33, 1467–1483. [Google Scholar] [CrossRef]
- Bassanini, I.; Ferrandi, E.E.; Riva, S.; Monti, D. Biocatalysis with Laccases: An Updated Overview. Catalysts 2021, 11, 26. [Google Scholar] [CrossRef]
- van Bloois, E.; Pazmiño, D.E.T.; Winter, R.T.; Fraaije, M.W. A Robust and Extracellular Heme-Containing Peroxidase from Thermobifida Fusca as Prototype of a Bacterial Peroxidase Superfamily. Appl. Microbiol. Biotechnol. 2009, 86, 1419–1430. [Google Scholar] [CrossRef]



| Methods | Advantage | Disadvantage | References | |
|---|---|---|---|---|
| Thermochemical depolymerization | Pyrolysis Microwave-assisted lignin depolymerization | Simple and fast operation Efficient and easy to handle | Poor selectivity and many by-products High energy consumption | [30,31,43,45] |
| Chemical depolymerization | Corrosive equipment, environmental pollution | [47,49,51,55,56,62,64,67,68] | ||
| Acid or base catalysts | Efficient and fast | High cost | ||
| Metal catalysts | High selectivity | High cost and difficult product separation | ||
| Ionic liquids | Highly adjustable reaction | Difficulty in recovery | ||
| Deep eutectic solvents | Strong designability | Difficulty in solvent recovery and product separation | ||
| Photocatalytic depolymerization | Sole photocatalysis | Strong selectivity, low energy consumption, environmentally friendly | High catalyst cost and poor stability | [72,74,79,87] |
| Electrochemical depolymerization | Combined strategy for electrocatalytic lignin depolymerization | Strong selectivity, low energy consumption, environmentally friendly | High requirements for equipment, high cost, difficult industrialization | [88,89,92,95] |
| Biological depolymerization | Fungi Bacteria Enzymes | Environmentally friendly Environmentally friendly Environmental protection and directional depolymerization | Low efficiency Long cycle and high cost Long cycle, high cost, vulnerable to environmental impact | [101,102,106,107,108,109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Z.; Liu, X.; Chen, J.; Wang, H.; Li, H. Research Progress on Lignin Depolymerization Strategies: A Review. Polymers 2024, 16, 2388. https://doi.org/10.3390/polym16172388
Pei Z, Liu X, Chen J, Wang H, Li H. Research Progress on Lignin Depolymerization Strategies: A Review. Polymers. 2024; 16(17):2388. https://doi.org/10.3390/polym16172388
Chicago/Turabian StylePei, Zhengfei, Xiaofang Liu, Jiasheng Chen, Huan Wang, and Hu Li. 2024. "Research Progress on Lignin Depolymerization Strategies: A Review" Polymers 16, no. 17: 2388. https://doi.org/10.3390/polym16172388






